Abstract
AIM
To identify potential anti-cancer constituents in natural extracts that inhibit cancer cell growth and migration.
METHODS
Our experiments used high dose thymoquinone (TQ) as an inhibitor to arrest LoVo (a human colon adenocarcinoma cell line) cancer cell growth, which was detected by cell proliferation assay and immunoblotting assay. Low dose TQ did not significantly reduce LoVo cancer cell growth. Cyclooxygenase 2 (COX-2) is an enzyme that is involved in the conversion of arachidonic acid into prostaglandin E2 (PGE2) in humans. PGE2 can promote COX-2 protein expression and tumor cell proliferation and was used as a control.
RESULTS
Our results showed that 20 μmol/L TQ significantly reduced human LoVo colon cancer cell proliferation. TQ treatment reduced the levels of p-PI3K, p-Akt, p-GSK3β, and β-catenin and thereby inhibited the downstream COX-2 expression. Results also showed that the reduction in COX-2 expression resulted in a reduction in PGE2 levels and the suppression of EP2 and EP4 activation. Further analysis showed that TG treatment inhibited the nuclear translocation of β-catenin in LoVo cancer cells. The levels of the cofactors LEF-1 and TCF-4 were also decreased in the nucleus following TQ treatment in a dose-dependent manner. Treatment with low dose TQ inhibited the COX-2 expression at the transcriptional level and the regulation of COX-2 expression efficiently reduced LoVo cell migration. The results were further verified in vivo by confirming the effects of TQ and/or PGE2 using tumor xenografts in nude mice.
CONCLUSION
TQ inhibits LoVo cancer cell growth and migration, and this result highlights the therapeutic advantage of using TQ in combination therapy against colorectal cancer.
Keywords: Thymoquinone, LoVo cell, Cyclooxygenase 2, Prostaglandin E2, Migration
Core tip: Prostaglandin E2 (PGE2) induces migration of human LoVo colon cancer cells, and the major mechanism involves the activation of the p-Akt/p-PI3K/p-GSK3β/β-catenin/LEF-1/TCF-4 pathway that ultimately up-regulates cyclooxygenase 2 (COX-2) expression. Thymoquinone (TQ) suppresses cancer cell migration and represents a potential therapeutic target for colon adenocarcinoma metastasis. PGE2 activation of COX-2 and β-catenin to induce human LoVo colon cancer cell migration was blocked by TQ. Our study used cell proliferation assay, immunoblotting assay, immunofluorescence assay, nuclear extraction and in vivo experiments to examine the COX2 protein, which affects the metastasis of highly metastatic LoVo cancer cells treated with TQ.
INTRODUCTION
Colorectal cancer is one of the most universally diagnosed gastrointestinal cancers and among the main causes of cancer-related death in western developed countries[1,2]. Despite advanced chemotherapeutic treatments, more than 130000 new cases of colon cancer are diagnosed each year[3], causing more than 56000 deaths/year in America[4]. Thymoquinone (TQ) is a phytochemical compound isolated from Nigella sativa that possesses anti-carcinogenic activity and induces apoptosis in tumor cells, and it can interfere with cancer cell survival through different mechanisms[5,6]. Available treatments for cancer include surgical removal, chemotherapy and adjuvant chemotherapy for patients who are strong enough to undergo it. To date, the surgical removal of cancer tissue is considered the most appropriate way to address colon cancer. Our present study investigated the use of phytochemical drugs as a supplementary chemotherapy approach. Laboratory studies have shown that TQ significantly inhibits oral cancer through the p38β MAPK family[7]. Among the hereditary colon cancers, hereditary non-polyposis colon cancer (HNPCC) patients present a particularly high risk for synchronous metastasis via the lymphatic system[8,9]. In this study, we used the colorectal cancer cell line LoVo, which was developed from a 56-year-old colon cancer patient. Many previous studies have verified that prostaglandin E2 (PGE2) promotes cancer development and have considered it a cancer marker; therefore, we used PGE2 as a control[10-12]. PGE2 seems to assist cell survival in colorectal cancer cells by augmenting Ras-MAPK signaling[13].
