Abstract
A robust high-throughput screening (HTS) strategy has been developed to discover small-molecule effectors targeting the sarco/endoplasmic reticulum calcium ATPase (SERCA), based on a fluorescence microplate reader that records both the nanosecond decay waveform (lifetime mode) and the complete emission spectrum (spectral mode), with high precision and speed. This spectral unmixing plate reader (SUPR) was used to screen libraries of small molecules with a fluorescence resonance energy transfer (FRET) biosensor expressed in living cells. Ligand binding was detected by FRET associated with structural rearrangements of green (GFP, donor) and red (RFP, acceptor) fluorescent proteins fused to the cardiac-specific SERCA2a isoform. The results demonstrate accurate quantitation of FRET along with high precision of hit identification. Fluorescence lifetime analysis resolved SERCA’s distinct structural states, providing a method to classify small-molecule chemotypes on the basis of their structural effect on the target. The spectral analysis was also applied to flag interference by fluorescent compounds. FRET hits were further evaluated for functional effects on SERCA’s ATPase activity via both a coupled-enzyme assay and a FRET-based calcium sensor. Concentration-response curves indicated excellent correlation between FRET and function. These complementary spectral and lifetime FRET detection methods offer an attractive combination of precision, speed, and resolution for HTS.
Introduction
The preceding article reports the performance of a novel microplate-reader that records fluorescence emission spectra with an unprecedented combination of speed and precision. That study indicated that this spectral-unmixing plate-reader (SUPR), when combined with a previously described fluorescence lifetime plate-reader (FLTPR) that achieves a similarly high level of performance using nanosecond time resolution, is ready for HTS. This study demonstrates an initial application.
The specific target in this work is sarco/endoplasmic reticulum calcium ATPase (SERCA)1,2. which has therapeutic relevance for a wide range of diseases, including heart failure2, multidrug-resistant leukemia3, and type II diabetes4. SERCA is a critical enzyme, as it maintains calcium homoeostasis by actively pumping calcium from the cytosol into the endoplasmic or sarcoplasmic reticulum. Over a dozen human SERCA isoforms have been described, each with tissue-specific expression and distinct structural and functional characteristics. Specialized SERCA isoforms are predominantly found in electrically-excitable cells, such as myocytes and cardiomyocytes, where calcium-cycling is necessary for the contractile apparatus to function properly. 5
Recently, SERCA-based therapy based on calcium up-regulation by percutaneous administration of gene therapy was tested in cardiac disease clinical trials (CUPID study). SERCA overexpression by administration of adeno-associated virus (AAV), delivered directly to the hearts of patients experiencing end-stage heart failure, was shown to correct deficits in SERCA2a (cardiac-isoform) expression and activity, known to be correlated to impairment in cardiac (diastolic) function.6 Despite encouraging early results, SERCA AAV gene therapy failed to meet primary end goals in phase IIb clinical trials. This failure was attributed to the limitations of AAV gene therapy, including the development of neutralizing antibodies 7, and difficulties of maintaining constant, long-term expression of the large 110 kD enzyme. We continue to explore alternative SERCA-based gene therapy strategies8–10 but are also actively pursuing the search for small-molecule SERCA effectors capable of ameliorating the SERCA malfunction found in numerous degenerative diseases. 5
Initial screening campaigns evaluated structural perturbations using a reconstituted membrane system and fluorescence resonance energy transfer (FRET) detection between SERCA and its regulatory partner phospholamban (PLB). In these studies, conventional fluorescence emission spectral recording was utilized for large-scale screening and resulted in the discovery of small-molecule activators of SERCA.1 We continued our development of SERCA biosensors by engineering a genetically-encoded intramolecular FRET sensor; donor and acceptor fluorescent proteins were fused to specific locations on SERCA’s cytoplasmic headpiece, known to undergo large-scale, physiologically-relevant, structural changes (5–10 nm), as depicted by the known crystal structures.11,2 This type of assay lends itself naturally to a structure-based screening campaign, in which the results are not only related to variation of compound structure, but also to variation of specific structural changes in the labeled target.
This two-color SERCA (2CS) biosensor utilizes green and red fluorescent proteins as a FRET pair. These red-shifted fluorescent proteins are less sensitive to cell autofluorescence and fluorescent compound interference, compared with their blue-shifted CFP and YFP counterparts. The existence of multiple 2CS FRET populations was previously identified using single-molecule fluorescence lifetime microscopy 12. These studies elucidated SERCA’s sensitivity to PLB and calcium, revealing insights into residues involved in this structure-activity relationship.13 This set the stage for a proof-of-principle structure-based small-molecule screen, using a prototype fluorescence lifetime (FLT) microplate reader.2,14 This seminal study proved that a genetically-encoded FRET sensor could be stably expressed in human embryonic kidney (HEK293) cells and utilized for HTS in a microplate format.
The present work utilizes a novel top-read fluorescence lifetime plate reader, which involves an epi-illumination geometry, thereby allowing for temperature control and the use of inexpensive black-bottom 384- or 1536-well plates. The preceding article established the technical feasibility of decomposing the fluorescence emission spectra into a linear combination of component spectra, from which the FRET efficiency (FRET) can be calculated. That study demonstrated high screening quality (high Z′ value) and the ability to resolve minute FRET changes (0.5%), even when the cellular autofluorescence was artificially increased. This study involves screening a small-molecule library (National Clinical Collections 1 & 2), which contains a collection of compounds that have already been evaluated in pre-clinical and clinical trials. The ability to accurately determine changes in FRET from the 2CS biosensor from two independent fluorescence measurements (spectral and lifetime) increases the confidence of hit selection.
Materials and Methods
Cell Culture
HEK293 (originally derived from human embryonic kidney) cells were maintained in phenol red-free DMEM from Gibco (Waltham, MA) supplemented with 2 mM GlutaMAX (Gibco), 10% fetal bovine serum (FBS), from Atlanta Biologicals (Lawrenceville, GA), and 1 IU/mL penicillin/streptomycin (Gibco) and grown at 37° C with 5% CO2. HEK293 cell lines were used to generate stable clones overexpressing the FRET-based biosensors and corresponding donor and acceptor labeled control cell lines.2 Three days prior to screening, the stable cell lines were expanded in five T225 flasks from Corning Inc. (Corning, NY). On each day of screening and FRET hit retesting, approximately 300 million cells were harvested by treatment of Tryple from Invitrogen (Carlsbad, CA), washed three times in phosphate buffer solution (PBS) with no magnesium or calcium from Thermo Scientific (Waltham, MA) by centrifugation at 300 g, filtered using 70 μm cell strainers (Corning), and diluted to 106 cells/mL using an automated countess cell counter (Invitrogen). On each day of screening, cell viability was assessed using the trypan blue assay.
