Abstract
It is well known that estrogen deficiency induces a deterioration of bone strength in aged females. The aim of this study is to determine the effect of estrogen depletion on tibia bone strength in sexually mature mice that are still undergoing skeletal maturation. At 8 weeks of age, C57BL/6 female mice underwent an ovariectomy (OVX) or sham (SHAM) surgery. Mice were killed at 2, 4, or 8 weeks post-surgery. Tibia length and cross-sectional area continued to increase in both treatment groups until 4 weeks post-surgery. Compared to SHAM mice, OVX mice demonstrated a significant reduction in uterine weight and plasma estrogen levels. Three-point bending was used to quantify the mechanical properties (breaking point, stress, stiffness, and elasticity) of the tibia. The tibias from the SHAM mice had a higher breaking point than all the age-matched OVX mice. At 8 weeks post-surgery, the tibias from the SHAM mice demonstrated higher elasticity, stress, and stiffness than the younger SHAM mice and the age-matched OVX mice. Compared to the SHAM mice, our study suggests that (1) there is a reduction in the mechanical strength of tibias from young OVX mice, and (2) the greatest decline in tibia strength of the OVX mice was once they reached skeletal maturity.
Keywords: Elasticity, Stress, Stiffness, Bone, Three-point bending, Ovariectomy, Tibia
Introduction
Osteoporosis is characterized by reduced bone strength and is often seen in postmenopausal women and those who have age-related estrogen deficiencies. However, there are conditions when women experience depleted estrogen levels prior to menopause. For example, it is common for a woman to undergo a surgical ovariectomy (OVX) as a preventative measure against ovarian cancer or other ovarian pathologies [1]. Furthermore, individuals born with a deficiency of the cytochrome P450 gene for aromatase/estrogen synthase (CYP19 mutation), or estrogen insensitivity (estrogen receptor α gene mutation) lack the ability to produce adequate levels of estrogen or generate a normal in vivo response to estrogen, respectively [2, 3]. The objective of this study is to determine how the depletion of gonadal estrogen affects bone strength in young females. It is well known that estrogen stimulates and regulates pubertal bone growth, and thus is responsible for minimizing bone resorption and remodeling while promoting the thickening of the cortical long bone [4–6]. Therefore, females that have an estrogen deficiency or insensitivity (surgically induced or from a genetic disorder), demonstrate reduced bone mineral density, incomplete epiphyseal growth plate fusion, and an overall diminishment in skeletal integrity [4, 5].
In this study, we used the C57BL/6 J inbred mouse stain, which has been commonly used to evaluate the mechanical properties of the skeletal system [7–10]. These mice become sexually mature at approximately 6 weeks of age [11], and bone maturity is reached at 16 weeks of age [12–14]. Therefore, studies that evaluate adult bone strength and morphology utilize these mice at approximately 16 weeks of age [10, 15]. However, to our knowledge, no studies have evaluated the effect of circulating in vivo estrogen in sexually mature mice that are still undergoing skeletal maturation.
We used three-point bending to quantify the mechanical strength of the tibia [16]. The tibia is commonly used in three-point bending because of its relative cylindrical shape. With this technique, force can be applied to the bone until reaching its breaking point (i.e., maximal load), and the degree to which a bone will bend to resist facture (i.e., elastic modulus) can be calculated [16–18]. Additional properties used to measure the resistance to fracture of a compact bone are stiffness, stress, and strain [19]. This study improves upon previous studies [20], which typically quantify the mechanical strength of compact bone without regard to body size (i.e., body weight and tibial length). Body composition should be considered because the removal of gonadal estrogen production (i.e., ovariectomy) is typically associated with an increase in abdominal adipose deposition [21, 22]. An increase in adipose mass can induce strain on a bone, which will ultimately influence its mechanical strength [8].
In this study, we desired to determine the mechanical strength of the tibia in ovariectomized mice over an 8-week period until skeletal maturity is reached at 16 weeks of age. Using three-point bending, we determined the maximal load, elasticity, stress, and stiffness of each tibia. Also, the cortical bone area and marrow cavity area were determined. We hypothesized that the in vivo depletion of estrogen in young mice would reduce bone strength, as has been similarly shown in skeletally mature mice.
Materials and methods
Animals
Six-week-old C57BL/6 female mice were acquired from Envigo (Indianapolis, IN, USA) and housed in the animal facility at the University of Central Arkansas (Conway, AR, USA). The mice were maintained on a 12-h light/dark cycle, kept at room temperature, and given food (Tekland 8640 rodent diet; Envigo) and water ad libitum. Mice were acclimated for 1–2 weeks before surgery. When the mice were 8 weeks old, a standard dorsal ovariectomy (OVX) or sham (SHAM) surgery was conducted. For surgery, mice were anesthetized with inhaled isoflurane (1–4%) and weighed. The mice were killed at 2, 4, or 8 weeks post-surgery using CO2 asphyxiation. All procedures were approved by the Institutional Animal Care and Use Committee.
