Abstract
Variable and low egg quality is a major limiting factor for the development of efficient aquaculture production. This stems from limited knowledge on the mechanisms underlying egg quality in cultured fish. Molecular analyses, such as transcriptomic studies, are valuable tools to identify the most important processes modulating egg quality. However, very few studies have been devoted to this aspect so far. Within this study, the microarray-based transcriptomic analysis of eggs (of different quality) of sea bass (Dicentrarchus labrax) was performed. An Agilent oligo microarray experiment was performed on labelled mRNA extracted from 16 batches of eggs (each batch obtained from a different female) of sea bass, in which over 24,000 published probe arrays were used. We identified 39 differentially expressed genes exhibiting a differential expression between the groups of low (fertilization rate < 60 %) and high (fertilization rate > 60 %) quality. The mRNA levels of eight genes were further analyzed by quantitative PCR. Seven genes were confirmed by qPCR to be differentially expressed in eggs of low and high quality. This study confirmed the importance of some of the genes already reported to be potential molecular quality indicators (mainly rnf213 and irf7), but we also found new genes (mainly usp5, mem-prot, plec, cenpf), which had not yet been reported to be quality-dependent in fish. These results suggest the importance of genes involved in several important processes, such as protein ubiquitination, translation, DNA repair, and cell structure and architecture; these probably being the mechanisms that contribute to egg developmental competence in sea bass.
Keywords: Microarray, Genomics, Transcriptomics, Aquaculture, Controlled reproduction
Introduction
One of the biggest obstacles in intensive aquaculture practice is highly variable and unpredictable gamete quality, with eggs being the highest concern (Bobe and Labbé 2010; Żarski et al. 2011). Up to now, there has been no clear husbandry protocol that could lead to the selection of spawners yielding high quality eggs. This is due to the great difficulty in identifying factors affecting egg quality in finfishes. This, in turn, is caused by the lack of clear and reliable egg quality indicators which, when available, could help in understanding the mechanisms determining eggs of high or low quality and identifying the traits which characterize “good” or “bad” spawners (Bobe and Labbé 2010; Żarski et al. 2012; Migaud et al. 2013).
By definition, egg quality is the ability of the egg to be fertilized and subsequently developed into a viable “normal embryo” (Bobe and Labbé 2010). The objective and precise evaluation of egg quality is one of the most important steps of the culture process, allowing the allocation/discarding of particular batches of eggs for/from further culture procedures (Migaud et al. 2013; Schaerlinger and Żarski 2015). Additionally, it enables the investigation of the effects of specific farming practices (e.g., the feeding regime of the broodstock, photothermal regimes applied, or the procedure of the induction of ovulation) on egg quality (Bromage et al. 1992; Brooks et al. 1997; Bobe and Labbé 2010). This has caused the development of several biological and biochemical indicators of egg quality, allowing the general discrimination of eggs of high or low quality. These include, among others, blastomere morphology (Bobe and Labbé 2010), oil droplet distribution (Mansour et al. 2007), oil droplet fragmentation (Żarski et al. 2011), cortical reaction intensity (Żarski et al. 2012), egg swelling intensity (Lahnsteiner et al. 2001), pH and electrical conductivity of the ovarian fluid (Lahnsteiner et al. 1999, 2001; Skaalsvik et al. 2015), causing the turbidity of water by the batch of eggs (Mansour et al. 2007), the floating ability of pelagic eggs (Carrillo et al. 1989), as well as egg nutritional constituents, including fatty acid profiles (Lahnsteiner et al. 1999; Henrotte et al. 2010). Most of these indicators are, however, valid only in particular species or under particular farming conditions (Ciereszko et al. 2009) or both. Therefore, the only generally reliable true assessment of egg quality may still be performed at different steps of embryonic development to evaluate developmental success and conformity.
The transcriptomic profile of eggs is one of the emerging tools allowing the improvement of husbandry practices in aquaculture (Bonnet et al. 2007; Bobe and Labbé 2010; Mommens et al. 2014; Chapman et al. 2014). Considering that the entire oogenesis in finfishes is a long-lasting process during which the oocytes are subjected to a number of morphological, biochemical, and molecular changes, the final characteristics of an ovulated egg are a specific “summary” of the oogenesis course (Lubzens et al. 2010; Chapman et al. 2014). This also applies to transcriptomic profiles, which were found to significantly differ between particular phases of oogenesis (Lanes et al. 2013) and in response to different life history traits or different husbandry practices (Bonnet et al. 2007). Therefore, the structure of maternally inherited messenger RNAs (mRNAs) contained in the egg upon ovulation can be considered as a result of previous events (Gohin et al. 2010). Until now, only a few studies have focused on the transcriptomic profile of ovulated eggs and its relation to the developmental competence of the eggs. This research was performed in rainbow trout, Oncorhynchus mykiss (Walbaum) (Aegerter et al. 2005; Bonnet et al. 2007), Atlantic halibut, Hippoglossus hippoglossus (L.) (Mommens et al. 2010, 2014), Atlantic cod, Gadus morhua L. (Lanes et al. 2012; Rise et al. 2014), and recently in striped bass, Morone saxatilis (Walbaum) (Chapman et al. 2014).
To investigate the molecular determinants of egg quality in finfishes, highly expressed genes with the highest differences in expression value (fold change) between eggs of high and low quality were searched for. It is generally agreed that the expression of a single gene is unlikely to be a reliable egg quality indicator valid in a wide range of situations. Some authors have already indicated that at the molecular level egg quality is probably characterized by a suite of genes for which variation and/or a particular pattern expression would reveal the real transcriptomic profile of a “good” and/or “bad” egg (Chapman et al. 2014; Rise et al. 2014; Sullivan et al. 2015). To this end, further large scale transcriptomic studies are required to identify the best candidate genes, presumably contributing to our better understanding of the molecular mechanisms standing behind the developmental competence of eggs.
Sea bass, Dicentrarchus labrax, is one of the most important cultured species in the Mediterranean Sea basin (Shields 2001). Its aquaculture has been constantly developing for the last 30 years, and a major production increment was observed within the last two decades. From the very beginning, controlled reproduction was one of the most important constraints and was therefore intensively studied in this species (e.g., Mylonas et al. 2003), while egg quality was specifically investigated in several studies (Devauchelle and Coves 1988; Carrillo et al. 1989; Cerdá et al. 1994, 1995). The protein composition (Carnevali et al. 1998) of eggs of varying quality, as well as comparable proteomic analysis of eggs obtained from domesticated and wild females (Crespel et al. 2008), was also studied in sea bass. To date, there has been no large scale (i.e., genome wide) transcriptomic analysis of the eggs of this species characterized by different quality.
In the present study, the comparative analysis of the transcriptomic profile (microarray analysis of total mRNA obtained from 16 egg batches) of sea bass eggs, obtained after the application of standardized reproductive protocol, and biological egg quality was performed. Additionally, the relative abundance of the mRNAs of eight genes was verified by quantitative PCR analysis. Gene ontology analysis of differentially expressed genes was performed to identify the main biological processes regulated at the transcriptomic level, as being possibly related to egg quality.
