Abstract
Purpose
SWOG S0702 was a cohort study of patients with cancer with bone metastases due to any cancer. Using baseline data from S0702 this report characterizes the oral health and oral health related quality of life (OHRQoL) of patients with advanced cancer.
Methods
S0702 case report forms captured dental assessment and patient reported outcomes (PRO) data. This analysis compares PRO dental discomfort with selected clinical assessments of dental health. This analysis focuses on the 2294 patients who underwent baseline dental examination prior to study registration, but also reports on the 1235 patients for whom only OHRQol data are available. Dental characteristics including number of teeth and the presence of gingivitis and periodontal disease were examined for correlation with PRO of oral pain, interference with eating, smiling, speech or quality of life.
Results
The median age of the study participants was 62. Greater than 60% of the 2294 patients with baseline dental assessments had none to mild plaque, calculus, gingivitis or periodontal disease, suggesting that most of this cohort had good oral hygiene. However, in each of these same categories, approximately 6% had dental findings classified as severe conditions (poor oral hygiene). There was strong evidence that the presence of periodontal disease, gingivitis and number of teeth was correlated with lower OHRQoL across multiple domains, including pain (mouth or jaw), interference with eating, smiling and speech, and overall quality of life.
Conclusions
This report characterizes the oral health and OHRQoL of patients with advanced bone metastases receiving palliative therapy.
Structured Abstract
This report characterizes the oral health and OHRQoL of patients with advanced bone metastases receiving palliative therapy. These novel data serve as a foundation for future studies of interventions to maximize oral health and to positively impact OHRQol in this patient population
Keywords: oral health, cancer, bone metastases, patient reported outcomes
Introduction
In the US, it is estimated that well over one and a half million new cases of cancer will be diagnosed in 20161; of those who are not cured, many will develop distant metastases. Bone is a common site of distant spread in advanced disease, particularly in tumors originating in the breast, prostate and lung.2 Data generated from Medicare and MarketScan databases indicate that in 2008 there were approximately 280,000 adults in the US with metastatic bone disease within the previous five years.3 Metastases are the major cause of death from advanced cancer.4
Palliative care is a critical component of cancer care and is structured to support the physical, emotional, spiritual and social needs of the patient (5). Oral health-related quality of life (OHRQoL)6,7 is a component of overall health that is often noted when cancer therapy induced mucositis or osteonecrosis of the jaw (ONJ) is present. Yet little is known about the general oral health and quality of life in patients with advanced cancer other than in tumors affecting the head and neck or undergoing stem cell transplantation.
Zoledronic acid (a bisphosphonate) is an FDA-approved drug that reduces skeletal-related events in patients with metastatic bone disease due to multiple myeloma and solid tumor such as breast cancer. Use of bisphosphonates reduces the risk of pathological fractures, hypercalcemia of malignancy, spinal cord compression, and need for surgery or radiation therapy to bone. However, for over a decade there have been reported cases of ONJ in cancer patients receiving intravenous bisphosphonates, resulting in painful exposed necrotic bone in the mandible, maxilla, or both. Treatment of this condition has proven to be difficult and often unsuccessful. SWOG, a cooperative group within the National Clinical Trials Network, initiated study S0702, “A Prospective Observational Multicenter Cohort Study to Assess the incidence of Osteonecrosis of the Jaw in Cancer Patients with Bone Metastases Starting Zoledronic Acid Treatment”. S0702 was a large cohort study to assess the incidence and predictors of ONJ in cancer patients receiving zoledronic acid for bone metastases due to any cancer (clinicaltrials.gov identifier NCT00874211). For this study, patients’ dentists were asked to submit data from dental evaluations using a study specific case report form. Patients were also regularly asked about their perception of dental discomfort. Thus S0702 provides an excellent resource to examine issues surrounding OHRQoL in patients with cancer involving the bone. OHRQoL issues can be critically important for cancer patients if dental health affects a patient’s ability and willingness to continue cancer treatment. Interventions can improve the status of oral health, which, in turn, might improve a patient’s quality of life overall and improve adherence to treatment. Using baseline data from S0702, this report characterizes the oral health and oral health related quality of life OHRQoL of patients with advanced cancer. We believed this information on the oral health and dental discomfort of these patients with advanced cancer is both valuable and novel.