After euthanization, the body weight was recorded and the uteri dissected from the mice. The tibias were dissected from the lower limbs of the mice, cleaned of any soft tissue, and stored at 20 °C until experimentation. Blood was collected via cardiac puncture (tuberculin syringe, 26G) and placed in a microtube coated with heparin (Sarstedt AG & Co, Germany). The blood samples were centrifuged at 2000 × g for 10 min. After centrifugation, the plasma was removed and stored at −20 °C. The plasma was shipped on dry ice to the Reproductive Ligand Assay and Analysis Core at the University of Virginia Medical School (Charlottesville, VA, USA) for the analysis of plasma estrogen (17β-estradiol; Calbiotech mouse estradiol assay, CA, USA).
Three-point bending
The modified, three-point bending apparatus used in this study has recently been described [16]. Before experimentation, the bones were soaked in a phosphate buffer solution for 24 h. The tibia was placed in a holder that fixed the ends of the bone in place while a measured amount of force was applied perpendicular to the midpoint of the anterior side of the tibial diaphysis. Force was applied (at a rate of 0.498 mm · s−1) using a flat-tipped wedge that was connected to a motorized force transducer (World Precision Instruments, FL, USA), which was controlled by a Hayden Kerk IDEA Drive Interface program. Force was amplified by a transbridge amplifier (World Precision Instruments) and recorded by WinDaq data acquisition software (DATAQ Instruments, OH, USA).
Structural properties
The tibial length was measured using a digimatic digital caliper (Mitutoyo, IL, USA). The midpoint of the tibia was sectioned using an IsoMet1000 Precision Saw (Buehler, IL, USA) equipped with an IsoMet 15HC diamond wafering blade. Each bone section was imaged using a Leica MZ6 microscope (IL, USA) equipped with an OptixCam digital camera. The image analysis program, ImageJ (NIH, USA) was used to measure the lateral and medial diameter, cortical area, and cavity area.
Using the bone dimensions and three-point bending data, the elasticity of the bone was calculated. Elasticity (1) is defined as the bone’s ability to deform in response to an applied force where F avg is the maximal load, L is the length between endpoints, V max is the bone’s horizontal displacement, and I is the moment of inertia (2) [16]. The mice tibias were treated as elliptical tubes when calculating moment of inertia.
| 1 |
| 2 |
Stress (3) essentially represents the area of bone over which the force was applied, and strain (4) is the deformation of the bone due to stress.
| 3 |
| 4 |
| 5 |
The bone toughness, or resistance to bending, is described by stiffness (5). The V max was important for calculating both elasticity and stiffness and was not able to be experimentally measured. We defined the V max as the distance the wedge traveled after 25% of the experimental time had elapsed. Our V max is comparable to those obtain by others [7, 17]. The maximal load, elasticity, stress, and stiffness data were normalized for body weight and tibia length.
Statistical analysis
All data are expressed as the mean ± S.E. for the number of mice used (Table 1). A log transformation was used on nonparametric data. Data were analyzed using a two-way analysis of variance to detect the effect of age and treatment, and p < 0.05 was considered significant. Post hoc analysis was conducted on the effect of age (Tukey HSD) and treatment (Student’s t test with a Bonferroni adjusted alpha) with p < 0.05. Statistical analysis was conducted with JMP 12.1.0 (SAS Institute Inc., Cary, NC).
Table 1.
