Abstract
AIM
To assess the rate of matrix Gla-protein carboxylation in patients with small intestinal bacterial overgrowth (SIBO) and to decipher its association with subclinical atherosclerosis.
METHODS
Patients with suspected SIBO who presented with a low risk for cardiovascular disease and showed no evidence of atherosclerotic plaques were included in the study. A glucose breath test was performed in order to confirm the diagnosis of SIBO and vascular assessment was carried out by ultrasound examination. Plasma levels of the inactive form of MGP (dephosphorylated-uncarboxylated matrix Gla-protein) were quantified by ELISA and vitamin K2 intake was estimated using a food frequency questionnaire.
RESULTS
Thirty-nine patients were included in the study. SIBO was confirmed in 12/39 (30.8%) patients who also presented with a higher concentration of dephosphorylated-uncarboxylated matrix Gla-protein (9.5 μg/L vs 4.2 μg/L; P = 0.004). Arterial stiffness was elevated in the SIBO group (pulse-wave velocity 10.25 m/s vs 7.68 m/s; P = 0.002) and this phenomenon was observed to correlate linearly with the levels of dephosphorylated-uncarboxylated matrix Gla-protein (β = 0.220, R2 = 0.366, P = 0.03). Carotid intima-media thickness and arterial calcifications were not observed to be significantly elevated as compared to controls.
CONCLUSION
SIBO is associated with reduced matrix Gla-protein activation as well as arterial stiffening. Both these observations are regarded as important indicators of subclinical atherosclerosis. Hence, screening for SIBO, intestinal decontamination and supplementation with vitamin K2 has the potential to be incorporated into clinical practice as additional preventive measures.
Keywords: Small intestinal bacterial overgrowth, Vitamin K, Dysbiosis, Atherosclerosis, Cardiovascular disease risk
Core tip: The matrix Gla-protein is involved in maintaining vascular health and vitamin K2 is a prerequisite for its activation and function. Intestinal bacteria are the main source of vitamin K2 in humans and small intestinal bacterial overgrowth (SIBO) is associated with altered vitamin K2 metabolism. This study demonstrates that SIBO is associated with increased plasma levels of inactive matrix Gla-protein, which, in turn, correlates directly with early markers of atherosclerotic disease such as increased arterial stiffness. Therefore, SIBO has the potential to serve as an indicator for increased risk of developing an overt cardiovascular disease.
INTRODUCTION
The presence of bacteria in the gastrointestinal tract is crucial for maintaining host health. One of the most important functions of gut bacteria is to metabolize carbohydrates, lipids, and proteins derived from food and to synthesize nutrients that are not adequately supplied by the diet.
Vitamin K is a lipophilic vitamin that is present in two main forms: (1) phylloquinone or vitamin K1; and (2) menaquinone (MK) or vitamin K2; the nomenclature is representative of the number of prenyl units contained in these isoforms[1]. Studies conducted on European populations have shown that the bulk of vitamin K intake in Western diets is in the form of phylloquinone (90%) with menaquinones accounting for only 10% (about 7.5% by MK-5 through to MK-13 and 2.5% by MK-4)[2]. This can be partially accounted for the fact that menaquinones are found only in meat, dairy-based foods and fermented soybeans (known as “natto”) that are a part of the Japanese diet. Therefore, while MK-4 is obtained by peripheral tissue conversion of menadione, which is produced by intestinal cleavage of phylloquinone[2], the bulk of menaquinones is derived from gut bacteria biosynthesis. In particular, bacterial species, such as Eggerthella lenta and Veillonella, are involved in the production of MK-7 while Enterobacteriaceae, such as Escherichia coli and Shigella, are responsible for the production of MK8. Bacteroides produce MK9-11 and Prevotella synthesize MK-5 and MK11-13[2,3].
Vitamin K2 is a cofactor that is involved in the carboxylation of several proteins, such as the matrix Gla-protein (MGP)[4]. The MGP is expressed by chondrocytes, vascular smooth muscle cells, endothelial cells and fibroblasts, and its primary function is to bind calcium crystals present in the vessel wall thereby preventing their nucleation on elastin fibers. Additionally, MGP also prevents osteoblastic differentiation of vascular smooth muscle cells and maintains the composition of the extracellular matrix[5]. Hence it can be concluded that MGP plays a crucial role in maintaining both arterial structure as well as function. Increased levels of the inactive form of MGP (dephosphorylated-uncarboxylated MGP, dp-ucMGP) is widely regarded as one of the best marker for low vitamin K2 status[6] and has been known to be associated with the signs of early vascular disease (intima-media thickening, arterial stiffness, and vascular calcifications)[7-14] and cardiovascular morbidity and mortality[15-17].
Although several recent studies have highlighted that patients with small intestinal bacterial overgrowth (SIBO) have low circulating levels of vitamin K2[18], investigations exploring the clinical relationship between SIBO, MGP activity, and arterial structure and function are still lacking.
The aim of this study was to investigate the rate of MGP carboxylation in patients with SIBO and to decipher its association with the risk of developing subclinical atherosclerosis.