Material and Methods
Artificial Spawning and Fertilization
For the experiment, 48 females of cultured European sea bass females (with average weight of 2.90 kg ± 1.1 SD) were reproduced at IFREMER experimental station of Palavas, France (experimentation agreement C34-19266), and resulting from a standard protocol including female stimulation, artificial spawning, artificial fertilization, and incubation of the embryos as follows. During the spawning season, female genital state was regularly controlled through the observation of an ovarian biopsy. The maturation stage of each female was determined according to Fauvel and Suquet (1998). Only females at postvitellogenic stage were chosen for further procedures and subject to intramuscular (at the dorsal musculature, behind the dorsal fin) injection of LHRHa (D-Trp6, SIGMA) solution at a dose of 10 μg kg−1.
After induction, females were individually kept indoor in 1-m3 tanks at a temperature of 12.9–13.5 °C, salinity of 34.8–36.2 ppt, pH of 7.9–8.1, and photoperiod of 8/16 (light/dark). After ovulation (occurring between 68 and 75 h following hormonal injection), the eggs from each female separately were stripped exhaustively into dry containers the time after stimulation and the total volume of the collected egg batch was recorded. Then, eggs were dispatched into aliquots for different treatments. One portion of 500 μl (approx. 500 eggs) was deep frozen in duplicate in liquid nitrogen and stored at −80 °C until molecular analysis. Batches of 5 ml of eggs were fertilized in triplicates with an aliquot of cryopreserved sperm. For that purpose, a mixture of sperm obtained from 20 males was dispatched into straws and cryopreserved according to the method of Fauvel et al. (1998) what allowed to cover the needs for all the crosses in order to exclude possible paternal effect in the analysis of embryonic development course (Saillant et al. 2001). During fertilization, the amount of sperm was always adjusted to 2 · 105 of spermatozoa per egg, as recommended by Fauvel et al. (1999). Fertilization was performed with seawater as an activating solution.
Investigation of the Early Development of Embryos and Larvae and Determination of Egg Biological Quality
Just after the fertilization (approx. 2 min), aliquots of 2.5 ml of eggs from each female were transferred into beakers and incubated in seawater at 14 °C for 3 h. After that period, the second cleavage was already completed and all the developing embryos had reached the four-cell stage. At this stage (3 h post-fertilization, HPF), developing and non-developing eggs were counted allowing first estimation of fertilization rate. From each batch, 72 developing eggs were separated and dispatched into microwell plates as described by Panini et al. (2001) in order to determine the developmental success of each egg separately after 3 and 4 days of incubation (3 and 4 days post-fertilization, DPF) as well as hatching rate (5 DPF). The incubation was conducted in seawater at a temperature ranging between 13.3 and 14.4 °C and with salinity of 35.7–36.1 ppt.
For the transcriptomic analysis, 16 egg batches were chosen, among which 8 batches represented high and 8 batches low egg quality groups. Each group was chosen taking into account two evaluation steps. The first step involved morphological evaluation of the external appearance of the eggs aimed at avoiding taking for further analysis the ones characterized by extreme abnormalities such as overripening signs. This allowed to consider for further analysis egg batches having “normal” morphology (as it is typically performed in aquaculture practice). Next, the choice of the egg samples for further analysis was based on evaluation of developmental competence of the eggs, where finally fertilization rate at 3 HPF, with 60 % of fertilization rate considered as the threshold value between the groups, was used. This threshold was chosen, however, on the basis of the developmental competence observed throughout embryonic development and hatching. For example, the neighboring samples (L7 and H1) were characterized by ∼10 % differences in fertilization rate but also over 25 % in hatching rate. The differences between the groups were checked with Mann-Whitney U test (Statistica, v.12, StatSoft, USA) at a significance level of 5 % (p < 0.05).
RNA Extraction
RNA extraction was performed with the use of TRI Reagent as previously described for rainbow trout (Bonnet et al., 2007). Briefly, the RNA from 16 batches of eggs was extracted each time from about 0.2 ml of eggs by using 3 ml of TRI Reagent. A NanoDrop® NP-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA) was used to measure the quantity of the RNA samples. The quality of the RNA samples was checked using a 2100 Bioanalyzer (Agilent Technologies Inc., Santa Clara, USA), and samples with RIN value higher than 8.5 were used for further analysis.
Microarray Analysis
Sea bass gene expression profiling was conducted using an Agilent 8x60K high-density oligonucleotide microarray (GEO platform no. GPL22152). Labeling and hybridization steps were performed following the “One-Color Microarray-Based Gene Expression Analysis (Low Input Quick Amp labeling)” Agilent protocol. Briefly, for each sample, 150 ng of total RNA was amplified and labeled using Cy3-CTP. Yield (>1.65 μg cRNA) and specific activity (>9 pmol of Cy3 per μg of cRNA) of Cy3-cRNA produced were checked with the Nanodrop. Of Cy3-cRNA, 1.65 μg was fragmented and hybridized on a sub-array. Hybridization was carried out for 17 h at 65 °C in a rotating hybridization oven prior to washing and scanning with an Agilent Scanner (Agilent DNA Microarray Scanner, Agilent Technologies, Massy, France) using the standard parameters for a gene expression 8x60K oligoarray (3 μm and 20 bits). Data were then obtained with the Agilent Feature Extraction software (10.7.1.1) according to the appropriate GE protocol (GE1_107_Sep09) and imported into GeneSpring GX software (Agilent Technologies, Santa Clara, CA, USA) for analysis. Data were published at the NCBI’s Gene Expression Omnibus (Edgar et al. 2002) and are accessible through GEO series accession number GSE84559. Samples were randomly distributed on the microarray for hybridization. The gene expression data was scale normalized and log(2) transformed before the statistical analysis.
Differentially expressed genes between the groups representing high (samples H1–H8) and low (samples L1–L8) egg quality were identified with the use of GeneSpring GX software. Due to the hybridization failure of two egg samples (one sample representing low and one sample representing high egg quality) for further analysis of the microarray data, only 14 samples were taken into account. The differences between the groups were analyzed with unpaired t test after application of minimum twofold change filter with the significance level of 5 % (p < 0.05). Next, normalized log(2)-transformed expression values as well as raw signal data were exported to MS Excel software where they were filtered according to the raw signal, i.e., only the genes for which raw signal of more than 50 % samples was above 50 was taken for further analytical steps. Average linkage clustering analysis (Gene Cluster 3.0) was performed for the differentially abundant genes (unsupervised linkage) and in relation to egg quality (supervised linkage).
Reverse Transcription and Real-Time qPCR
Reverse transcription PCR was performed with the Maxima® First Strand cDNA Synthesis Kit (Thermo Scientific) according to the manufacturer’s protocol. Briefly, 2 μg of total RNA was mixed with 4 μl of 5X Reaction Mix and 2 μl Maxima Enzyme Mix. Next, the content of each tube was filled up to 20 μl with nuclease-free water. The samples were then gently mixed, centrifuged, and incubated for 10 min at 25 °C followed by 30 min at 50 °C. The reaction was terminated at 85 °C for 5 min.