Methods
Study Population
To be eligible, patients must have had cancer involving the bone from any malignancy, must have been planning to receive zoledronic acid treatment within 30 days after registration, and must not have had a pre-existing diagnosis of ONJ. Patients who received prior bisphosphonates for low bone mass up to three years prior to registration, or who received prior bisphosphonates for metastatic bone disease up to 180 days prior to registration, were also allowed. The accrual goal was 3500 patients. Patients enrolled in S0702 remain in follow-up and the data are still maturing. The participating sites obtained institutional review board approval. Informed, written consent was obtained from all patients prior to enrollment. The primary analysis of study results will be reported in a later manuscript.
Patients were registered beginning January 30, 2009 and ending December 13, 2013. Before November 1, 2011, patients were required to undergo a baseline dental examination prior to registration. A study specific letter to the oral health care provider, dental evaluation case report forms, and OHRQoL questions were included. The study letter to the dental provider defined a dental exam to include dental history and exam, periodontal exam and dental imaging. After November 1, 2011, the protocol was amended to more accurately reflect community standard (which at most only recommend regular dental care) and, among other changes, the baseline dental examination was no longer required. For those patients undergoing dental care, the study specific dental communication and dental evaluation case report form were used. All enrolled patients were provided the OHRQoL questions. The objective of this analysis is to compare patient-reported dental discomfort at registration with selected clinical assessments of dental health. Baseline data on the S0702 cohort are provided in this report.
Patient-Reported Outcomes
The Brief Pain Inventory (BPI) is a validated patient-reported outcome measure of pain in patients with cancer8; we adapted questions from the BPI for use patients with oral health complications. To collect patient-reported measures of dental discomfort, study site personnel were given five specific questions to ask patients about problems experienced in the patient’s mouth or jaw over the 3-month period prior to joining the study: (1) “Please rate your pain by selecting the one number that best describes your pain on the AVERAGE” [“Average pain”], (2) “Please rate how your oral health has interfered with your eating on AVERAGE” [“Interference with eating”], (3) “Please rate how oral health has interfered with how you smile on AVERAGE” [“Interference with smile”], (4) “Please rate how oral health has interfered with how you speak on AVERAGE” [“Interference with speech”], (5) “Please rate how your oral health has interfered with your overall quality of life on AVERAGE” [“Interference with quality of life”]. All measures of dental discomfort were collected on an 11-point rating scale (0 to 10), with 0 indicating no problems (pain or interference) and 10 indicating extreme problems (pain or interference). Based on prior literature examining 11-point pain rating scales, we considered a difference of two or more points to be clinically meaningful.9
Clinical measures of dental health
The dental communication informed the provider of the patient’s participation on S0702 and advised the provider to treat as clinically indicated. The S0702 dental case report form captured data regarding the number of teeth, presence of dentures, and periodontal examination (dental plaque, calculus, gingivitis, pocket depth). As clinical measures of dental health, we selected periodontal disease assessment (none, mild, moderate, severe), gingivitis assessment (none, mild, moderate, severe), number of maxillary teeth, number of mandibular teeth, and total number of teeth. The reporting was done by checking boxes on the case report form. A comment box was to be used as needed. These data were collected as part of the study dental assessments. This analysis focuses on periodontal and gingival assessments. To aid in interpretation, all clinical measures were dichotomized; periodontal disease and gingivitis were categorized as none, mild, or moderate (grouped together) versus severe, in order to reflect how the worst cases of disease might correlate with patient-reported outcomes on average. The number of maxillary, mandibular, and total teeth were categorized as greater or equal to the median versus less than the median.
