Abstract
Background and Purpose
The ultra‐rapidly activating delayed rectifier K+ current I Kur (encoded by Kv1.5 or KCNA5) plays an important role in human atrial repolarization. The present study investigates the regulation of this current by protein tyrosine kinases (PTKs).
Experimental Approach
Whole‐cell patch voltage clamp technique and immunoprecipitation and Western blotting analysis were used to investigate whether the PTK inhibitors genistein, tyrphostin AG556 (AG556) and PP2 regulate human atrial I Kur and hKv1.5 channels stably expressed in HEK 293 cells.
Key Results
Human atrial I Kur was decreased by genistein (a broad‐spectrum PTK inhibitor) and AG556 (a highly selective EGFR TK inhibitor) in a concentration‐dependent manner. Inhibition of I Kur induced by 30 μM genistein or 10 μM AG556 was significantly reversed by 1 mM orthovanadate (a protein tyrosine phosphatase inhibitor). Similar results were observed in HEK 293 cells stably expressing hKv1.5 channels. On the other hand, the Src family kinase inhibitor PP2 (1 μM) slightly enhanced I Kur and hKv1.5 current, and the current increase was also reversed by orthovanadate. Immunoprecipitation and Western blotting analysis showed that genistein, AG556, and PP2 decreased tyrosine phosphorylation of hKv1.5 channels and that the decrease was countered by orthovanadate.
Conclusion and Implications
The PTK inhibitors genistein and AG556 decrease human atrial I Kur and cloned hKv1.5 channels by inhibiting EGFR TK, whereas the Src kinase inhibitor PP2 increases I Kur and hKv1.5 current. These results imply that EGFR TK and the soluble Src kinases may have opposite effects on human atrial I Kur.
Abbreviations
- EGFR
EGF receptor
- IKur
ultra‐rapidly activating delayed rectifier potassium current
- PP2
3‐(4‐chlorophenyl) 1‐(1,1‐dimethylethyl)‐1H–pyrazolo[3,4‐d] pyrimidin‐4‐amine
- PTK
protein tyrosine kinase
- TK
protein kinase
- tyrphostin AG556
AG556
Tables of Links
| TARGETS | |
|---|---|
| Voltage‐gated ion channels a | Catalytic receptors c |
| Kv1.5 (IKur) channels | EGFR tyrosine kinases |
| Enzymes b | Protein tyrosine phosphatases |
| Src tyrosine kinases |
These Tables list key protein targets and the ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,b,cAlexander et al., 2015a,b,c)
Introduction
The ultra‐rapidly activating delayed rectifier current I Kur was initially reported in human atrial myocytes (Wang et al., 1993) and later found in atria, but not in the ventricles of the human heart (Li et al., 1996b). This current contributes to the repolarization of human atrial action potentials (Feng et al., 1997; Wettwer et al., 2004; Li et al., 2008). I Kur is carried by Kv1.5 (KCNA5) channels (Fedida et al., 1993; Snyders et al., 1993; Feng et al., 1997). Loss‐of‐function and/or gain‐of‐function Kv1.5 mutations may increase atrial fibrillation susceptibility (Olson et al., 2006; Christophersen et al., 2013) and so Kv1.5 channels are attractive targets for the treatment of atrial arrhythmias (Li et al., 2008; Schumacher et al., 2009; Tamargo et al., 2009; Loose et al., 2014). Our earlier study demonstrated that stimulation of β‐adrenoceptors increases, whereas α‐adrenoceptor stimulation decreases, human atrial I Kur via activating protein kinase A and protein kinase C respectively (Li et al., 1996a). The up‐regulation by protein kinase A and the down‐regulation by protein kinase C have been confirmed in Kv1.5 channels expressed in Xenopus laevis oocytes with Kvβ1.3 (Kwak et al., 1999) or Kvβ1.2 subunits (Williams et al., 2002). A recent study showed that AMP‐activated protein kinase down‐regulated Kv1.5 channels via activating the ubiqutin ligase Nedd4‐2 with subsequent clearance of channel protein from the cell membrane (Mia et al., 2012).