After resuspension and dilution in PBS, the cells were constantly and gently stirred using a magnetic stir bar at room temperature, keeping the cells in suspension and evenly distributed to avoid clumping. During screening, cells were then dispensed into five 384 well assay plates, one containing no compound, one containing eight-point concentration curves of three known SERCA effectors, and three containing the NCC libraries 1 and 2. The same methods were applied for subsequent FRET testing of the reproducible hits identified in the pilot screen. Concentration-response curves (CRC) of the FRET hits were assessed at multiple time points by repeatedly scanning the 384-well plates. HEK293 stable clones expressing either the D1ER calcium FRET sensor (CFP/YFP) or the 2CS biosensor was used to evaluate the hits. The D1ER calcium sensor monitored changes in endoplasmic reticulum [Ca2+].15,16 The methods and protocols for the D1ER cells were identical to those for 2CS, with the exception that they were evaluated only in spectral mode and the 384-well plates containing compound CRC were scanned every three minutes for two-hours.
Liquid dispensing
Cells were dispensed using a Multidrop Combi liquid dispenser from Thermo (Pittsburg, PA), at a density of 106 cells/mL. Compounds were diluted in DMSO and dispensed either using an automated Echo acoustic liquid dispenser from Labcyte (Sunnyvale, CA) or a Mosquito liquid dispenser from TTP Labtech (Melbourn, UK).
Cells and compound mixtures were dispensed into 384-well flat, black-bottom polypropylene plates from Greiner (Kremsmünste, Austria). The cells were dispensed at room temperature into plates containing test compounds. They were incubated with compound for 20, 60, 90, and 120 minutes and then scanned in both lifetime and spectral modes. 727 compounds from the NCC 1 and 2 compound libraries were purchased from Evotec (Hamburg, Germany), formatted into 96-well mother plates using a Biomek FX liquid dispenser from Beckman Coulter (Brea, CA), and subsequently formatted across three 384-well plates at 50 nL (10 μM final concentration per well) using an Echo liquid dispenser from Labcyte. Control wells containing matching %v/v DMSO were formatted into unused wells and columns 1, 2, 23, and 24 of the assay plates. The eleven reproducible FRET hits were purchased from three different vendors Sequoia Sciences (Saint Louis, MO), Tocris (Minneapolis, MN), or Santa Cruz (Santa Cruz, CA) depending on their availability.
Instrumentation and Data Analysis
An in-depth description of the fluorescence instrumentation is described in the previous article (Schaaf et al. 2016a) and in the supplemental material (Figure). For lifetime mode, the observed fluorescence waveform was convolved with the instrument response function, and the average energy transfer efficiency (E = 1− τDA/τD) was calculated from the average lifetimes of donor τD and donor-acceptor, τDA, FRET cell lines. The structural correlates for FRET were modeled as previously described 17–19 assessing the nanosecond time dependence of the TR-FRET waveforms according to (Eq. 1)–(Eq. 6):
| (Eq. 1) |
| (Eq. 2) |
| (Eq. 3) |
| (Eq. 4) |
| (Eq. 5) |
| (Eq. 6) |
where FD is the time-resolved fluorescence decay function of the GFP donor (Eq. 1), best-fit by a two exponential decay (Figure S6). FDA (Eq. 2) is the time-resolved fluorescence decay function of the GFP donor-acceptor FRET sample. FD and FDA were fit to a linear combination of mole fractions of xD and xDA and xD equals zero for the intramolecular FRET sensor (Eq. 3). FDA is a linear combination with molar fraction Xj of two FRET-affected fluorescence decays Tj(t) (Eq. 4). ρj is the probability of each distance distribution, determined by least-squares minimization of the distance (nm) R associated with each donor-acceptor lifetime species τ1 (Eq. 5). (σj ) Gaussian interprobe distance distributions centered at Rj = 5.5 nm and 10.2 nm with distribution widths defined by the standard deviation and full-width half-maximum (Eq. 6). The Förster distance (R0 ) for the eGFP and tagRFP FRET pair is 5.8 nm. The parameters in this system of equations were optimized utilizing simultaneous least-squares minimization to waveforms from donor-only and donor-acceptor cell lines. The best-fit model was indicated by minimized χ2 and by evaluation of the parameter error surface as described in our previous publications 17–19.
For spectral detection, the observed fluorescence emission spectrum was fitted by least-squares minimization to a linear combination of component spectra:
| (Eq. 7) |
where D is donor, A is acceptor, C is cell autofluorescence, and W is water Raman, and a, b, c, d are the coefficients determined from the fit.
| (Eq. 8) |
For an intramolecular FRET sensor, having both donor D and acceptor A, FRET was determined from (Eq. 8), where QR is the ratio of quantum yields (QD/QA) in the absence of FRET, AR is the ratio of molar absorptivities (εA/εD), both obtained from reported values.20 QR is corrected for spectrograph sensitivity at the appropriate wavelength (Figure S5). The only experimental observable in (Eq. 8) was FR.
| (Eq. 9) |
Full derivation of (Eqs. 7–. 9)- can be found in the supplementary material.
HTS Data Analysis
Fluorescent compounds were identified and flagged as potential false-positives by evaluating the similarity index (SI) between an observed compound spectrum and DMSO control spectra :
| (Eq. 10) |
The spectra of 192 DMSO control wells (%v/v DMSO) were averaged for each screen to generate a single control spectrum (I(b)). The fluorescence spectra of the two-color SERCA biosensor, screened against 727 separate compounds (I(a1–727)), was compared with the single DMSO control spectrum. The similarity index between the spectra was computed over the GFP emission wavelength (i = 500–540nm). The fluorescent compound threshold was set to flag potential false-positives with an SI greater than 2×10−4 throughout the screens.
Enzymatic SERCA activity assays of FRET hits
Functional assays were performed using rabbit light skeletal sarcoplasmic (SR) vesicles 2. An enzyme-coupled, NADH-linked ATPase assay was used to measure SERCA ATPase activity in 96-well microplates. Each well contained 50 mM MOPS (pH 7.0), 100 mM KCl, 5 mM MgCl2, 1 mM EGTA, 0.2 mM NADH, 1 mM phosphoenol pyruvate, 10 IU/mL of pyruvate kinase, 10 IU/mL of lactate dehydrogenase, 1 μM of the calcium ionophore A23187 from Sigma (St. Louis, MO), and CaCl2 added to set free [Ca2+] to 10 μM.21 4 μg/mL of SR vesicle, calcium, compound, and assay mix were incubated for 20 min. The assay was started upon the addition of ATP, at a final concentration of 5 mM (total volume to 200 μL), and absorbance read in a SpectraMax Plus microplate spectrophotometer from Molecular Devices (Sunnyvale, CA).
