Abstract
Succinate dehydrogenase (SDH) is a key mitochondrial enzyme complex composed of 4 subunits. SDH histochemistry is routinely utilized in the assessment of muscle biopsies to reveal underlying pathology such as subsarcolemmal mitochondrial aggregates. In this study we evaluated the utility of SDHB immunohistochemistry (IHC) in 27 muscle biopsies, including 13 mitochondrial myopathies (MMs), 9 inflammatory myopathies (IMs), and 5 controls. SDHB IHC was performed on FFPE tissue sections with a mouse MAb (Abcam 21A11AE7) in parallel with histochemical SDH stains on fresh-frozen tissue. In all muscle biopsies SDHB IHC exhibited granular immunoreactivity and highlighted the dark type 1 and lighter type 2 staining pattern observed by histochemistry. In all cases of MM SDHB IHC showed subsarcolemmal granular aggregates involving the entire periphery of the fibers that were more distinct than those seen by SDH histochemistry. In three extraocular muscle biopsies SDHB immunoreactive speckles of various sizes were distributed throughout the entire sarcoplasm that were more prominent than those seen on SDH histochemistry. Subsarcolemmal and cytoplasmic granular aggregates seen on SDHB IHC correlated with mitochondrial pathology on electron microscopy. In cases of IM, there was diffuse sarcoplasmic SDHB immunoreactivity in degenerating fibers but no evidence of subsarcolemmal aggregates. This study demonstrates that SDHB IHC is highly sensitive and specific in the identification of MM. The automation, reproducibility, and cost efficiency of SDHB IHC offer advantages over the labor intensive histochemical method requiring frozen sections. Since this technique is performed on FFPE tissues, it can be easily applied for retrospective studies.
INTRODUCTION
Succinate dehydrogenase (SDH) is a mitochondrial enzyme complex located in the inner mitochondrial membrane that has dual roles in the oxidation of succinate to fumarate in the Krebs cycle and in electron transport during oxidative phosphorylation (1). SDH is a heterodimeric complex composed of four protein subunits (SDHA, SDHB, SDHC, and SDHD) and SDH assembly factor A2 (SDHA2), which is required for stabilization of the SDH complex (2). SDHA and SDHB form the catalytic core, whereas SDHC and SDHD anchor the complex to the inner mitochondrial membrane (2).
A variety of mitochondrial enzymes, including SDH, may be affected in mitochondrial diseases. These disorders involve tissues generating high amounts of ATP in mitochondria, particularly skeletal muscle, myocardiocytes, and neurons (3). SDH histochemical assay is a key tool in the evaluation of muscle biopsies from patients with mitochondrial myopathies (4). SDH histochemistry highlights the oxidative capacity of muscle fibers and therefore allows for the differentiation of type I, or high oxidative capacity (dark staining), and type II, or low oxidative capacity (light staining), fibers. Additionally, abnormal subsarcolemmal mitochondrial aggregates are readily visualized on SDH histochemical stains in cases of mitochondrial myopathies. These abnormal fibers often have the appearance of “ragged-red fibers”, a term designated by the appearance on a Gomori-trichrome stain (5-7). Confirmation of the presence of abnormal mitochondria is usually done by electron microscopy. Characteristic ultrastructural changes include an increased number of mitochondria, altered mitochondrial size, shape, and formation of cristae, and the presence of crystalline or osmiophilic inclusions (8).
SDHB immunohistochemistry has emerged as a reliable method for diagnosing and screening of SDH-deficient tumors, including SDH-deficient pheochromocytoma/paraganglioma, SDH-deficient GIST, and SDH-deficient renal cell carcinoma (9,10). If any of the SDH subunits are inactivated, the entire complex becomes unstable, resulting in degradation of the SDHB subunit (10). SDHB immunohistochemistry may be performed on archival, formalin fixed paraffin embedded tissues using a commercially available mouse monoclonal antibody (10). The goal of this study is to evaluate the staining patterns of SDHB immunoreactivity in muscle biopsies of patients with myopathies and compare these staining patterns to those seen using the traditional SDH histochemical assay.