Compared to normal intestinal tissues, COX-2 expression is 80%-90% higher in colorectal cancers. Cancers of the head, breast, cervix, bladder and gastrointestinal system have also shown high levels of COX-2 expression[14-16]. COX-2/PGE2 signaling affects cell physiology in multiple tumor types and maintains colorectal tumorigenesis[12,17]. PGE2 as a proangiogenic factor is associated with transformed vascular permeability and angiogenesis[18]. COX-2 expression is thought to contribute to the principal PGE2 metabolic product[19,20]. Some non-steroidal anti-inflammatory drugs (NSAIDs) and vegetables produce anti-tumor effects that reduce PGE2 synthesis or inhibit COX-2[21-24]. In our experiments, we sought to identify compounds similar to NSAIDs or adjunct drugs to increase the effectiveness of cancer chemotherapy. Our experimental drug, TQ, has promising anti-tumor effects, and it inhibited the incidence of fore-stomach tumors and fibrosarcoma tumors and increased cellular longevity[25,26]. We previously evaluated PGE2-induced migration in human LoVo cancer cells, and the major mechanism involves the activation of the p-Akt/p-PI3K/p-GSK3β/β-catenin pathway that ultimately up-regulates COX-2 expression (unpublished data). After the addition of TQ, the exact anticancer mechanism produced by PGE2 was determined. Previous studies have demonstrated that β-catenin translocation, which includes co-interaction with and activation of the promoters LEF-1 and TCF-4, subsequently modulates downstream gene expression[27]. The nuclear cofactors LEF and TCF were triggered to initiate the transcription and translation of COX-2[28]. Cell metastasis efficiency is a focus of our work because it correlates with COX-2 activity[29,30]. Moreover, cell migration is promoted due to COX-2 expression[31]. Numerous studies of animals treated with TQ have demonstrated that TQ is not toxic[32-34].
Our study used immunoblotting assays, immunofluorescence assays, nuclear extraction and in vivo experiments to examine the COX2 protein, which affects the metastasis of highly metastatic LoVo cancer cells treated with TQ.
MATERIALS AND METHODS
Cells, antibodies, reagents, and enzymes
The human colon cancer cell line LoVo was obtained from the American Tissue Culture Collection (ATCC) (Rockville, MD, United States). LoVo cells were established from metastatic nodules that were resected from a 56-year-old colon adenocarcinoma patient.
We utilized antibodies against the following proteins: phospho-PI3K, phospho-Akt, COX-2, phospho-GSK3β, β-catenin, LEF-1, HADAC-1 (Santa Cruz Biotechnology, Inc. Santa Cruz, CA, United States), and TCF-4 (Cell Signaling Technology, Inc. Beverly, MA, United States). α-tubulin and β-actin (Santa Cruz Biotechnology, Inc. Santa Cruz, CA, United States) were used as loading controls. The following horseradish peroxidase-conjugated antibodies were purchased from Santa Cruz Biotechnology, Inc. (CA, United States): goat anti-mouse IgG, goat anti-rabbit IgG, and rabbit anti-goat IgG. Nude mice were purchased from the National Laboratory Animal Center (NLAC).
Cell culture
The colon cancer cell line LoVo was cultured in 10-cm2 culture dishes in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 100 μg/mL penicillin, 100 μg/mL streptomycin, 2 mmol/L glutamine, 1 mmol/L HEPES buffer, and 10% fetal bovine serum (FBS) in humidified air (5% CO2) at 37 °C.
Cell proliferation assay
LoVo cells were seeded at a density of 1.5 × 104 cells per well in 24-well plates, and after 24 h the cells were treated with different concentrations of TQ varying from 0 to 20 μmol/L (Sigma Aldrich, St Louis MO, United States) dissolved in DMSO. An MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay was used to determine living cells 24 h after treatment. Culture supernatants were removed, and MTT (Sigma, United States) in phosphate-buffered saline (PBS, pH 7.4) was added to each well. After 4 h of incubation at 37 °C, the MTT solution was removed, and DMSO was added to dissolve the resultant formazan crystals. Absorbance was read at 540 nm in a Flexstation 3 device (MDS Analytical Technologies, Canada). The percentage viability was calculated as (test-background)/(control-background) × 100.
Immunoblotting assay
Cultured LoVo cells were washed with cold PBS and resuspended in lysis buffer [50 mmol/L Tris (pH 7.5), 0.5 mol/L NaCl, 1.0 mmol/L EDTA (pH 7.5), 10% glycerol, 1 mmol/L BME, 1% IGEPAL-630, and a proteinase inhibitor cocktail (Roche Molecular Biochemical)] to isolate total proteins. After incubation for 30 min on ice, the supernatant was collected by centrifugation at 12000 × g for 15 min at 4 °C. Protein concentration was then determined using the Bradford method. Samples containing equal protein amounts (60 μg) were loaded and analyzed using immunoblotting analysis. Proteins were separated using 10% SDS-PAGE and transferred onto PVDF membranes (Millipore, Belford, MA, United States). The membranes were blocked with blocking buffer (5% non-fat dry milk, 20 mmol/L Tris-HCl, pH 7.6, 150 mmol/L NaCl, and 0.1% Tween 20) for at least 1 h at room temperature. The membranes were incubated with primary antibodies in the above solution on an orbital shaker at 4 °C overnight. Following the primary antibody incubations, the membranes were incubated with horseradish peroxidase-linked secondary antibodies (anti-rabbit, anti-mouse, or anti-goat IgG). Detection was performed using ECL reagents and a digital imaging system.