MATERIALS AND METHODS
During a six month period, all outpatients at the Gastroenterology Division of the Agostino Gemelli Hospital in Rome that presented with clinical signs of SIBO (e.g., bloating, abdominal pain, and diarrhea) were included in the study.
Patient history with an emphasis on the personal and family history of coronary heart disease, lifestyle, and pharmacotherapy was stringently recorded.
Only subjects that were between 40 and 60 years without any previous cardiovascular events (including myocardial infarction or angina, congestive heart failure, intermittent claudication, previous arterial revascularization, thromboembolic disease and stroke) and with a low cardiovascular disease risk (as per the Framingham risk score up to 8 points) were considered eligible for inclusion in this investigation[19]. The exclusion criteria were (1) current heavy smoking (> 10 cigarettes/d by self-report); (2) morbid obesity (body mass index > 30 kg/m2); (3) dyslipidemia (LDL cholesterol > 160 mg/dL); (4) hypertriglyceridemia (triglycerides > 400 mg/dL); and (5) uncontrolled hypertension (systolic blood pressure > 140 mmHg and/or diastolic blood pressure > 95 mmHg). The patients suffering from diabetes, chronic kidney disease (at any stage), those receiving oral anticoagulant treatment and those afflicted with any carotid, aortic, femoral, and popliteal plaque (according to the Mannheim criteria as detected at the time of ultrasound examination) were also excluded from the protocol[20].
The patients fulfilling the selection criteria underwent a glucose breath test (GBT), filled in a food frequency questionnaire for the assessment of daily vitamin K2 intake, provided a blood sample for the quantification of circulating dp-ucMGP, and underwent ultrasound (US) examination of the non-coronary arterial system.
The study was performed in agreement with the Declaration of Helsinki and subsequent amendments. Written informed consent was obtained from all patients.
GBT test for SIBO diagnosis
All patients included in the study underwent a GBT to confirm the diagnosis of SIBO[21]. In order to avoid false positive or negative results, each patient was advised to strictly adhere to certain instructions before taking the test. These conditions were (1) laxatives, antibiotics, and prokinetics to be prohibited for at least four weeks before the GBT; (2) consumption of a low-fiber dinner was allowed but the patients were prohibited from eating or drinking eight hours before the test; (3) washing of mouth with antiseptics was done immediately before administration of the test; and (4) smoking, chewing gum, and doing physical exercise were prohibited before and during the test.
The GBT procedure was performed according to the recommendations of the Rome consensus[22]. The procedure was as follows: after the administration of 50 g glucose dissolved in 250 mL of water, hydrogen and methane excretion in the expired air was assessed at 15-min intervals for a total time period of 120 min. These values were then compared to baseline values. SIBO diagnosis was regarded as confirmed if the expired air exhibited an increase of at least 12 parts per million (ppm) above the baseline in hydrogen and/or methane activity.
Quantification of vitamin K2 daily intake
In order to assess the daily intake of vitamin K2, a food frequency questionnaire obtained from the European Prospective Investigation on Cancer and Nutrition (EPIC) nutrient database project was readapted and used[23].
The questionnaire was structured into three sections. The first section addressed the type and frequency of foods that were consumed by the subjects and included a list of more than 100 items divided into categories (fruit, vegetables, cereals, pasta/bread/rice, soups, meat, fish, eggs, milk/dairy products, fast food products, condiments, sweets, beverages, and vitamins). The frequency of intake of each food item was recorded and ranked (daily, weekly, monthly, yearly, or never).
The second section estimated the serving size and ranked it according to food weight (small, medium, and large portions).
Finally, the last section of the questionnaire investigated cooking habits (e.g., addition of salt to foods, preferred cooking method, etc.).
For the purpose of the study, scientific literature was reviewed in order to identify vitamin K2 rich foods that are commonly present in a Western diet. The results of the literature survey were taken into consideration while composing the food questionnaire so as to avoid underestimation of vitamin K2 intake by the study subjects. A list of vitamin K2 content in food is provided as Supplementary Table 1.
Table 1.
Patients’ baseline characteristics
| Overall | SIBO (n = 12) | no-SIBO (n = 27) | P value | |
| Age | 53 (41-60) | 55 (41-60) | 57 (43-60) | 0.306 |
| Sex (males/females) | 14 (35.9)/25 (64.1) | 2 (16.7)/10 (83.3) | 12 (44.4)/15 (55.6) | 0.095 |
| Vitamin K2 intake (μg/d) | 29.5 (8-103.6) | 21.2 (8-49.7) | 31.9 (10.5-103.6) | 0.111 |
| Males: 25.7 (8-103.6) | ||||
| Females 29.6 (10.4-91.8) | ||||
| Framingham risk score | 5.4 (2-8) | 5.5 (2-7) | 5.2 (2-8) | 0.897 |
| Males: 5.8 (2-8) | ||||
| Females: 6.9 (0-8) |
Data are expressed as median (range) or frequency (%). Comparisons have been performed between SIBO and no-SIBO group. Framingham score was built on age, sex, race, arterial pressure, smoking, diabetes, total blood cholesterol, HDL-cholesterol. SIBO: Small intestinal bacterial overgrowth.
Although the food frequency questionnaire only referred to the dietary intake of the previous 12 mo, all subjects participating in the study attested to having followed a stable dietary regimen for the last five years.