Statistical methods
The primary analysis for this report was conducted in all eligible patients with available baseline dental status and patient-reported outcomes. We also considered that patients likely to have dentures (as indicated by self-report of full dentures or zero total teeth) might have a qualitatively different oral symptom experience. For instance, such patients might no longer have gingivitis or periodontal disease but might experience discomfort due to their denture. As such, a secondary sensitivity analysis excluded these patients in the examination of the correlations between gingivitis and periodontal disease with patient-reported outcomes. Differences between groups were assessed with chi-squared tests (when categorical) or t-tests (when linear). Analyses were performed with SAS statistical software version 9.4 (SAS Institute, Cary, NC) and p values are two-sided. This analysis is considered descriptive and hypothesis generating. As such, we did not control for multiple comparisons; p<0.05 was considered statistically significant.
Results
In total, S0702 registered 3571 patients, of whom 42 were known to be ineligible or were missing the Pre-study form, leaving 3529 patients eligible for this analysis. Patients were recruited from over 100 institutions in the United States. There were 2294 patients who underwent a baseline dental examination prior to registration, and who also completed a patient-reported Pre-study form. This cohort represents the evaluable patient set for this analysis and the focus of this report.
Patient Demographic, Behavioral, and Clinical Characteristics
Table 1 shows characteristics of patients registered to S0702. The characteristics of all eligible patients are shown in column A. Forty-two percent of patients were >65 years with a median age of 62 years and slightly more than half were female. Black patients comprised nearly 11% of the cohort and Hispanic patients nearly 6%. The most common cancer types were breast (32%) and prostate (20%). Most patients were not current smokers (87%), rarely drank (80% drank ≤3/month), and had good performance status (0–1; 88%). Results to assess whether evaluable patients differed from non-evaluable patients are shown in columns B and C, respectively. The evaluable patients were less likely to be black and current smokers, but were more likely to have ≥1 drink per week and to have better performance status. However, distributions by age, sex, ethnicity, and presence of ≥1 comorbid condition were approximately similar between the groups.
Table 1.
Baseline Patient Characteristics
| A.All patients(n=3,529) | B.Analysis set(n=2,294) | C.Not in analysis set(n=1,235) | B vs C | ||||
|---|---|---|---|---|---|---|---|
| Characteristic | n | % | n | % | n | % | χ2 p-value |
| Age | 0.048 | ||||||
| ≤55 | 876 | 24.8% | 536 | 23.4% | 340 | 27.5% | 0.006 |
| 56–65 | 1,167 | 33.1% | 771 | 33.6% | 396 | 32.1% | 0.35 |
| 66–75 | 978 | 27.7% | 644 | 28.1% | 334 | 27.0% | 0.51 |
| >75 | 508 | 14.4% | 343 | 15.0% | 165 | 13.4% | 0.20 |
