Abstract
Background and purpose
Astroglia contribute to the pathophysiology of major depression and antidepressant drugs act by modulating synaptic plasticity; therefore, the present study investigated whether the fast antidepressant action of ketamine is reflected in a rapid alteration of the astrocytes’ morphology in a genetic animal model of depression.
Experimental Approach
S‐Ketamine (15 mg·kg−1) or saline was administered as a single injection to Flinders Line (FSL/ FRL) rats. Twenty‐four hours after the treatment, perfusion fixation was carried out and the morphology of glial fibrillary acid protein (GFAP)‐positive astrocytes in the CA1 stratum radiatum (CA1.SR) and the molecular layer of the dentate gyrus (GCL) of the hippocampus was investigated by applying stereological techniques and analysis with Imaris software. The depressive‐like behaviour of animals was also evaluated using forced swim test.
Key Results
FSL rats treated with ketamine exhibited a significant reduction in immobility time in comparison with the FSL‐vehicle group. The volumes of the hippocampal CA1.SR and GCL regions were significantly increased 1 day after ketamine treatment in the FSL rats. The size of astrocytes in the ketamine‐treated FSL rats was larger than those in the FSL‐vehicle group. Additionally, the number and length of the astrocytic processes in the CA1.SR region were significantly increased 1 day following ketamine treatment.
Conclusions and Implications
Our results support the hypothesis that astroglial atrophy contributes to the pathophysiology of depression and a morphological modification of astrocytes could be one mechanism by which ketamine rapidly improves depressive behaviour.
Abbreviations
- BDNF
brain‐derived neurotrophic factor
- CA1.SR
CA1 stratum radiatum
- EAAT
excitatory amino acid transporter
- FRL
Flinders resistant line
- FSL
Flinders sensitive line
- GCL
granular cell layer of dentate gyrus
- GFAP
glial fibrillary acid protein
- MDG
molecular layer of dentate gyrus
Tables of Links
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,bAlexander et al., 2015a,b).
Introduction
Major depressive disorder is a serious and costly psychiatric condition (Bielczyk et al., 2015), and severe depression with suicidal ideation is a particular concern in the treatment of depression (Zilles et al., 2015; Kessler et al., 2003). The plasticity of the hippocampal structure is believed to be one of the central mechanisms underlying severe depression (Duman, 2002; Cotter et al., 2001a). Importantly, glial cells play a dynamic role in modifying brain structure (Volterra and Meldolesi, 2005) and significantly, clinical and preclinical observations converge on the hypothesis that the structural changes as well as the functional abnormality of glial cells contribute to the pathophysiology of major depression (Liu et al., 2011). Cotter and co‐workers observed a reduction in glial cell density in the frontal cortex of patients with major depressive disorder (Cotter et al., 2001b). Specifically, one of the different types of glial cells, astrocytes play a key role in the maintenance of brain homeostasis, supplying energy to the neurons, recycling neurotransmitters, and by connecting to the blood vessels promoting their development. In particular, astrocytes participate in a dynamic structural framework for supporting synaptic function (Christopherson et al., 2005; Ma et al., 2012). Earlier findings have shown that astrocytes significantly contribute to the regulation of the function of the excitatory glutamate synapses in the hippocampus (Anderson and Swanson, 2000; Lehre and Rusakov, 2002). Interestingly, preclinical studies suggested that stress decreases the number and volume of astroglia soma in the hippocampus, an effect which may be reversed by fluoxetine (Czeh et al., 2006). Therefore, it is most likely that the therapeutic effect of conventional antidepressant drugs is not only as a consequence of the modification of neuronal plasticity but also as a result of a change in the morphology and number of astrocytes, which subsequently affects synaptogenesis, synaptic strength and stability (Czeh and Di Benedetto, 2013).
Currently, the treatment of major depression is not ideal due to the slow onset of action and only partial therapeutic effect of traditional antidepressant drugs (Gaynes et al., 2009). Clinical studies from the last decade indicate that ketamine; primarily acting on the glutamatergic NMDA receptor, quickly (within hours) improves depressive symptoms especially suicidal ideation in patients with treatment‐resistant depression (Serafini et al., 2014; Drewniany et al., 2015). However, several novel undiscovered mechanisms of action of the rapid‐acting interventions exist and identification of more specific targets related to the fast antidepressant effect of ketamine could help in the development of novel antidepressant treatments with a fast action and fewer side effects. Accordingly, in the current study, we sought to examine the assumption that an alteration in glial plasticity, especially of astrocytes, may be evident in the acute (24 h) phase following the administration of ketamine.
Methods
Animals
This study was performed on an adult male Flinders sensitive line (FSL), a genetic animal model of depression, with a Flinders resistant line (FRL) rats (FSL, n = 12; FRL, n = 12) as the control group. The FSL/FRL animals, originating from the colonies at Karolinska Institute (Sweden) and University of North Carolina (NC, USA), were bred at the Translational Neuropsychiatry Unit, Aarhus University, Denmark. The average age of the animals was 95 days. All animal care and procedures were carried out in accordance with the guidelines issued by the Danish national committee on animal ethics (permission id 2012‐15‐2934‐00254). Animals were pairwise housed in groups of two in a temperature‐controlled environment (20–22°C), having a normal 12 h light : dark cycle (lights on at 06:00). The animals had free access to food and water. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015).
Antidepressant drug treatments
Four experimental groups were set up: FRL.vehicle (n = 6), FRL.ketamine (n = 6), FSL.vehicle (n = 6) and FSL.ketamine (n = 6). S‐Ketamine hydrochloride (5 mg·mL−1, Pfizer ApS, Denmark, ATC‐code N01AX14) was obtained from the local hospital pharmacy. Rats received a single i.p. injection of ketamine or saline (15 mg·kg−1) (Muller et al., 2013) and were perfused 24 h after treatment as described below.