Real-time qPCR was performed using the Step One Plus system (Applied Biosystems, Foster City, USA). Reverse transcription products were diluted to 1:20 and 4 μl was used for real-time PCR, using GoTag® qPCR Master Mix (Promega, Madison, WI, USA) and 1500 pmol of each primer (Table 1). The enzyme was activated 2 min at 95 °C, followed by 40 cycles of denaturation at 95 °C for 15 s, and annealing and elongation at 60 °C for 1 min. After amplification, a melting curve was performed according to the manufacturer’s recommendations to check the amplification specificity. Relative expression was normalized using the geometric mean of expression values recorded by qPCR of the six genes exhibiting the most stable expression level. For each sample, the expression level of each gene was analyzed in triplicates. Finally, the data between the groups (representing low and high egg quality) were analyzed with the Mann-Whitney U test (Statistica, v.12, StatSoft, USA). Differences between groups were considered significant when p < 0.05. With real-time qPCR, 8 genes for all the 16 samples were analyzed.
Table 1.
The primer sequences used for real-time qPCR of RNA obtained from the eggs of European sea bass characterized by different quality
| Symbol | Forward sequence | Reverse sequence |
|---|---|---|
| Differentially expressed genes | ||
| polk | GCAAAGAAACTCTGCCCCAA | CCGGATCTCATTGCACACAG |
| rab2a | GGCTTTCATCAACACAGCCA | GGAGTTGGTGGTAGGATGCT |
| irf7 | CTAACCGTCTCCAGCTCCAT | CAGTGGATGGGAAGCAGAGA |
| usp5 | AGAAGGATCAGCAGTGGGTC | GCTCCCTCCCTTGTCTCATT |
| kctd12 | GGAGTGTGCTTGCATTGTCA | CTTATCCACCACACCCCTCA |
| plec | TCCTCTCAGTCCAGCAAAGG | ATCCTGTCCTTGAGCCAGTC |
| hy-prot(1) | GGTAGCCCTGCCCTTCTTAA | TTAGTGGGCTGCATCCTCAA |
| mem-prot | ACGTTCTGTCGTGCCTCTCT | TGCAGAGGGCTTTTGCTATT |
| Most stable genes | ||
| hspa14 | GTGCCTGAGGAAGAGTCTGT | CCCGATGGAAGGAGAACAGT |
| cops3 | GCCTTGGAGCAGTTTGTGAA | TTGATCAACTCGCACAGCTG |
| mtif3 | CACCATGCACCGTCTAGTTG | TTTGTGTTCACTGACCAGCG |
| eif3ea | CTCACCACCAAAATTGCCCA | GCGAAGTCCACCATGTTTGT |
| hectd3 | AGCCGCACTCAAAGGAAAAG | CAGAGTCTACAGCGGGGAAA |
| eif3e | AGAGCACCAAGAACGAGACA | TCCATCTTGCTTGAGTCGCT |
Differentially expressed genes refer to the ones exhibiting different mRNA abundance between the groups representing “low” and “high” egg quality. Most homogenized genes are the ones used for the data normalization. For each gene symbol, the protein is provided according to the iHOP database or specific abbreviation was introduced (explained in the footnote of the table). Numbers in parenthesis represent number of subsequent gene, when more than one gene with the same name was identified
hy-prot hypothetical protein, un-prot uncharacterized protein, n-f protein was not found, mem-prot membrane protein
Data Mining
All the differentially expressed genes as well as genes which expression level highly correlated with the fertilization rate were identified using Basic Local Alignment Search Tool (BLAST). For this purpose, expressed sequence tag (taken for the microarray design) search was performed with standard nucleotide BLAST tool (BLASTN) followed by translated BLAST tool (BLASTX). The official gene symbol ontology of the identified genes was obtained using the UniProt Knowledgebase (UniProtKB).
Results
Biological Quality of Eggs
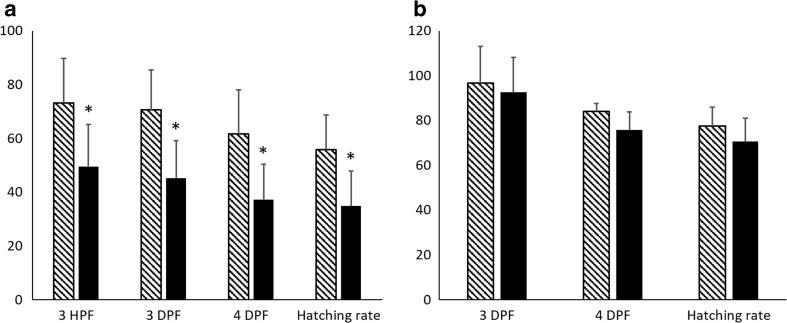
The mean fertilization rate observed at 3 HPF in the group characterized by low egg quality was significantly lower (p < 0.05) than in the group representing high egg quality. This was observed throughout development until hatching, where significant differences between the groups were also observed (Fig. 1a). The fertilization and hatching rates, when only fertilized eggs (the first cell cleavages were noticed at 3 HPF) were taken for further incubation, did not reveal any differences in terms of the developmental competence of the embryos up to the larval stage (Fig. 1b). The results of the biological evaluation of egg quality confirmed the relevance of the estimation of the fertilization rate at 3 HPF, which was then considered as the quality indicator used for the identification of groups of high and low egg quality.
Fig. 1.
Fertilization (HPF hours post-fertilization, DPF days post-fertilization) and hatching rates of embryos of European sea bass representing high (gray bars) and low (black bars) egg quality. a Fertilization and hatching rate was calculated taking into account all the eggs used for fertilization from each batch. b Fertilization and hatching rate was calculated only for the eggs which started to develop at third HPF. Data marked with asterisk were statistically different (p < 0.05)
Microarray Analysis
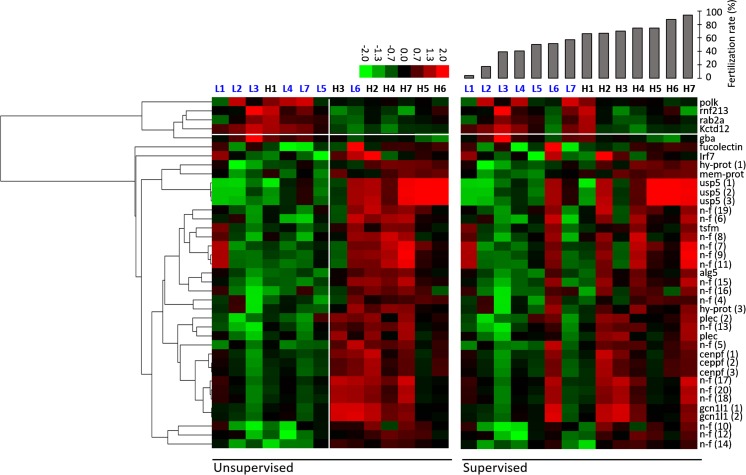
The microarray analysis resulted in the identification of 461 differentially expressed genes between the groups representing low and high egg quality. However, in only 39 genes more than 50 % of the samples exhibited raw signal above 50. Therefore, only those 39 genes were considered in further analysis. Unsupervised average linkage clustering revealed four clusters distinguishing groups of low and high egg quality, as well as under- and over-expression patterns of the analyzed genes. Two samples were clustered into not originally assigned groups, i.e., sample L6, initially assigned to the group of low egg quality, was clustered together with the high egg quality samples. Similarly, sample H1, which was originally assigned to the high egg quality group, was clustered together with low egg quality samples (Fig. 2). Supervised (according to the fertilization rate) average linkage clustering of the same genes clustered genes in the same order (Fig. 2). Three samples characterized by the lowest (L1–L3) and four by the highest quality (H4–H7) were grouped at the extreme edges of the cluster, being therefore in agreement with the unsupervised linkage. Among the samples characterized by moderate quality (between 50 and 75 % of FR), changes in positioning were observed, in comparison to unsupervised linkage, with only two samples (L6 and H1) changing their position for more than two (Fig. 2). Descriptions and ontology of all the differentially expressed genes are presented in Table 2.