| Sex | 0.15 | ||||||
| Female | 1,821 | 51.6% | 1,204 | 52.5% | 617 | 50.0% | |
| Male | 1,708 | 48.4% | 1,090 | 47.5% | 618 | 50.0% | |
| Race | <.0001 | ||||||
| White | 2,970 | 84.2% | 1,956 | 85.3% | 1,014 | 82.1% | 0.014 |
| Black | 381 | 10.8% | 194 | 8.5% | 187 | 15.1% | <.0001 |
| Asian | 57 | 1.6% | 46 | 2.0% | 11 | 0.9% | 0.012 |
| Other | 121 | 3.4% | 98 | 4.3% | 23 | 1.9% | 0.0002 |
| Ethnicity | 0.46 | ||||||
| Not Hispanic/Latino | 3,218 | 94.3% | 2,083 | 94.1% | 1,135 | 94.7% | |
| Hispanic/Latino | 193 | 5.7% | 130 | 5.9% | 63 | 5.3% | |
| Unknown | 118 | 81 | 37 | ||||
| Current Smoker | <.0001 | ||||||
| Yes | 435 | 12.3% | 214 | 9.3% | 221 | 17.9% | |
| No | 3,091 | 87.7% | 2,077 | 90.7% | 1,014 | 82.1% | |
| Not answered | 3 | 3 | 0 | ||||
| Cancer Type | <.0001 | ||||||
| Breast | 1,134 | 32.1% | 765 | 33.3% | 369 | 29.9% | 0.035 |
| Multiple Myeloma | 587 | 16.6% | 385 | 16.8% | 202 | 16.4% | 0.75 |
| Prostate | 714 | 20.2% | 508 | 22.1% | 206 | 16.7% | 0.0001 |
| Lung | 673 | 19.1% | 386 | 16.8% | 287 | 23.2% | <.0001 |
| Other | 421 | 11.9% | 250 | 10.9% | 171 | 13.8% | 0.010 |
| Performance Status | <.0001 | ||||||
| 0–1 | 3,092 | 87.6% | 2,072 | 90.3% | 1,020 | 82.6% | |
| ≥2 | 437 | 12.4% | 222 | 9.7% | 215 | 17.4% | |
| Alcoholic drinks in past 3 months | <.0001 | ||||||
| Never or < 1/month | 2,258 | 64.1% | 1,401 | 61.2% | 857 | 69.4% | <.0001 |
| 1–3 per month | 549 | 15.6% | 373 | 16.3% | 176 | 14.3% | 0.11 |
| ≥1 per week | 718 | 20.4% | 516 | 22.5% | 202 | 16.4% | <.0001 |
| Not answered | 4 | 4 | 0 | ||||
| Comorbidities reported | |||||||
| Reports any condition below | 1,169 | 33.1% | 767 | 33.4% | 402 | 32.6% | 0.59 |
| Auto-immune disease | 81 | 2.3% | 51 | 2.2% | 30 | 2.4% | 0.70 |
| Chronic infection (including HIV/AIDS) | 33 | 0.9% | 20 | 0.9% | 13 | 1.1% | 0.59 |
| Coagulopathy or blood clotting problems | 156 | 4.4% | 103 | 4.5% | 53 | 4.3% | 0.78 |
| Diabetes | 546 | 15.5% | 345 | 15.0% | 201 | 16.3% | 0.33 |
| Malnutrition | 20 | 0.6% | 8 | 0.3% | 12 | 1.0% | 0.019 |
| Osteoporosis or osteopenia | 430 | 12.2% | 320 | 13.9% | 110 | 8.9% | <.0001 |
| Paget’s disease of bone or metabolic bone disease | 15 | 0.4% | 11 | 0.5% | 4 | 0.3% | 0.50 |
| Renal failure or renal insufficiency | 122 | 3.5% | 76 | 3.3% | 46 | 3.7% | 0.52 |
Patient Dental Characteristics
Table 2 shows clinical assessments of dental health for those patients in the analysis set. Of note, half of patients reported having 26 or more natural teeth (with 297 patients (13%) reporting either 0 maxillary or 0 mandibular teeth), and 22% reported partial or complete dentures.
Table 2.
Baseline Dental Characteristics
| Characteristic | Analysis set(n=2,294) | |
|---|---|---|
| n | % | |
| Dental visit within the last 6 months | ||
| No | 114 | 5.0% |
| Yes | 2,180 | 95.0% |
| Number of dental cleanings within the past 2 years | ||
| 0 | 555 | 25.3% |
| 1 | 325 | 14.8% |
| 2 | 276 | 12.6% |
| 3 | 272 | 12.4% |
| 4 | 550 | 25.0% |
| >4 | 220 | 10.0% |
| Unknown | 96 | |
| Prior oral surgery | ||