Tibial bone structural dimensions
| Age (weeks) | Weeks post-surgery | Treatment | No. of animals | Tibial length (mm) | Cortical area (mm2) | Cavity area (mm2) |
|---|---|---|---|---|---|---|
| 10 | 2 | SHAM | 8 | 16.59 ± 0.11a | 0.47 ± 0.01 a,b | 0.30 ± 0.02 |
| OVX | 4 | 16.52 ± 0.18 a | 0.31 ± 0.06 a,b | 0.31 ± 0.06 | ||
| 12 | 4 | SHAM | 16 | 16.98 ± 0.11 | 0.55 ± 0.02 | 0.29 ± 0.01 |
| OVX | 12 | 17.24 ± 0.12 | 0.59 ± 0.02 | 0.33 ± 0.02 | ||
| 16 | 8 | SHAM | 10 | 17.06 ± 0.13 | 0.54 ± 0.01 | 0.30 ± 0.02 |
| OVX | 7 | 17.23 ± 0.19 | 0.56 ± 0.01 | 0.32 ± 0.03 |
Two-way ANOVA followed by Bonferroni post hoc test, α = 0.05
aBoth treatments at 2 weeks post-surgery are different (p = 0.001) than 4 and 8 weeks post-surgery
bTreatments are different (p = 0.001) at 2 weeks post-surgery
Results
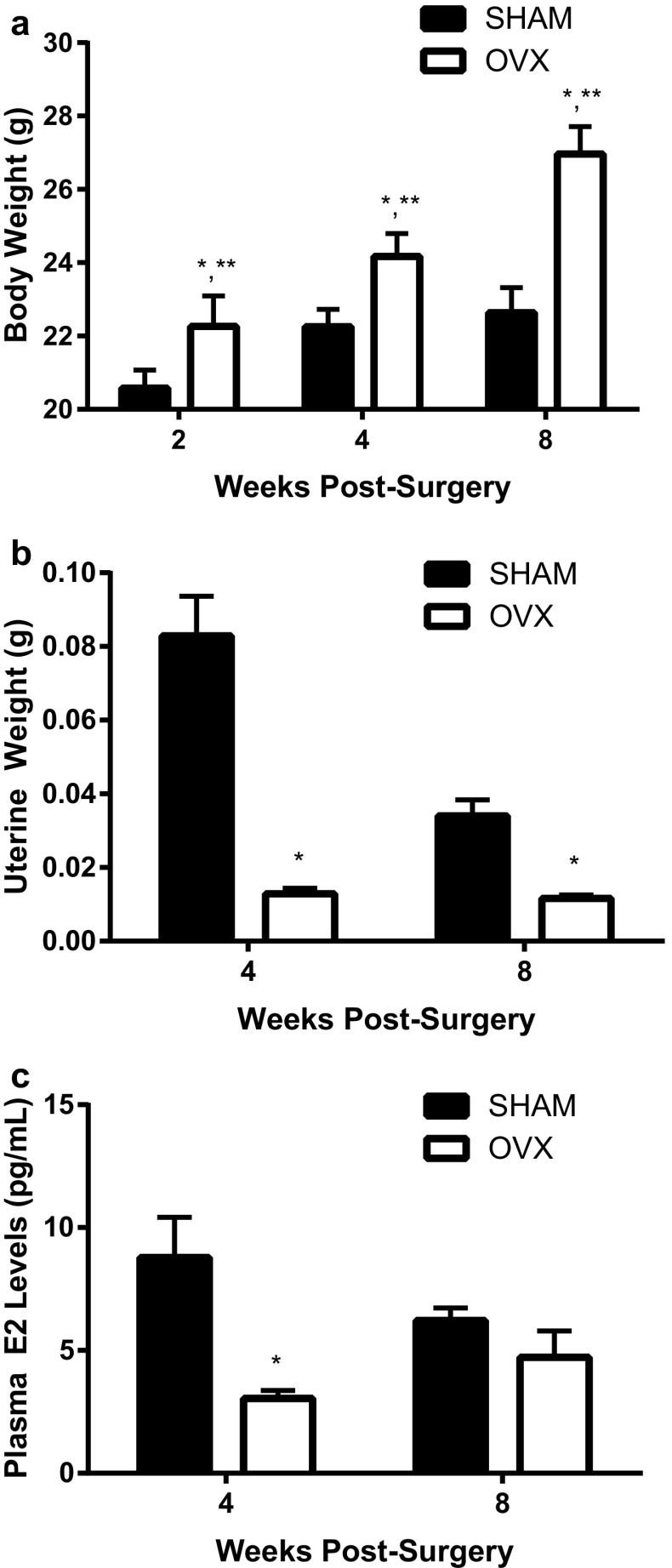
The surgical removal of the ovaries (OVX) at 8 weeks of age resulted in a progressive increase (p < 0.001) in body weight over the next 8 weeks compared to SHAM mice (Fig. 1a). This increase in body weight was also due to the age of the mice in both OVX and SHAM mice (p < 0.001). In both surgical treatment groups, there was an increase in the length of the tibia (p = 0.001) until 12 weeks of age (i.e., 4 weeks post-surgery); after 12 weeks there was no additional increase in length (Table 1). Similarly, when each tibia was cross-sectioned at its mid-diaphysis, the area of the cortical/compact bone increased until 12 weeks of age in both treatment groups (p = 0.001, Table 1). In addition, mice 2 weeks post-OVX demonstrated less tibial cortical area than their age-matched SHAM mice. Even with the increase in compact bone structure, the area medullary cavity was similar between ages and surgical treatment groups. Figure 1b shows that ovariectomization (OVX) of the mice resulted in a decrease (p < 0.001) in uterine weight compared to SHAM mice. Similarly, as shown in Fig. 1c, the plasma estrogen (E2, 17β-estradiol) levels also declined with OVX at 12 weeks; however, at 16 weeks of age the SHAM mice estrogen levels declined to similar levels as the OVX mice. The correlation between uterine weight and plasma estrogen levels was significant (p < 0.001).
Fig. 1.