Plasma assay dp-ucMGP
Citrated plasma was separated from whole blood by centrifuging at 1500 × g for 10 min. The aliquots of 2 mL each were frozen at -20 °C or -80 °C within 30 min of blood sampling. For long-term storage exceeding 2 months, all samples were kept at -80 °C till use. dp-ucMGP concentration was assessed using a dual-antibody test based on the sandwich ELISA methodology developed by VitaK (inaKtif MGP iSYS kit, Immunodiagnostic Systems Ltd, Boldon, United Kingdom).
Ultrasound examination
The US and Doppler US (D-US) examination of the non-coronary arterial system was conducted in order to examine for early signs of vascular dysfunction (arterial stiffening) and for the presence of early vascular lesions (intima-media thickening, arterial calcifications, and subclinical plaques).
Arterial stiffness was assessed by applying an automated radiofrequency-based method (Quality Arterial Stiffness (RF-QAS); Esaote Medical Systems, Genova, Italy) to the D-US examination of the left common carotid artery. Local pulse-wave velocity (PWV) was calculated by combining arterial distension with local distending pressure measure. Assuming a constant difference between mean arterial pressure and diastolic pressure along the arterial tree, the QAS system is able to detect systo-diastolic changes in the arterial diameter following arterial wall movements during the cardiac cycle and to convert local distension variations in modifications of local distending pressure (pulse pressure). PWV was calculated by the Bramwell-Hill equation[24,25], as follows:
PWV = √ΔP · V/ΔV · ρ
where ΔV and ΔP are changes in volume and pressure, respectively, and ρ is the density of blood. As per the equation, PWV increases when there is an increase in arterial stiffness.
Using the same radiofrequency-based technology outlined above, intima-media thickness (IMT) was measured (Quality Intima-Media Thickness (RF-QIMT); Esaote Medical Systems, Genova, Italy) in a 1 cm long segment of the left common carotid artery, i.e., 1 cm before the bifurcation. To preserve measurement quality, mean IMT values calculated over 6 cardiac cycles were recorded only if the standard error was lower than 20 μm.
The presence of vascular calcifications was investigated by B-mode US in 11 vascular segments (common carotid arteries, common femoral arteries, popliteal arteries, posterior tibial arteries, anterior tibial arteries, and subrenal abdominal aorta) as has been previously described in the literature[26].
Statistical analysis
The Shapiro-Wilk test was performed to verify the normality of data distribution and statistical analysis was carried out using non-parametric tests.
Continuous variables were expressed as median and range (minimum and maximum value) while categorical variables were expressed as frequencies and percentages.
For comparing patients with SIBO with those without, the Mann-Whitney and chi-square tests were applied in order to highlight differences, if any, in baseline characteristics such as sex, age, and vitamin K2 intake. The same tests were also employed to test discrepancies in variables under study (plasma levels of dp-ucMGP, PWV, IMT, vascular calcifications).
Due to the presence of repeat values in the dataset, Kendall’s tau-b correlation coefficient was used to investigate the association between dp-ucMGP levels, PWV, IMT, and vascular calcifications. Additionally, data distribution was explored in order to find the best-fit regression model for elucidating the relationship between dp-ucMGP levels and US/D-US parameters. A linear regression was constructed using PWV as dependent variable, and the assumptions were verified by the appropriate diagnostics. Correlations and regression analyses were performed on both the overall population as well as the SIBO group separately.
Statistical analysis was conducted using the R statistics program version 3.1.2[27]. All statistical tests were two-sided and differences were considered significant at P-values below 0.05.
RESULTS
Amongst the 189 patients that were initially recruited into the study, 44 were deemed ineligible as a result of moderate or high Framingham risk score. A further 86 were ruled out as they were found to be afflicted with one or more of the comorbidities listed among the exclusion criteria. Twenty patients refused consent for being a part of this investigation (Figure 1). Eventually, 39 patients were selected to be a part of this study.
Figure 1.
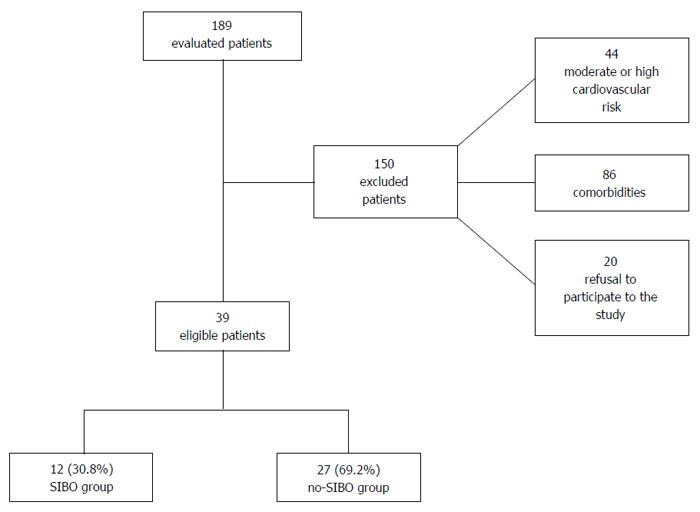
Study workflow.