MATERIALS AND METHODS
Twenty nine muscle biopsies, including 13 cases of mitochondrial myopathy (MMs), 9 cases of inflammatory myopathy (5 cases of polymyositis and 4 of dermatomyositis), and 5 normal controls (NC) were retrieved form the archives of the Departments of Pathology at the Rhode Island Hospital and The Miriam Hospital. Controls were defined as biopsies with no evidence of mitochondrial abnormality and no significant inflammation or other pathologic finding. In the MM group, biopsies were taken most commonly from the quadriceps but also from the vastus lateralis, gluteus maximus, and posterior neck. In three cases of MM the biopsies were taken from the extraocular levator palpebrae muscle. In the inflammatory myopathy group, biopsies were most commonly taken from the deltoid muscle but also from the quadriceps and gluteus maximus muscles. Biopsies in the control group were taken from the deltoid or quadriceps muscle.
The original hematoxylin and eosin (H&E) sections were reviewed by 2 pathologists (M.P. and E.S.). Diagnoses were supported by adjunctive histochemistry including Gomori trichrome, ATPase, SDH, COX, NADH, and immunohistochemical staining for slow and fast myosin using mouse monoclonal antibodies at 1:100 and 1:200 dilution respectively (clone WB-MHCs at 1:100 and clone WB-MHCf at 1:200 dilution, respectively; Leica, Newcastle, UK) in all cases.
Ultrastructural examination of mitochondria was assessed by electron microscopy in all cases of MM. In two cases genomic analysis of mitochondrial DNA was performed.
Histochemical SDH staining
Histochemical SDH staining was performed on fresh-frozen tissue (FFT). Appropriate cross-sections of muscle were selected, rolled in talc, and submerged in liquid nitrogen. The tissue was then stored in a minus 80° C freezer until used. All samples were sectioned on a cryostat at 8-12 micrometers. Enzymatic activity of SDH was assayed by placing the slides in SDH incubating solution, containing sodium succinate as a substrate and nitro-blue tetrazolium (NBT) for visualization of reaction for one hour at 37°C (11). Reduced NBT forms a highly colored formazan dye that is finely granular blue. The pattern of staining was evaluated by light microscopy and compared to control muscle tissue. The staining intensity was scored as strong when speckled pattern of staining was easily detectable at low magnification (×40), or as weak when the staining was seen at higher-power magnifications (×200 and ×400) but not clearly observed at lower magnifications.
Immunohistochemical SDHB staining
IHC staining was performed on 4-μm-thick formalin-fixed, paraffin-embedded (FFPE) whole tissue sections using a Dako Autostainer, with a polymer-based detection system and a Dako EnVision FLEX High pH kit (Dako, Carpinteria, CA). A mouse monoclonal antibody against SDHB (clone 21A11AE7; Abcam, Cambridge, MA) was used at a dilution of 1:200. Appropriate positive and negative controls were stained in parallel. Staining results were assessed by two pathologists (M.P. and E.S.) in a blinded manner. The staining intensity was scored similarly to the histochemical assay.
RESULTS
Clinical characteristics
The patients included 13 males and 14 females and ranged in age from 3 to 91 years (Table 1). Most patients had a combination of myopathic features, such as weakness, pain, exercise intolerance, or hypotonia. Medical histories were significant in some cases for diabetes, hypertension, obesity, and seizures. Eight patients with MM presented with ptosis or ophthalmoplegia. Of these, two were diagnosed with Kearns-Sayre syndrome, a form of mitochondrial myopathy characterized by adult onset chronic progressive external opthalmoplegia (12). Mitochondrial DNA testing was performed in one case, a vastus lateralis biopsy from a 3 year old female, and showed a homoplasmic variant, 619 T>C. A second patient, a 47 year old female with adult onset motor and sensory neuropathy and myopathy, had a prior diagnosis of mitochondrial DNA polymerase (POLG1) gene mutation.
Table 1.