Receptor protein tyrosine kinases (PTKs) such as the EGF receptor (EGFR) kinase, and non‐receptor PTKs (e.g. the Src family kinases) (Hubbard and Till, 2000) play crucial roles in mediating cell growth, embryonic development, differentiation, metabolism, immune system function and oncogenesis. In addition, PTKs regulate transmembrane ion channels (Davis et al., 2001; Levitan, 1994). EGFR PTKs regulate several ion channels, including those carrying the cardiac voltage‐gated sodium current (INa) (Liu et al., 2007), Kir2.3 and Kir2.1 (Zhang et al., 2011a,b), recombinant human cardiac IKs (hKCNQ1/hKCNE1) (Dong et al., 2010) and the human EAG1 (Kv10.1) channels (Wu et al., 2012). Src family kinases also participate in regulation of hERG channels (Zhang et al., 2008) and cardiac transient outward potassium current (Ito, carried by hKv4.3 channels) (Zhang et al., 2012). An earlier report demonstrated that Src TK was associated with native hKv1.5 channels in human myocardium and cloned hKv1.5 channels. Tyrosine phosphorylation of hKv1.5 channels suppressed the channel current in cells coexpressing v‐Src (Holmes et al., 1996).
The present study investigated effects of EGFR TK and Src family kinases on I Kur/hKv1.5 channels using pharmacological tools. Our results demonstrated that genistein (a broad spectrum PTK inhibitor) and AG556 (a highly selective EGFR kinase inhibitor) inhibited I Kur/hKv1.5 current, whereas the inhibitor of Src family kinases, PP2, increased the current, by decreasing phosphorylation of hKv1.5 channels.
Methods
Human atrial myocyte isolation
The protocols for obtaining human atrial tissues was approved by the Ethics Committee of the University of Hong Kong (UW‐10‐174) with patients' consent. The investigation follows the principles outlined in the Declaration of Helsinki (see Cardiovascular Research 1997;35:2–4) for using human tissue. Human right atrial tissues were collected from patients undergoing coronary artery bypass grafting. Human atrial myocytes were enzymatically dissociated using the procedure as previously described (Li et al., 1996a, 2008). The isolated myocytes were kept at room temperature in a high‐potassium medium for at least 2 h before the electrophysiological recordings and randomly used for testing the effects of genistein, AG556, PP2 and/or orthovanadate on human atrial I Kur.
Cell culture, mutagenesis and gene transfection
The HEK 293 cell line (Tang et al., 2007; Wu et al., 2011) stably expressing hKv1.5/pBKCMV vector provided by Dr M. Tamkun (Colorado State University, CO, USA) was cultured with DMEM (Invitrogen, Hong Kong) containing 400 μg·mL−1 G418 (Sigma‐Aldrich, St Louis, MO, USA) and 10% fetal bovine serum. Cells for electrophysiological recording were seeded on a glass cover slip.
The high‐potential tyrosine phosphorylation sites of hKv1.5 channels were predicted with NetPhos 2.0 software (www.cbs.dtu.dk/cgi‐bin). Mutants of hKv1.5 channels were generated using the site‐directed mutagenesis kit (Stratagene, La Jolla, CA, USA), and each mutant was confirmed via full DNA sequencing analysis (Gene Centre, University of Hong Kong) as described previously (Zhang et al., 2011b; Wu et al., 2013). The mutants Y155F, Y521F, Y601F or Y155F–Y521F–Y601F were transiently transfected (10 μL of Lipofectamine 2000 with 4 μg of the plasmid) into HEK 293 cells for electrophysiological recording.
Electrophysiology
I Kur and hKv1.5 current were recorded using the experimental conditions and procedures as described previously (Li et al., 1996a; Wu et al., 2011). Briefly, atrial myocytes or HEK 293 cells were transferred into the cell chamber and superfused with Tyrode's solution. Whole‐cell configuration was established by a gentle suction after obtaining a gigaohm seal. Series resistance (3–5 MΩ) was compensated by 50–80% to reduce voltage errors. The data of membrane electrical signals were acquired using an EPC‐10 amplifier and Pulse software (Heka Elektronik, Lambrecht, Germany). All the experiments were conducted at room temperature (22–24°C). The data obtained in cells with unstable Rs and/or leakage current increased during experiments were discarded for analysis.