Nuclear extraction
Cytoplasmic and nuclear fractions were obtained using extraction reagents containing membrane lysis buffer [10 mmol/L HEPES (pH 8.0), 1.5 mmol/L MgCl2, 10 mmol/L KCl, 1 mmol/L DDT, and proteinase inhibitor] and nuclear lysis buffer [20 mmol/L HEPES (pH 8.0), 1.5 mmol/L MgCl2, 10 mmol/L NaCl, 1 mmol/L DDT, 0.2 mmol/L EDTA, 0.25 mol/L glycerol, and proteinase inhibitor]. Following treatment, the cells were resuspended in PBS, incubated with ice-cold membrane lysis buffer for 10 min, and centrifuged at 12000 rpm for 2 min to pellet nuclei. The supernatant was stored for use as a cytoplasmic fraction, and the nuclear pellet was lysed with nuclear lysis buffer to obtain a nuclear fraction.
Immunofluorescence assay
LoVo cells were treated with increasing TQ dosages (5, 10, and 20 μmol/L) for 24 h, and PGE2 (5 μmol/L) was added for the last six hours. An immunofluorescence assay was performed on the LoVo cells using a β-catenin antibody (1:250) directed against a target of interest. A fluorophore-conjugated secondary antibody (green) that was directed against the primary antibody was used for detection, and a blue fluorescent DAPI nuclear acid counterstain that preferentially stains dsDNA was included. Anti-β-catenin (green) merged with DAPI (blue) is also shown in the resultant confocal microscopy images.
Migration assay
A migration assay was performed using a 48-well Boyden chamber (Neuro Probe) plate with 8-μm pore size polycarbonate membrane filters[35]. The lower compartment was filled with DMEM containing 10% FBS. LoVo cells were placed in the upper part of the Boyden chamber, which contained serum-free medium, and incubated for 4-8 h. After incubation, cells on the membrane filter were fixed with methanol and stained with 0.05% Giemsa for 1 h. The cells on the upper filter surface were removed with a cotton swab. The filters were then rinsed in double-distilled water for additional stain leaching. The cells were then air-dried for 20 min. Migratory phenotypes were determined by counting the cells that migrated to the lower side of the filter using microscopy at 200 × and 400 × magnification. Ten fields were blindly selected and counted as the mean cell number in each filter. The sample was assayed in triplicate.
Study animals
Twelve nude mice were bred in the animal care facility of the Chinese Medicine Library (Taichung, Taiwan) and were purchased from The National Laboratory Animal Center. The animals received subcutaneous injections of LoVo colorectal cancer cells (1 × 106 cells per injection) in the back. Ambient temperature was maintained at 25 °C, and the mice were kept on an artificial 12-h light-dark cycle, with the light period beginning at 7:00 am. All of the protocols were approved by the Institutional Animal Care and Use Committee of China Medical University, Taichung, Taiwan, ROC.
LoVo cells were treated with PGE2 (5 μmol/L) for 24 h and were then injected subcutaneously into the mice (1 × 106 cells). After one week, various concentrations (0.5, 10 and 20 μmol/L) of TQ were administered for three weeks (3 times per week) by intraperitoneal injection. All protocols were reviewed and approved by the Institutional Review Board (IRB, ethical clearance number 104-223-N), Animal Care and Use Committee of China Medical University, Taichung, Republic of China, and the study was conducted in accordance with the principles of laboratory animal care[36].
Statistical analysis
Each experiment was duplicated at least three times. The results are presented as the mean ± SE, and statistical comparisons were made using Student’s t-test. Significance was defined at P < 0.05 or 0.01.
RESULTS
Effect of TQ on viability of LoVo cells
We first tested the effect of TQ on the viability of LoVo cells. Several TQ concentrations were used to evaluate cell viability after co-culture for 24 h. The results indicated that 20 μmol/L TQ can significantly reduce LoVo cell proliferation (Figure 1).
Figure 1.
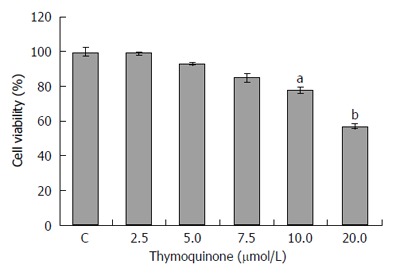
Thymoquinone affects the viability of LoVo colon cancer cells. To explore the effect of thymoquinone (TQ) on the viability of human LoVo colon cancer cells, we first treated LoVo cells with various concentrations (2.5, 5, 7.5, 10 and 20 μmol/L) of TQ for 24 h and subsequently measured cell viability by MTT assay. The results showed a significant reduction of cell viability of approximately 60% following treatment (20 μmol/L) for 24 h. Each value represents the mean ± SE. aP < 0.05; bP < 0.01.
p-PI3K-, p-Akt-, p-GSK3β- and β-catenin-induced COX-2 expression is down-regulated by TQ
Previous reports have demonstrated that PGE2 increases COX-2 expression via activation of the EP4/β-catenin pathway, suggesting that PGE2 regulates p-PI3K, p-AKT, and p-GSK-3β expression in LoVo cells. PGE2 has been reported to activate downstream signaling via EP2 and EP4 to elicit various biological responses. As shown in Figure 2, TQ treatment reduced the up-regulation of COX-2 expression by PGE2, and β-catenin had an obvious effect on EP2 and EP4 regulation by PGE2.