Clinical Features, Staining Characteristics, and Pathologic Diagnosis
| N | Sex | Age | Biopsy Site | Medical History | SDH Histoche mistry |
SDHB IHC |
Fiber type | Red Ragged Fibers |
Patholo gic Diagno sis |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 3 | Vastus Lateralis |
Seizures, severe developmental delay, homoplasmic variant 619 T>C |
SA | SA | Marked type 1 predominance |
No | MM |
| 2 | M | 73 | Quadriceps | Chronic progressive external opthalmoplegia |
SA | SA | No predominance | Rare | MM |
| 3 | F | 47 | Gluteus Maximus/Qua driceps |
Adult onset motor and sensory neuropathy, myopathy, ptosis, early diplopia. mDNA polymerase (POLG1) gene mutation |
SA | SA | Marked type 1 predominance |
Rare | MM |
| 4 | M | 84 | Quadriceps | Progressive lower extremity weakness p-ANCA and MPO + vasculitis, HTN |
SA | SA | Type 2 predominance | No | MM |
| 5 | M | 53 | Quadriceps | Seizures, memory loss, migraines, HTN. Jerking of limbs and decreased strength |
SA | SA | Type 1 predominance | No | MM |
| 6 | F | 53 | Leg (not specified) |
Ophthalmoplegia, rhabdomyolysis. Chronic ptosis and muscle weakness |
SA | SA | No predominance | No | MM |
| 7 | M | 13 | Quadriceps | Kearns-Sayre Syndrome, ptosis, retinitis pigmentosa |
SA | SA | Type 1 predominance | Rare | MM |
| 8 | F | 70 | Quadriceps | HTN, dementia | SA | SA | No predominance | Numerous | MM |
| 9 | F | 52 | Posterior Neck |
CAD, HTN, Type 1 Diabetes Proximal muscle weakness |
SA | SA | Type 1 predominance | Rare | MM |
| 10 | M | 35 | Thigh | Chronic regional pain syndrome, ptosis since childhood |
SA | SA | No predominance | Numerous | MM |
| 11 | M | 56 | Levator Palpebrae |
Oculopharyngeal dystrophy. Ptosis, dysphagia |
Focal SDA |
SDA | No predominance | Scattered | MM |
| 12 | M | 32 | Levator Palpebrae |
Chronic back pain, anxiety, HTN. B/L levator ptosis |
Focal SDA |
SDA | No predominance | No | MM |
| 13 | F | 13 | Levator Palpebrae |
Kearns-Sayre Syndrome. Ptosis and opthalmoplegia |
Focal SDA |
SDA | Type 1 predominance | Numerous | MM |
| 14 | F | 67 | Gluteus maximus |
Weakness and dysphagia | Rare DF | Rare DF | Type 2 atrophy | No | PM |
| 15 | F | 21 | Deltoid | Progressive weakness | Rare DF | Rare DF | No predominance | No | PM |
| 16 | M | 32 | Quadriceps | Weakness of upper and lower extremities |
Rare DF | Rare DF | Type 2 predominance | No | PM |
| 17 | M | 45 | Muscle unspecified |
Severe bilateral calf pain and weakness |
Rare DF | Rare DF | Type 1 predominance | No | PM |
| 17 | F | 62 | Quadriceps | Proximal muscle pain and dysphagia | Rare DF | Rare DF | Type 1 predominance | No | PM |
| 19 | F | 65 | Thigh | Muscle pain with heliotrope rash | Rare DF | Rare DF | No predominance | No | DM |
| 20 | M | 79 | Deltoid | Arm and leg weakness with chest and arm rash |
No SA | No SA | No predominance | No | DM |
| 21 | F | 91 | Deltoid | Severe muscle weakness and rash on extremities and trunk |
Rare DF | Rare DF | Type 1 predominance | No | DM |
| 22 | F | 88 | Deltoid | Progressive muscle wasting and weakness |
Rare DF | Rare DF | Type 1 predominance | No | DM |
| 23 | M | 33 | Quadriceps | Muscle pain and weakness | No SA | No SA | No predominance | No | NC |
| 24 | M | 74 | Thigh | Muscle pain and weakness | No SA | No SA | Type 2 predominance | No | NC |
| 25 | F | 58 | Quadriceps | Muscle and joint pain | No SA | No SA | Slight type 2 predominance |
No | NC |
| 26 | M | 33 | Deltoid | Chronic shoulder pain | No SA | No SA | No predominance | No | NC |
| 27 | F | 65 | Deltoid | Progressive muscle weakness | No SA | No SA | Type 1 predominance | No | NC |
IHC, immunohistochemistry; SA, subsarcolemmal aggregates; SDA, subsarcolemmal and diffuse aggregates; DF, degenerative fibers; MM, mitochondrial myopathy; PM, polymyositis; DM, dermatomyositis; NC, normal control.