| No | 548 | 24.8% |
| Yes | 1,663 | 75.2% |
| Number of extractions | ||
| 1–3 | 602 | 38.1% |
| >3 | 980 | 61.9% |
| Unknown number of extractions | 81 | |
| Unknown | 83 | |
| Prior periodontal treatments | ||
| No | 1,599 | 75.0% |
| Yes | 534 | 25.0% |
| Unknown | 161 | |
| Dental implants | ||
| No | 2,115 | 94.4% |
| Yes | 126 | 5.6% |
| Number of implants | ||
| 1–3 | 86 | 69.9% |
| >3 | 37 | 30.1% |
| Unknown number of implants | 3 | |
| Unknown | 53 | |
| Tori present | ||
| No | 1,655 | 80.8% |
| Yes | 393 | 19.2% |
| Unknown | 246 | |
| Plaque | ||
| None | 210 | 9.8% |
| Mild | 1,241 | 58.0% |
| Moderate | 558 | 26.1% |
| Severe | 132 | 6.2% |
| Unknown | 153 | |
| Calculus | ||
| None | 324 | 15.1% |
| Mild | 1,187 | 55.4% |
| Moderate | 511 | 23.9% |
| Severe | 120 | 5.6% |
| Unknown | 152 | |
| Gingivitis | ||
| None | 541 | 25.3% |
| Mild | 1,048 | 49.1% |
| Moderate | 421 | 19.7% |
| Severe | 126 | 5.9% |
| Unknown | 158 | |
| Periodontal disease | ||
| None | 623 | 32.6% |
| Mild | 801 | 41.9% |
| Moderate | 355 | 18.6% |
| Severe | 131 | 6.9% |
| Unknown | 384 | |
| Number of teeth | ||
| 0–20 | 594 | 26.6% |
| 21–25 | 526 | 23.6% |
| 26–28 | 871 | 39.1% |
| >28 | 238 | 10.7% |
| Unknown | 65 | |
| Dentures, partial or complete | ||
| No | 1,745 | 78.1% |
| Yes | 489 | 21.9% |
| Unknown | 60 | |
Patient-Reported Outcomes
Table 3 shows patient-reported outcomes for all eligible patients and contrasts those in the evaluable analysis set with those not evaluable. Overall, most patients reported no problems, with over 75% of those reporting 0 (no problem) on all questions in all subgroups. Those included in the analysis set had slightly lower average discomfort ratings on all questions than those not included in the analysis set (for example, 0.90 Average pain not in Analysis Set vs 0.78 Average pain Analysis Set, p=0.06); however, none of these differences were clinically significant (all were less than two points on an 11-point scale) and none of these differences were statistically significant.
Table 3.
Baseline Patient Related outcomes
| A. All patients with Prestudy Form(n=3,529) | B. Analysis set(n=2,294) | C. Not in analysis set(no dental exam)(n=1,235) | B vs C | ||||
|---|---|---|---|---|---|---|---|
| Patient-reported outcome | Mean (SD) | % 0 scores(no problem) | Mean (SD) | % 0 scores(no problem) | Mean (SD) | % 0 scores(no problem) | t-test p value |
| Average pain | 0.82 (1.85) | 76.3% | 0.78 (1.78) | 76.8% | 0.90 (1.98) | 75.5% | 0.06 |
| Interference with eating | 0.50 (1.54) | 86.4% | 0.49 (1.52) | 86.8% | 0.53 (1.58) | 85.8% | 0.48 |
| Interference with smile | 0.32 (1.37) | 92.0% | 0.30 (1.33) | 92.7% | 0.36 (1.43) | 90.7% | 0.24 |
| Interference with speech | 0.23 (1.07) | 93.2% | 0.23 (1.07) | 93.3% | 0.24 (1.06) | 93.0% | 0.96 |
| Interference with quality of life | 0.35 (1.29) | 89.4% | 0.34 (1.27) | 89.8% | 0.38 (1.34) | 88.6% | 0.37 |
For all questions and groups of patients shown, the median value was 0 (no problem), and the range 0 to 10.