Behavioural testing
Depression‐like behaviour of the rats was assessed by the modified forced swim test (Slattery and Cryan, 2012) 30 min before transcardial perfusion (i.e. 23.5 h after the injection). The behavioural pattern of animals was categorized according to mobility and immobility (floating) as described previously (Liebenberg et al., 2015).
Tissue preparation
The procedures were as described previously (Kaae et al., 2012). Briefly, the rats were anaesthetised deeply with an i.p. injection of pentobarbital sodium/lidocaine, and were subsequently perfused with heparin (10 U·mL−1)‐treated 0.9% saline (pH = 7.3) for 4 min, followed by ice‐cold 4% paraformaldehyde (pH = 7.2–7.4) for 7 min. The right or left hemisphere was selected randomly, and they were placed in a cryoprotective solution containing 30% (w.v‐1) sucrose for 48 h, followed by placement on the copper blocks and frozen in the liquid nitrogen. Brains were then cut coronally at 40 μm thickness on a cryostat (Leica, Germany). The first section of each series was assigned randomly by using a random table, and two sets of sections were chosen based on a systematic sampling principle and a section sampling fraction of 1/12 (Gundersen, 2002). Therefore, we had 8–10 sections per series with a fixed distance 480 μm (12 × 40). One set of sections was used for thionin staining, and the second one was used for glial fibrillary acid protein (GFAP) staining.
Glial fibrillary acid protein immunohistochemistry
Free‐floating sections were washed in Tris buffer saline (TBS) containing 0.1% Triton X‐100 for 30 min followed by blocking endogenous peroxidase (30% H2O2 and methanol dissolved in TBS) for 30 min. Antigen retrieval prior to immunohistochemistry was performed by heating the sections in the retrieval solution (Dako, Ref# S1699, Denmark) dissolved in distilled water in the oven for 30 min. Thereafter, sections were washed three times in 1% BSA and 0.3% Triton‐X in TBS solution for 10 min. Following this step, sections were incubated with a polyclonal rabbit anti‐GFAP (Dako, Ref# Z0334, Denmark) at 1:500 dilutions in 1% BSA, TB buffer 50 mM overnight at 4°C. Subsequently, sections were washed in TBS with 0.1% BSA and Triton X‐100 for 10 min, and then incubated in polyclonal secondary goat anti‐rabbit IgG antibody/HRP (1:200 dilutions; Dako, Ref# P0448, Denmark) for 2 h. Afterwards, sections were rinsed three times in TBS for 10 min. The immunolabelling was done by using 3,3′‐diaminobenzidine solution (Sigma, USA) for 1 min. Finally, the sections were mounted on the gelatin‐coated slides and counterstained with 0.25% thionin solution (thionin, Sigma T3387).
Stereological quantification of the volumes of the hippocampal regions
The volumes of two subregions of the rat hippocampus [CA1 stratum radiatum (CA1.SR) and granular cell layer of dentate gyrus (GCL)] were estimated on Nissl‐stained sections by applying the Cavalieri estimator with point counting (Gundersen et al., 1988) using the newCAST software (Visiopharm, Hørsholm, Denmark), an Olympus light microscope (Olympus BX50, Olympus, Denmark) modified for stereology with a digital camera (Olympus DP72, Olympus, Denmark), a motorized microscope stage (Prior H138 with controller H29, Cambridge, UK) and a 10× lens (Olympus, Splan, N.A. 0.45).
The following formula was used for estimating the volume of hippocampal subregions:
where ΣP is the total number of the points hitting the region of interest, (a/p) is the area per point (0.007 mm2), T is the section thickness (40 μm) and SSF is the section sampling fraction (1/12).
Acquisition of images for three‐dimensional reconstructions of astrocytes in the CA1.SR area
A systematic set of Z‐stacks of the 40 μm thick GFAP‐stained sections of the CA1.SR area was captured by an Olympus scanner VS120 (using software VS‐ASW 2.5, Build 9483) by using a 60× oil objective (Olympus, Splan, N.A. 1.35). The advantage of this method of image acquisition was the possibility of Z‐stack capturing of more than one astrocyte at the same time. The height of the Z‐stacks was 20–25 μm, and the acquisition of images was performed in steps of 0.5 μm (Figure 1A).
Figure 1.
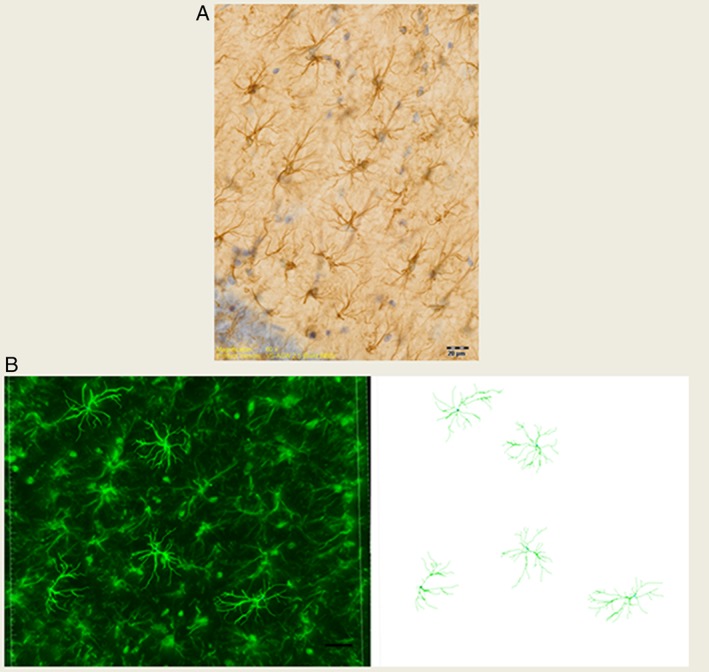
(A) This GFAP immunostained image was captured from the CA1.SR area of rat hippocampus using a 60× objective. Scale bar is 20 μm. (B) 3D reconstruction of GFAP positive astrocytes in the CA1.SR subregion of the rat hippocampus by using Filament Tracers Algorithm in Imaris software. Scale bar is 20 μm.