Fig. 2.
Unsupervised (on the left) and supervised (on the right) average linkage clustering of 39 differentially expressed genes. Supervised clustering was performed according to the fertilization rate (diagram above the respective samples) recorded 3 HPF. Each row represents the same gene with symbol given on the right-hand side. Each column represents a RNA sample with its symbol provided above the respective column. Samples annotated with black font (H1–H7) represented high quality group, whereas samples annotated with blue font (L1–L7) represented low egg quality group. Expression level for each gene is presented using color intensity scale, where red and green represents over- and under-expression levels, respectively. Black color represents median abundance of the gene
Table 2.
Gene symbol, description, GenBank number (or NCBI Reference Sequence number for the PREDICTED proteins), and Gene Ontologies (with UniProtKB accession no.) of all the genes found to be differentially expressed (on the base of microarray analysis) when groups of “high” and “low” egg quality were compared, genes which were highly correlated with the egg fertilization rate as well as the most homogenized genes (used for qPCR data normalization)
| Symbola | Descriptionb | GenBank accession no. | GenBank/NCBI reference sequence | UniProtKB/Swiss-Prot Gene Ontology | UniProtKB accession no. | ||
|---|---|---|---|---|---|---|---|
| function | Process | Component | |||||
| Differentially expressed genes | |||||||
| polk | DNA polymerase kappa | FM009023 | KKF10166 | DNA binding, DNA-directed DNA polymerase activity, transferase activity | DNA repair, translesion synthesis, nucleotide-excision repair, DNA gap filling | Nucleus, nucleoplasm | Q9UBT6 |
| rnf213 | E3 ubiquitin-protein ligase RNF213 | FM016314 | XP_008298790 | Ubiquitin-protein transferase activity, protein binding | Protein ubiquitination | Cytoplasm, nucleolus, membrane | Q63HN8 |
| rab2a | Ras-related protein Rab-2A | FM000715 | XP_008332898 | GTPase activity, protein binding, GTP binding | Mitotic cell cycle, intracellular protein transport, metabolic process | Nucleus, Golgi membrane, endoplasmic reticulum, membrane | P61019 |
| kctd12 | BTB/POZ domain-containing protein KCTD12 | FM024677 | XP_008308481 | Poly(A) RNA binding | Regulation of G protein-coupled receptor protein signaling pathway, protein homooligomerization | Plasma membrane, presynaptic membrane, synapse | Q96CX2 |
| gba | Glucosylceramidase | FM021788 | XP_010748685 | Glucosylceramidase activity, receptor binding, hydrolase activity | Response to hormones (testosterone, estrogen, glucocorticoid) and pH, metabolic process (lipid, carbohydrate, sphingolipid, glycosphingolipid) | Lysosome membrane, lysosome lumen | P04062 |
| plec(2) | Plectin-like isoform X5 | FM008272 | XP_011603659 | Protein binding, poly(A) RNA binding | Cellular component disassembly involved in execution phase of apoptosis, programmed cell death, apoptotic process | Cytoplasm, plasma membrane | Q15149 |
| plec(1) | Plectin-like isoform X3 | FM004961 | XP_010767028 | Protein binding, poly(A) RNA binding | Cellular component disassembly involved in execution phase of apoptosis, programmed cell death, apoptotic process | Cytoplasm, plasma membrane | Q15149 |
|
usp5(1)
usp5(2) usp5(3) |
Ubiquitin carboxyl-terminal hydrolase 5 | FM016194 | XP_010733307 | Ubiquitin-specific protease activity, protein binding, metal ion binding, hydrolase activity, cysteine-type peptidase activity | Ubiquitin-dependent protein catabolic process, positive regulation of proteasomal ubiquitin-dependent protein catabolic process, protein K48-linked deubiquitination | Lysosome | P45974 |
|
cenpf(1)
cenpf(2) cenpf(3) |
Centromere protein F (No. 1) | FM016762 | XP_010730622 | Protein binding, protein homodimerization activity, transcription factor binding | Regulation of G2/M transition of mitotic cell cycle, chromosome segregation, metaphase plate congression, kinetochore assembly, regulation of striated muscle tissue development | Chromosome centromeric region, kinetochore, nucleus, cytosol | P49454 |
| mem-prot | – | FM024907 | WP_038038408 | n/a | n/a | n/a | n/a |
| hy-prot(3) | – | FM013574 | WP_033116978 | n/a | n/a | n/a | n/a |
| hy-prot(1) | – | FM005046 | WP_030404274 | n/a | n/a | n/a | n/a |
| tsfm | TSFM Ts translation elongation factor, mitochondrial | FM024343 | KKF32528 | Translation elongation factor activity | Translational elongation | Mitochondrion | B5X5B4 |
| irf7 | Interferon regulatory factor 7 | FM005989 | KKF30244 | Protein binding, DNA binding, RNA polymerase II core promoter proximal region sequence-specific DNA binding transcription factor activity | Regulation of adaptive immune response, regulation of transcription, DNA-templated, regulation of innate immune response | Nucleus, cytoplasm | P70434 |
|
gcn1l1(1)
gcn1l1(2) |
Translational activator GCN1 (No. 1) | FM024347 | KKF24933 | Translation factor activity, RNA binding | Regulation of translation | Cytoplasm, ribosome, membrane | Q92616 |