Patient characteristics are described in Table 1. The median age of the study group was 53 (41-60) years, and 14 (35.9%) of the participants were male. SIBO was diagnosed in 12/39 (30.8%) patients and the 27 patients without SIBO were regarded as the control group (no-SIBO group). The analysis of the data contained in the food questionnaires revealed that the median vitamin K2 daily intake was approximately 29.5 (8-103.6) μg/d. No differences in median age, sex, and Framingham score could be observed between the SIBO and no-SIBO groups.
Vitamin K2 intake was determined to be 21.2 (8-49.7) μg/d in the SIBO group, which was similar to 31.9 (10.5-103.6) μg/d consumed by the control subjects in the no-SIBO group (P = 0.111). The median dp-ucMGP serum level was 4.76 μg/L (1.75–25.7) (Table 2); dp-ucMGP was observed to be significantly increased in patients with SIBO (9.5 μg/L vs 4.2 μg/L, P = 0.02; Table 2 and Figure 2).
Table 2.
Comparison of circulating levels of dephosphorylated-uncarboxylated matrix Gla-protein and ultrasound parameters (pulse-wave velocity, intima-media thickness, and vascular calcifications) in patients with or without small intestinal bacterial overgrowth
| Overall | SIBO (n = 12) | no-SIBO (n = 27) | P value | |
| dp-ucMGP (μg/L) | 4.73 (1.75-23.8) | 9.5 (3.6-23.8) | 4.2 (1.75-14.5) | 0.004a |
| PWV (m/s) | 8.5 (5.7-14) | 10.25 (5.7-14) | 7.68 (5.6-11) | 0.002a |
| IMT (μm) | 661 (467-1009) | 596 (467-1009) | 663 (506-932) | 0.465 |
| Calcifications | 16 (41) | 6 (50) | 10 (37) | 0.609 |
Data are expressed as median (range) or frequency (%), significant comparisons (aP < 0.05). SIBO: Small intestinal bacterial overgrowth; dp-ucMGP: Dephosphorylated-uncarboxylated matrix Gla-protein; PWV: Pulse-wave velocity; IMT: Intima-media thickness.
Figure 2.
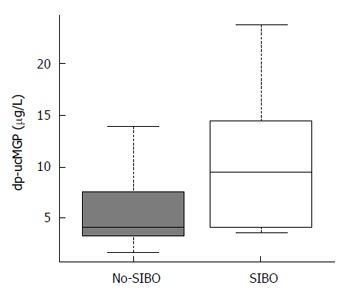
Plasma concentration of dephosphorylated-uncarboxylated matrix Gla-protein in patients under study. Patients with small intestinal bacterial overgrowth (SIBO) exhibited significantly higher levels as compared to the patients without SIBO (9.5 μg/L vs 4.2 μg/L; P = 0.02). Median values are represented by boxplot internal lines and ranges by whiskers. dp-ucMGP: Dephosphorylated-uncarboxylated matrix Gla-protein.
The daily intake of vitamin K2 was not associated with plasma levels of dp-ucMGP (τ = -0.08, P = 0.441).
Sixteen of the 39 patients (41%) presented with at least one or more microcalcifications in the explored arterial districts (Supplementary Table 2). It is to be noted however that no arterial plaque was detected. In the overall population, median carotid artery PWV was 8.5 m/s (5.69-14) and median IMT was 661 μm (467-1009). In patients with SIBO, the median PWV was significantly higher than that observed in the no-SIBO group (10.25 m/s vs 7.68 m/s; P = 0.002; Figure 3) but the median IMT value and the rate of early arterial calcifications did not differ substantially (Table 2). dp-ucMGP levels correlated with arterial stiffness as measured by PWV (τ = 0.339, P = 0.002). In particular, regression analysis showed that there was a significant direct linear correlation between dp-ucMGP and PWV (β = 0.219, R2 = 0.293, P = 0.0004; Figure 4).
Figure 3.
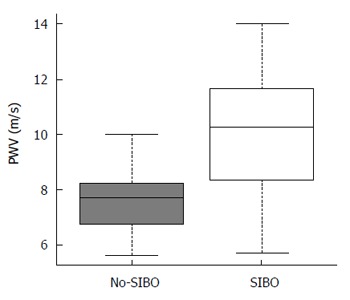
Pulse-wave velocity values observed in the study population. Pulse-wave velocity (PWV) was increased in patients with small intestinal bacterial overgrowth (SIBO) compared to the no-SIBO group (10.25 m/s vs 7.68 m/s; P = 0.002). Median values are represented by boxplot internal lines and ranges by whiskers.
Figure 4.
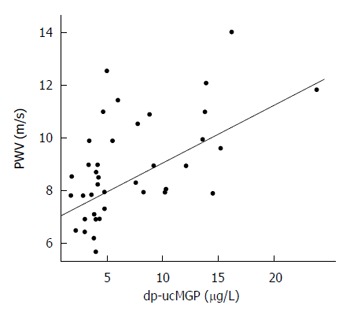
Linear regression model highlighting the direct correlation between plasma concentration of dephosphorylated-uncarboxylated matrix Gla-protein and pulse-wave velocity (β = 0.219, R2 = 0.293, P = 0.0004). dp-ucMGP: Dephosphorylated-uncarboxylated matrix Gla-protein; PWV: Pulse-wave velocity.