Correlations between Baseline Dental Characteristics and Patient-Reported Outcomes
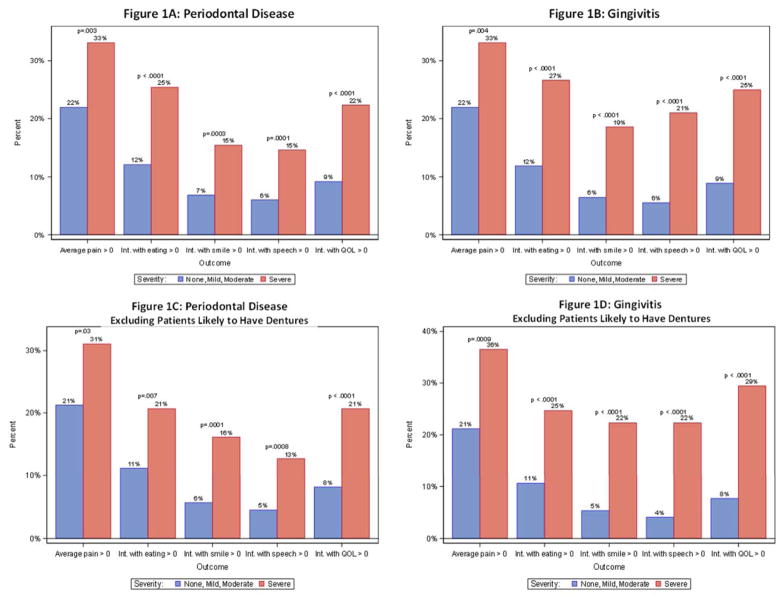
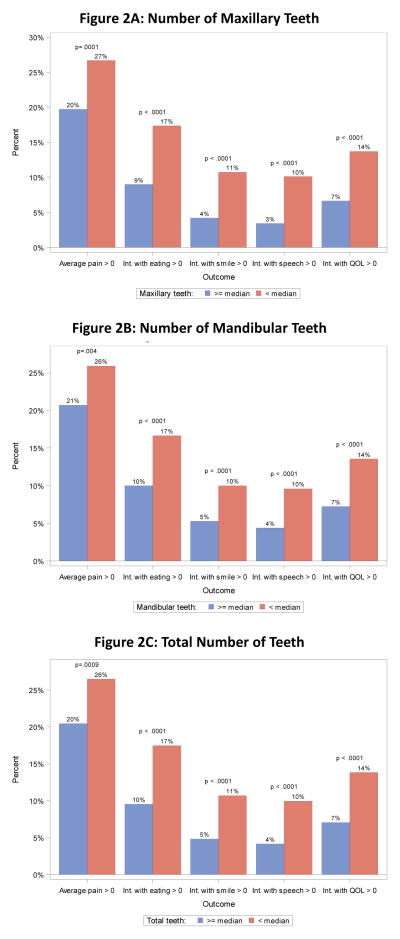
We examined the clinical correlations between dental status and patient-reported outcomes. Given that more than 75% of patients reported no problems for all of the patient-reported outcomes (Table 3), to aid in the interpretation, we dichotomized the measures of patient-reported discomfort, contrasting 0 scores (no problem) with any report of problems (>0). For each patient-reported outcome, patients with severe periodontal disease or gingivitis were much more likely (p<.01 in all cases) to have worse patient-reported outcomes (Figures 1A and 1B). While power to analyze correlations within in the five major groups of cancers on S0702 (breast, prostate, lung, multiple myeloma and other) was limited, a sensitivity analysis showed similar patterns of association in all five cancer subgroups (data not shown). When patients likely to have full dentures were excluded, the results were very similar (Figures 1C and 1D). Similarly, patients with fewer maxillary or mandibular teeth were much more likely (p<.01 in all cases) to have worse patient-reported outcomes (Figures 2A and 2B). Finally, we examined the correlations between patient-reported outcomes and the total number of teeth (Figure 2C); again, there was a strong correlation between fewer teeth and worse patient-reported outcomes. Thus the finding of a very strong correlation between baseline dental health and patient-reported outcomes was consistently strong across all correlative analyses.
Figure 1.
Periodontal disease and patient reported outcomes
Figure 2.
Teeth and patient reported outcomes
Discussion
This report defines the OHRQoL and associated clinical dental assessments in patients with metastatic bone disease. The study population was drawn from community and academic oncology clinics across the United States with the median age of 62 and with 15% of participants being non-white. In the 2,294 patients who had dental evaluations, the majority (>60%) had none to mild plaque, calculus, gingivitis or periodontal disease, suggesting that most of this cohort had good oral hygiene. However, in each of these same categories approximately 6% had dental findings classified as severe conditions (poor oral hygiene). There was strong evidence that the presence of periodontal disease, gingivitis and fewer teeth was correlated with lower OHRQoL across multiple domains, including pain (mouth or jaw), interference with eating, smiling and speech, and overall quality of life. The data generated from S0702 are consistent with what would be empirically expected, yet are novel data for this patient population. A recent publication estimated the prevalence of severe periodontitis in the US to be slightly less than 9% in adults aged 30 to 79 years;10 approximately 7% of S0702 participants had severe periodontitis. The results of this analysis illustrate an area where additional attention may be given to aid palliative interventions for patients with metastatic bone disease.