Morphological analysis of astrocytes
The images captured were transferred to Imaris software (Version 7.7, Bitplane A.G., Zurich, Switzerland), and by using the Filament Tracers algorithm, the morphology (number of branches, total length of the branches, area of the branches area and sholl analysis of intersections) of astrocytes was quantified. The centre of the astrocyte soma was used as a reference point, and the length of the processes was measured based on the radial distance from the reference point in 10 μm. The sum of the number of branching intersections for all circles, the intersection of the branches and the circle with the maximal radial distance were measured for each astrocyte.
Morphological analysis was performed on 30 GFAP positive astrocytes in the CA1.SR area of the hippocampus from each animal. To obtain a reliable reconstruction, the sampling/selection criteria for the astrocytes were: (i) the cell bodies must be located in the middle of the section thickness with the clear border; (ii) the cell must have intact branches; and (iii) the branches of the selected cell should not be obscured by the branches of nearby cells or the background staining. We used the semi‐automatic option in the Filament Tracer module for quantifying the morphology of astrocytes. For 3D reconstruction of astrocytic branches, the diameter of the starting point was determined based on the longest diameter of the cell body. By identifying the branch endpoints and bifurcations, the complexity of the astrocytic branches was quantified (Figure 1B).
Stereological quantification of the volume of astrocytes
The volume of astrocytes was investigated in two subregions of the hippocampus: the CA1.SR and molecular layer of the dentate gyrus (MDG) on GFAP stained sections. Based on the division of GCL along the transverse axis into supra‐pyramidal blade (located between the CA3 and the CA1 areas) and infra‐pyramidal blade (located below the CA3 field) (Amaral et al., 2007), MDG was divided into supra.MDG and infra.MDG areas.
The astrocytic volume was calculated by applying 3D nucleator (NewCast Ver 4.1, Visiopharm, Hørsholm, Denmark). The number of half‐lines was set at 6, and the mode was vertical uniform random (VUR) based on the assumed rotational symmetry of astrocytes. The volume of GFAP‐immunopositive astrocytes was estimated with a 100× oil‐immersion objective lens (Olympus, Plan Apochromat, N.A. 1.25) (Figure 2A). We randomly sampled 50–80 astrocytes per animal with the optical disector set at a height of 15 μm.
Figure 2.
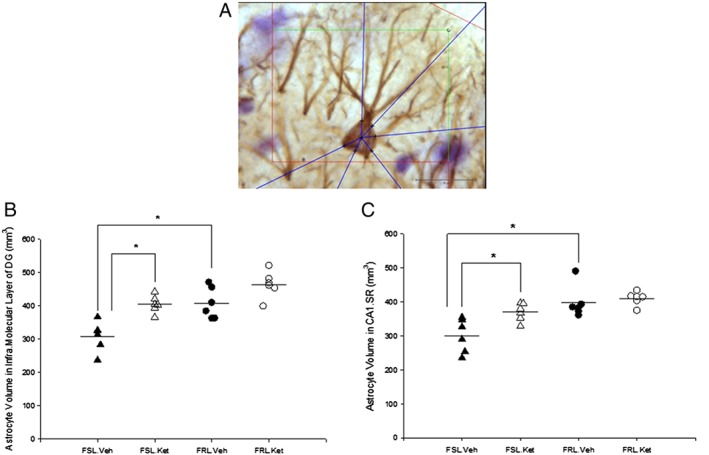
(A) Illustration of the 3D nucleator tool for estimating the volume of GFAP positive astrocytes in the CA1.SR, supra‐ and infra‐MDG subfields of rat hippocampus. Six half‐lines (blue half‐lines) were used and the intersections between the half‐lines and the border of the cell soma were assigned by using a 100× objective. Scale bar is 20 μm; (B) Volume of the astrocytes in the infra‐MDG in the FSL and FRL rats 1 day after a single ketamine or saline administration.*P < 0.05; (C) Volume of the astrocytes in the CA1.SR area of the hippocampus in FSL and FRL rats 1 day after a single ketamine or saline administration, *P < 0.05.
All investigations were performed by the same researcher, who was unaware of the identity of the rat groups.
Statistical analysis
Statistical analysis of data was performed by using the IBM SPSS Statistics 22 programme and graphs were created by Sigmaplot 12.5 (SYSTAT, San Jose, CA). To compare the behaviour of animals, the volumes of the hippocampal subregions and the morphological alterations of the astrocytes between four groups of animals, two‐way ANOVA followed by a post hoc Bonferroni test was employed. For assessing the normal distribution of variables before doing the two‐way ANOVA, a Q‐Q plot and histogram for each variable was generated.
Two tailed Pearson analysis tested for a correlation between the different structural parameters of the hippocampus. A two‐tailed probability level of P < 0.05 was used as the significance level. The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015).
Results
The animal behaviour in the forced swim test
The results from the forced swim test revealed a rapid, clear antidepressant‐like effect of ketamine on immobility time, with a strain × treatment interaction (F 1, 20 = 6.32; P < 0.05), a significant effect of strain (F 1, 20 = 54.73; P < 0.05) and ketamine treatment (F 1, 20 = 5.96; P < 0.05). The duration of the immobility time was significantly higher in the FSL‐vehicle rats compared to the FRL‐vehicle rats. A meaningful decrease in the duration of immobility behaviour in the FSL rats 1 day after a single injection of ketamine was observed (P < 0.05) (Figure 3).
Figure 3.