Additionally, in patients with SIBO, a direct linear correlation between dp-ucMGP levels and PWV could be verified (β = 0.220, R2 = 0.366, P = 0.03) but no such relation was found between dp-ucMGP and IMT (P = 0.507).
In the no-SIBO group, no significant relationship between dp-ucMGP, arterial stiffness, and IMT could be observed (P = 0.08 and P = 0.415, respectively).
DISCUSSION
In recent years, the studies on identifying early markers of atherosclerosis have garnered a significant amount of scientific interest. The early identification of vascular dysfunction and lesions has the potential to help individuals that stand to benefit from prevention of disease progression policies[28-30].
For the successful implementation of strategies aimed at prevention of disease formation and progression, it is crucial to recognize pathologies that function as risk factors for the development of atherosclerosis. Several diseases of the gastrointestinal tract, wherein gut bacteria act in a pathogenic capacity, are associated with vascular dysfunction and increase the risk of atherosclerosis in the host[31-33]. Nevertheless, it has been recently demonstrated that metabolic products generated by gut bacteria are implicated in the development of atherosclerotic lesions[34].
MGP is a vitamin K2 dependent protein that helps in preventing calcium accumulation in the arterial wall. Humans need gut bacteria in order to fulfill their vitamin K2 requirement as dietary intake is often insufficient. This is especially true in the case of the Western population. SIBO, a condition that is characterized by gut bacteria dysbiosis, is associated with impaired vitamin K metabolism in humans[18,35]. For this reason, patients afflicted with SIBO and/or low vitamin K2 status could hypothetically be at an increased risk for the development of atherosclerotic disease.
To the best of our knowledge, the present study is the first to investigate the consequences of vitamin K2 metabolism derangement on MGP activity in patients with SIBO. When compared to the control group, the SIBO group presented with higher dp-ucMGP serum levels, which is suggestive of either a reduced dietary vitamin K2 intake or an altered vitamin K2 production by intestinal bacteria. It is to be noted that the daily median vitamin K2 intake of patients included in the study was comparable to that of other European populations[36,37] and was similar between patients with SIBO and those without (21.1 μg/d vs 31.9 μg/d, P = 0.111; Table 2). As plasma vitamin K2 levels are difficult to assess with accuracy[38], we adopted dp-ucMGP levels as surrogate biomarkers for estimating the vitamin K2 nutritional status. Our results strongly indicated that dietary vitamin K2 intake does not correlate with dp-ucMGP serum levels. This observation indirectly confirms the previously reported theory that food is not the primary source of vitamin K2 supplementation in humans and that the gut microbiota is crucial for overcoming dietary insufficiencies under physiologic conditions[2,3].
In all subjects including those in the SIBO group, the serum levels of inactive dp-ucMGP were found to directly correlate with PWV, which is a surrogate parameter of arterial stiffness. However, no significant association was found with either IMT or the presence of calcifications. Atherosclerosis is a progressive disease characterized by a wide spectrum of vascular changes[39]. While arterial stiffening is an early marker of vascular dysfunction, intima-media thickening and calcifications are the first structural changes that can be detected in atherosclerotic vessels; these changes are usually a manifestation of the adaptive remodeling to flow, wall tension, and lumen diameter alterations[40]. As a result of this, even in the cases that are potentially at low risk for the development of cardiovascular diseases, SIBO appears to cause early vascular dysfunction possibly due to reduced MGP activity. This observation holds true even in the absence of clear signs of structural alterations. The absence of a statistical correlation between dp-ucMGP values and initial signs of vascular remodeling can be explained by the absence of study subjects with medium and high Framingham risk scores. It is reasonable to argue that in the presence of other cardiovascular risk factors, patients with SIBO may be at increased risk for developing structural arterial lesions as compared to patients without SIBO, and that SIBO itself may confer an increased risk of developing an overt cardiovascular disease. Therefore, based on the results obtained in our study, we propose that even asymptomatic patients should be screened for SIBO so as to rule out an additional factor that predisposes patients to cardiovascular diseases. This screening is especially important for patients with known atherosclerotic lesions or previous cardiovascular events as it offers a chance for therapeutic intervention so as to correct a condition that can potentially contribute to disease progression. In patients with SIBO vitamin K2 supplementation and intestinal decontamination, along with additional preventive measures, may be therefore recommended.
The present study suffers from certain limitations. Firstly, information regarding the specific composition of the small intestinal bacteria was not collected due to the invasiveness of the procedure. In addition, at our institution, SIBO is diagnosed by GBT as per the Rome consensus recommendations[18]. We were, therefore, unable to perform metagenomic or metabolomic analyses to assess if the abundance of bacteria specifically involved those involved in vitamin K2 production.
Secondly, instead of computed tomography (CT) scans, US and D-US were used to investigate the presence of vascular calcifications and flow parameters. This was done because US is less harmful, easily reproducible and allows for the quantification of parameters useful for assessing arterial stiffness whereas CT scans require radiation exposure and are also more expensive[41].