Figure 2.
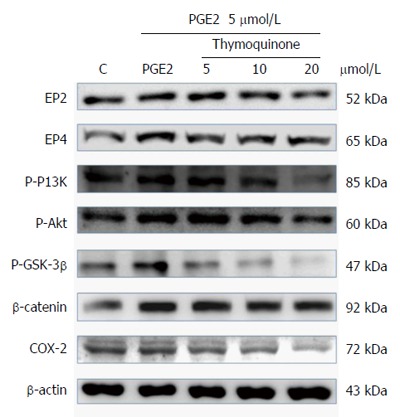
Thymoquinone inhibits the expression of PI3K, Akt, p-GSK3β, β-catenin and COX-2 proteins in LoVo cells. LoVo cells were cultured in medium that was treated with TQ (5, 10 and 20 μmol/L) for 24 h; PGE2 (5 μmol/L) was added for the last 6 h. Following this, the cells were immunoblotted with the indicated antibodies. The cells were lysed, and the extracts were separated by 10% SDS-PAGE, transferred to PVDF membranes and immunoblotted with antibodies against p-PI3K, p-Akt, p-GSK3β, β-catenin and COX-2.
Effect of TQ on β-catenin translocation
β-catenin is a key protein that affects nuclear COX-2 transcription. We used an immunofluorescence assay to evaluate β-catenin protein levels in the cytosol and nucleus. Confocal microscopy confirmed that TQ treatment led to the translocation of β-catenin into the LoVo cell nucleus (Figure 3).
Figure 3.
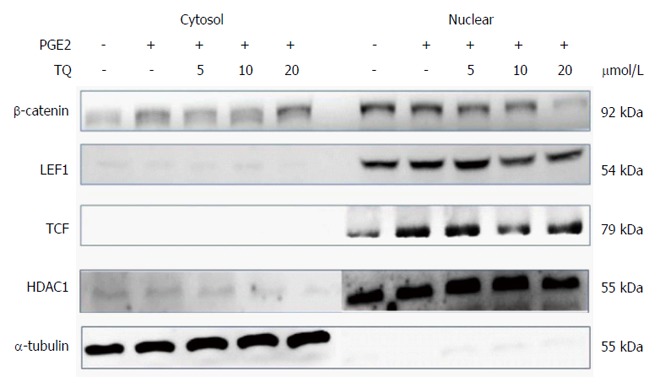
Thymoquinone inhibits β-catenin nuclear translocation in the LoVo cancer cell line. Nuclear isolation showed a decrease in β-catenin translocation into the nucleus. The cofactors LEF-1 and TCF-4 decreased in the nucleus following TQ treatment in a dose-dependent manner. TQ: Thymoquinone.
Nuclear translocation inhibition of β-catenin in the LoVo cancer cell line
Previous studies have demonstrated that the translocation of β-catenin involves co-interaction with and activation of the promoters LEF-1 and TCF-4, which subsequently modulate downstream gene expression. Evaluation of isolated LoVo cell nuclei showed that β-catenin translocation decreased in the nucleus, but its interactions with the cofactors LEF-1 and TCF-4 did not change. We observed a concentration-dependent decrease in the levels of β-catenin and the proteins LEF-1 and TCF-4 in the nucleus following TQ treatment (Figure 4).
Figure 4.
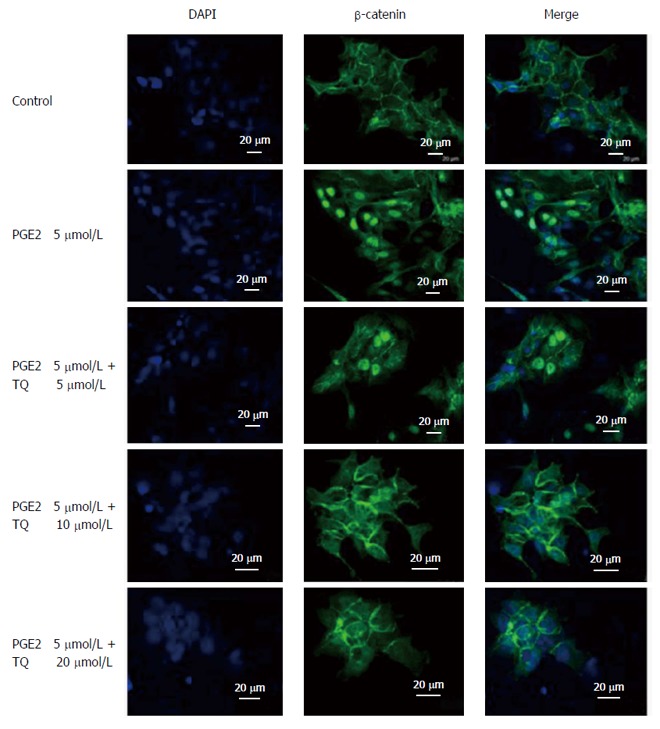
Thymoquinone treatment inhibits β-catenin translocation into LoVo cell nuclei. An immunofluorescence assay was performed on LoVo cells using a β-catenin primary antibody and a secondary antibody (1:250) producing green fluorescence; DAPI (blue fluorescence) was included to stain cell nuclei. Merged β-catenin and DAPI (green and blue, respectively) signals are shown. The indicated treatments were assessed. PGE2: Prostaglandin E2; TQ: Thymoquinone.