Results
High-precision FRET efficiency determinations from two independent fluorescence measurements
The sarco/endoplasmic reticulum calcium ATPase (SERCA) cycles through multiple conformations as it pumps calcium into the sarcoplasmic reticulum. Briefly, a green fluorescent protein (GFP) was fused to the N-terminus of SERCA and a red fluorescent protein (RFP) was fused to a flexible intrasequence loop located on the nucleotide-binding domain of SERCA. The distance between these two fluorescent proteins can be measured by determining the rate of fluorescence resonance energy transfer (FRET). This two-color SERCA (2CS) biosensor was stably expressed in a human embryonic kidney (HEK293) cell line and grown in sufficient quantities for high-throughput screening.2 The binding of potential small-molecule effectors is directly evaluated as changes in FRET (Figure 1B).
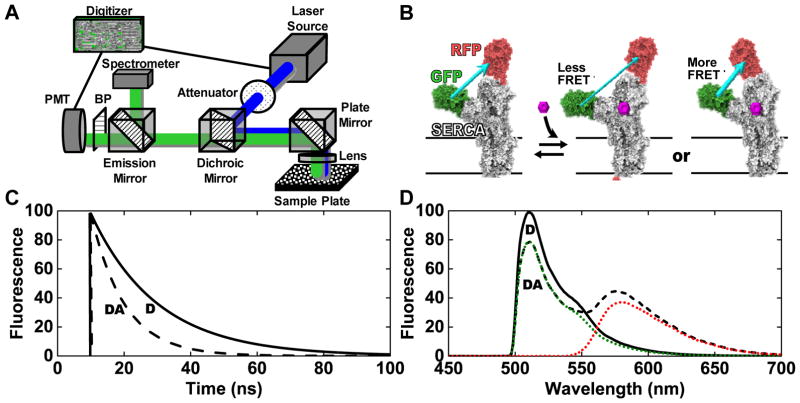
Figure 1.
(A) Diagram of the instrument. (B) The two-color SERCA (2CS) intramolecular FRET biosensor.2,12,22 As depicted, the FRET efficiency is dependent on the structural status of SERCA’s domains, which may be affected by the binding of small molecules. Conceptual data (C, lifetime mode) and (D, spectral mode) illustrate the dependence of fluorescence signals on FRET. Solid black curve (D): donor only (no FRET). Dashed black curve (DA): donor plus acceptor (FRET). In (D) dotted curves show the resolution of the spectrum into components corresponding to donor (GFP) and acceptor (RFP) emission.
Conceptual fluorescence lifetime waveforms are depicted in Figure 1C. In the actual lifetime measurements, typically 1000 laser pulses are averaged over a 200 ms interval, to generate an entire decay waveform for each well of a 384-well plate. The fluorescence lifetimes τDA (FRET biosensor with donor and acceptor) and τD (donor-only control) are used to determine the energy transfer efficiency FRET = 1 − τDA/τD. A representative complete fluorescence emission spectrum acquired with a 100 ms integration time at 0.5 nm spectral resolution in spectral mode is shown in Figure 1D. The high-quality emission spectrum, acquired from a single well was decomposed into a linear combination of its spectral components green fluorescent protein (GFP) and red fluorescent protein (RFP). These components were then used to solve for the contribution of the fluorescence emission from GFP (donor fit) and RFP (acceptor fit), allowing for a high-precision determination of an ensemble-averaged FRET from the 2CS biosensor.
Global lifetime analysis resolves structural status of 2CS biosensor
Global analysis of the fluorescence intensity decay rate (lifetime mode) was used to resolve two distinct structural states of the 2CS biosensor (Eqs. 1–6). These distinct structural states of 2CS were previously resolved using single-molecule fluorescence microscopy12, which is not a high-throughput detection method. Here, we demonstrate analogous structural resolution of the 2CS biosensor, except with an ensemble-averaged FRET measurement, acquired in 200 ms per well from live-cell suspensions.
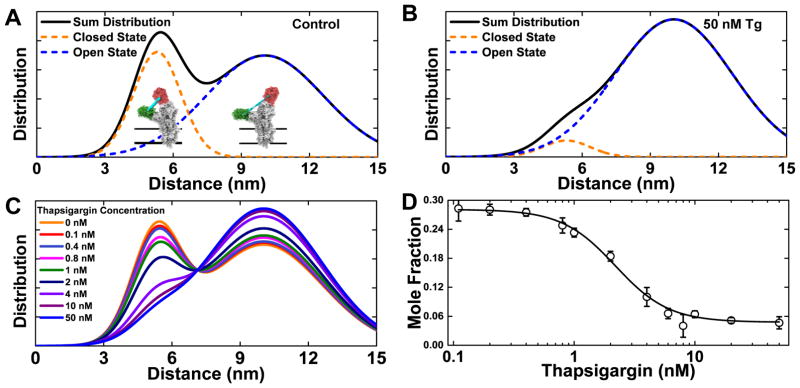
FRET is a sensitive spectroscopic molecular ruler, due to the R−6 distance dependence of the rate of energy transfer from an excited donor fluorophore (GFP) to an acceptor (RFP) in the ground state.23 The exceptionally good precision of direct waveform recording (DWR) and global analysis of the fluorescence decay waveforms produced in this fashion allow FRET measurements to be evaluated in terms of distance distributions and mole fractions of physiologically-relevant structural states. Fluorescence lifetime waveforms were analyzed using a global two-component model(Eqs. 1–6), yielding a two-state structural model for SERCA’s cytosolic headpiece, describing an equilibrium between the open (10.2 nm) and closed (5.5 nm) structural states with full-width at half-maximum (FWHM) of 6.9 and 2.2 nm; respectively. The Gaussian distance distribution was determined for each structural state and plotted as a histogram in Figure 2A. The distance R and FWHM fitting parameters were allowed to vary globally, and the mole fraction of each state was determined according to the two-component global fit (Eqs. 1–6). Thapsigargin inhibits SERCA at nanomolar concentration, and perturbs SERCA2a’s cytosolic headpiece, greatly increasing the population of the more open and disordered structural state at 50 nM (Figure 2B). The full concentration dependence is illustrated in (Figure 2C), and the concentration-response curve of the closed state mole fraction (Figure 2D) yields an EC50 value of 2.2 nM thapsigargin in agreement with the known EC50 for SERCA inhibition.24
Figure 2.