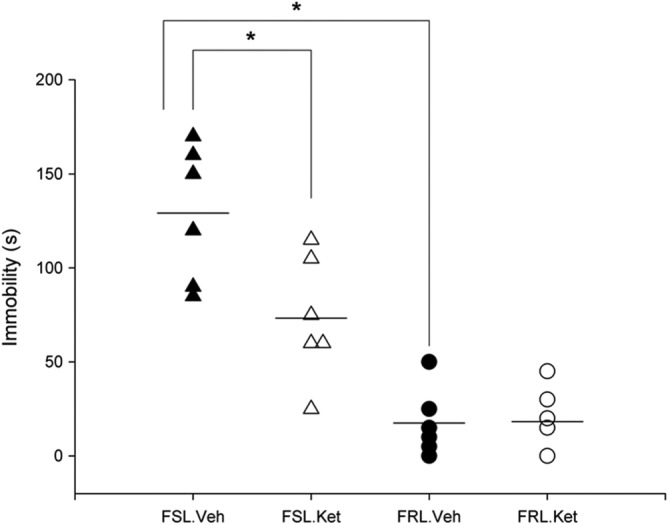
Fast effect of a single i.p. injection of ketamine on the immobility behaviour in the forced swim test in the FSL and FRL rats, *P < 0.05.
Rapid effect of ketamine treatment on the volume of the astrocytes in the hippocampal subregions
The stereological investigation showed that the volume of the GFAP positive astrocytes in the CA1.SR area was significantly influenced by strain (F 1, 20 = 19.23; P < 0.05) and ketamine treatment (F 1, 20 = 6.97; P < 0.05). In the FSL.vehicle rats, the volume of astrocytes in the CA1.SR area was significantly smaller than the FRL.vehicle rats (P < 0.05). Similarly, the astrocytes’ volume in the supra‐ and infra‐molecular layers of the DG was significantly larger in the FRL.vehicle rats compared with the FSL.vehicle rats. Notably, our results showed that astrocytes in the supra‐ and infra‐molecular layers of the DG from FSL rats with ketamine treatment are significantly larger in comparison with the FSL rats without treatment (Figure 2B). The results also demonstrated a quantitative augmentation of the volume of astrocytes in the CA1.SR area of the hippocampus 1 day after a single injection of ketamine in FSL rats (P < 0.05) (Figure 2C). Pearson correlation analysis demonstrated a positive correlation between the volume of the CA1.SR subregion and the size of the astrocytes in this area (r = 0.60; P < 0.05). Additionally, we found a positive correlation between the volume of astrocytes and total length of astrocytic processes in the CA1.SR area (r = 0.65; P < 0.05).
Rapid effect of ketamine treatment on the volume of the hippocampal subregions
By applying the Cavalieri estimator, a significant strain × treatment interaction on the volume of CA1.SR area was found (F 1, 20 = 13.65; P < 0.05). Additionally, a significant influence of ketamine treatment on the volume of the CA1.SR was observed (F 1, 20 = 49.11; P < 0.05).
Moreover, a significant influence of strain (F 1, 20 = 4.7; P < 0.05) and treatment (F 1, 20 = 8.42; P < 0.05) on the volume of the GCL was observed. FRL‐vehicle rats showed considerably larger CA1.SR and GCL areas compared to those of the FSL‐vehicle rats (P < 0.05). These results indicate that the volume of both hippocampal subregions (CA1.SR and GCL) is markedly increased in the FSL rats 1 day after a single injection of ketamine (P < 0.05) (Figure 4).
Figure 4.
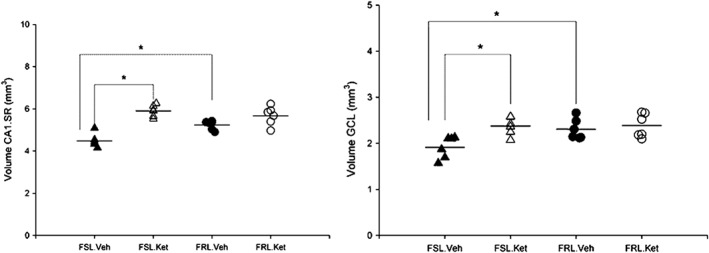
(Left) Volume of the CA1.SR area in the FSL (n = 12) and FRL (n = 12) rats 1 day after a single ketamine or saline administration. *P < 0.05. (Right) Volume of the GCL area in the FSL (n = 12) and FRL (n = 12) rats 1 day after a single ketamine or saline administration, *P < 0.05.
Rapid effect of ketamine treatment on the morphology of astrocytes in the CA1.SR subregion of the hippocampus
Total length of astrocytic processes
Two‐way ANOVA showed a significant strain × treatment interaction (F 1, 20 = 11.79; P < 0.05). The total length of the astrocytic processes was significantly different between the FSL.vehicle and FRL.vehicle rats (P < 0.05). Interestingly, 1 day after a single injection of ketamine, the length of the astrocytic branches was significantly increased in the FSL rats and similarly, ketamine had a significant effect on the total length of the astrocytic branches in the FRL rats (P < 0.05). Our results showed a comparable correlation between the total length of the astrocytic processes and the volume of the CA1.SR (r = 0.835; P < 0.05). Furthermore, the total length of the astrocytic processes was negatively correlated with the duration of immobility time (r = −0.64; P < 0.05).
Total number of astrocytic processes
The total number of astrocytic branches was influenced significantly by strain (F 1, 20 = 22.01; P < 0.05), treatment (F 1, 20 = 97.79; P < 0.05) and strain × treatment interaction (F 1, 20 = 7.74; P < 0.05). Astrocytes in the CA1.SR area of the hippocampus showed a significantly higher number of branches in the FRL rats in comparison with the FSL rats (31 ± 2.4 vs 24 ± 2.1; P < 0.05). Moreover, ketamine treatment significantly induced branching of astrocytes in the FSL and FRL rats (24 ± 2.1 vs 38 ± 1.8, P <0.05; 31 ± 2.4 vs 41 ± 5.3; P < 0.05). Correlation analysis demonstrated a significant association between the total number of astrocytic branches and the volume of the CA1.SR (r = 0.78; P < 0.05). Furthermore, correlation analysis showed that the duration of the immobility behaviour was negatively correlated with the total number of astrocytic branches (r = −0.610; P < 0.05).