TQ treatment inhibits LoVo cell migration
Migration is an important step in LoVo colon cancer progression because this cancer metastasizes supraclavicular lymph nodes. We performed a Boyden chamber migration assay to determine the effect of TQ on LoVo cells and found that TQ inhibited LoVo cell migration in a dose-dependent manner (Figure 5).
Figure 5.
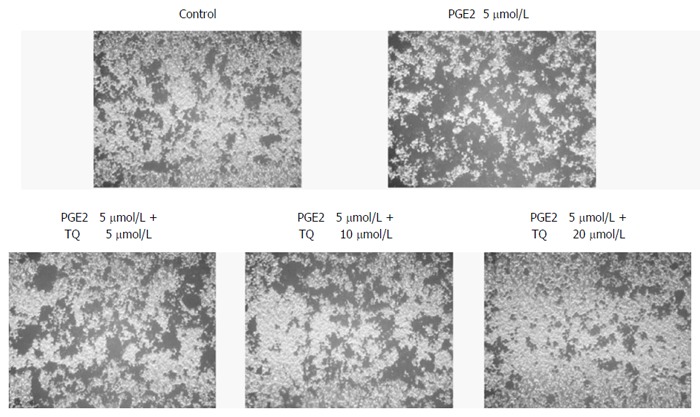
Thymoquinone efficiently inhibits LoVo cell migration. LoVo cells were pretreated with increasing dosages (5, 10, and 20 μmol/L) of TQ for 24 h. A Boyden chamber migration assay was performed to assess cell migration ability. The responses to different treatments were analyzed via microscopy. PGE2: Prostaglandin E2; TQ: Thymoquinone.
Effect of TQ on tumor growth in nude mouse xenografts
To verify previous results on the growth inhibition and anti-metastatic effects of TQ in human LoVo cells, the therapeutic potential of TQ in a pre-subcutaneous LoVo cell model in nude mice was determined. TQ was administered for 30 consecutive days. The drug did not induce death or weight loss of more than 15% to 20% of original weight, which is a very sensitive parameter for perniciousness in mice. The effects of TQ on PGE2-associated protein markers for inflammation and metastasis in the nude mouse xenograft model were then determined. COX-2 and β-catenin protein levels in different tissue groups in the nude mice were down-regulated by TQ treatment in a concentration-dependent manner (Figure 6).
Figure 6.
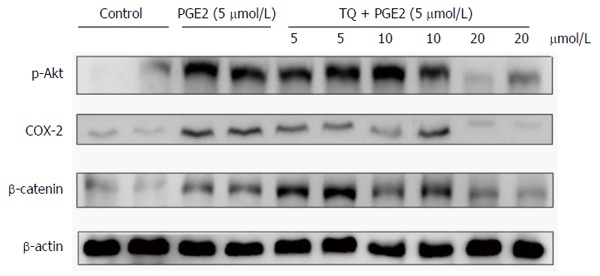
Impact of thymoquinone and/or prostaglandin E2 administration on tumor growth in nude mice xenografts. Tumor tissues were harvested from nude mice and lysed. Protein content was quantified and analyzed by immunoblotting. p-Akt, β-catenin and COX-2 levels in LoVo cells were detected. PGE2: Prostaglandin E2; TQ: Thymoquinone.
DISCUSSION
TQ was shown to be an anticancer agent by virtue of its anti-proliferative potential and capacity to induce cell cycle arrest[37]. In our previous studies, COX-2 protein was found to be regulated by PGE2, resulting in cell proliferation and migration. Here, a 20 μmol/L concentration of TQ, which is 50% of the non-cytotoxic concentration determined by MTT assay, arrested the migration of LoVo colorectal cancer cells. We tested TQ on LoVo colorectal cancer cells that were already induced by PGE2 (5 μmol/L); these cells exhibited significantly enhanced performance over the control group. Our experimental drug TQ inhibited the survival pathway proteins p-PI3K, p-Akt, p-GSK3β, β-catenin and COX-2.