Physiological measurements due to bilateral ovariectomy (OVX). Mice underwent OVX or SHAM surgery at 8 weeks of age. In all graphs (a–c), significance is defined as p < 0.05 using a two-way ANOVA followed by post hoc analysis. *Represents OVX is p < 0.05 from the SHAM treatment group at each age group. **Indicates p < 0.05 from both SHAM and OVX treatment groups across ages
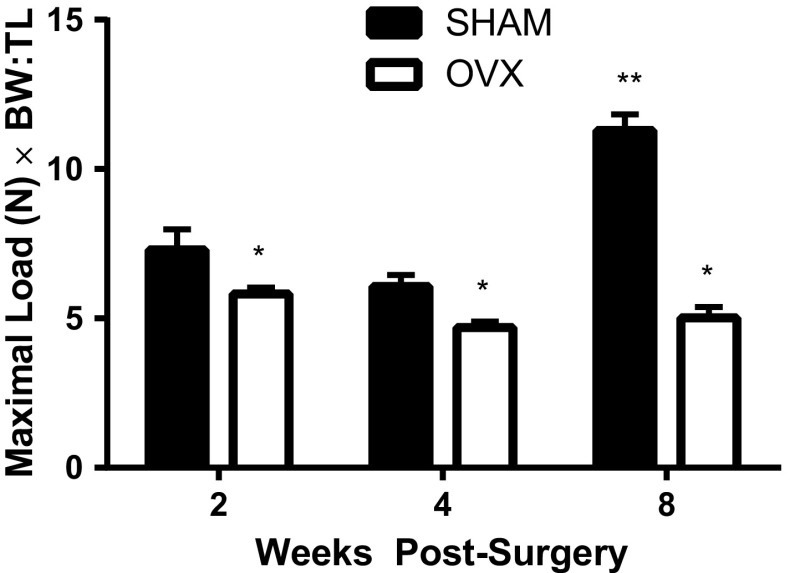
The three-point bending technique was used to assess the mechanical properties (fracture point, stiffness, and elasticity) of the tibia. A constant amount of force was applied to the midpoint of the tibia diaphysis to determine the maximum load that could be placed on the tibia until fracture/breakage occurred. Both the age (p < 0.001) and surgical treatment (p < 0.001) of the mice had a significant effect on the maximum load withstood by the tibias; thus, all OVX mice had a lower breaking point than the age-matched SHAM mice (Fig. 2). However, at 16 weeks of age (i.e., 8 weeks post-surgery) a greater force was required to reach the SHAM mice breaking point than the 10- and 12-week-old SHAM mice and the age-matched OVX mice.
Fig. 2.
Using three-point bending, less force was required to reach the fracture/breaking point of the tibia from ovariectomized (OVX) than age-matched SHAM mice. The mice underwent OVX or SHAM surgery at 8 weeks of age. A constant force was applied (rate of 0.498 mm · s−1) to the mid-diaphysis of the tibia until fracture (max load) occurred. Data are normalized for the size of each individual mouse using the body weight-to-tibial length ratio (BW:TL). Significance is defined as p < 0.05 using a two-way ANOVA followed by post hoc analysis. *Represents OVX is p < 0.05 from the SHAM treatment groups at each age group. **Indicates p < 0.05 from both SHAM and OVX treatment groups across ages
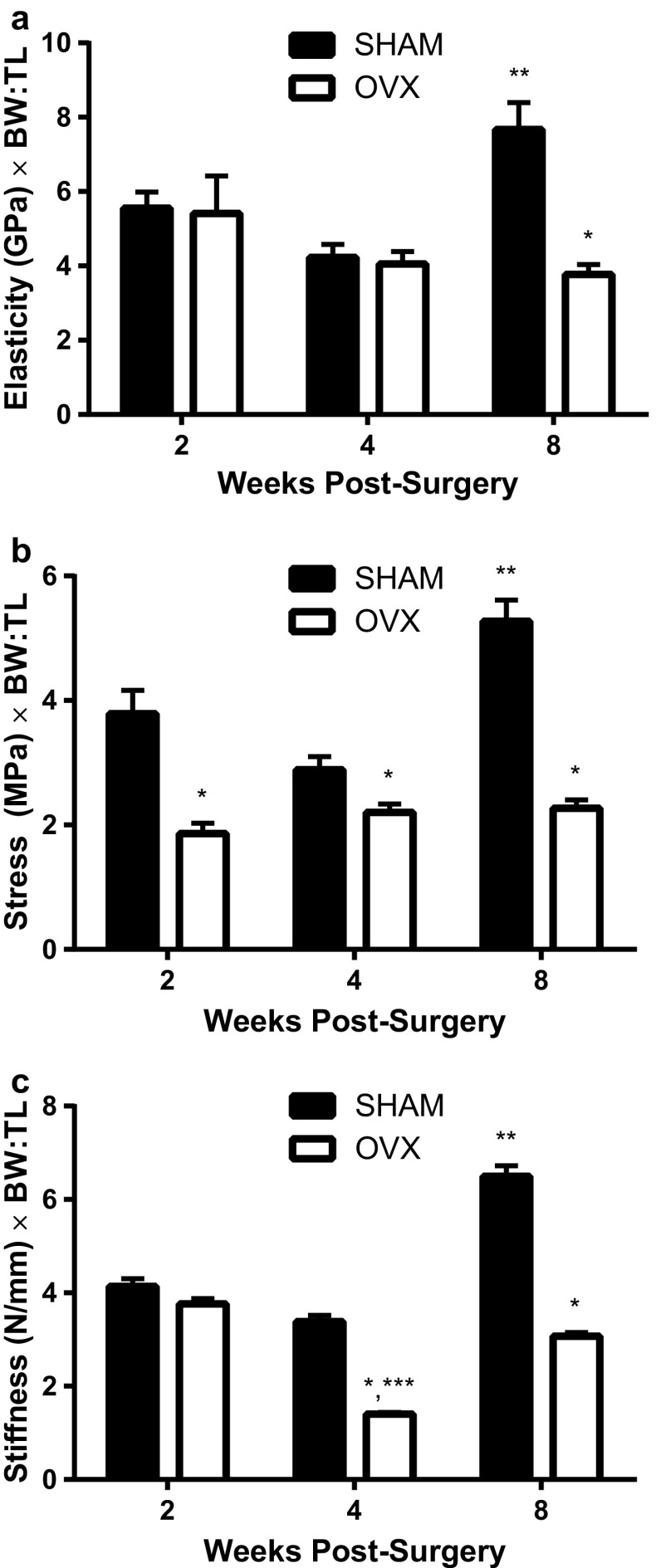
At 16 weeks of age, the tibias from the SHAM mice also demonstrated a higher elasticity, stress, and stiffness than the younger SHAM mice and the age-matched OVX mice (Fig. 3). For all three parameters (i.e., elasticity, stress, and stiffness), the age of the mice and surgical treatment had a significant effect. The elasticity of the compact bone was only different between the SHAM and OVX mice at 16 weeks of age (p < 0.001); otherwise there was no difference between the two treatment groups at younger ages. The tibias from the SHAM mice were able to withstand more stress at every age than the OVX mice tibias (p < 0.001). Furthermore, SHAM mice at 16 weeks resisted more stress than any other treatment group (p < 0.001). The SHAM mice had tibias that demonstrated greater stiffness than OVX mice at 4 and 8 weeks post-surgery (p < 0.001).
Fig. 3.
Biomechanical properties (elasticity, stress, and stiffness) of the tibia between ovariectomized (OVX) and SHAM mice. In all graphs (a–c), significance is defined as p < 0.05 using a two-way ANOVA followed by post hoc analysis. Data are normalized for the size of each individual mouse using the body weight-to-tibial length ratio (BW:TL). Significance is defined as p < 0.05 using a two-way ANOVA followed by post hoc analysis. *Represents OVX is p < 0.05 from the SHAM treatment groups at each age group. **Indicates p < 0.05 from both SHAM and OVX treatment groups across ages. ***Indicates OVX is p < 0.05 from the other OVX treatment groups