Lifetime analysis of time-resolved fluorescence decay waveforms resolves structural states of the 2CS biosensor. (A) Two structural states of SERCA are resolved, corresponding to two Gaussian interprobe distance distributions, consistent with a two-state structural model with an equilibrium between closed (5.5 nm FRET distance, orange) and open (10.2 nm FRET distance, blue) structural states. (B) The addition of a saturating dose (50 nM) of the known inhibitor thapsigargin (Tg) shifts this equilibrium substantially toward the open state. (C) Concentration dependence of Tg effect on the structural distribution (n= 8 wells for each concentration). (D) Plot, based on (C), of closed state mole fraction vs Tg.
Pilot screening of NCC libraries to evaluate both spectral and lifetime FRET detection
A small-molecule library (National Clinical Collections 1&2), consisting of 727 compounds previously evaluated in preclinical and clinical trials, was used to evaluate both spectral and lifetime modes of the spectral unmixing plate reader (SUPR). After an initial quality control check of the 2CS cell line on each day of screening (response to known effectors and signal level), a HEK stable clone overexpressing the 2CS biosensor was dispensed, using a Multidrop liquid dispenser into 384-well plates, and then scanned in both spectral and lifetime modes after 20, 60, 90, and 120 minutes of incubation with the compounds or control wells.
A single-exponential fit (Eq. 1) was used to determine the lifetime τDA from 2CS and τD from the one-color SERCA2a donor-only control cell line. These lifetimes were used to determine FRET = 1− τDA/τD. In spectral mode, the observed spectrum acquired from each well was decomposed into a linear combination of components (GFP, RFP, cellular autofluorescence, and water Raman). The reference spectrum of each was used to solve for the total contribution of fluorescence emission from each component (Eq. 7). These values were used to calculate a fitted ratio of the total fluorescence emission of RFP/GFP (Eq. 9) and then FRET from the 2CS biosensor was determined using the simplified FRET equation for intramolecular FRET sensors as described in the preceding article (Eq. 8).
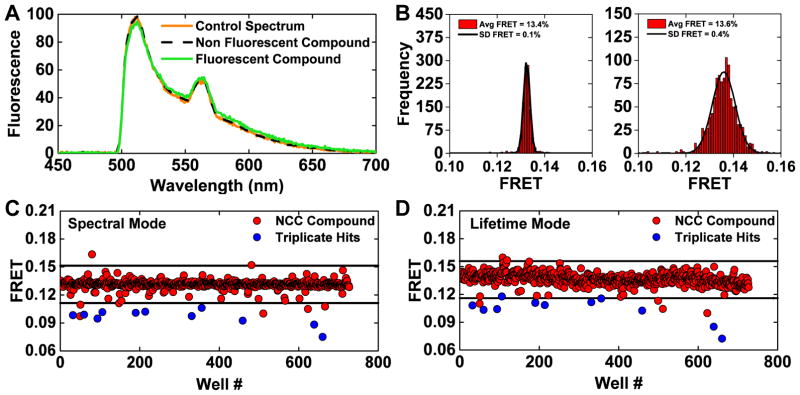
Both lifetime and spectral fluorescence measurements are prone to interference from fluorescent compounds. We took advantage of the information contained in the full emission spectrum to develop a streamlined process to flag these potential false-positives. A spectral similarity index (Eq. 10), which monitors differences from the spectra of control wells with no compound added, was computed in the donor only region. A stringent similarity index threshold (2×10−4) was used to flag 44 compounds as potential false-positives due to interference from compound fluorescence (Figure 3A).
Figure 3.
(A) Fluorescence emission spectra were used to identify and flag potential interference from fluorescent compounds by assessing the similarity index (Eq. 1) of each well from a pilot screen of the NCC1 & 2 small-molecule libraries. A control spectrum (%v/v DMSO well) and non-fluorescent (compound not identified as a FRET hit during screening with 2CS) have a high degree of similarity, as shown as direct overlap of orange and black spectrum. A slightly fluorescent compound is depicted by the green spectrum and was flagged as a potential false-positive hit. The fluorescent profile of all 1152 wells from one NCC screen was assessed using a stringent similarity index threshold; 44 compounds were flagged as potential false-positives due to interference from compound fluorescence. (B) Histogram plots of the wells from one NCC screen after removing potential fluorescent compounds. Gaussian fits depict an increase in precision from spectral mode (left) in comparison to lifetime mode (right), shown as the frequency of FRET efficiency determined by either method and a narrower distribution from spectral mode (average FRET calculated by spectral unmixing or lifetime and the standard deviation determined from the Gaussian fit). (C) One 2CS pilot NCC screen (spectral mode) is shown with a hit threshold set at a 0.02 change in 2CS FRET (4 SD). 16 FRET hits were identified in this screen. 11 of these 2CS FRET hits were found to be reproducible across three independent screens (blue). (D) The same 384-well plates were scanned in lifetime mode. 16 hits were identified using the same threshold set at a 0.02 change in 2CS FRET efficiency (3 SD). In this screen, nine of the reproducible 2CS FRET hits identified in spectral mode were also FRET hits as assessed by lifetime mode (blue).
Histogram plots from all wells that passed the fluorescence compound filter, from a single NCC screen demonstrate a three-fold increase in precision from the spectral mode in comparison to lifetime mode, as exemplified by a narrower distribution (Figure 3B). These two complementary determinations of FRET were used to identify hits, quickly rule out false-positives, and increase reproducibility across screens.
From three independent screens of the NCC libraries at a time point of 20 min after compound incubation, eleven reproducible FRET hits were found. These hits were identified using the spectral unmixing method and a hit threshold set at a 0.02 change in FRET. The results from one 2CS NCC screen are depicted in Figure 3C and D. Based on triplicate screens, 11 out of 16 compounds were identified as reproducible FRET hits (shown as blue circles in (Figure 3C). In lifetime mode, 9 of the 11 reproducible FRET hits found using spectral mode were also reproducible lifetime hits (Figure 3D).