SDHB immunohistochemcal and histochemical staining
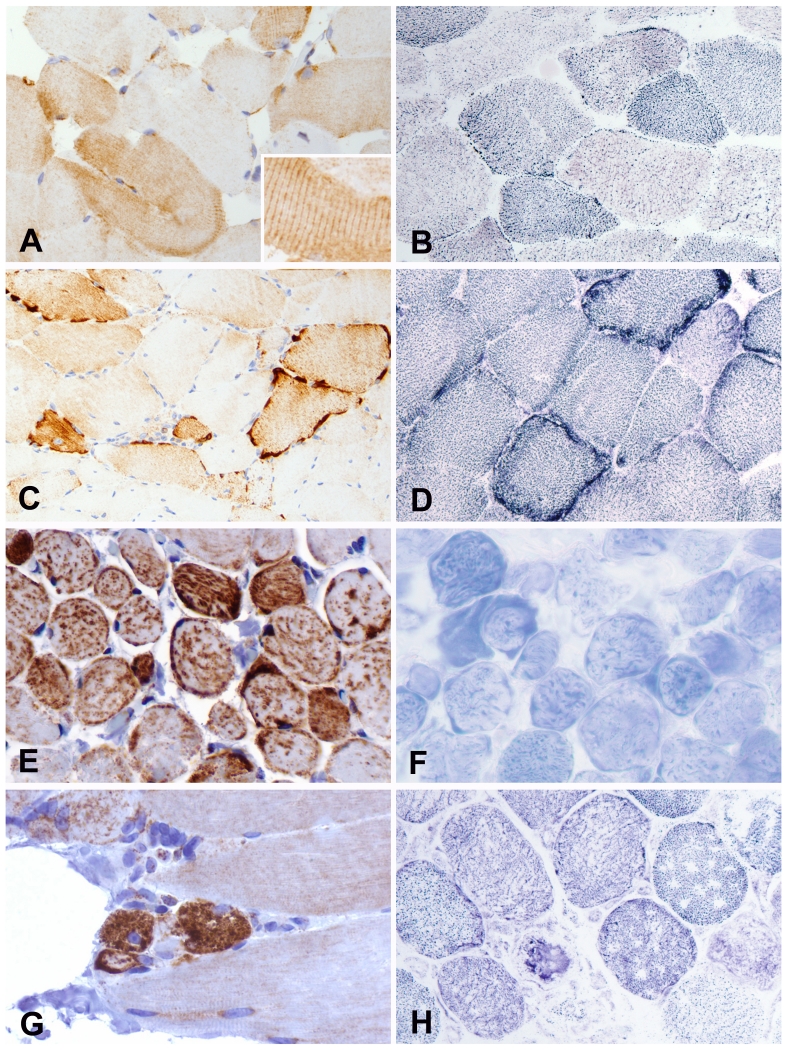
In all muscle biopsies including study and control groups SDHB IHC exhibited granular cytoplasmic staining. SDHB IHC differentially expressed according to the muscle fiber type with stronger immunoreactivity in the type 1 oxidative fibers and weak staining in type 2 non-oxidative fibers in all study groups and in normal controls (Figure 1A). This staining pattern closely resembled the type 1 (dark) and type 2 (light) staining seen on SDH histochemistry (Figure 1B). In contrast to SDH histochemistry, sarcomeric cross-striations were readily seen on SDHB IHC (Figures 1A).
FIGURE1.
Paired SDHB IHC (left) and SDH histochemistry (right) images. A,B, Fiber-type staining pattern with stronger reactivity in the type 1 oxidative fibers and weak staining in type 2 non-oxidative fibers. Sarcomeric cross-striations were readily seen on SDHB IHC (inset) but not on SDH histostain. C, Strongly immunoreactive subsarcolemmal granular aggregates involving the entire periphery of the fibers in case of mitochondrial myopathy D, Two fibers with dark blue subsarcolemmal mitochondrial aggregates on SDH histochemistry in the same case. E, SDHB immunoreactive speckles of various sizes are diffusely distributed throughout the entire sarcoplasm in a case of mitochondrial myopathy biopsied from extraocular muscle. F, These speckles were less prominent on SDH histochemistry in the same case. G, Small degenerative fibers with diffuse rather than granular sarcoplasmic SDHB immunoreactivity in case of inflammatory myopathy, but no subsarcolemmal aggregates. H, SDH histochemistry in the same case with small darker degenerative fiber and fibers with empty spaces as a result of freezing artifact.