| fucolectin | Fucolectin-4 | FM025664 | KKF16053 | Fucose binding, carbohydrate binding, calcium ion binding, metal ion binding | Regulation of complement activation, lectin pathway, regulation of cellular defense response, regulation of innate immune response | Extracellular region | Q9I928 |
| alg5 | Dolichyl-phosphate beta-glucosyltransferase | FM024334 | KKF15163 | Transferase activity, transferring glycosyl groups | Protein glycosylation, post-translational protein modification, transferase activity | Endoplasmic reticulum, endoplasmic reticulum membrane | Q9Y673 |
| n-f(4) | – | FM024309 | – | – | – | – | – |
| n-f(5) | – | FM027632 | – | – | – | – | – |
| n-f(6) | – | FM028757 | – | – | – | – | – |
| n-f(7) | – | FM019745 | – | – | – | – | – |
| n-f(8) | – | FM015144 | – | – | – | – | – |
| n-f(9) | – | FM021366 | – | – | – | – | – |
| n-f(10) | – | AM984356 | – | – | – | – | – |
| n-f(11) | – | FM021366 | – | – | – | – | – |
| n-f(12) | – | FM007989 | – | – | – | – | – |
| n-f(13) | – | FM000334 | – | – | – | – | – |
| n-f(14) | – | FM002847 | – | – | – | – | – |
| n-f(15) | – | FM010821 | – | – | – | – | – |
| n-f(16) | – | FM001796 | – | – | – | – | – |
| n-f(17) | – | FM014863 | – | – | – | – | – |
| n-f(18) | – | FM014863 | – | – | – | – | – |
| n-f(19) | – | FM020519 | – | – | – | – | – |
| n-f(20) | – | FM014863 | – | – | – | – | – |
| Genes exhibiting the most stable expression level | |||||||
| hspa14 | Heat shock 70 kDa protein 14 | FM023807 | KKF23225 | Protein and ATP binding | “De novo” cotranslational protein folding | Cytoplasm, cytosol, membrane, ribosome | Q0VDF9 |
| cops3 | COP9 signalosome complex subunit 3 | FM027858 | XP_008300965 | Protein binding | Ubiquitin-dependent protein catabolic process, in utero embryonic development, signal transduction, protein binding | Nucleus, cytoplasm | Q9UNS2 |
| mtif3 | Translation initiation factor IF-3, mitochondrial | FM022743 | KKF15983 | Translation initiation factor activity | Mitochondrial translational initiation | Mitochondrion | Q9H2K0 |
| eif3ea | Eukaryotic translation initiation factor 3 subunit E-A | FM026913 | KKF20815 | Translation initiation factor activity | Regulation of translational initiation | Nucleus, cytoplasm | Q6DRI1 |
| hectd3 | E3 ubiquitin-protein ligase HECTD3 | FM020929 | KKF23624 | Ubiquitin-protein transferase activity | Protein ubiquitination involved in ubiquitin-dependent protein catabolic process | Cytoplasm | Q3U487 |
| eif3e | Eukaryotic translation initiation factor 3 subunit E | FP237239 | XP_010738667 | Translation initiation factor activity, protein binding | Regulation of translational initiation | Nucleus, cytoplasm, cytosol | P60228 |
Numbers in parenthesis represent number of subsequent gene, when more than one gene with the same name was identified
Specific, not described symbols: n-f protein was not found, hy-prot only sequence of hypothetical protein could be found, mem-prot refer to unspecified membrane protein, un prot only sequence for uncharacterized protein was found
aFor all the genes, when possible, gene symbol identical to human one was given
bFor all the genes, when possible, gene description identical to human one was given
Real-Time qPCR Analysis
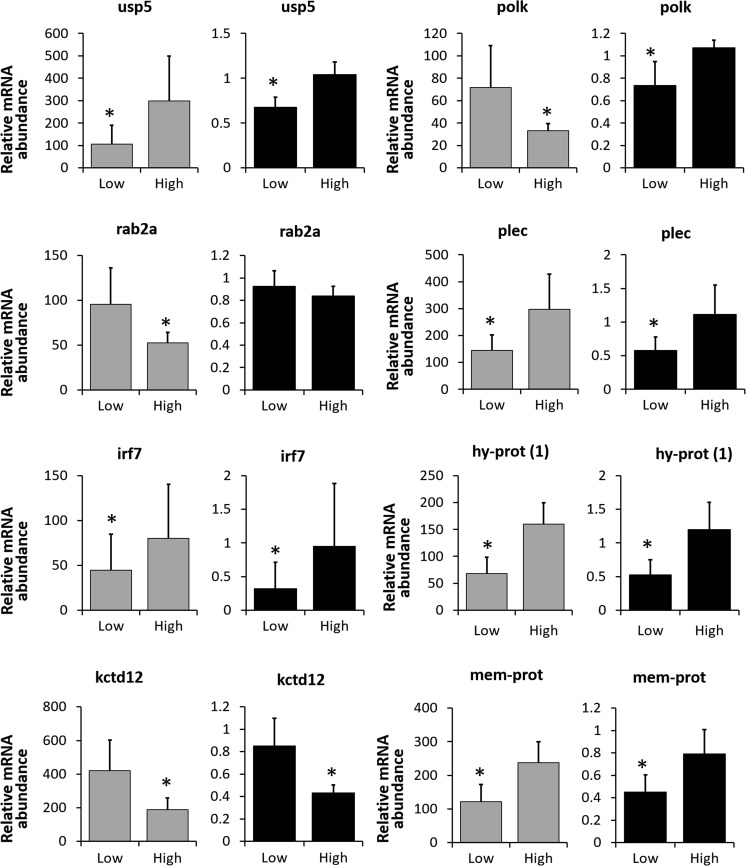
Among the eight genes chosen for the real-time qPCR analysis, significant differences in relative expression between the low and high egg quality groups were recorded in seven of them. However, for one gene—DNA polymerase kappa (polk)—qPCR revealed the opposite expression pattern from the one found during the microarray analysis (Fig. 3). For ras-related protein Rab-2A (rab2a), qPCR did not confirm significant differences between the experimental groups. Descriptions and ontology of all the analyzed genes are presented in Table 2.
Fig. 3.
The relative gene expression level in “low” and “high” egg quality groups obtained after microarray (gray bars) and real-time qPCR (black bars) analysis. Data marked with an asterisk were statistically different (p < 0.05). Description and ontology of all the genes are presented in Table 2
Genes Exhibiting the Most Stable Expression Level
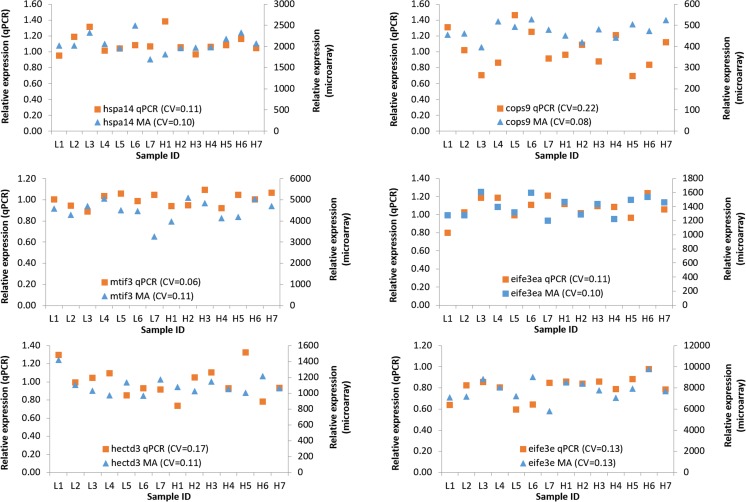
The real-time qPCR of genes exhibiting the most stable expression level during the microarray analysis revealed similar expression patterns and stability in all the six analyzed genes. Only in the case of COP9 signalosome complex subunit 3 (cops9) did the coefficient of variation after qPCR exhibits an over 10 % higher value as compared to the microarray data. On the other hand, the results of qPCR analysis of mitochondrial translation initiation factor IF-3 (mtif3) indicate that expression level was more stable than that recorded during the microarray analysis (Fig. 4). Nonetheless, the qPCR validation confirmed the stability of those genes, which were subsequently used for qPCR data normalization.