Immunoprecipitation and Western blots
The immunoprecipitation and Western blots were performed following the procedure described previously (Liu et al., 2007; Wu et al., 2012). Briefly, samples of HEK 293 cells stably expressing hKv1.5 channels and grown to 70–80% confluence, were treated with the different compounds (30 min at room temperature) and then detached and centrifuged (4°C). After the cell pellet was lysed (Liu et al., 2007; Wu et al., 2012), the protein lysate was quantified with a protein assay reader (Bio‐Rad Laboratories, Hercules, CA, USA) and diluted to equal concentrations. Proteins were immunoprecipitated with 2 μg anti‐hKv1.5 channel antibody (NeuroMab, Davis, CA, USA) and protein A/G beads (100 μL, Upstate) overnight at 4°C. Immunoprecipitated proteins bound to pelleted protein A/G beads were washed with PBS, denatured in Laemmli sample buffer, separated with SDS‐PAGE and electroblotted onto nitrocellulose membranes. The immunoblots were probed with anti‐phosphotyrosine antibody (1:2000; Cell Signaling Technology Inc., Danvers, MA, USA) overnight at 4°C in the blocking medium containing 5% BSA in Tris buffered saline (TBS) and Tween 20 and subsequently treated with goat anti‐mouse IgG‐HRP antibody (1:5000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 1 h at room temperature. Blots were developed with enhanced chemiluminescence (GE Healthcare, Hong Kong) and exposed on X‐ray film (Fuji Photo Film GmbH). The blots were then stripped and reprobed with the anti‐hKv1.5 antibody to determine total hKv1.5 channel proteins. The film was scanned, imaged by a Bio‐Imaging System (Syngene, Cambridge, UK) and analysed via Gene Tools software (Syngene).
Data and statistical analysis
The data collection and statistical analysis in this study comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015). Data are presented as mean ± SEM. A group size of number of 5 or more was determined based on previous experience (Li et al., 2008; Zhang et al., 2012) with SigmaPlot 12.5 (SPSS Science, Chicago, IL, USA). Paired and/or unpaired Student's two‐tailed t‐test was used as appropriate to determine the statistical significance of differences between two group means. One‐way ANOVA for multiple groups was followed by Tukey's test. A value of P < 0.05 was considered to indicate statistical significance.
Materials
3‐(4‐Chlorophenyl) 1‐(1,1‐dimethylethyl)‐1H‐pyrazolo[3,4‐d] pyrimidin‐4‐amine (PP2) was obtained from Tocris Bioscience (Bristol, UK). All other compounds were obtained from Sigma‐Aldrich (St. Louis, MO, USA). Stock solutions of genistein (100 mM), daidzein (100 mM), AG556 (100 mM) and PP2 (10 mM) were prepared in DMSO and then aliquoted and stored at −20°C. Aqueous stock solutions of sodium orthovanadate (100 mM) was prepared and pH was adjusted to 9.0 with HCl.
Tyrode's solution contained the following: (mM) NaCl 140, KCl 5.4, MgCl2 1.0, CaCl2 1.8, HEPES 5.0 and glucose 10 (pH adjusted to 7.3 with NaOH). The pipette solution contained the following: (mM) KCl 20, K‐aspartate 110, MgCl2 1.0, HEPES 10, EGTA 5, GTP 0.1, Na2‐phosphocreatine 5 and Mg‐ATP 5 (pH adjusted to 7.2 with KOH).
Results
Effect of genistein on I Kur
The effect of genistein (a broad spectrum PTK inhibitor) on I Kur was investigated in human atrial myocytes. The time course of I Kur at +50 mV (Figure 1A) was determined in a representative human atrial myocyte with the voltage protocol (inset) in the absence and presence of 30 μM genistein. The current was gradually decreased by genistein, and the inhibition reached a steady‐state level in 5 min and almost fully recovered on washout. Figure 1B displays the family of voltage‐dependent I Kur recorded in a representative cell with the voltage steps as shown in the inset in the absence (control) and presence of 10, 30 and 100 μM genistein. The current was inhibited by genistein in a concentration‐dependent manner. The percentage values of current inhibition, measured (+50 mV, n = 6) at the steady‐state current of I Kur at end of voltage step in cells treated with 3, 10, 30 and 100 μM genistein, are illustrated in Figure 1C. Significant inhibition was observed at 10–100 μM (n = 6, P < 0.05 vs. control).