In all cases of MM SDHB IHC exhibited scattered fibers with strongly immunoreactive subsarcolemmal granular aggregates that were more distinct than those seen by SDH histochemistry (Figure 1C and 1 D). The subsarcolemmal aggregates involved the entire periphery of the fibers (Figure 1C). In three ocular biopsies from the levator palpebral muscle SDHB immunoreactive speckles of various sizes were distributed throughout the entire sarcoplasm (Figure 1E). These speckles were less prominent on SDH histochemistry (Figure 1F).
Subsarcolemmal and cytoplasmic granular aggregates seen on SDHB immunostain correlated with mitochondrial pathology on EM. Cases of MM showed classic ultrastructural findings of mitochondrial abnormalities including increased mitochondrial number, abnormal mitochondrial architecture, and intramitochondrial osmiophilic inclusions.
In cases of inflammatory myopathy including both polymyositis and dermatomyositis degenerating fibers were characteristically of smaller size when compared to the neighboring fibers with no degenerative changes (Figure 1G,H). There was diffuse sarcoplasmic SDHB immunoreactivity rather than granular SDHB immunoreactivity in degenerating fibers but none had evidence of subsarcolemmal aggregates (Figure 1G,H).
DISCUSSION
We evaluated immunohistochemical SDHB staining in FFPE muscle biopsies of patients with MM, inflammatory myopathies, and normal muscle. We demonstrated that SDHB IHC is highly sensitive and specific and may prove useful for the identification of MM.
SDH plays a vital role by forming a link in the chain of biochemical reactions required for the oxidation of lipids, carbohydrates, and proteins (13). More than 6 decades ago Kun and Abood were the first to utilize the tetrazolium compound for the estimation of SDH enzymatic activity (14). By using the tetrazoilum reagent Rutenberg et al estimated SDH activity in tissue homogenates (15). Seligman and Ruthenberg showed that SDH enzyme activity may be demonstrated histochemically in frozen sections of heart, kidney, liver, and brain using blue tetrazolium (16). High enzymatic SDH activity was subsequently found in skeletal muscle and localized to the mitochondria (17). SDH histochemical staining is now routinely utilized in the assessment of muscle biopsies and provides important information regarding the distribution of skeletal muscle fiber types and the presence of subsarcolemmal mitochondrial aggregates in cases of mitochondrial myopathies (8). While this assay is very useful for assessing mitochondrial abnormalities, it has several disadvantages. The histochemical method is more time-consuming and technician-dependent. Indeed the stain has to be prepared just prior to the assay and a prepared batch cannot be stored for later use. In addition, it requires frozen tissue, which may have numerous artifacts if the muscle tissue is frozen too slowly or too quickly (18). In this study, SDHB IHC on FFPE tissue was found to be a highly sensitive and highly specific in the identification of MM.
Immunohistochemical staining distinguished muscle fiber type with an accuracy very similar to histochemical staining, and is therefore useful for analyzing the distribution of fiber types in skeletal muscle. Moreover, compared to the histochemical staining method, SDHB immunohistochemistry provided more distinct visualization of mitochondrial aggregates in cases of MM. The automation, reproducibility, and cost efficiency of SDHB offer other advantages over the labor-intensive histochemical approach. The immunohistochemical technique works well on FFPE tissues and can be easily used to perform retrospective archival studies.
Historically, time consuming methods have been used to differentiate between slow and fast fibers via myosin ATPase activity at different pH levels (19), and determine the oxidative activity using cytochrome C oxidase (COX), and nicotinamide adenine dinucleotide (NADH) in addition to SDH in fresh frozen muscle tissues (20,21). Recently, immunohistochemistry with fast myosin antibody became available to provide a faster and more reliable means to identify all fiber types in a single muscle section. It is now considered to be the gold standard (22,23). COX immunohistochemistry may provide information on specific COX subunit alterations (24). SDHB immunohistochemistry is a reliable alternative to the classic histochemical staining method. As noted above, a further advantage is obtained since these antibodies have a stored shelf life of one year compared to the need to immediately use the histochemical stains that rely on enzymatic activity once they are prepared. Clearly, from a practical standpoint, application of these IHC methodologies provide potential benefits for triaging of tissue and laboratory workflow that translates into overall cost benefits.