Sholl analysis
In this study, sholl analysis revealed that irrespective of ketamine treatment, the branching patterns of astrocytes in the CA1.SR subregion of the hippocampus were significantly different between the FSL.vehicle and the FRL.vehicle rats and taken together; ketamine treatment had a significant effect on the astrocytic arborization in both the FSL and FRL rats 1 day after a single injection.
The number of branching intersections 10 μm and 20 μm away from the cell soma were significantly lower in the FSL.vehicle rats compared to the FRL.vehicle rats (P < 0.05); however, no difference was detected between the FSL.vehicle and the FRL.vehicle rats within 30 and 40 μm sholl spheres (P > 0.05). Our results demonstrated that acute ketamine treatment is associated with a significant increase in the length of the astrocytic branches at distances of 10, 20 and 30 μm away from the cell body of astrocytes in the FSL group. The number of intersections within the 40 μm sholl spheres around the cell soma did not change significantly in the FSL rats after ketamine treatment (P > 0.05) (Figure 5).
Figure 5.
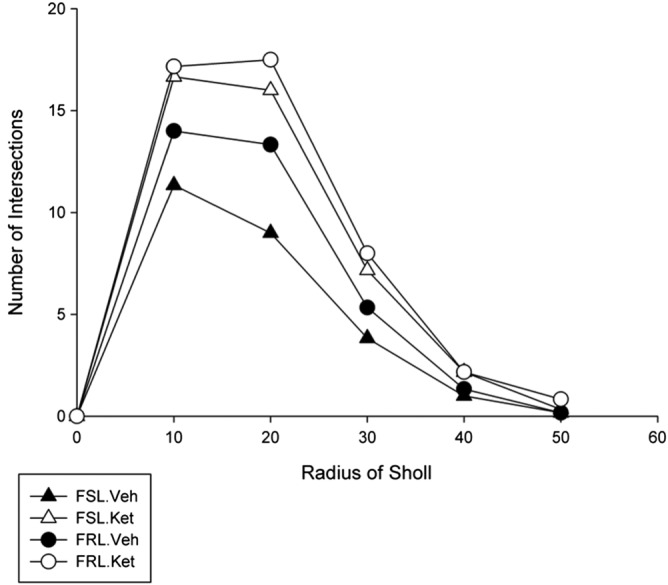
Effect of ketamine treatment 1 day after a single injection on the branching pattern of astrocytes in the CA1.SR subregion of the hippocampus using sholl analysis in the FSL and FRL rats. The number of branching intersections 10 and 20 μm away from the cell soma were significantly lower in the FSL.vehicle rats compared to the FRL.vehicle rats (P < 0.01). Significant increase in the length of the astrocytic branches at 10, 20 and 30 μm away from the cell body of astrocytes in the FSL group 1 day after treatment (P < 0.05).
Discussion
The main finding of the present study is that ketamine modulates several structural parameters related to astroglial plasticity in the hippocampus in the following 24 h. These findings go hand‐in‐hand with the behavioural observations, where ketamine acts as a potent antidepressant.
Ketamine has been shown to have behavioural antidepressant‐like effects in several other studies (60 min to weeks after the treatment) (Garcia et al., 2008; Dutta et al., 2015; Kavalali and Monteggia, 2015; Monteggia and Zarate, 2015). The recent clinical studies have focused on the rapid reduction of the suicidal ideation in patients with severe depression following a single ketamine administration. Our findings that show ketamine has an ameliorative effect on the immobility behaviour 24 h after a single injection are in agreement with these previous observations.
Earlier neuroimaging studies documented that one of the prominent structural alterations in the limbic areas of patients with major depressive disorder is a reduction in the volume of the hippocampus (Sheline et al., 1996; Campbell et al., 2004; Cole et al., 2011). Our findings are also in agreement with an earlier study from our laboratory, where it was shown that the volume of the hippocampus, in particular subregions of the hippocampus such as the CA1.SR and GCL, is significantly smaller in the FSL rats than the corresponding control group (Ardalan et al., 2016b; Kaae et al., 2012). Intriguingly, results obtained from using a non‐rodent model (spontaneously appearance of behavioural depression in captive cynomolgus macaques) suggested that the difference in the anterior hippocampal volume of depressed monkeys primarily was related to the changes in the volume of the CA1 and DG areas (Willard et al., 2013).
With regard to the effects of interventions, it was elucidated earlier that conventional antidepressant drugs produce a normalization of the structural plasticity of the hippocampus, by altering the volume of the area, the number of cells (for example through decreasing apoptosis or inducing cellular genesis), the volume of the cells and inducing morphological changes of the dendrites and axons (Boldrini et al., 2012; 2013; Joshi et al., 2015). In the present work, we found evidence for the hypothesis that ketamine rapidly alters the volume of the CA1.SR and GCL areas (within 24 h) in the depressed animals. One possible explanation for the larger volume of the GCL in the ketamine‐treated animals is an increase in the number of granule cells in this layer as a result of the stimulation of the rate of the neurogenesis without inducing a deficit in the maturation of the newborn neurons. Another explanation could be a reduction in the rate of apoptosis in this area after ketamine treatment. We postulated that in the CA1.SR area, alterations in the morphology of pyramidal cell dendrites, as well as the number and morphology of glial cells could be responsible for normalizing the volume of the area after ketamine treatment. The results of the present study coupled with previous observation on female depressed monkeys all corroborate the hypothesis that a reduction in the volume of the CA1 subregion of the hippocampus is mainly associated with the changes in the number of glial cells and the extent of neuropil in this area (Willard et al., 2013).