Reproducibility of hit identification across independent screens and time course studies
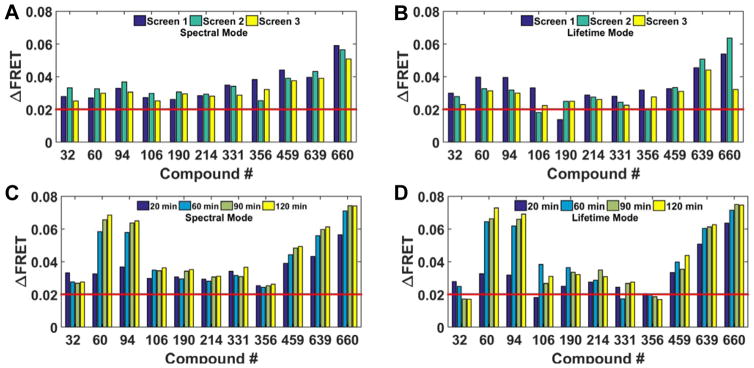
The reproducibility of 2CS FRET hits identified across three independent screens in spectral mode, after 20 min compound incubation at 10 μM concentration, is depicted in Figure 4A. The change in 2CS FRET (Δ FRET) of each hit from three independent NCC screens remained nearly constant from one screen to the next. For purposes of directly evaluating the use of the spectral recording method, a hit threshold of a 0.02 negative change in FRET (red bar) was applied and will be used from here on. When these same hits were evaluated in lifetime mode, nine of the eleven spectral FRET hits were found to be reproducible hits (Figure 4B). Compounds #106 and190 were not identified as lifetime FRET hits in one of the three independent screens.
Figure 4.
Reproducible FRET hits assessed across independent screens and time course studies. (A) Spectral mode identified eleven reproducible 2CS FRET hits using a threshold of 0.02 change in FRET (red line). The reproducibility of each 2CS FRET hit, after 20 min incubation, across three independent screens is shown. The ΔFRET from each compound remains consistent from screen to screen. ΔFRET was calculated by assessing the change in FRET of 2CS from the average FRET, determined by the Gaussian fit of all wells not flagged as fluorescence compounds. (B) Lifetime mode assessment of the eleven reproducible spectral FRET hits. Nine of the eleven FRET hits were reproducible (triplicate) hits using a 0.02 FRET threshold. Compounds #106 and190 were not identified as lifetime FRET hits in one of the three independent screens. (C) Spectral FRET hits evaluated by time-course studies. Each independent pilot screen was scanned in spectral mode after 20, 60, 90, and 120 minutes of compound incubation. The change in 2CS FRET efficiency (ΔFRET) of each compound is plotted and each reproducible FRET hit, identified as a hit using the spectral unmixing method, remained a hit over multiple time points. The 2CS FRET hits depicted here were from screen 2 (turquoise bars in A and B). Compounds #60, 94, 459, 639, and 660 exhibited an increased FRET change over time. (D) Lifetime FRET change evaluated by time-course studies. Ten compounds from screen 2 were identified as hits, after 20 minutes of compound incubation, using a threshold set at 0.02 FRET change (red bar). Compounds #32 and 356 displayed a modest reduction in 2CS ΔFRET at the later time points. Compounds # 60, 94, 459, 639, and 660 again exhibited an increased FRET change over time.
The ability to acquire lifetime and spectral measurements with scan times under three minutes for an entire 384-well microplate, allowed for the examination of 2CS FRET changes in response to the full NCC library of compounds at multiple compound incubation time points. Time-course screening may not be directly amenable to large-scale screening but is potentially highly applicable for assessing the reproducibility of a large number of FRET hits identified during a large-scale HTS campaign, at multiple concentrations. Time-dependent compound effects may also elucidate compounds with low binding affinities or delayed effects from low membrane permeability. These types of studies may also be useful for other fluorescence bioassays solely based on monitoring time-dependent effects, as we will depict later using the cameleon calcium FRET sensor. For these 2CS pilot screening studies, time-dependent screening was used to determine the inter-screen reproducibility of FRET hit identification as assessed both spectral and lifetime modes.
Time-dependent scans of reproducible 2CS FRET hits were consistent over multiple time points (20, 60, 90, and 120 min after compound incubation). The 2CS FRET hits analyzed here were from screen 2 (turquoise bars in Figure 4A and B) and show excellent reproducibility across time points. Five compounds # 60, 359, 459, and 639 exhibited an increased FRET change over time (Figure 4C). Reproducible hits were evaluated in lifetime mode (Figure 4D) Very subtle differences in the 2CS FRET changes were found across spectral and lifetime methods. Ten compounds from screen 2 were identified as hits, after 20 minutes of compound incubation. Compounds #32 and 356 displayed a modest reduction in 2CS ΔFRET at the later time points. Compounds #60, 94, 459, 639, and 660 again exhibited an increased FRET change over time. Overall, both methods show excellent agreement in terms of the direction and magnitude of FRET change.
Multi-parameter concentration-dependent effects of FRET hits
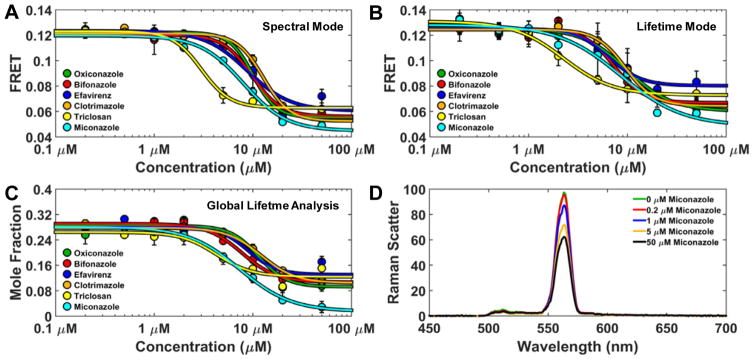
The reproducible 2CS FRET hits were further evaluated as a function of concentration. Compounds were dispensed into 384-well plates, across an eight-point concentration-gradient (n=4 for each concentration). Three independent dose-dependent FRET tests were performed on the 2CS FRET hits. Six compounds that produced the largest reproducible FRET change after 120 minute compound incubation are depicted. These compounds dose-dependently decreased 2CS FRET as determined by spectral mode (Figure 5A). Each FRET curve was fit using the Hill equation. Decreased 2CS FRET was observed at micromolar concentrations with notable differences in the apparent EC50 (half maximal effective concentration) of the FRET curve. The same 384 well plate, containing the reproducible hits, was evaluated in lifetime mode with a simple, single exponential fit (Eq. 1–2) and demonstrated great agreement between the FRET change across the two modes of independent FRET measurements. (Figure 5B).
Figure 5.
Multi-parameter concentration-dependent effect of FRET hits (A) Spectral mode analysis of the reproducible 2CS FRET hits. Compounds were dispensed into 384 well plates, across an eight-point concentration-gradient (n=4 for each concentration). Six representative compounds produced a dose-dependent FRET change as evaluated in spectral mode. These compounds altered FRET with micromolar affinities with subtle differences across the compounds. (B) The same 384-well plate was evaluated using lifetime mode and demonstrated excellent agreement in the dose-dependent FRET change across two independent FRET measurements. (C) Global analysis of the lifetime data depicts a dose-dependent change in the mole fraction of the closed 2CS headpiece (5.5 nm distance distribution). Using this distance distribution model, the confirmed reproducible hits perturbed the 2CS structural equilibrium between open and closed states. All of the hits decreased 2CS FRET, indicating increased distance between GFP and RFP. (D) Water Raman spectrum acquired from compound-only wells of the known compound aggregator miconazole demonstrates ultra-high-sensitivity of spectral recording. Compound aggregation dose-dependently causes more light to be absorbed and decreases inelastic light scattering (Raman band).