Discussion
This study demonstrates that the loss of gonadal estrogen production (via an ovariectomy) induces a reduction of tibial strength in young female mice that are skeletally immature. Because most studies utilize skeletally mature mice (≥16 weeks old) to evaluate the decline in mechanical strength of compact bone, the objective of this study was to determine if estrogen depletion in skeletally immature mice could induce a similar decrease in cortical bone strength. In maturing females, the loss of estrogen is typically associated with increased bone turnover, whereby osteoclast activity will exceed osteoblast activity [10]. This is the first study to evaluate the effect of an ovariectomy on compact bone strength using skeletally immature mice.
Our results indicate that the tibia was still developing in 10-week-old mice (when compared to 12 and 16 weeks of age); this was suggested by the shorter tibial length and smaller cortical area. In addition, the body weight of our SHAM mice did not stabilize until 12 weeks of age. Similarly, Somerville et al. [13] used the tibia from C57BL/6 female mice and found that the tibial length, cortical area, and bone mineral density peaked and stabilized by 12 weeks of age. Both Somerville et al. [13] and Beamer et al. [14] suggest that skeletal maturity in these mice is reached by 16 weeks of age. In young mice, cortical bones will grow in length and diameter. An increase in tibial length will occur via endochondral ossification, in which, osteoclasts will facilitate the resorption of cartilage and osteoblasts will build the bone matrix. Osteoblasts will also deposit bone matrix along the periosteum [23]. After 2 weeks post-surgery (10 weeks of age), only the maximal load and stress withstood by the tibias was reduced in the ovariectomized mice. In contrast, once the mice reached skeletal maturity at 16 weeks of age, all four parameters (i.e., maximal load, elasticity, stress, and stiffness), which evaluate the mechanical strength of compact bone, were reduced in ovariectomized mice. As similarly reported by Somerville et al. [13], results from our SHAM mice show that age does increase the maximum load, elasticity, stress, and stiffness of the tibia. Ovariectomization of the mice appears to negate this age effect. Iwaniec and Turner [23] have recently described that with growth, weight gain, and the subsequent increase in bone mass, there is an adaptive skeletal response that is induced by the mechanical strain on the skeletal system. This adaptive response, which is sometimes referred to as the mechanostat theory and is not well understood, provides some insight into our results, which demonstrate that an increase in age increases the maximum load, elasticity, stress, and stiffness of bone.