Our results on the structure of astrocytes in the CA1.SR area demonstrate that in depressed animals the morphology of astrocytes including the size of the cells and the branching patterns of astrocytic processes are significantly reduced. It is well documented that the interaction between the astrocytes and neurons is crucial for modulating the excitatory glutamate hippocampal synapses (Jessen, 2004) by regulating the amount of glutamine, an essential factor for releasing neuronal glutamate (Laming et al., 2000) and D‐serine factor (Ben Achour and Pascual, 2010). It has been shown that astrocytic foot processes are the main source of D‐serine and that activation of NMDA receptors needs the binding of both glutamate and D‐serine (Panatier et al., 2006). Additionally, it has been demonstrated that intermediate filament protein (GFAP) has an important role in regulating the astrocytic and neuronal glutamate transporter, as a loss of astrocytic GFAP resulted in decreases in the activity of both the astrocytic excitatory amino acid transporter 1 (EAAT1) and neuronal EAAT3 (Hughes et al., 2004). Therefore, it is reasonable to believe that disruption of the morphology of the astrocytes contributes to the pathogenesis of major depression by perturbing synaptic plasticity.
The results of the present study indicated that a single dose of ketamine has a rapid conspicuous effect on the soma size and on the astrocytic arborization in the CA1.SR area of the hippocampus in a rat model of depression. This finding is in agreement with previous results showing that astroglial process endings are dynamic and change their morphology rapidly (within minutes) (Hirrlinger et al., 2004). Crucially, one of the principle tasks of the astrocytes is the synthesis and release of neurotrophic factors such as brain‐derived neurotrophic factor (BDNF) (Althaus and Richter‐Landsberg, 2000) and the antidepressant effect of traditional antidepressant drugs and ketamine have been shown to be dependent on the hippocampal level of BDNF (Duman and Monteggia, 2006; Monteggia et al., 2013). Accordingly, one possible explanation for the rapid therapeutic effect of ketamine is the rapid activation of astrocytes and substantial stimulation of BDNF synthesis by activated hypertrophic astrocytes. Additionally, earlier studies tried to identify the role of astrocytes as one of the cellular mechanisms underlying the therapeutic effect of fluoxetine by studying the effect of fluoxetine on the expression of 5‐HT receptor subtypes, such as 5‐HT2B, on the astrocytes; they discovered that fluoxetine had a significant effect on the glutamatergic function of astrocytes (Li et al., 2011). However, other studies reported contradictory results on the morphological alterations of astrocytes induced by fluoxetine treatment. For instance, Czeh et al. (2006) demonstrated that stress reduced the volume of astrocytes in the hippocampus and fluoxetine treatment did not prevent this somal volume shrinkage of astrocytes. We also anticipated that after ketamine administration, glutamate activates astrocytes quickly and as a consequence the concentration of astrocytic Ca2+ is increased. This enhancement in the intracellular levels of Ca2+ has a significant effect on the synaptic plasticity (Hassinger et al., 1995). It has been discovered that some distal astrocytic branches and, rarely, astrocytic cell bodies in the cerebral cortex of adult rats contain NMDA receptors. Therefore, it has been suggested that some functions of the NMDA receptor depend on the activation of astrocytic NMDA receptors (Conti et al., 1996). Thus, the results of the present study greatly increase our knowledge about the involvement of astrocytes in the fast synaptic plasticity observed after ketamine treatment. Consistent with the results of the present study, we recently published a paper about the stimulation of rapid synaptogenesis by ketamine. We found that 1 day after a single dose of S‐ketamine, the number of excitatory synapses in the CA1.SR area of hippocampus significantly increased (Ardalan et al., 2016a). Additionally, we observed a significant sustained effect on the synaptic plasticity of the hippocampus 1 week after a single dose of S‐ketamine (Ardalan et al., 2016b).
Parallel with the astrocytic changes in the CA1.SR area, our study showed that the size of the GFAP‐positive astrocytes in the MDG in the FSL rats was significantly smaller than that of the control group and ketamine counteracted this effect of depression on the morphology of astrocytes. The cellular modifications in this hippocampal subregion are important, as the microvasculature of the DG is a key contributor to adult hippocampal neurogenesis. There is experimental evidence indicating that angiogenesis and neurogenesis are modulated by the signalling of VEGF, one of the factors expressed by astrocytes in hippocampus, and the overexpression of VEGF induces angiogenesis and neurogenesis in the adult hippocampus (Licht et al., 2011; Egeland et al., 2015). Accordingly, we noted that impairment of the morphology of astrocytes in the MDG subregion of the hippocampus inhibits VEGF signalling and this is one of the possible mechanisms underlying the impairment in neurogenesis and angiogenesis in depression. In contrast, ketamine by changing the morphology of astrocytes and consequently the function of astrocytes has a counter effect on the hippocampal microvasculature and neurogenesis. Looked at from another aspect, local vasoconstriction or vasodilator responses in the brain are triggered by neuronal activity, which is dependent on the glutamate‐mediated [Ca2+] oscillations in the astrocytes. Thus, another possible consequence of the morphological changes of astrocytes in depression is the impairment of activity‐dependent vasodilatation as a result of inhibiting these Ca2+ responses (Parri and Crunelli, 2003; Zonta et al., 2003), which is reversed by ketamine treatment.
Conclusion
Our results support the hypothesis that the morphological impairment of hippocampal astrocytes contributes to the pathogenesis of depression. One of the mechanisms underlying the fast antidepressant effect of ketamine is its ability to modify the morphology of astrocytes, so that they can optimally modulate the synaptic micro‐environment, neurogenesis and vascularization.