The compounds found to be reproducible hits and that exhibited dose-dependent FRET changes all decreased 2CS FRET. The imidazole antifungal clotrimazole was a hit and has been previously shown to inhibit SERCA function.25 The related compounds oxiconazole, bifonazole, and miconazole were also hits. The antibacterial triclosan has been shown to increase cytosolic calcium26 but to our knowledge not through interaction with SERCA.
Global analysis of the lifetime data (Eqs. 1–6)resolved a dose-dependent change in the mole fractions of the open and closed structural states. Using this distance distribution model, the reproducible hits perturbed the 2CS structural equilibrium between open and closed states in Figure 5C (5.5 nm distance distribution shown). The high sensitivity of spectral mode is shown by the fluorescence signal of the water Raman spectrum. Raman scattering was acquired from compound-only wells of the known aggregator miconazole.27 Compound aggregation dose-dependently causes more light to be absorbed and decreases inelastic light scattering (Raman band) (Figure 5D). This information may become useful for flagging potential false-positives due to compound aggregation.
Functional characterization of FRET hits on SERCA ATPase activity and ER calcium content
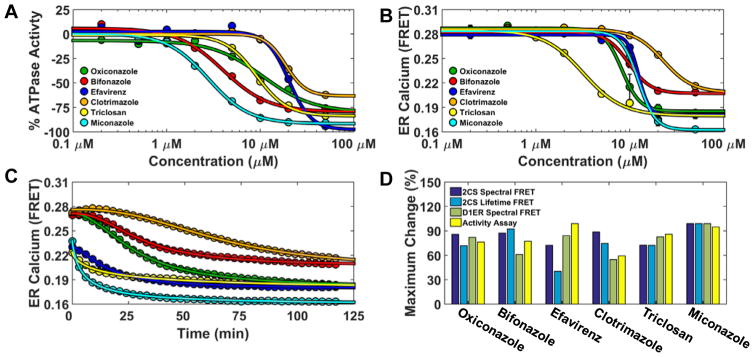
Functional assays of the confirmed reproducible NCC hits were used to assess the relationship of hits that perturb 2CS structure and their effects on SERCA function. The ATPase activity of purified SERCA was measured after 20 minute incubation with a saturating dose of buffered free calcium (10 μM) and titration of each compound. Experiments were performed in triplicate with eight-point concentration curves. The top six hits were found to dose-dependently inhibit SERCA’s ATPase function (Figure 6A). The antifungal Miconazole shows almost complete inhibition (92.4%) with a Ki of 2.8 μM. Clotrimazole’s ability to inhibit SERCA’s ATPase was slightly reduced in comparison with a Ki of 17.3 μM which is in agreement with previous steady-state measurements (7–35 μM).25
Figure 6.
Functional characterization of FRET hits on SERCA ATPase activity and ER calcium content. (A) 2CS FRET hits inhibit SERCA ATPase activity. NADH-enzyme coupled activity assay of purified SERCA was measured at eight different concentrations of the reproducible FRET hits. The maximal rate of SERCA activity was measured at saturating calcium (10 μM) after 20 minute incubation with compounds and dose-dependent inhibition was observed. (B) Endoplasmic reticulum calcium was depleted by the 2CS FRET hits. ER calcium was monitored in live-cells overexpressing the endoplasmic reticulum localized calcium FRET sensor (D1ER). D1ER FRET is dependent on calcium concentration, where less calcium causes a reduction in FRET. ER calcium levels were monitored over time in response to an eight-point concentration gradient of each hit compound. A 384 well plate was repeatedly scanned (every three minutes) with D1ER cells. The dose-dependent FRET change (ER calcium depletion) after 120 minutes compound incubation is shown and depicts differential depletion at each compound concentration. (C) Maximal ER calcium depletion in the presence of a saturating dose (50 μM) of each compound (decreased D1ER FRET) was assessed over a 120 minute period. The 2CS FRET hits displayed time-dependent and compound-specific ER calcium depletion. Miconazole (turquoise) exhibited both maximal SERCA ATPase Vmax inhibition and the largest amount of ER depletion. (D) Structure and activity assay correlation of 2CS FRET hits. The maximal change (percent change) of the structural FRET change from the 2CS FRET biosensor as well as the maximal change from two different functional assay (ATPase activity assay and D1ER calcium depletion). are shown.
SERCA malfunction can result in decreased ATPase activity and/or calcium pumping efficiency. ER calcium content was monitored over time using the endoplasmic-localized cameleon calcium FRET sensor (D1ER).15 As demonstrated in the preceding article, known SERCA inhibitors deplete ER calcium in a time and dose-dependent manner and can be monitored using live-cells expressing D1ER. Briefly, D1ER FRET changes were monitored over time by repeatedly (every 3 minutes) scanning a 384-well plate containing varying concentrations of the 2CS FRET hits. D1ER cells were assessed immediately after compound incubation. These plates were scanned only in spectral mode using 434 nm excitation with a laser-driven light-source, to acquire a full emission spectrum from each well. The appropriate CFP/YFP reference spectra were used to determine FRET using (Eq. 7).
D1ER FRET curves were determined for each time-point scan, after compound incubation, over a period of 120 minutes (40 scans total). The 2CS FRET hits displayed time-dependent and compound-specific ER calcium depletion. Miconazole (turquoise) exhibited both maximal SERCA ATPase Vmax inhibition and the largest amount of ER calcium depletion as depicted at the final 120 min time point in Figure 6B. Maximal ER calcium depletion in the presence of a saturating dose (50 μM) of each compound (decreased D1ER FRET) was assessed over a 120 minute period (Figure 6C). The 2CS FRET hits displayed time-dependent and compound-specific ER calcium depletion. The KI and EC50’s from the ATPase activity and D1ER FRET curves showed good agreement at the 20 minute time points. The structure-activity relationship of the 2CS FRET hits was further analyzed by comparing 2CS FRET (both spectral and lifetime mode), ATPase activity, and ER calcium depletion (Figure 6D). The maximal change (shown as percent change) of the structural FRET change from the 2CS FRET biosensor had excellent agreement with the maximal change from two different functional assays (ATPase activity assay and ER calcium depletion). Structural perturbation of the 2CS FRET biosensor directly relates to a compound’s affect on SERCA function.