It is estimated that over a quarter million patients in the US have metastatic bone disease3 and the S0702 study population sheds insight into the oral health of this patient population. If the overall population with metastatic bone disease has a similar frequency of poor oral hygiene (6%), then approximately 15,000 patients would be affected. The Surgeon General’s Report on Oral Health in America highlights the importance of oral health for its ability to impact the ability to eat, food choices, appearance, and communication.11 Indeed, the Surgeon General’s Reports identifies the mouth as the center of vital functions that are critical to total health and well-being. With this in mind, patients with advanced cancer may benefit from optimizing oral health as a palliative intervention.
Conditions affecting the oral cavity can be wide ranging. In the general population, there is a correlation between oral health and overall health as noted in patients with cardiovascular disease and diabetes.11 Tooth loss has been shown to affect food choice and overall nutritional status.11 Gingivitis and periodontal disease can lead to tooth loss, but the direct impact of gingivitis and periodontal disease on food choice has not been documented. Sensitive teeth hurt when exposed to hot, cold, and/or acidic foods and beverages; those with sensitive teeth may avoid foods that cause pain.11 In addition the condition of the oral cavity may influence speech, socialization and self-confidence.11
Data on the general US population suggest that in 2013 approximately 40% of the adult population did not have a dental visit within the past year.12 In S0702 approximately one third of enrolled patients did not have a dental exam within 6 months of study entry (1,227/3,529), which is roughly consistent with the typical US experience. Moreover, S0702 was designed to capture a broad spectrum of patients receiving zoledronic acid that was representative of the cancer treatment population. To that end, eligibility criteria were broadly written. Within the study, however, there were differences between those patients who did (N=2,294) and did not (N= 1,277) have a baseline dental assessment, suggesting that the analyzed cohort was a somewhat selected patient group. Most notably, S0702 participants not in the analysis set were more likely to be black, current smokers, and to have a higher performance status. S0702 does not permit analysis by insurance type but it is notable that S0702 provided funds for the baseline dental assessment on an as needed basis. The reason why 34% of overall S0702 enrolled patients did not undergo baseline assessments is not evaluable from the study data.
This study has limitations. The patient-reported outcomes may not have been collected synchronously with the dental assessments because S0702 allowed a 6 month time window for the dental assessments, which is within the American Dental Association standard time frame for dental care.13 The significance of this 6 month time window on OHRQoL is unknown. S0702 did not collect data on dental insurance, income or education. Patients who participate in SWOG clinical trials may reflect higher education and income levels than the general population.14, 15 These factors in the general population correlate with oral health.12, 13 How these factors impact the study population cannot not be analyzed from the present S0702 data. But the study also has notable strengths. Unique findings in the S0702 baseline dental assessments paired with the patient-reported outcomes add to the OHRQoL and heath care literature. The sample was large, including over 2000 evaluable patients. The data were collected prospectively. Also, the sample represented a demographically and clinically diverse range of patients. Although the study did not formally adjust for multiple comparisons, nearly all of the observed correlations between dental status and patient-reported outcomes would have been statistically significant even under a conservative Bonferroni multiple comparisons approach. We plan to extend our examinations of these data to include clinical outcomes, including survival, after follow up for the study has been completed.
Baseline assessments of the 2,294 patients enrolled in S0702 who had dental care within 6 months of study entry confirms the importance of OHRQoL in patients with advanced cancer involving the bone. Across all patient-reported outcomes the more severe the dental periodontal or gingivitis condition, the greater negative effect on pain, eating, smiling, speech and overall OHRQoL. There are established, standard interventions to improve oral health,12, 16, 17 however, insurance coverage, personal preference, access to care, as well as other factors may serve as barriers for seeking dental care.11 This report serves as a foundation for future studies to further assess how oral health and interventions to maximize oral health can impact Qol, OHRQol, financial expenditures (personal and other), as well as whether factors associate with oral health interfere with care of the underlying cancer.