Lastly, this study included a relatively small number of patients. A strict adherence to the selection criterion was followed so as to avoid the effect of confounding factors such as treatment with oral vitamin K antagonists, diabetes and kidney disease on MGP carboxylation, or the influence of the previous history of vascular disease and of moderate/high cardiovascular disease risk on the prevalence of vascular calcifications and on the measurement of D-US parameters. The exclusion of patients with comorbidities limited external interactions which allowed us to exclusively evaluate the correlation between SIBO, vitamin K2 metabolism, MGP carboxylation and early arterial dysfunction or vascular lesions without the interference of any confounding factors.
In conclusion, patients affected by SIBO have higher levels of inactive MGP as well as increased arterial stiffness both of which are early markers for vascular dysfunction. This condition is not influenced by vitamin K2 intake from diet confirming that bacteria are the main source of this vitamin in humans and that vitamin K2 metabolism may be altered as a consequence of small intestinal dysbiosis. Longitudinal studies assessing the role of SIBO as a condition that predisposes patients to the development of atherosclerosis are needed; for this category of patients, vitamin K2 supplementation and the treatment of intestinal dysbiosis may be therapeutic alternatives of significant utility.
COMMENTS
Background
Small intestinal bacterial overgrowth (SIBO) is associated with altered vitamin K2 metabolism. Vitamin K2 deficiency leads to a reduced carboxylation of the matrix Gla-protein, which is crucial for maintaining the integrity of the vascular system. Intestinal bacteria are involved in vitamin K2 metabolism.
Research frontiers
Little is known about vitamin K2 metabolism in patients with intestinal dysbiosis and its association with vascular disease.
Innovations and breakthroughs
SIBO is associated with both reduced matrix Gla-protein activation as well as arterial stiffening both of which are important signs of subclinical atherosclerosis.
Applications
Patients should be screened for SIBO so as to rule out an additional factor that predisposes to cardiovascular disease or accelerates its progression. Vitamin K2 supplementation and intestinal decontamination are a valid therapeutic option in case of patients with SIBO.
Terminology
SIBO is a condition characterized by the presence of more than 105 CFU/mL of bacteria in the small intestine. Matrix Gla-protein binds calcium crystals present in the vessel wall thereby preventing their nucleation on elastin fibers and maintains the composition of the extracellular matrix, preserving optimum arterial structure and function.
Peer-review
In this manuscript the authors have assessed the role of matrix Gla-protein carboxylation in patients with SIBO and its association with subclinical atherosclerosis.Authors have used non-invasive Glucose Breath test to diagnose SIBO. This is an important study. The idea is novel but the number of patients enrolled is too less to come to this conclusion as mentioned by the authors also.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: This study was approved by the Local Institutional Review Board.
Informed consent statement: Patients gave their written consent to participate to this study.
Conflict-of-interest statement: The Authors have no conflicts of interest to declare.
Data sharing statement: the anonymous dataset is available from the corresponding author (francesca.ponziani@yahoo.it) and will be provided on request after obtaining all Authors’ agreement.
Peer-review started: October 31, 2016
First decision: December 19, 2016
Article in press: January 18, 2017
P- Reviewer: Osawa S, Rana SV S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH
References
- 1.Booth SL. Vitamin K: food composition and dietary intakes. Food Nutr Res. 2012:56. doi: 10.3402/fnr.v56i0.5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shearer MJ, Newman P. Metabolism and cell biology of vitamin K. Thromb Haemost. 2008;100:530–547. [PubMed] [Google Scholar]
- 3.Shearer MJ, Newman P. Recent trends in the metabolism and cell biology of vitamin K with special reference to vitamin K cycling and MK-4 biosynthesis. J Lipid Res. 2014;55:345–362. doi: 10.1194/jlr.R045559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gröber U, Reichrath J, Holick MF, Kisters K. Vitamin K: an old vitamin in a new perspective. Dermatoendocrinol. 2014;6:e968490. doi: 10.4161/19381972.2014.968490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evrard S, Delanaye P, Kamel S, Cristol JP, Cavalier E. Vascular calcification: from pathophysiology to biomarkers. Clin Chim Acta. 2015;438:401–414. doi: 10.1016/j.cca.2014.08.034. [DOI] [PubMed] [Google Scholar]
- 6.Schurgers LJ, Cranenburg EC, Vermeer C. Matrix Gla-protein: the calcification inhibitor in need of vitamin K. Thromb Haemost. 2008;100:593–603. [PubMed] [Google Scholar]