Discussion
This study illustrates the complementary combination of spectral and lifetime fluorescence detection for the purposes of HTS. The spectral unmixing method increases the precision of hit identification and reproducibility of the hits in concentration-response curves (Figure 4). The fluorescence lifetime detection mode offers excellent precision and offers the additional advantage of structural resolution, revealed by multi-exponential global lifetime fitting. This approach resolves multiple FRET populations and assesses them in terms of distance-distributions and mole fractions, assigned to structurally-relevant perturbations of SERCA effectors (Figure 2). This resolution of multiple FRET-detected structural states from a live-cell biosensor is highly advantageous for screening, offering the potential to elucidate chemotypes or classes of compounds, identified in large-scale screens, which differentially alter the structural status of a biosensor. This high-content information can be used to generate structure-activity relationships based on binding-affinities, structural dynamics, and disorder.
The capability to couple two independent measurements of FRET, thereby substantially decreasing the false-positive rate, would be of significant value to the high-throughput screening community. Spectral recording does not offer the resolution of structural information, in terms of resolving multiple structural states, but can be used to identify fluorescent compounds, eliminate artifacts due to dispenser error or contaminated samples, and increase assay precision across screens (Figure 3).
This is the first microplate reader capable of direct waveform recording in both lifetime and spectral domains. A recent review of fluorescence lifetime imaging (FLIM) plate readers demonstrates the medium-throughput capabilities currently offered by other technologies (20 min scan times per 96 well plate).28 The approach described here is considerably faster, yet offers very high precision.
Beyond developing new fluorescence technology, the overarching goal of this research is to identify novel small-molecule SERCA effectors with therapeutic potential for multiple disease states. These studies employed a 2CS biosensor based on the SERCA2a isoform2, which is the primary isoform expressed in the heart. We have engineered constructs based on the other human isoforms, with the intent of performing drug-discovery campaigns to identify isoform-specific SERCA effectors. Further, we are currently developing new synthetic analogues based on our previously identified SERCA activators and inhibitors, where our HTS approach allows us to quickly assess and triage the most prominent candidates from a large pool of synthetically-derived analogues. We concluded this assay-based demonstration by investigating hits identified through structural-based screening, using an NADH-enzyme coupled ATPase activity assay and the D1ER endoplasmic reticulum-targeted calcium sensor to evaluate the correlation between the structure and function of hits identified in this pilot screening campaign (Figure 6).
The compounds identified during these pilot screens all decreased FRET from the 2CS biosensor, corresponding to opening of SERCA’s cytoplasmic headpiece. This may be a consequence of their similarity in the mode of binding or mechanism of inhibition. However, the 2CS biosensor is not limited to detection of decreases in FRET. In broken cells, we have previously shown that ligands such as calcium increase FRET, due to closure of SERCA’s cytoplasmic headpiece.22 It is plausible that the maximal FRET effect is essentially reached for the HEK293 live-cells, in which calcium and ATP maintain SERCA in its closed structural state.
The novel paradigm used in the present study enables the measurement of multiple FRET parameters. These high-content assays are ideally suited for high-throughput screening campaigns, with potential to discover novel allosteric effectors, which may differentially perturb FRET. This strategy is now being evaluated for use on homogenate and microsomal cellular preparations using FRET-based biosensors. These applications allow for fine control of environment (calcium, nucleotide, pH, etc.). Preliminary results have demonstrated that these purified preparations of FRET-based biosensors are suitable for counter screens and also in-depth structural evaluations of novel SERCA effectors.
The high-resolution FRET approach, coupled to functional assays, is applicable to a wide range of protein targets, including the ryanodine receptor29, myosin18,30, phospholamban10, multiple-drug resistance receptor31, and the tumor necrosis receptor32. The ability to quickly and reliably assess structural perturbations from biosensors in relation to physiologically-relevant functional changes holds high promise for the development of allosteric effectors and potentially valuable lead compounds.
Supplementary Material
Acknowledgments
Jesse E. McCaffrey, Bengt Svensson, Razvan L. Cornea, and J. Michael Autry provided helpful discussions, and Octavian Cornea prepared the manuscript for publication. Simon J. Gruber and Seth L. Robia developed many of the reagents and materials used. Fluorescence microscopy was performed at the UMN Imaging Center, flow cytometry at the UMN Lillehei Heart Institute, compound dispensing at the UMN Institute of Therapeutic Drug Discovery and Development, and spectroscopy was performed at the UMN Biophysical Technology Center. pcDNA-D1ER was a gift from Amy Palmer & Roger Tsien (Addgene plasmid # 36325).
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH grants R42DA037622 (to G.D.G and D.D.T.), R01GM27906 (to D.D.T.), and R01HL129814 (to D.D.T.). T.M.S. was supported by the NIH Chemistry-Biology Interface Training Grant (5T32GM008700), and by predoctoral fellowships from 3M and Arnold H. Johnson.
Footnotes
Declaration of Conflicting Interests
Dr. Thomas holds equity in and serves as an executive officer for Photonic Pharma LLC. This relationship has been reviewed and managed by the University of Minnesota.