Fig. 4.
Relative expression level of the most stable genes (which were used for data normalization) recorded during the microarray (MA) analysis and qPCR analysis. For expression level of each gene, coefficient of variation (CV) was calculated and provided. Description and ontology of all the genes are given in Table 2. Sample ID represents egg samples assigned to different quality groups (L1–L7 for low egg quality group, H1–H7 for high egg quality group)
Discussion
Biological Egg Quality
Biological egg quality in finfishes is classically assessed through the monitoring of embryonic development at different stages and/or at hatching (Forniés et al. 2001; Żarski et al. 2011, 2012). For many fish species, the hatching rate has been considered more reliable than embryonic survival at earlier stages (Żarski et al. 2011; Schaerlinger and Żarski 2015). This is due to successive embryo mortality during incubation, which can be associated with impairment of important events occurring during embryogenesis, such as, for example, activation of the zygotic genome or the exhibition of serious developmental abnormalities (e.g., Schaerlinger and Żarski 2015). However, it should be highlighted that poor egg quality is very often manifested during early embryonic development (Chapman et al. 2014), which enables the use of early developmental stages (such as, e.g., the eight-cell stage) as a reliable quality indicator (Mommens et al. 2014). The data obtained in our study clearly indicate that embryonic survival at 3 HPF was reliable enough to estimate the overall quality of eggs in sea bass. This suggests that eggs without any developmental competence failed to develop at the earliest possible stage. A similar observation was also reported for striped bass, for which comparable survival of viable embryos was recorded up to 5 days post-hatch, even if originated from batches characterized by different quality (Chapman et al. 2014). In the case of sea bass, it was also previously reported that determination of embryonic survival was a reliable quality indicator (Saillant et al. 2001). It can thus be concluded that, in the case of sea bass, the embryos exhibiting high developmental competence at the earliest embryonic stages exhibited similar developmental competence later on.
Differences Between the Groups of High and Low Quality
The results of our study clearly suggest the possible importance of genes responsible for intracellular protein degradation in the formation of egg developmental competence. One of the genes is ubiquitin carboxyl-terminal hydrolase 5 (usp5), whose expression was significantly higher in high quality eggs (confirmed also by qPCR). It encodes one of the deubiquitinating enzymes (DUB), which are enzymes involved in ubiquitin-mediated proteolysis of proteins (Nandi et al. 2006; Dayal et al. 2009). Through their important function, DUBs play a key regulatory role in many processes, from hereditary cancer to neurodegeneration (Nijman et al. 2005). Interestingly, another ubiquitin-related gene, E3 ubiquitin-protein ligase RNF213 (rnf213), encoding a probable ligase catalyzing the final phase of the formation of protein-ubiquitin complex (e.g., Liu et al. 2011), was upregulated in eggs of low quality, which is in accordance with the quality-related expression pattern of rnf213 reported for Atlantic halibut (Mommens et al. 2014). The mRNA level of the other DUBs (e.g., ubiquitin-specific peptidase 11 and 14—usp11 and usp14, respectively), as well as other genes related to the ubiquitin-related processes, was also reported to be positively correlated with egg quality in striped bass (Chapman et al. 2014). This suggests that DUBs and other ubiquitin-related genes can be considered a very important group of genes involved in the developmental competence of the egg in sea bass and should be considered important candidates for the overall transcriptomic profile of a “good” egg.
Both microarray and qPCR analysis revealed significant differences between the groups of low and high egg quality in terms of abundance of mRNA of interferon regulatory factor 7 (irf7) and fucolectin-4 (fucolectin), both involved in innate immune response (Bianchet et al. 2002; Mommens et al. 2014). Interestingly, irf7 and another gene related with immune response (mhc class II antigen alpha chain—mhc2a) were found to be very important candidate markers of egg quality in Atlantic halibut (Mommens et al. 2014). Therefore, it may be suggested that the genes related with the immune system (including irf7 and fucolectin) may constitute good candidates for molecular markers of egg quality.
To the best of our knowledge, the remaining successfully annotated genes [polk, BTB/POZ domain-containing protein KCTD12 (kctd12), glucosylceramidase (gba), centromere protein F (cenpf), mitochondrial TSFM Ts translation elongation factor (tsfm), translational activator GCN1 (gcnl1l), dolichyl-phosphate beta-glucosyltransferase (alg5), and plectin-like isoform (plec)] have not yet been reported to be egg quality-dependent in any fish species. However, their involvement in a variety of important processes, such as DNA repair (polk; Lone et al. 2007) or enzymatically dependent processes (regulated by gba; Church et al. 2004; Beavan et al. 2015), indicates their possible significant contribution to the transcriptomic profile of a “good” egg. This also refers to overexpressed cenpf in high egg quality—a gene encoding one of the centromere proteins and involved in the cell division process—with a biological function similar to the other centromere protein (centromere protein k—cenpk) found to be upregulated in low egg quality in striped bass (Chapman et al. 2014). This allows the suggestion that genes involved in physical cell division (such as cenpf and cenpk) should be more closely studied in terms of their contribution to egg quality-dependent transcriptomic profile.
One of the very interesting genes overexpressed in the high egg quality group, as revealed by microarray (MA) and qPCR, is plec. This gene encodes plectin, which is a very large (>500 kDa) protein and an important cytolinker able to interact with a variety of cytoskeletal structures (Ackerl et al. 2007; Wiche and Winter 2011). In mice, the knockout of plectin (namely plec1) led to early lethality just after birth with signs of starvation and growth retardation (Ackerl et al. 2007). In humans, plectin (PLEC1) deficiency led to muscular dystrophy and pyloric atresia (Natsuga et al. 2010). Interestingly, the expression levels of the genes involved in cytoskeleton organization have already been found to be egg quality-dependent in rainbow trout (Bonnet et al. 2007). Considering that the assembly of the cytoskeleton also often involves membrane proteins (Herrmann and Aebi 2000), the importance of membrane protein (mem-prot), found to be overexpressed in high egg quality, could be additionally highlighted. This suggests that plec, together with other structural proteins (including mem-prot), may be considered in future studies on the profiling of the molecular “picture” of a “good” egg.
In the overall transcriptomic profile of the egg, genes responsible for the translational processes were reported to be very important elements securing proper embryonic development (Lanes et al. 2013; Sullivan et al. 2015). Therefore, MA analysis in our study revealed three genes (tsfm, gcnl1l, alg5) which have direct involvement in the translational process, and for the first time, it is suggested that they have a relation with egg quality and may constitute a good basis for the determination of the transcriptomic profile of a “good” egg. However, further investigation is still needed.