- 7.Pivin E, Ponte B, Pruijm M, Ackermann D, Guessous I, Ehret G, Liu YP, Drummen NE, Knapen MH, Pechere-Bertschi A, et al. Inactive Matrix Gla-Protein Is Associated With Arterial Stiffness in an Adult Population-Based Study. Hypertension. 2015;66:85–92. doi: 10.1161/HYPERTENSIONAHA.115.05177. [DOI] [PubMed] [Google Scholar]
- 8.Mayer O, Seidlerová J, Wohlfahrt P, Filipovský J, Vaněk J, Cífková R, Windrichová J, Topolčan O, Knapen MH, Drummen NE, et al. Desphospho-uncarboxylated matrix Gla protein is associated with increased aortic stiffness in a general population. J Hum Hypertens. 2016;30:418–423. doi: 10.1038/jhh.2015.55. [DOI] [PubMed] [Google Scholar]
- 9.Dalmeijer GW, van der Schouw YT, Vermeer C, Magdeleyns EJ, Schurgers LJ, Beulens JW. Circulating matrix Gla protein is associated with coronary artery calcification and vitamin K status in healthy women. J Nutr Biochem. 2013;24:624–628. doi: 10.1016/j.jnutbio.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Liabeuf S, Bourron O, Vemeer C, Theuwissen E, Magdeleyns E, Aubert CE, Brazier M, Mentaverri R, Hartemann A, Massy ZA. Vascular calcification in patients with type 2 diabetes: the involvement of matrix Gla protein. Cardiovasc Diabetol. 2014;13:85. doi: 10.1186/1475-2840-13-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delanaye P, Krzesinski JM, Warling X, Moonen M, Smelten N, Médart L, Pottel H, Cavalier E. Dephosphorylated-uncarboxylated Matrix Gla protein concentration is predictive of vitamin K status and is correlated with vascular calcification in a cohort of hemodialysis patients. BMC Nephrol. 2014;15:145. doi: 10.1186/1471-2369-15-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schurgers LJ, Barreto DV, Barreto FC, Liabeuf S, Renard C, Magdeleyns EJ, Vermeer C, Choukroun G, Massy ZA. The circulating inactive form of matrix gla protein is a surrogate marker for vascular calcification in chronic kidney disease: a preliminary report. Clin J Am Soc Nephrol. 2010;5:568–575. doi: 10.2215/CJN.07081009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parker BD, Schurgers LJ, Vermeer C, Schiller NB, Whooley MA, Ix JH. The association of uncarboxylated matrix Gla protein with mitral annular calcification differs by diabetes status: The Heart and Soul study. Atherosclerosis. 2010;210:320–325. doi: 10.1016/j.atherosclerosis.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hermans MM, Vermeer C, Kooman JP, Brandenburg V, Ketteler M, Gladziwa U, Rensma PL, Leunissen KM, Schurgers LJ. Undercarboxylated matrix GLA protein levels are decreased in dialysis patients and related to parameters of calcium-phosphate metabolism and aortic augmentation index. Blood Purif. 2007;25:395–401. doi: 10.1159/000108629. [DOI] [PubMed] [Google Scholar]
- 15.Mayer O, Seidlerová J, Vaněk J, Karnosová P, Bruthans J, Filipovský J, Wohlfahrt P, Cífková R, Windrichová J, Knapen MH, et al. The abnormal status of uncarboxylated matrix Gla protein species represents an additional mortality risk in heart failure patients with vascular disease. Int J Cardiol. 2016;203:916–922. doi: 10.1016/j.ijcard.2015.10.226. [DOI] [PubMed] [Google Scholar]
- 16.Mayer O, Seidlerová J, Bruthans J, Filipovský J, Timoracká K, Vaněk J, Cerná L, Wohlfahrt P, Cífková R, Theuwissen E, et al. Desphospho-uncarboxylated matrix Gla-protein is associated with mortality risk in patients with chronic stable vascular disease. Atherosclerosis. 2014;235:162–168. doi: 10.1016/j.atherosclerosis.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 17.Dalmeijer GW, van der Schouw YT, Magdeleyns EJ, Vermeer C, Verschuren WM, Boer JM, Beulens JW. Circulating desphospho-uncarboxylated matrix γ-carboxyglutamate protein and the risk of coronary heart disease and stroke. J Thromb Haemost. 2014;12:1028–1034. doi: 10.1111/jth.12609. [DOI] [PubMed] [Google Scholar]
- 18.Giuliano V, Bassotti G, Mourvaki E, Castellani D, Filippucci E, Sabatino G, Gizzi S, Palmerini F, Galli F, Morelli O, et al. Small intestinal bacterial overgrowth and warfarin dose requirement variability. Thromb Res. 2010;126:12–17. doi: 10.1016/j.thromres.2009.11.032. [DOI] [PubMed] [Google Scholar]
- 19.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 20.Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S, Hernandez Hernandez R, et al. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012;34:290–296. doi: 10.1159/000343145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ponziani FR, Gerardi V, Gasbarrini A. Diagnosis and treatment of small intestinal bacterial overgrowth. Expert Rev Gastroenterol Hepatol. 2016;10:215–227. doi: 10.1586/17474124.2016.1110017. [DOI] [PubMed] [Google Scholar]
- 22.Gasbarrini A, Corazza GR, Gasbarrini G, Montalto M, Di Stefano M, Basilisco G, Parodi A, Usai-Satta P, Vernia P, Anania C, et al. Methodology and indications of H2-breath testing in gastrointestinal diseases: the Rome Consensus Conference. Aliment Pharmacol Ther. 2009;29 Suppl 1:1–49. doi: 10.1111/j.1365-2036.2009.03951.x. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. EPIC study. Available from: http://epic.iarc.fr/about/background.php.