In our study, the OVX mice did not demonstrate the increase in cortical bone strength due to skeletal maturity shown by the SHAM mice. There have been conflicting results that suggest that the elevation in follicle stimulating hormone (FSH) due to estrogen deficiency is associated with a decline in bone mass [24, 25]. However, Ozbek et al. [26] recently found that elevated levels of FSH due to hypogonadotropic and hypergonadotropic hypogonadism in adolescent girls did not have an impact on femur bone mineral density. Many of the estrogenic effects concerning the osteogenesis of bone mass have been shown to be mediated by estrogen receptor α (ERα) signaling mechanisms in osteoclasts. Therefore, we hypothesize that the blunted mechanical strength once the OVX mice reach skeletal maturity at 16 weeks of age is due to a downregulation of the intracellular events associated with ERα.
Nakamura et al. [27] demonstrated that the selective knockout of ERα (ERα−/−) in osteoclasts caused apoptosis and bone resorption via the Fas ligand (FASL) system in trabecular bone. Fas is a transmembrane protein that is associated with the tumor necrosis family (TNF) of receptors that induces apoptosis. Recently, using 12-week-old C57BL/6 mice, Wang et al. [28] found that OVX causes a downregulation of FASL in osteoblasts from the femur trabeculae via the proinflammatory cytokines, interferon-γ and TNF-α. They also demonstrated that FASL knockout osteoblasts caused an increase in osteoclast number, which led to a reduction in bone mass. It is unclear, but hypothesized, that these same mechanisms exist in cortical bone to promote osteoclast apoptosis with estrogen withdrawal [27]. ERα has also been implicated in the Wnt/Lrp/Fzd intracellular signaling mechanism that controls cortical bone mass; it has little effect on trabecular bone. Wnt16 is a glycoprotein that is derived from osteoblasts and binds to frizzled (Fzd) receptors and low-density lipoprotein 5/6 co-receptors [29, 30]. Todd et al. [30] found that OVX C57BL/6 mice demonstrated a 50% reduction in Wnt16 expression in the cortex of the femur. This reduction was prevented in OVX mice given 17β-estradiol supplementation.
As has been similarly described by others [10, 30], the ovariectomized mice demonstrated weight gain, a decline in uterus weight, and a decline in plasma estrogen levels. Most of our data are reported at 2, 4, and 8 weeks post-surgery; unfortunately, we were not able to report the plasma estrogen concentration at 2 weeks because the red blood cells were lysed in both experimental groups and an accurate reading could not be obtained. Even though there was an insignificant difference in plasma estrogen levels between the OVX and SHAM mice at 8 weeks post-surgery, our results indicated a significant correction between uterine weight and plasma estrogen levels at 4 and 8 weeks post-surgery. LeBlanc et al. [31] has found similar results using a SHAM and OVX rat model. Therefore, it appears that in intact mice and rats, there is a decline in plasma estrogen and uterine weight with an increase in age.
Conclusions
Overall, estrogen deficiency does reduce bone strength in skeletally immature mice. However, the effects are exacerbated once bones reach full maturity. This study provides foundational knowledge about how the inability to produce in vivo estrogen, or the bones’ insensitivity to estrogen effects compact bone in younger females.
Acknowledgments
Support was provided by grants from the National Institutes of Health (NIH) National Institute of General Medical Sciences (NIGMS) (P20 GM103429) to B.J.F.H. and the Arkansas Space Grant Consortium to A.W. Assistance was provided by Dr. Rahul Mehta (Dept of Physics & Astronomy) and Otis Perkins (undergraduate student). Analysis of plasma estrogen was conducted by The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core, which is supported by the Eunice Kennedy Shriver NICHD/NIH (SCCPIR) Grant U54-HD28934.