Conclusions
In our study, we have presented for the first time data on the transcriptomic profiling of sea bass eggs characterized by different quality. We confirmed the relevance of some of the genes already reported to be potential molecular quality determinants (mainly rnf213 and irf7), but we also found new genes (mainly usp5, mem-prot, plec, cenpf), whose expression patterns were not reported to be quality-dependent in any fish species. Together, our results stress the importance of genes, or groups of genes, being involved in protein ubiquitination, translation, DNA repair, and cell structure and architecture, probably the mechanisms that contribute to egg developmental competence in sea bass. This study also indicates the importance of further genomic research (for different species and with high numbers of samples characterized by a high variation in egg quality) in order to verify whether these processes are involved in the determination of egg quality competence in other species and in other culture environments, which may be an important contribution for both science and aquaculture. Additionally, it must be emphasized that there is still a high number of uncharacterized genes (with unknown ontology) that may offer a very important contribution to our knowledge. Therefore, the efforts undertaken for the characterization of these genes will have significant importance for future studies. However, apart from many unanswered questions remaining, and new questions arising, the findings of our study may contribute to a better understanding of the molecular mechanisms behind egg quality in teleosts and may help in the determination of future research priorities for the determination of the molecular profile of a “good” egg.
Acknowledgements
This work was supported by the French National Research Agency (ANR-13-BSV7-0015-Maternal Legacy to JB and CF) and by COST Action (FA1205 AQUAGAMETE).
Compliance with Ethical Standards
Conflict of Interest
Authors have no conflict of interest to declare.
References
- Ackerl R, Walko G, Fuchs P, Fischer I, Schmuth M, Wiche G (2007) Conditional targeting of plectin in prenatal and adult mouse stratified epithelia causes keratinocyte fragility and lesional epidermal barrier defects. J Cell Sci 120:2435–2443 [DOI] [PubMed]
- Aegerter S, Jalabert B, Bobe J (2005) Large scale real-time PCR analysis of mRNA abundance in rainbow trout eggs in relationship with egg quality and post-ovulatory ageing. Mol Reprod Dev 72:377–385 [DOI] [PubMed]
- Beavan M, Uk M, Mcneill A, Uk M, Proukakis C, Europe PMC Funders Group (2015) Evolution of prodromal clinical markers of Parkinson disease in a glucocerebrosidase mutation positive cohort. JAMA Neurol 72:201–208 [DOI] [PMC free article] [PubMed]
- Bianchet MA, Odom EW, Vasta GR, Amzel LM (2002) A novel fucose recognition fold involved in innate immunity. Nat Struct Biol 9:628–634 [DOI] [PubMed]
- Bobe J, Labbé C (2010) Egg and sperm quality in fish. Gen Comp Endocrinol 165:535–548 [DOI] [PubMed]
- Bonnet E, Fostier A, Bobe J (2007) Microarray-based analysis of fish egg quality after natural or controlled ovulation. BMC Genomics 8:55 [DOI] [PMC free article] [PubMed]
- Bromage N, Jones J, Randall C, Thrush M, Davies B, Springate J, Duston J, Barker G (1992) Broodstock management, fecundity, egg quality and the timing of egg production in the rainbow trout (Oncorhynchus mykiss). Aquaculture 100:141–166
- Brooks S, Tyler CR, Sumpter JP (1997) Quality in fish: what makes a good egg? Rev Fish Biol Fish 7:387–416
- Carnevali O, Mosconi G, Centonze F, Navas J, Zanuy S, Carrillo M, Bromage NR (1998) Influence of dietary lipid composition on yolk protein components in sea bass, Dicentrarchus labrax. Sci Mar 62:311–318
- Carrillo M, Bromage N, Zanuy S, Serrano R, Prat F (1989) The effect of modifications in photoperiod on spawning time, ovarian development and egg quality in the sea bass (Dicentrarchus labrax L.). Aquaculture 81:351–365
- Cerdá J, Carrillo M, Zanuy S, Ramos J, de la Higuera M (1994) Influence of nutritional composition of diet on sea bass, Dicentrarchus labrax L., reproductive performance and egg and larval quality. Aquaculture 128:345–361
- Cerdá J, Zanuy S, Carrillo M, Ramos J, Serrano R (1995) Short- and long-term dietary effects on female sea bass (Dicentrarchus labrax): seasonal changes in plasma profiles of lipids and sex steroids in relation to reproduction. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 111:83–91
- Chapman RW, Reading BJ, Sullivan CV (2014) Ovary transcriptome profiling via artificial intelligence reveals a transcriptomic fingerprint predicting egg quality in striped bass, Morone saxatilis. PLoS One 9:e96818 [DOI] [PMC free article] [PubMed]
- Church HJ, Cooper A, Stewart F, Thornton CM, Wraith JE (2004) Homozygous loss of a cysteine residue in the glucocerebrosidase gene results in Gaucher’s disease with a hydropic phenotype. Eur J Hum Genet 12:975–978 [DOI] [PubMed]
- Ciereszko A, Wojtczak M, Dietrich GJ, Kuźmiński H, Dobosz S (2009) A lack of consistent relationship between distribution of lipid droplets and egg quality in hatchery-raised rainbow trout, Oncorhynchus mykiss. Aquaculture 289:150–153
- Crespel A, Rime H, Fraboulet E, Bobe J, Fauvel C (2008) Egg quality in domesticated and wild seabass (Dicentrarchus labrax): a proteomic analysis. Cybium 32:205
- Dayal S, Sparks A, Jacob J, Allende-Vega N, Lane DP, Saville MK (2009) Suppression of the deubiquitinating enzyme USP5 causes the accumulation of unanchored polyubiquitin and the activation of p53. J Biol Chem 284:5030–5041 [DOI] [PMC free article] [PubMed]
- Devauchelle N, Coves D (1988) Sea bass (Dicentrarchus labrax) reproduction in captivity: gametogenesis and spawning. Aquat Living Resour 1:215–222
- Edgar R, Domrachev M, Lash AE (2002) Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 30:207–210 [DOI] [PMC free article] [PubMed]
- Fauvel C, Suquet M (1998) La qualité des gametes chez le bar et quelques déterminants en aquaculture. La Piscic Française 134:5–10
- Fauvel C, Suquet M, Dreanno C, Zonno V, Menu B (1998) Cryopreservation of sea bass (Dicentrarchus labrax) spermatozoa in experimental and production simulating conditions. Aquat Living Resour 11:387–394
- Fauvel C, Savoye O, Dreanno C, Cosson J, Suquet M (1999) Characteristics of sperm of captive seabass in relation to its fertilization potential. J Fish Biol 54:356–369