Author contributions
The main idea of this study was from G. W. and M. A.; M. A. and G. W. designed the study and wrote the protocol together. Data collection has been done by M. A. and A. H. R.; J. R. N. mainly contributed to the applied stereological methods of this study. The statistical analysis of data and the first draft of this manuscript was done by M. A. All authors contributed to and have approved the final manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Acknowledgements
We are grateful to Maj‐Britt Lundorf and Helene M. Andersen for their excellent technical assistance. This work was supported by the Lundbeck Foundation. The Centre for Stochastic Geometry and Advanced Bioimaging is supported by the Villum Foundation. MA was supported by the Lundbeck Foundation.
Ardalan, M. , Rafati, A. H. , Nyengaard, J. R. , and Wegener, G. (2017) Rapid antidepressant effect of ketamine correlates with astroglial plasticity in the hippocampus. British Journal of Pharmacology, 174: 483–492. doi: 10.1111/bph.13714.
References
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: Overview. Br J Pharmacol 172: 5729–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015b). The concise guide to PHARMACOLOGY 2015/16: Transporters. Br J Pharmacol 172: 6110–6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althaus HH, Richter‐Landsberg C (2000). Glial cells as targets and producers of neurotrophins. Int Rev Cytol 197: 203–277. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Scharfman HE, Lavenex P (2007). The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies). Prog Brain Res 163: 3–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CM, Swanson RA (2000). Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia 32: 1–14. [PubMed] [Google Scholar]
- Ardalan M, Wegener G, H.Rafati A, Randel Nyengaard J (2016a). S‐ketamine rapidly reverses synaptic and vascular deficits of hippocampus in genetic animal model of depression. Int J Neuropsychopharmacol. doi: 10.1093/ijnp/pyw098 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardalan M, Wegener G, Polsinelli B, Madsen TM, Nyengaard JR (2016b). Neurovascular plasticity of the hippocampus one week after a single dose of ketamine in genetic rat model of depression. Hippocampus 26: 1414–1423. [DOI] [PubMed] [Google Scholar]
- Ben Achour S, Pascual O (2010). Glia: the many ways to modulate synaptic plasticity. Neurochem Int 57: 440–445. [DOI] [PubMed] [Google Scholar]
- Bielczyk NZ, Buitelaar JK, Glennon JC, Tiesinga PH (2015). Circuit to construct mapping: a mathematical tool for assisting the diagnosis and treatment in major depressive disorder. Front Psych 6: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Hen R, Underwood MD, Rosoklija GB, Dwork AJ, Mann JJ et al. (2012). Hippocampal angiogenesis and progenitor cell proliferation are increased with antidepressant use in major depression. Biol Psychiatry 72: 562–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Santiago AN, Hen R, Dwork AJ, Rosoklija GB, Tamir H et al. (2013). Hippocampal granule neuron number and dentate gyrus volume in antidepressant‐treated and untreated major depression. Neuropharmacology 38: 1068–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S, Marriott M, Nahmias C, MacQueen GM (2004). Lower hippocampal volume in patients suffering from depression: a meta‐analysis. Am J Psychiatry 161: 598–607. [DOI] [PubMed] [Google Scholar]
- Christopherson KS, Ullian EM, Stokes CCA, Mullowney CE, Hell JW, Agah A et al. (2005). Thrombospondins are astrocyte‐secreted proteins that promote CNS synaptogenesis. Cell 120: 421–433. [DOI] [PubMed] [Google Scholar]
- Cole J, Costafreda SG, McGuffin P, Fu CH (2011). Hippocampal atrophy in first episode depression: a meta‐analysis of magnetic resonance imaging studies. J Affect Disord 134: 483–487. [DOI] [PubMed] [Google Scholar]
- Conti F, DeBiasi S, Minelli A, Melone M (1996). Expression of NR1 and NR2A/B subunits of the NMDA receptor in cortical astrocytes. Glia 17: 254–258. [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Landau S, Kerwin R, Everall I (2001a). Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry 58: 545–553. [DOI] [PubMed] [Google Scholar]
- Cotter DR, Pariante CM, Everall IP (2001b). Glial cell abnormalities in major psychiatric disorders: the evidence and implications. Brain Res Bull 55: 585–595. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al. (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeh B, Di Benedetto B (2013). Antidepressants act directly on astrocytes: evidences and functional consequences. Eur Neuropsychopharmacol 23: 171–185. [DOI] [PubMed] [Google Scholar]
- Czeh B, Simon M, Schmelting B, Hiemke C, Fuchs E (2006). Astroglial plasticity in the hippocampus is affected by chronic psychosocial stress and concomitant Fluoxetine treatment. Neuropharmacology 31: 1616–1626. [DOI] [PubMed] [Google Scholar]
- Drewniany E, Han J, Hancock C, Jones RL, Lim J, Nemat Gorgani N et al. (2015). Rapid‐onset antidepressant action of ketamine: potential revolution in understanding and future pharmacologic treatment of depression. J Clin Pharm Ther 40: 125–130. [DOI] [PubMed] [Google Scholar]
- Duman RS (2002). Pathophysiology of depression: the concept of synaptic plasticity. Eur Psychiatry 17 (Suppl 3): 306–310. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM (2006). A neurotrophic model for stress‐related mood disorders. Biol Psychiatry 59: 1116–1127. [DOI] [PubMed] [Google Scholar]
- Dutta A, McKie S, Deakin JF (2015). Ketamine and other potential glutamate antidepressants. Psychiatry Res 225: 1–13. [DOI] [PubMed] [Google Scholar]
- Egeland M, Zunszain PA, Pariante CM (2015). Molecular mechanisms in the regulation of adult neurogenesis during stress. Nature reviews . Neuroscience 16: 189–200. [DOI] [PubMed] [Google Scholar]
- Garcia LS, Comim CM, Valvassori SS, Reus GZ, Barbosa LM, Andreazza AC et al. (2008). Acute administration of ketamine induces antidepressant‐like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry 32: 140–144. [DOI] [PubMed] [Google Scholar]