In addition to subsarcolemmal mitochondrial aggregates, in three ocular biopsies immunoreactive aggregates filled the entire fiber. Although the anatomic pattern of muscle involvement in MM is variable, the extraocular muscles are commonly involved (3). The reason that extraocular muscles are particularly sensitive to mitochondrial disease is uncertain, but it may be that these muscles have exceptionally high requirements for ATP. In agreement with this, extraocular muscles have the most mitochondria per mass than any other body muscles. This may explain diffuse aggregates of mitochondria seen on SDHB IHC in biopsies from extraocular muscles in this study.
In this study, we did not find decrease or loss of SDHB expression in any of the MM cases. Recently, Alston and colleagues described a child who regressed rapidly after 1 year of age and presented with unsteadiness, repeated falls, eventual loss of walking ability, and loss of muscle tone (25). A diagnostic muscle biopsy showed severely reduced SDH and complex II activity, and genetic analysis identified a novel homozygous SDHB gene mutation (c.143A>T, p.Asp48Val). Further analysis revealed almost complete absence of the SDHB subunit by SDS-PAGE. Clearly, it would be of interest to assess cases with mutations in SDHB or other subunits of mitochondrial complex II that lead to deterioration of mitochondrial function by SDHB immunohistochemistry.
In conclusion, this study expands the diagnostic utility of SDHB immunohistochemistry from SDH-deficient and proficient tumors to non-neoplastic muscle biopsies. SDHB immunohistochemistry proved to be sensitive and specific for identification of MM and should be considered as a reliable alternative to the histochemical technique.
Acknowledgments
This study was supported by the Molecular Pathology Core of the COBRE Center for Cancer Research Development, funded by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number P20GM103421. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
This study was presented in part at the annual meeting of the United States and Canadian Academy of Pathology in Seattle, WA in 2016.
References
- 1.Rutter J, Winge DR, Schiffman JD. Succinate dehydrogenase - Assembly, regulation and role in human disease. Mitochondrion. 2010;10:393–401. doi: 10.1016/j.mito.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yankovskaya V, Horsefield R, Törnroth S, et al. Architecture of succinate dehydrogenase and reactive oxygen species generation. Science. 2003;299:700–4. doi: 10.1126/science.1079605. [DOI] [PubMed] [Google Scholar]
- 3.Pytel P, Anthony DC. Peripheral nerves and skeletal muscles. In: Kumar V, Abbas AK, Aster JC, editors. Robbins and Cotran Pathologic Basis of Disease. 9th ed. Elsevier Saunders; Philadelphia: 2015. pp. 1227–1250. [Google Scholar]
- 4.Bancroft JD, Stevens A. Theory and Practice of Histological Techniques. 3rd ed. Churchill Livingstone; New York: 1990. [Google Scholar]
- 5.Rifai Z, Welle S, Kamp C, et al. Ragged red fibers in normal aging and inflammatory myopathy. Annals of neurology. 1995;37:24–29. doi: 10.1002/ana.410370107. [DOI] [PubMed] [Google Scholar]
- 6.Miles L, Miles MV, Horn PS, et al. Importance of muscle light microscopic mitochondrial subsarcolemmal aggregates in the diagnosis of respiratory chain deficiency. Hum Pathol. 2012;43:1249–1257. doi: 10.1016/j.humpath.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 7.Zierz CM, Joshi PR, Zierz S. Frequencies of myohistological mitochondrial changes in patients with mitochondrial DNA deletions and the common m.3243A>G point mutation: Frequencies of myohistological. Neuropathology. 2015;35:130–136. doi: 10.1111/neup.12173. [DOI] [PubMed] [Google Scholar]