References
- 1.Cornea RL, Gruber SJ, Lockamy EL, et al. High-throughput FRET assay yields allosteric SERCA activators. J Biomol Screen. 2013;18:97–107. doi: 10.1177/1087057112456878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gruber SJ, Cornea RL, Li J, et al. Discovery of enzyme modulators via high-throughput time-resolved FRET in living cells. J Biomol Screen. 2014;19:215–222. doi: 10.1177/1087057113510740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bleeker NP, Cornea RL, Thomas DD, et al. A Novel SERCA Inhibitor Demonstrates Synergy with Classic SERCA Inhibitors and Targets Multidrug-Resistant AML. Mol Pharm. 2013;10(11):4358–66. doi: 10.1021/mp400458u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang S, Dahl R, Hsieh W, et al. Small Molecular Allosteric Activator of the Sarco/Endoplasmic Reticulum Ca2+-ATPase (SERCA) Attenuates Diabetes and Metabolic Disorders. J Biol Chem. 2015;291(10):5185–98. doi: 10.1074/jbc.M115.705012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brini M, Carafoli E. Calcium pumps in health and disease. Physiol Rev. 2009;89:1341–1378. doi: 10.1152/physrev.00032.2008. [DOI] [PubMed] [Google Scholar]
- 6.Zsebo K, Yaroshinsky A, Rudy JJ, et al. Long-term effects of AAV1/SERCA2a gene transfer in patients with severe heart failure: analysis of recurrent cardiovascular events and mortality. Circ Res. 2014;114:101–108. doi: 10.1161/CIRCRESAHA.113.302421. [DOI] [PubMed] [Google Scholar]
- 7.Greenberg B, Butler J, Felker GM, et al. Prevalence of AAV1 neutralizing antibodies and consequences for a clinical trial of gene transfer for advanced heart failure. Gene Ther. 2015;23(3):313–9. doi: 10.1038/gt.2015.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lockamy EL, Cornea RL, Karim CB, et al. Functional and physical competition between phospholamban and its mutants provides insight into the molecular mechanism of gene therapy for heart failure. Biochem Biophys Res Commun. 2011;408(3):388–92. doi: 10.1016/j.bbrc.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ha KN, Traaseth NJ, Verardi R, et al. Controlling the Inhibition of the Sarcoplasmic Ca2+-ATPase by Tuning Phospholamban Structural Dynamics. J Biol Chem. 2007;282:37205–37214. doi: 10.1074/jbc.M704056200. [DOI] [PubMed] [Google Scholar]
- 10.Gruber SJ, Haydon S, Thomas DD. Phospholamban mutants compete with wild type for SERCA binding in living cells. Biochem Biophys Res Commun. 2012;420:236–240. doi: 10.1016/j.bbrc.2012.02.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hou Z, Hu Z, Blackwell DJ, et al. 2-Color calcium pump reveals closure of the cytoplasmic headpiece with calcium binding. PLoS One. 2012;7:e40369. doi: 10.1371/journal.pone.0040369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pallikkuth S, Blackwell DJ, Hu Z, et al. Phosphorylated phospholamban stabilizes a compact conformation of the cardiac calcium-ATPase. Biophys J. 2013;105:1812–1821. doi: 10.1016/j.bpj.2013.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smolin N, Robia SL. A structural mechanism for calcium transporter headpiece closure. J Phys Chem B. 2015;119:1407–1415. doi: 10.1021/jp511433v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen KJ, Peterson KC, Muretta JM, et al. Fluorescence lifetime plate reader: resolution and precision meet high-throughput. Rev Sci Instrum. 2014;85:113101. doi: 10.1063/1.4900727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palmer AE, Jin C, Reed JC, et al. Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc Natl Acad Sci U S A. 2004;101:17404–17409. doi: 10.1073/pnas.0408030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmer AE, Tsien RY. Measuring calcium signaling using genetically targetable fluorescent indicators. Nat Protoc. 2006;1:1057–1065. doi: 10.1038/nprot.2006.172. [DOI] [PubMed] [Google Scholar]
- 17.Muretta JM, Jun Y, Gross SP, et al. The structural kinetics of switch-1 and the neck linker explain the functions of kinesin-1 and Eg5. Proc Natl Acad Sci U S A. 2015;112:E6606–6613. doi: 10.1073/pnas.1512305112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muretta JM, Rohde JA, Johnsrud DO, et al. Direct real-time detection of the structural and biochemical events in the myosin power stroke. Proc Natl Acad Sci U S A. 2015;112:14272–14277. doi: 10.1073/pnas.1514859112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, James ZM, Dong X, et al. Structural and functional dynamics of an integral membrane protein complex modulated by lipid headgroup charge. J Mol Biol. 2012;418:379–389. doi: 10.1016/j.jmb.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thorn TLaK: Fluorescent protein properties. Retrieved from http://nic.ucsf.edu/FPvisualization/
- 21.Mueller B, Karim CB, Negrashov IV, et al. Direct detection of phospholamban and sarcoplasmic reticulum Ca-ATPase interaction in membranes using fluorescence resonance energy transfer. Biochemistry. 2004;43:8754–8765. doi: 10.1021/bi049732k. [DOI] [PubMed] [Google Scholar]
- 22.Hou Z, Hu S, Blackwell DJ, et al. Two-color calcium pump reveals closure of the cytoplasmic headpiece with calcium binding. PLoS ONE. 2012 doi: 10.1371/journal.pone.0040369. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lakowicz J. Principles of Fluorescence Spectroscopy. 3. 2006. [Google Scholar]
- 24.Rogers TB, Inesi G, Wade R, et al. Use of thapsigargin to study Ca2+ homeostasis in cardiac cells. Biosci Rep. 1995;15:341–349. doi: 10.1007/BF01788366. [DOI] [PubMed] [Google Scholar]
- 25.Bartolommei G, Tadini-Buoninsegni F, Hua S, et al. Clotrimazole inhibits the Ca2+-ATPase (SERCA) by interfering with Ca2+ binding and favoring the E2 conformation. J Biol Chem. 2006;281:9547–9551. doi: 10.1074/jbc.M510550200. [DOI] [PubMed] [Google Scholar]
- 26.Cherednichenko G, Zhang R, Bannister RA, et al. Triclosan impairs excitation-contraction coupling and Ca2+ dynamics in striated muscle. Proc Natl Acad Sci U S A. 2012;109:14158–14163. doi: 10.1073/pnas.1211314109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seidler J, McGovern SL, Doman TN, et al. Identification and prediction of promiscuous aggregating inhibitors among known drugs. Journal of medicinal chemistry. 2003;46:4477–4486. doi: 10.1021/jm030191r. [DOI] [PubMed] [Google Scholar]
- 28.Guzman C, Oetken-Lindholm C, Abankwa D. Automated High-Throughput Fluorescence Lifetime Imaging Microscopy to Detect Protein-Protein Interactions. J Lab Autom. 2016;21:238–245. doi: 10.1177/2211068215606048. [DOI] [PubMed] [Google Scholar]
- 29.Svensson B, Oda T, Nitu FR, et al. FRET-based trilateration of probes bound within functional ryanodine receptors. Biophys J. 2014;107:2037–2048. doi: 10.1016/j.bpj.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colson BA, Thompson AR, Espinoza-Fonseca LM, et al. Site-directed spectroscopy of cardiac myosin-binding protein C reveals effects of phosphorylation on protein structural dynamics. Proc Natl Acad Sci U S A. 2016;113:3233–3238. doi: 10.1073/pnas.1521281113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iram SH, Gruber SJ, Raguimova ON, et al. ATP-Binding Cassette Transporter Structure Changes Detected by Intramolecular Fluorescence Energy Transfer for High-Throughput Screening. Mol Pharmacol. 2015;88:84–94. doi: 10.1124/mol.114.096792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis AK, James ZM, McCaffrey JE, et al. Open and closed conformations of the isolated transmembrane domain of death receptor 5 support a new model of activation. Biophys J. 2014;106:L21–24. doi: 10.1016/j.bpj.2014.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.