- Forniés M, Mañanós E, Carrillo M, Rocha A, Laureau S, Mylonas C, Zohar Y, Zanuy S (2001) Spawning induction of individual European sea bass females (Dicentrarchus labrax) using different GnRHa-delivery systems. Aquaculture 202:221–234
- Gohin M, Bobe J, Chesnel F (2010) Comparative transcriptomic analysis of follicle-enclosed oocyte maturational and developmental competence acquisition in two non-mammalian vertebrates. BMC Genomics 11:18 [DOI] [PMC free article] [PubMed]
- Henrotte E, Mandiki RSNM, Prudencio AT, Vandecan M, Mélard C, Kestemont P (2010) Egg and larval quality, and egg fatty acid composition of Eurasian perch breeders (Perca fluviatilis) fed different dietary DHA/EPA/AA ratios. Aquac Res 41:e53–e61
- Herrmann H, Aebi U (2000) Intermediate filaments and their associates: multi-talented structural elements specifying cytoarchitecture and cytodynamics. Curr Opin Cell Biol 12:79–90 [DOI] [PubMed]
- Lahnsteiner F, Weismann T, Patzner RA (1999) Physiological and biochemical parameters for egg quality determination in lake trout, Salmo trutta lacustris. Fish Physiol Biochem 20:375–388
- Lahnsteiner F, Urbanyi B, Horvath A, Weismann T (2001) Bio-markers for egg quality determination in cyprinid fish. Aquaculture 195:331–352
- Lanes CFC, Fernandes JMO, Kiron V, Babiak I (2012) Profiling of key apoptotic, stress, and immune-related transcripts during embryonic and postembryonic development of Atlantic cod (Gadus morhua L.). Theriogenology 78:1583–1596.e2 [DOI] [PubMed]
- Lanes CFC, Bizuayehu TT, de Oliveira Fernandes JM, Kiron V, Babiak I (2013) Transcriptome of Atlantic cod (Gadus morhua L.) early embryos from farmed and wild broodstocks. Mar Biotechnol 15:677–694 [DOI] [PubMed]
- Liu W, Morito D, Takashima S, Mineharu Y, Kobayashi H, Hitomi T, Hashikata H, Matsuura N, Yamazaki S, Toyoda A, Kikuta KI, Takagi Y, Harada KH, Fujiyama A, Herzig R, Krischek B, Zou L, Kim JE, Kitakaze M, Miyamoto S, Nagata K, Hashimoto N, Koizumi A (2011) Identification of RNF213 as a susceptibility gene for moyamoya disease and its possible role in vascular development. PLoS One. doi:10.1371/journal.pone.0022542 [DOI] [PMC free article] [PubMed]
- Lone S, Townson SA, Uljon SN, Johnson RE, Brahma A, Nair DT, Prakash S, Prakash L, Aggarwal AK (2007) Human DNA polymerase kappa encircles DNA: implications for mismatch extension and lesion bypass. Mol Cell 25:601–614 [DOI] [PubMed]
- Lubzens E, Young G, Bobe J, Cerdà J (2010) Oogenesis in teleosts: how fish eggs are formed. Gen Comp Endocrinol 165:367–389 [DOI] [PubMed]
- Mansour N, Lahnsteiner F, Patzner RA (2007) Distribution of lipid droplets is an indicator for egg quality in brown trout, Salmo trutta fario. Aquaculture 273:744–747
- Migaud H, Bell G, Cabrita E, McAndrew B, Davie A, Bobe J, Herráez MPP, Carrillo M (2013) Gamete quality and broodstock management in temperate fish. Rev Aquac 5:S194–S223
- Mommens M, Fernandes JM, Bizuayehu TT, Bolla SL, Johnston IA, Babiak I (2010) Maternal gene expression in Atlantic halibut (Hippoglossus hippoglossus L.) and its relation to egg quality. BMC Res Notes 3:138 [DOI] [PMC free article] [PubMed]
- Mommens M, Fernandes JM, Tollefsen K, Johnston IA, Babiak I (2014) Profiling of the embryonic Atlantic halibut (Hippoglossus hippoglossus L.) transcriptome reveals maternal transcripts as potential markers of embryo quality. BMC Genomics 15:829 [DOI] [PMC free article] [PubMed]
- Mylonas CC, Sigelaki I, Divanach P, Mananõs E, Carrillo M, Afonso-Polyviou A (2003) Multiple spawning and egg quality of individual European sea bass (Dicentrarchus labrax) females after repeated injections of GnRHa. Aquaculture 221:605–620
- Nandi D, Tahiliani P, Kumar A, Chandu D (2006) The ubiquitin-proteasome system. J Biosci 31:137–155 [DOI] [PubMed]
- Natsuga K, Nishie W, Shinkuma S, Arita K, Nakamura H, Ohyama M, Osaka H, Kambara T, Hirako Y, Shimizu H (2010) Plectin deficiency leads to both muscular dystrophy and pyloric atresia in epidermolysis bullosa simplex. Hum Mutat 31:1687–1698 [DOI] [PMC free article] [PubMed]
- Nijman SMB, Luna-Vargas MPA, Velds A, Brummelkamp TR, Dirac AMG, Sixma TK, Bernards R (2005) A genomic and functional inventory of deubiquitinating enzymes. Cell 123:773–786 [DOI] [PubMed]
- Panini EB, Mylonas CC, Zanuy S, Carrillo M, Ramos J, Bruce MP (2001) Incubation of embryos and larvae of marine fish using microtiter plates. Aquac Int 9:189–195
- Rise ML, Nash GW, Hall JR, Booman M, Hori TS, Trippel EA, Gamperl AK (2014) Variation in embryonic mortality and maternal transcript expression among Atlantic cod (Gadus morhua) broodstock: a functional genomics study. Mar Genomics 18(Pt A):3–20 [DOI] [PubMed]
- Saillant E, Chatain B, Fostier A, Przybyla C, Fauvel C (2001) Parental influence on early development in the European sea bass. J Fish Biol 58:1585–1600
- Schaerlinger B, Żarski D. Evaluation and improvements of egg and larval quality in percid fishes. In: Kestemont P, Dabrowski K, Summerfelt RC, editors. Biology and culture of percid fishes. Dordrecht: Springer Netherlands; 2015. pp. 193–223. [Google Scholar]
- Shields R (2001) Larviculture of marine finfish in Europe. Aquaculture 200:55–88
- Skaalsvik TH, Bolla SL, Thornqvist P-O, Babiak I (2015) Quantitative characteristics of Atlantic halibut (Hippoglossus hippoglossus L.) egg quality throughout the reproductive season. Theriogenology 83:38–47 [DOI] [PubMed]
- Sullivan CV, Chapman RW, Reading BJ, Anderson PE. Transcriptomics of mRNA and egg quality in farmed fish: some recent developments and future directions. Gen Comp Endocrinol. 2015 doi: 10.1016/j.ygcen.2015.02.012. [DOI] [PubMed] [Google Scholar]
- Wiche G, Winter L (2011) Plectin isoforms as organizers of intermediate filament cytoarchitecture. BioArchitecture 1:14–20 [DOI] [PMC free article] [PubMed]
- Żarski D, Palińska K, Targońska K, Bokor Z, Kotrik L, Krejszeff S, Kupren K, Horváth Á, Urbányi B, Kucharczyk D (2011) Oocyte quality indicators in Eurasian perch, Perca fluviatilis L., during reproduction under controlled conditions. Aquaculture 313:84–91
- Żarski D, Krejszeff S, Palińska K, Targońska K, Kupren K, Fontaine P, Kestemont P, Kucharczyk D (2012) Cortical reaction as an egg quality indicator in artificial reproduction of pikeperch, Sander lucioperca. Reprod Fertil Dev 24:843 [DOI] [PubMed]