Acknowledgments
Funding/Support:
Funding for this work was provided National Institutes of Health, National Cancer Institute, NCI Community Oncology Research Program (NCORP) Research Base grant, 5UG1CA189974.
Footnotes
Trial Registration: ClinicalTrials.gov Identifier: NCT00874211
Additional Contributions:
Danika L. Lew, MD, provided statistical assistance; Lisa Hansen, RN, MS, provided clinical input; Kimberly Kaberle and Diane Liggett, BS, were responsible for protocol coordination and data management; No compensation was received beyond their salary for this work.
Conflict of Interest Statement:
There are no conflicts of interest to report. SWOG and the authors have full control of the primary data. We agree to allow the journal to review aggregate level data, if requested.
References
- 1.NIH (statistics on cancer incidence) http://www.cancer.gov/about-cancer/what-is-cancer/statistics.
- 2.Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27(3):165–176. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 3.Li S, Peng U, Weinhandl ED, et al. Estimated number of prevalent cases of metastatic bone disease in the US adult population. Clinical Epidemiology. 2012;4:87–93. doi: 10.2147/CLEP.S28339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. [accessed 5-31-2016]; http://www.who.int/mediacentre/factsheets/fs297/en/
- 5.Smith TJ, Temin S, Alesi ER, et al. American Society of Clinical Oncology provisional clinical opinion: the integration of palliative care into standard oncology care. J Clin Oncol. 2012;30(8):880–7. doi: 10.1200/JCO.2011.38.5161. [DOI] [PubMed] [Google Scholar]
- 6.Sischo L, Broder HL. Oral health-related quality of life: What, why, how, and future implications. Critical Reviews in Oral Biology & Medicine. 2011;90(11):1264–1270. doi: 10.1177/0022034511399918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennadi D, Reddy CVK. Oral health related quality of life. Journal of international Society of Preventive & Community Dentistry. 2013;3(1):1–6. doi: 10.4103/2231-0762.115700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994 Mar;23(2):129–38. [PubMed] [Google Scholar]
- 9.Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001 Nov;94(2):149–58. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 10.Eke PI, Zhang X, Lu H, et al. Predicting Periodontitis at State and Local Levels in the United States. J Dent Res. 2016 May;95(5):515–22. doi: 10.1177/0022034516629112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.U.S. Department of Health and Human Services. Oral Health in America: A Report of the Surgeon General. Rockville, MD: U.S. Department of Health and Human Services, National Institute of Dental and Craniofacial Research, National Institutes of Health; 2000. [Google Scholar]
- 12.Center for Disease Control and Prevention. oral and dental health. http://www.cdc.gov/nchs/fastats/dental.htm.
- 13.American Dental Association guidelines; Update and recommendations. American Dental Association Council on Scientific Affairs. J Am Dent Assoc. 2006;137:1304–1312. doi: 10.14219/jada.archive.2006.0393. [DOI] [PubMed] [Google Scholar]
- 14.Unger JM, Hershman DL, Albain KS, Moinpour CM, Petersen JA, Burg K, Crowley JJ. Patient income level and cancer clinical trial participation. J Clin Oncol. 2013 Feb 10;31(5):536–42. doi: 10.1200/JCO.2012.45.4553. Epub 2013 Jan 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Unger JM, Gralow JR, Albain KS, Ramsey SD, Hershman DL. Patient Income Level and Cancer Clinical Trial Participation: A Prospective Survey Study. JAMA Oncol. 2016 Jan 1;2(1):137–9. doi: 10.1001/jamaoncol.2015.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Dental Association. http://www.ada.org.
- 17.Center for Disease Control and Prevention. [accessed 5-31-2016];oral health for adults. https://www.cdc.gov/oralhealth/publications/factsheets/adult_oral_health/adults.htm.