When β-catenin is translocated from the cytosol into the nucleus, it plays a role in the transcription and translation of COX-2[28]. The translocation of β-catenin from the cytosol into the nucleus also plays a role in the transcription and translation of COX-2.
Nuclear isolation techniques and immunofluorescence were used to determine the localization of β-catenin and the COX-2 transcription cofactors LEF-1 and TCF-4[38]. Our experiments showed that TQ suppresses β-catenin translocation induced by PGE2, and the expression of both LEF-1 and TCF-4 was also suppressed. This result suggests that β-catenin loses its ability to translocate into the nucleus and bind to LEF-1 and TCF-4 following TQ treatment. These cofactors were also affected by TQ with a gradually declining, concentration-dependent trend. Immunofluorescence confocal microscopy showed fluorescent images of anti-β-catenin (green) and DAPI staining (blue) in the panel representing the cell nucleus. These results indicate that TQ treatment inhibited β-catenin translocation into the nucleus and subsequently reduced COX-2 activation.
We can observe the impact of TQ on COX-2 by assessing these translation- and transcription-associated proteins. COX-2 plays a central role in cell migration. These results showed that decreasing COX-2 levels will correspondingly reduce cell migration. In this context, we demonstrated that TQ significantly reduces metastasis in vivo. However, we investigated the therapeutic potential of TQ using a highly aggressive human LOVO cancer cell xenograft nude mouse model. p-Akt, β-catenin and COX-2 expression substantially decreased. TQ inhibited LoVo colorectal cancer cell growth and inhibited migration. In the future, we will evaluate the therapeutic advantage of combining chemotherapeutic agents for colorectal cancer.
COMMENTS
Background
Several types of phytochemicals are used for cancer chemotherapy. The aim was to identify potential anti-cancer constituents in natural extracts that inhibit cancer cell growth and migration. Thymoquinone (TQ) is a phytochemical compound isolated from Nigella sativa. Previous data show that TQ suppresses the activation of AKT, inhibits cellular proliferation, and shows anti-oxidant/anti-inflammatory effects.
Research frontiers
Prostaglandin E2 (PGE2) induces migration of human LoVo colon cancer cells, and the major mechanism involves the activation of the p-Akt/p-PI3K/p-GSK3β/β-catenin/LEF-1/TCF-4 pathway that ultimately up-regulates cyclooxygenase 2 (COX-2) expression.
Innovations and breakthroughs
TQ suppresses cancer cell migration and represents a potential therapeutic target for colon adenocarcinoma metastasis. PGE2 activation of COX-2 and β-catenin to induce human LoVo colon cancer cell migration was blocked by thymoquinone.
Applications
The results reveal that TQ can be considered a potential treatment strategy for advanced stage colon cancer treatment.
Peer-review
The study described in this paper investigated the use of phytochemical drugs as a supplementary chemotherapy approach. For this purpose the authors used the high dose of TQ as an inhibitor to arrest LoVo (a human colon adenocarcinoma cell line) cell growth. The authors used immunoblotting assays, immunofluorescence assays, nuclear extraction and in vivo experiments to examine the COX2 protein, which affects the metastasis of highly metastatic LoVo cancer cells treated with TQ.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Taiwan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by the Institutional Review Board of China Medical University, Taiwan.
Institutional animal care and use committee statement: All protocols were reviewed and approved by the Institutional Review Board (IRB, ethical clearance number 104-223-N), Animal Care and Use Committee of China Medical University, Taichung, China, and the study was conducted in accordance with the principles of laboratory animal care.
Conflict-of-interest statement: We declare that there are no conflicts of interest to disclose.
Data sharing statement: No additional data are available.