- 24.Van Bortel LM, Balkestein EJ, van der Heijden-Spek JJ, Vanmolkot FH, Staessen JA, Kragten JA, Vredeveld JW, Safar ME, Struijker Boudier HA, Hoeks AP. Non-invasive assessment of local arterial pulse pressure: comparison of applanation tonometry and echo-tracking. J Hypertens. 2001;19:1037–1044. doi: 10.1097/00004872-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Bramwell JC, Hill AV. The velocity of the pulse wave in man. Proc R Soc Lond B. 1922;93:298–306. [Google Scholar]
- 26.Flore R, Ponziani FR, Tinelli G, Arena V, Fonnesu C, Nesci A, Santoro L, Tondi P, Santoliquido A. New modalities of ultrasound-based intima-media thickness, arterial stiffness and non-coronary vascular calcifications detection to assess cardiovascular risk. Eur Rev Med Pharmacol Sci. 2015;19:1430–1441. [PubMed] [Google Scholar]
- 27.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available from: http://www.R-project.org/2014.
- 28.Vlachopoulos C, Xaplanteris P, Aboyans V, Brodmann M, Cífková R, Cosentino F, De Carlo M, Gallino A, Landmesser U, Laurent S, et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis. 2015;241:507–532. doi: 10.1016/j.atherosclerosis.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Koivistoinen T, Virtanen M, Hutri-Kähönen N, Lehtimäki T, Jula A, Juonala M, Moilanen L, Aatola H, Hyttinen J, Viikari JS, et al. Arterial pulse wave velocity in relation to carotid intima-media thickness, brachial flow-mediated dilation and carotid artery distensibility: the Cardiovascular Risk in Young Finns Study and the Health 2000 Survey. Atherosclerosis. 2012;220:387–393. doi: 10.1016/j.atherosclerosis.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Boesen ME, Singh D, Menon BK, Frayne R. A systematic literature review of the effect of carotid atherosclerosis on local vessel stiffness and elasticity. Atherosclerosis. 2015;243:211–222. doi: 10.1016/j.atherosclerosis.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 31.De Marchi S, Chiarioni G, Prior M, Arosio E. Young adults with coeliac disease may be at increased risk of early atherosclerosis. Aliment Pharmacol Ther. 2013;38:162–169. doi: 10.1111/apt.12360. [DOI] [PubMed] [Google Scholar]
- 32.Singh S, Kullo IJ, Pardi DS, Loftus EV. Epidemiology, risk factors and management of cardiovascular diseases in IBD. Nat Rev Gastroenterol Hepatol. 2015;12:26–35. doi: 10.1038/nrgastro.2014.202. [DOI] [PubMed] [Google Scholar]
- 33.He C, Yang Z, Lu NH. Helicobacter pylori-an infectious risk factor for atherosclerosis? J Atheroscler Thromb. 2014;21:1229–1242. doi: 10.5551/jat.25775. [DOI] [PubMed] [Google Scholar]
- 34.Org E, Mehrabian M, Lusis AJ. Unraveling the environmental and genetic interactions in atherosclerosis: Central role of the gut microbiota. Atherosclerosis. 2015;241:387–399. doi: 10.1016/j.atherosclerosis.2015.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scarpellini E, Gabrielli M, Za T, Lauritano EC, Santoliquido A, Rossi E, Ojetti V, Cammarota G, De Stefano V, Gasbarrini A. The interaction between small intestinal bacterial overgrowth and warfarin treatment. Am J Gastroenterol. 2009;104:2364–2365. doi: 10.1038/ajg.2009.288. [DOI] [PubMed] [Google Scholar]
- 36.Walther B, Karl JP, Booth SL, Boyaval P. Menaquinones, bacteria, and the food supply: the relevance of dairy and fermented food products to vitamin K requirements. Adv Nutr. 2013;4:463–473. doi: 10.3945/an.113.003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geleijnse JM, Vermeer C, Grobbee DE, Schurgers LJ, Knapen MH, van der Meer IM, Hofman A, Witteman JC. Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: the Rotterdam Study. J Nutr. 2004;134:3100–3105. doi: 10.1093/jn/134.11.3100. [DOI] [PubMed] [Google Scholar]
- 38.Schurgers LJ, Vermeer C. Differential lipoprotein transport pathways of K-vitamins in healthy subjects. Biochim Biophys Acta. 2002;1570:27–32. doi: 10.1016/s0304-4165(02)00147-2. [DOI] [PubMed] [Google Scholar]
- 39.Sakakura K, Nakano M, Otsuka F, Ladich E, Kolodgie FD, Virmani R. Pathophysiology of atherosclerosis plaque progression. Heart Lung Circ. 2013;22:399–411. doi: 10.1016/j.hlc.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 40.Simova I. Intima-media thickness: Appropriate evaluation and proper measurement, described. 2015. Curr Opin Cardiol. 2015;30:422–431. Available from: http://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-13/Intima-media-thickness-Appropriate-evaluation-and-proper-measurement-described. [Google Scholar]
- 41.Ikonomidis I, Makavos G, Lekakis J. Arterial stiffness and coronary artery disease. Curr Opin Cardiol. 2015;30:422–431. doi: 10.1097/HCO.0000000000000179. [DOI] [PubMed] [Google Scholar]