References
- 1.Adeel S, Singh K, Vydareny KH, Kumari M, Shah E, Weitzmann MN, Tangpricha V. Bone loss in surgically ovariectomized pre-menopausal women is associated with T lymphocyte activation and thymic hypertrophy. J. Investig. Med.: Off. Publ. Am Fed. Clin. Res. 2013;61(8):1178–1183. doi: 10.2310/JIM.0000000000000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baykan EK, Erdoğan M, Özen S, Darcan Ş, Saygılı LF. Aromatase deficiency, a rare syndrome: case report. J. Clin. Res. Pediatr. Endocrinol. 2013;5(2):129–132. doi: 10.4274/Jcrpe.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bulun SE. Aromatase and estrogen receptor alpha deficiency. Fertil. Steril. 2014;101(2):323–329. doi: 10.1016/j.fertnstert.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almeida OM, Jorgetti W, Oksman D, Jorgetti C, Rocha DL, Gemperli R. Comparative study and histomorphometric analysis of bone allografts lyophilized and sterilized by autoclaving, gamma irradiation and ethylene oxide in rats. Acta Cir. Bras. 2013;28(1):66–71. doi: 10.1590/S0102-86502013000100011. [DOI] [PubMed] [Google Scholar]
- 5.Riggs BL. Endocrine causes of age-related bone loss and osteoporosis. Novartis Found. Symp. 2002;242:247–259. doi: 10.1002/0470846542.ch15. [DOI] [PubMed] [Google Scholar]
- 6.Ornoy A, Giron S, Aner R, Goldstein M, Boyan BD, Schwartz Z. Gender-dependent effects of testosterone and 17 beta-estradiol on bone growth and modelling in young mice. Bone Miner. 1994;24(1):43–58. doi: 10.1016/S0169-6009(08)80130-4. [DOI] [PubMed] [Google Scholar]
- 7.Main RP, Lynch ME, van der Meulen MC. Load-induced changes in bone stiffness and cancellous and cortical bone mass following tibial compression diminish with age in female mice. J. Exp. Biol. 2014;217(Pt 10):1775–1783. doi: 10.1242/jeb.085522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lang DH, Sharkey NA, Lionikas A, Mack HA, Larsson L, Vogler GP, Vandenbergh DJ, Blizard DA, Stout JT, Stitt JP, McClearn GE. Adjusting data to body size: a comparison of methods as applied to quantitative trait loci analysis of musculoskeletal phenotypes. J. Bone Miner. Res. 2005;20(5):748–757. doi: 10.1359/JBMR.041224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Razi H, Birkhold AI, Zaslansky P, Weinkamer R, Duda GN, Willie BM, Checa S. Skeletal maturity leads to a reduction in the strain magnitudes induced within the bone: a murine tibia study. Acta Biomater. 2015;13:301–310. doi: 10.1016/j.actbio.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 10.Bouxsein ML, Myers KS, Shultz KL, Donahue LR, Rosen CJ, Beamer WG. Ovariectomy-induced bone loss varies among inbred strains of mice. J. Bone Miner. Res. 2005;20(7):1085–1092. doi: 10.1359/JBMR.050307. [DOI] [PubMed] [Google Scholar]
- 11.Nelson JF, Karelus K, Felicio LS, Johnson TE. Genetic influences on the timing of puberty in mice. Biol. Reprod. 1990;42(4):649–655. doi: 10.1095/biolreprod42.4.649. [DOI] [PubMed] [Google Scholar]
- 12.Glatt V, Canalis E, Stadmeyer L, Bouxsein ML. Age-related changes in trabecular architecture differ in female and male C57BL/6J mice. J. Bone Miner. Res. 2007;22(8):1197–1207. doi: 10.1359/jbmr.070507. [DOI] [PubMed] [Google Scholar]
- 13.Somerville JM, Aspden RM, Armour KE, Armour KJ, Reid DM. Growth of C57BL/6 mice and the material and mechanical properties of cortical bone from the tibia. Calcif. Tissue Int. 2004;74(5):469–475. doi: 10.1007/s00223-003-0101-x. [DOI] [PubMed] [Google Scholar]
- 14.Beamer WG, Donahue LR, Rosen CJ, Baylink DJ. Genetic variability in adult bone density among inbred strains of mice. Bone. 1996;18(5):397–403. doi: 10.1016/8756-3282(96)00047-6. [DOI] [PubMed] [Google Scholar]
- 15.Cao JJ, Gregoire BR. A high-fat diet increases body weight and circulating estradiol concentrations but does not improve bone structural properties in ovariectomized mice. Nutr. Res. 2016;36(4):320–327. doi: 10.1016/j.nutres.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Walker AH, Perkins O, Mehta R, Ali N, Dobretsov M, Chowdhury P. Changes in mechanical properties of rat bones under simulated effects of microgravity and radiation. Phys. Procedia. 2015;66:610–616. doi: 10.1016/j.phpro.2015.05.081. [DOI] [Google Scholar]