Peer-review started: September 8, 2016
First decision: October 20, 2016
Article in press: December 19, 2016
P- Reviewer: Kunac N S- Editor: Gong ZM L- Editor: Wang TQ E- Editor: Wang CH
References
- 1.Gali-Muhtasib H, Ocker M, Kuester D, Krueger S, El-Hajj Z, Diestel A, Evert M, El-Najjar N, Peters B, Jurjus A, et al. Thymoquinone reduces mouse colon tumor cell invasion and inhibits tumor growth in murine colon cancer models. J Cell Mol Med. 2008;12:330–342. doi: 10.1111/j.1582-4934.2007.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin TY, Fan CW, Maa MC, Leu TH. Lipopolysaccharide-promoted proliferation of Caco-2 cells is mediated by c-Src induction and ERK activation. Biomedicine (Taipei) 2015;5:5. doi: 10.7603/s40681-015-0005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jemal A, Thomas A, Murray T, Thun M. Cancer statistics, 2002. CA Cancer J Clin. 2002;52:23–47. doi: 10.3322/canjclin.52.1.23. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 5.Woo CC, Kumar AP, Sethi G, Tan KH. Thymoquinone: potential cure for inflammatory disorders and cancer. Biochem Pharmacol. 2012;83:443–451. doi: 10.1016/j.bcp.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 6.Towhid ST, Schmidt EM, Schmid E, Münzer P, Qadri SM, Borst O, Lang F. Thymoquinone-induced platelet apoptosis. J Cell Biochem. 2011;112:3112–3121. doi: 10.1002/jcb.23237. [DOI] [PubMed] [Google Scholar]
- 7.Abdelfadil E, Cheng YH, Bau DT, Ting WJ, Chen LM, Hsu HH, Lin YM, Chen RJ, Tsai FJ, Tsai CH, et al. Thymoquinone induces apoptosis in oral cancer cells through p38β inhibition. Am J Chin Med. 2013;41:683–696. doi: 10.1142/S0192415X1350047X. [DOI] [PubMed] [Google Scholar]
- 8.Kalady MF. Surgical management of hereditary nonpolyposis colorectal cancer. Adv Surg. 2011;45:265–274. doi: 10.1016/j.yasu.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Wu Z, Zhang S, Aung LH, Ouyang J, Wei L. Lymph node harvested in laparoscopic versus open colorectal cancer approaches: a meta-analysis. Surg Laparosc Endosc Percutan Tech. 2012;22:5–11. doi: 10.1097/SLE.0b013e3182432b49. [DOI] [PubMed] [Google Scholar]
- 10.Chang SH, Liu CH, Conway R, Han DK, Nithipatikom K, Trifan OC, Lane TF, Hla T. Role of prostaglandin E2-dependent angiogenic switch in cyclooxygenase 2-induced breast cancer progression. Proc Natl Acad Sci USA. 2004;101:591–596. doi: 10.1073/pnas.2535911100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eruslanov E, Kaliberov S, Daurkin I, Kaliberova L, Buchsbaum D, Vieweg J, Kusmartsev S. Altered expression of 15-hydroxyprostaglandin dehydrogenase in tumor-infiltrated CD11b myeloid cells: a mechanism for immune evasion in cancer. J Immunol. 2009;182:7548–7557. doi: 10.4049/jimmunol.0802358. [DOI] [PubMed] [Google Scholar]
- 12.Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C, Kaidi A. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30:377–386. doi: 10.1093/carcin/bgp014. [DOI] [PubMed] [Google Scholar]
- 13.Kaidi A, Qualtrough D, Williams AC, Paraskeva C. Direct transcriptional up-regulation of cyclooxygenase-2 by hypoxia-inducible factor (HIF)-1 promotes colorectal tumor cell survival and enhances HIF-1 transcriptional activity during hypoxia. Cancer Res. 2006;66:6683–6691. doi: 10.1158/0008-5472.CAN-06-0425. [DOI] [PubMed] [Google Scholar]
- 14.Voutsadakis IA. Pathogenesis of colorectal carcinoma and therapeutic implications: the roles of the ubiquitin-proteasome system and Cox-2. J Cell Mol Med. 2007;11:252–285. doi: 10.1111/j.1582-4934.2007.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sano H, Kawahito Y, Wilder RL, Hashiramoto A, Mukai S, Asai K, Kimura S, Kato H, Kondo M, Hla T. Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res. 1995;55:3785–3789. [PubMed] [Google Scholar]
- 16.Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 17.Brown JR, DuBois RN. COX-2: a molecular target for colorectal cancer prevention. J Clin Oncol. 2005;23:2840–2855. doi: 10.1200/JCO.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 18.Form DM, Auerbach R. PGE2 and angiogenesis. Proc Soc Exp Biol Med. 1983;172:214–218. doi: 10.3181/00379727-172-41548. [DOI] [PubMed] [Google Scholar]
- 19.Pugh S, Thomas GA. Patients with adenomatous polyps and carcinomas have increased colonic mucosal prostaglandin E2. Gut. 1994;35:675–678. doi: 10.1136/gut.35.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rigas B, Goldman IS, Levine L. Altered eicosanoid levels in human colon cancer. J Lab Clin Med. 1993;122:518–523. [PubMed] [Google Scholar]