- Gaynes BN, Warden D, Trivedi MH, Wisniewski SR, Fava M, Rush AJ (2009). What did STAR*D teach us? Results from a large‐scale, practical, clinical trial for patients with depression. Psychiatr Serv 60: 1439–1445. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ (2002). The smooth fractionator. J Microsc 207: 191–210. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N et al. (1988). The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS 96: 857–881. [DOI] [PubMed] [Google Scholar]
- Hassinger TD, Atkinson PB, Strecker GJ, Whalen LR, Dudek FE, Kossel AH et al. (1995). Evidence for glutamate‐mediated activation of hippocampal neurons by glial calcium waves. J Neurobiol 28: 159–170. [DOI] [PubMed] [Google Scholar]
- Hirrlinger J, Hulsmann S, Kirchhoff F (2004). Astroglial processes show spontaneous motility at active synaptic terminals in situ. Eur J Neurosci 20: 2235–2239. [DOI] [PubMed] [Google Scholar]
- Hughes EG, Maguire JL, McMinn MT, Scholz RE, Sutherland ML (2004). Loss of glial fibrillary acidic protein results in decreased glutamate transport and inhibition of PKA‐induced EAAT2 cell surface trafficking. Brain Res Mol Brain Res 124: 114–123. [DOI] [PubMed] [Google Scholar]
- Jessen KR (2004). Glial cells. Int J Biochem Cell Biol 36: 1861–1867. [DOI] [PubMed] [Google Scholar]
- Joshi SH, Espinoza RT, Pirnia T, Shi J, Wang Y, Ayers B et al. (2015). Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry 79: 282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaae SS, Chen F, Wegener G, Madsen TM, Nyengaard JR (2012). Quantitative hippocampal structural changes following electroconvulsive seizure treatment in a rat model of depression. Synapse 66: 667–676. [DOI] [PubMed] [Google Scholar]
- Kavalali ET, Monteggia LM (2015). How does ketamine elicit a rapid antidepressant response? Curr Opin Pharmacol 20: 35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR et al. (2003). The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS‐R). JAMA 289: 3095–3105. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: Reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laming PR, Kimelberg H, Robinson S, Salm A, Hawrylak N, Muller C et al. (2000). Neuronal‐glial interactions and behaviour. Neurosci Biobehav Rev 24: 295–340. [DOI] [PubMed] [Google Scholar]
- Lehre KP, Rusakov DA (2002). Asymmetry of glia near central synapses favors presynaptically directed glutamate escape. Biophys J 83: 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Zhang S, Zhang H, Hertz L, Peng L (2011). Fluoxetine affects GluK2 editing, glutamate‐evoked Ca(2+) influx and extracellular signal‐regulated kinase phosphorylation in mouse astrocytes. J Psychiatry Neurosci 36: 322–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht T, Goshen I, Avital A, Kreisel T, Zubedat S, Eavri R et al. (2011). Reversible modulations of neuronal plasticity by VEGF. Proc Natl Acad Sci U S A 108: 5081–5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebenberg N, Joca S, Wegener G (2015). Nitric oxide involvement in the antidepressant‐like effect of ketamine in the Flinders sensitive line rat model of depression. Acta Neuropsychiatrica 27: 90–96. [DOI] [PubMed] [Google Scholar]
- Liu Q, Li B, Zhu HY, Wang YQ, Yu J, Wu GC (2011). Glia atrophy in the hippocampus of chronic unpredictable stress‐induced depression model rats is reversed by electroacupuncture treatment. J Affect Disord 128: 309–313. [DOI] [PubMed] [Google Scholar]
- Ma S, Kwon HJ, Huang Z (2012). A functional requirement for astroglia in promoting blood vessel development in the early postnatal brain. PLoS One 7: e48001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteggia LM, Gideons E, Kavalali ET (2013). The role of eukaryotic elongation factor 2 kinase in rapid antidepressant action of ketamine. Biol Psychiatry 73: 1199–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteggia LM, Zarate C Jr (2015). Antidepressant actions of ketamine: from molecular mechanisms to clinical practice. Curr Opin Neurobiol 30: 139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller HK, Wegener G, Liebenberg N, Zarate CA Jr, Popoli M, Elfving B (2013). Ketamine regulates the presynaptic release machinery in the hippocampus. J Psychiatr Res 47: 892–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA et al. (2006). Glia‐derived D‐serine controls NMDA receptor activity and synaptic memory. Cell 125: 775–784. [DOI] [PubMed] [Google Scholar]
- Parri R, Crunelli V (2003). An astrocyte bridge from synapse to blood flow. Nat Neurosci 6: 5–6. [DOI] [PubMed] [Google Scholar]
- Serafini G, Howland RH, Rovedi F, Girardi P, Amore M (2014). The role of ketamine in treatment‐resistant depression: a systematic review. Curr Neuropharmacol 12: 444–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW (1996). Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A 93: 3908–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery DA, Cryan JF (2012). Using the rat forced swim test to assess antidepressant‐like activity in rodents. Nat Protoc 7: 1009–1014. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volterra A, Meldolesi J (2005). Astrocytes, from brain glue to communication elements: the revolution continues. Nature reviews . Neuroscience 6: 626–640. [DOI] [PubMed] [Google Scholar]
- Willard SL, Riddle DR, Forbes ME, Shively CA (2013). Cell number and neuropil alterations in subregions of the anterior hippocampus in a female monkey model of depression. Biol Psychiatry 74: 890–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles D, Wolff‐Menzler C, Wiltfang J (2015). Electroconvulsive therapy for the treatment of major depression. Nervenarzt 86: 549–556. [DOI] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T et al. (2003). Neuron‐to‐astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci 6: 43–50. [DOI] [PubMed] [Google Scholar]