- 17.Jiao F, Chiu H, Jiao Y, de Rijk WG, Li X, Eckstein EC, Beamer WG, Gu W. Quantitative trait loci for tibial bone strength in C57BL/6J and C3H/HeJ inbred strains of mice. J. Genet. 2010;89(1):21–27. doi: 10.1007/s12041-010-0007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tuukkanen J, Koivukangas A, Jamsa T, Sundquist K, Mackay CA, Marks SC., Jr Mineral density and bone strength are dissociated in long bones of rat osteopetrotic mutations. J. Bone Miner. Res. 2000;15(10):1905–1911. doi: 10.1359/jbmr.2000.15.10.1905. [DOI] [PubMed] [Google Scholar]
- 19.Ritchie RO, Koester KJ, Ionova S, Yao W, Lane NE, Ager JW., 3rd Measurement of the toughness of bone: a tutorial with special reference to small animal studies. Bone. 2008;43(5):798–812. doi: 10.1016/j.bone.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamal B, Russell D, Payne A, Constante D, Tanner KE, Isaksson H, Mathavan N, Cobb SR. Biomechanical properties of bone in a mouse model of Rett syndrome. Bone. 2015;71:106–114. doi: 10.1016/j.bone.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Windahl SH, Andersson N, Chagin AS, Martensson UE, Carlsten H, Olde B, Swanson C, Moverare-Skrtic S, Savendahl L, Lagerquist MK, Leeb-Lundberg LM, Ohlsson C. The role of the G protein-coupled receptor GPR30 in the effects of estrogen in ovariectomized mice. Am. J. Physiol. Endocrinol. Metab. 2009;296(3):E490–496. doi: 10.1152/ajpendo.90691.2008. [DOI] [PubMed] [Google Scholar]
- 22.Stubbins RE, Holcomb VB, Hong J, Nunez NP. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur. J. Nutr. 2012;51(7):861–870. doi: 10.1007/s00394-011-0266-4. [DOI] [PubMed] [Google Scholar]
- 23.Iwaniec UT, Turner RT. Influence of body weight on bone mass, architecture and turnover. J. Endocrinol. 2016;230(3):R115–130. doi: 10.1530/JOE-16-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rouach V, Katzburg S, Koch Y, Stern N, Somjen D. Bone loss in ovariectomized rats: dominant role for estrogen but apparently not for FSH. J. Cell. Biochem. 2011;112(1):128–137. doi: 10.1002/jcb.22908. [DOI] [PubMed] [Google Scholar]
- 25.Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, Papachristou DJ, Zaidi S, Zhu LL, Yaroslavskiy BB, Zhou H, Zallone A, Sairam MR, Kumar TR, Bo W, Braun J, Cardoso-Landa L, Schaffler MB, Moonga BS, Blair HC, Zaidi M. FSH directly regulates bone mass. Cell. 2006;125(2):247–260. doi: 10.1016/j.cell.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 26.Ozbek MN, Demirbilek H, Baran RT, Baran A. Bone mineral density in adolescent girls with hypogonadotropic and hypergonadotropic hypogonadism. J. Clin. Res. Pediatr. Endocrinol. 2016;8(2):163–169. doi: 10.4274/jcrpe.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, Harada Y, Azuma Y, Krust A, Yamamoto Y, Nishina H, Takeda S, Takayanagi H, Metzger D, Kanno J, Takaoka K, Martin TJ, Chambon P, Kato S. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell. 2007;130(5):811–823. doi: 10.1016/j.cell.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 28.Wang L, Liu S, Zhao Y, Liu D, Liu Y, Chen C, Karray S, Shi S, Jin Y. Osteoblast-induced osteoclast apoptosis by fas ligand/FAS pathway is required for maintenance of bone mass. Cell Death Differ. 2015;22(10):1654–1664. doi: 10.1038/cdd.2015.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moverare-Skrtic S, Wu J, Henning P, Gustafsson KL, Sjogren K, Windahl SH, Koskela A, Tuukkanen J, Borjesson AE, Lagerquist MK, Lerner UH, Zhang FP, Gustafsson JA, Poutanen M, Ohlsson C. The bone-sparing effects of estrogen and WNT16 are independent of each other. Proc. Natl. Acad. Sci. U. S. A. 2015;112(48):14972–14977. doi: 10.1073/pnas.1520408112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Todd H, Galea GL, Meakin LB, Delisser PJ, Lanyon LE, Windahl SH, Price JS. Wnt16 is associated with age-related bone loss and estrogen withdrawal in murine bone. PLoS ONE. 2015;10(10):e0140260. doi: 10.1371/journal.pone.0140260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LeBlanc AJ, Reyes R, Kang LS, Dailey RA, Stallone JN, Moningka NC, Muller-Delp JM. Estrogen replacement restores flow-induced vasodilation in coronary arterioles of aged and ovariectomized rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;297(6):R1713–1723. doi: 10.1152/ajpregu.00178.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]