- 8.Dubowitz V, Sewry CA, Oldfors A. Histological and Histochemical Stains and Reactions. In: Dubowitz V, Sewry CA, Oldfors A, editors. Muscle biopsy. A Practical Approach. 4th ed. Saunders Elsevier; Philadelphia: 2013. pp. 16–27. [Google Scholar]
- 9.van Nederveen FH, Gaal J, Favier J, et al. An immunohistochemical procedure to detect patients with paraganglioma and phaeochromocytoma with germline SDHB, SDHC, or SDHD gene mutations: a retrospective and prospective analysis. Lancet Oncol. 2009;10:764–71. doi: 10.1016/S1470-2045(09)70164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gill AJ. Succinate dehydrogenase (SDH) and mitochondrial driven neoplasia. Pathology. 2012;44:285–92. doi: 10.1097/PAT.0b013e3283539932. [DOI] [PubMed] [Google Scholar]
- 11.Sheehan DC, Hrapchak BB. Theory and Practice of Histotechnology. 2nd ed. Battelle Press; Columbus: 1980. Enzyme histochemistry; pp. 292–309. [Google Scholar]
- 12.Chen T, Pu C, Shi Q, et al. Chronic progressive external ophthalmoplegia with inflammatory myopathy. Int J Clin Exp Pathol. 2014;7:8887–92. [PMC free article] [PubMed] [Google Scholar]
- 13.Hoekstra AS, Bayley JP. The role of complex II in disease. Biochim Biophys Acta. 2013;1827:543–51. doi: 10.1016/j.bbabio.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Kun E, Abood LG. Colorimetric Estimation of Succinic Dehydrogenase by Triphenyltetrazolium Chloride. Science. 1949;109:144–6. doi: 10.1126/science.109.2824.144. [DOI] [PubMed] [Google Scholar]
- 15.Rutenburg AM, Gofstein R, Seligman AM. Preparation of a new tetrazolium salt which yields a blue pigment on reduction and its use in the demonstration of enzymes in normal and neoplastic tissues. Cancer Res. 1950;10:113–21. [PubMed] [Google Scholar]
- 16.Seligman AM, Rutenburg AM. The histochemical demonstration of succinic dehydrogenase. Science. 1951;113:317–20. doi: 10.1126/science.113.2934.317-a. [DOI] [PubMed] [Google Scholar]
- 17.Malaty HA, Bourne GH. Histochemistry of succinic dehydrogenase. Nature. 1953;171:295–7. doi: 10.1038/171295a0. [DOI] [PubMed] [Google Scholar]
- 18.Meng H, Janssen PML, Grange RW, et al. Tissue triage and freezing for models of skeletal muscle disease. J Vis Exp. 2014;89 doi: 10.3791/51586. doi: 10.3791/51586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hintz CS, Coyle EF, Kaiser KK, et al. Comparison of muscle fiber typing by quantitative enzyme assays and by myosin ATPase staining. J Histochem Cytochem. 1984;32:655–60. doi: 10.1177/32.6.6202737. [DOI] [PubMed] [Google Scholar]
- 20.Oldfors A, Larsson N, Holme E, et al. Mitochondrial DNA deletions and cytochrome c oxidase deficiency in muscle fibres. J Neurol Sci. 1992;110:169–177. doi: 10.1016/0022-510x(92)90025-g. [DOI] [PubMed] [Google Scholar]
- 21.Bourgeois JM, Tarnopolsky MA. Pathology of skeletal muscle in mitochondrial disorders. Mitochondrion. 2004;4:441–52. doi: 10.1016/j.mito.2004.07.036. [DOI] [PubMed] [Google Scholar]
- 22.Jay V, Becker LE. Fiber-type differentiation by myosin immunohistochemistry on paraffin-embedded skeletal muscle. A useful adjunct to fiber typing by the adenosine triphosphatase reaction. Arch Pathol Lab Med. 1994;118:917–8. [PubMed] [Google Scholar]
- 23.Rojiani AM, Cho ES. Neuropathologic applications of immunohistochemical fiber typing in the non-neoplastic muscle biopsy. Mod Pathol. 1998;11:334–8. [PubMed] [Google Scholar]
- 24.Rahman S, Lake BD, Taanman JW, et al. Cytochrome oxidase immunohistochemistry: clues for genetic mechanisms. Brain. 2000;123:591–600. doi: 10.1093/brain/123.3.591. [DOI] [PubMed] [Google Scholar]
- 25.Alston CL, Davison JE, Meloni F, van der Westhuizen FH, He L, Hornig-Do HT, Peet AC, Gissen P, Goffrini P, Ferrero I, Wassmer E, McFarland R, Taylor RW. Recessive germline SDHA and SDHB mutations causing leukodystrophy and isolated mitochondrial complex II deficiency. J Med Genet. 2012;49:569–77. doi: 10.1136/jmedgenet-2012-101146. [DOI] [PMC free article] [PubMed] [Google Scholar]