- 21.Hanif R, Pittas A, Feng Y, Koutsos MI, Qiao L, Staiano-Coico L, Shiff SI, Rigas B. Effects of nonsteroidal anti-inflammatory drugs on proliferation and on induction of apoptosis in colon cancer cells by a prostaglandin-independent pathway. Biochem Pharmacol. 1996;52:237–245. doi: 10.1016/0006-2952(96)00181-5. [DOI] [PubMed] [Google Scholar]
- 22.Elder DJ, Halton DE, Hague A, Paraskeva C. Induction of apoptotic cell death in human colorectal carcinoma cell lines by a cyclooxygenase-2 (COX-2)-selective nonsteroidal anti-inflammatory drug: independence from COX-2 protein expression. Clin Cancer Res. 1997;3:1679–1683. [PubMed] [Google Scholar]
- 23.Chan TA, Morin PJ, Vogelstein B, Kinzler KW. Mechanisms underlying nonsteroidal antiinflammatory drug-mediated apoptosis. Proc Natl Acad Sci USA. 1998;95:681–686. doi: 10.1073/pnas.95.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ciou SY, Hsu CC, Kuo YH, Chao CY. Effect of wild bitter gourd treatment on inflammatory responses in BALB/c mice with sepsis. Biomedicine (Taipei) 2014;4:17. doi: 10.7603/s40681-014-0017-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Badary OA, Gamal El-Din AM. Inhibitory effects of thymoquinone against 20-methylcholanthrene-induced fibrosarcoma tumorigenesis. Cancer Detect Prev. 2001;25:362–368. [PubMed] [Google Scholar]
- 26.Badary OA, Al-Shabanah OA, Nagi MN, Al-Rikabi AC, Elmazar MM. Inhibition of benzo(a)pyrene-induced forestomach carcinogenesis in mice by thymoquinone. Eur J Cancer Prev. 1999;8:435–440. doi: 10.1097/00008469-199910000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Kriegl L, Horst D, Reiche JA, Engel J, Kirchner T, Jung A. LEF-1 and TCF4 expression correlate inversely with survival in colorectal cancer. J Transl Med. 2010;8:123. doi: 10.1186/1479-5876-8-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nuñez F, Bravo S, Cruzat F, Montecino M, De Ferrari GV. Wnt/β-catenin signaling enhances cyclooxygenase-2 (COX2) transcriptional activity in gastric cancer cells. PLoS One. 2011;6:e18562. doi: 10.1371/journal.pone.0018562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu L, Stevens J, Hilton MB, Seaman S, Conrads TP, Veenstra TD, Logsdon D, Morris H, Swing DA, Patel NL, et al. COX-2 inhibition potentiates antiangiogenic cancer therapy and prevents metastasis in preclinical models. Sci Transl Med. 2014;6:242ra84. doi: 10.1126/scitranslmed.3008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Emenaker NJ, Zudaire E, St Croix B. Chemoprevention of metastasis. Oncotarget. 2014;5:6556–6557. doi: 10.18632/oncotarget.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao L, Wu Y, Xu Z, Wang H, Zhao Z, Li Y, Yang P, Wei X. Involvement of COX-2/PGE2 signalling in hypoxia-induced angiogenic response in endothelial cells. J Cell Mol Med. 2012;16:1840–1855. doi: 10.1111/j.1582-4934.2011.01479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirui PK, Cameron J, Benghuzzi HA, Tucci M, Patel R, Adah F, Russell G. Effects of sustained delivery of thymoqiunone on bone healing of male rats. Biomed Sci Instrum. 2004;40:111–116. [PubMed] [Google Scholar]
- 33.Hosseinzadeh H, Parvardeh S. Anticonvulsant effects of thymoquinone, the major constituent of Nigella sativa seeds, in mice. Phytomedicine. 2004;11:56–64. doi: 10.1078/0944-7113-00376. [DOI] [PubMed] [Google Scholar]
- 34.Attoub S, Sperandio O, Raza H, Arafat K, Al-Salam S, Al Sultan MA, Al Safi M, Takahashi T, Adem A. Thymoquinone as an anticancer agent: evidence from inhibition of cancer cells viability and invasion in vitro and tumor growth in vivo. Fundam Clin Pharmacol. 2013;27:557–569. doi: 10.1111/j.1472-8206.2012.01056.x. [DOI] [PubMed] [Google Scholar]
- 35.Hsieh YC, Hsieh SJ, Chang YS, Hsueh CM, Hsu SL. The lipoxygenase inhibitor, baicalein, modulates cell adhesion and migration by up-regulation of integrins and vinculin in rat heart endothelial cells. Br J Pharmacol. 2007;151:1235–1245. doi: 10.1038/sj.bjp.0707345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.U.S. National Institutes of Health. Laboratory animal welfare; proposed U.S. government principles for the utilization and care of vertebrate animals used in testing, research and training. Fed Regist. 1984;49:29350–29351. [PubMed] [Google Scholar]
- 37.Jrah-Harzallah H, Ben-Hadj-Khalifa S, Almawi WY, Maaloul A, Houas Z, Mahjoub T. Effect of thymoquinone on 1,2-dimethyl-hydrazine-induced oxidative stress during initiation and promotion of colon carcinogenesis. Eur J Cancer. 2013;49:1127–1135. doi: 10.1016/j.ejca.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Daniels DL, Weis WI. Beta-catenin directly displaces Groucho/TLE repressors from Tcf/Lef in Wnt-mediated transcription activation. Nat Struct Mol Biol. 2005;12:364–371. doi: 10.1038/nsmb912. [DOI] [PubMed] [Google Scholar]