Figure 1.
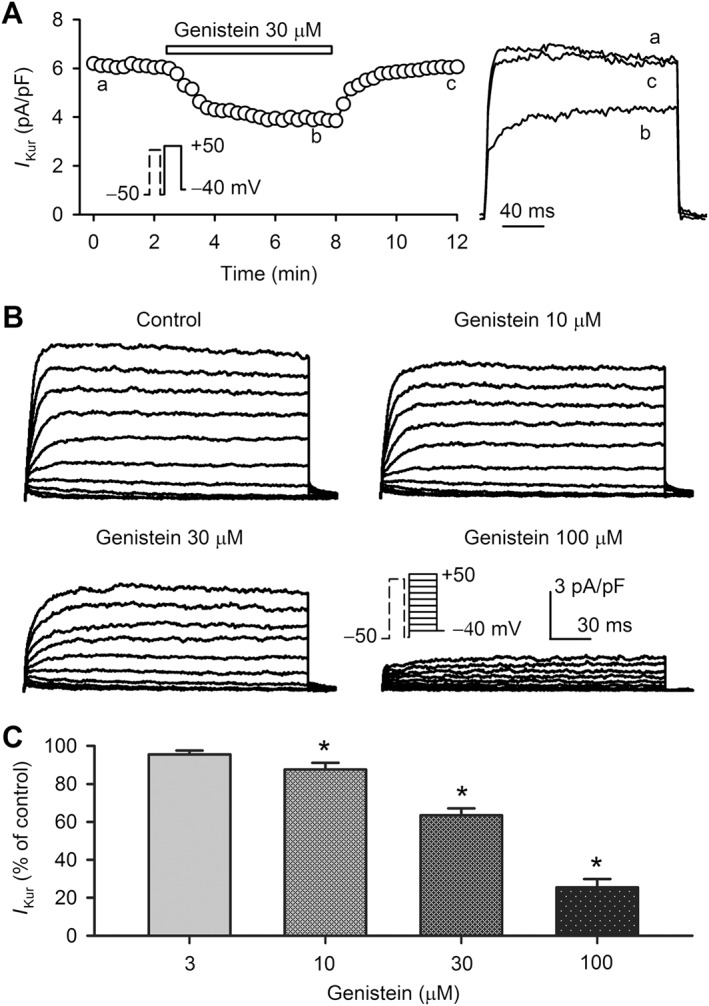
Inhibition of human atrial I Kur by genistein. (A) Time course of I Kur recorded in a typical experiment with a 100 ms prepulse to +40 mV from −50 mV to inactivate I to, followed by 200 ms test pulse to +50 mV (inset) after a 10 ms interval every 15 s. The cell was treated with 30 μM genistein. I Kur traces at corresponding time points are shown in right side of the panel. (B) Family of I Kur (capacitance compensated) at various depolarization voltages (a 100 ms prepulse to +40 mV to inactivate I to, followed by 200 ms test pulses to between −40 and +50 from −50 mV after a 10 ms interval and then to −40 mV in a typical experiment) in the absence and presence of 10, 30 and 100 μM genistein. (C) Percent values of I Kur at +50 mV in cells treated with 3, 10, 30 and 100 μM genistein (n = 6). *P < 0.05, significantly different from control.
To determine whether the I Kur reduction by genistein, as previously observed in rat cardiac Kv currents (Gao et al., 2004), is related to PTK inhibition, we tested daidzein (a PTK‐inactive analogue of genistein) in human atrial myocytes. Daidzein at 30 μM had no inhibitory effect on I Kur, whereas genistein at 30 μM markedly suppressed this current in the same myocyte (Figure 2A), suggesting that PTK inhibition is likely to be involved in the decreased I Kur induced by genistein.
Figure 2.
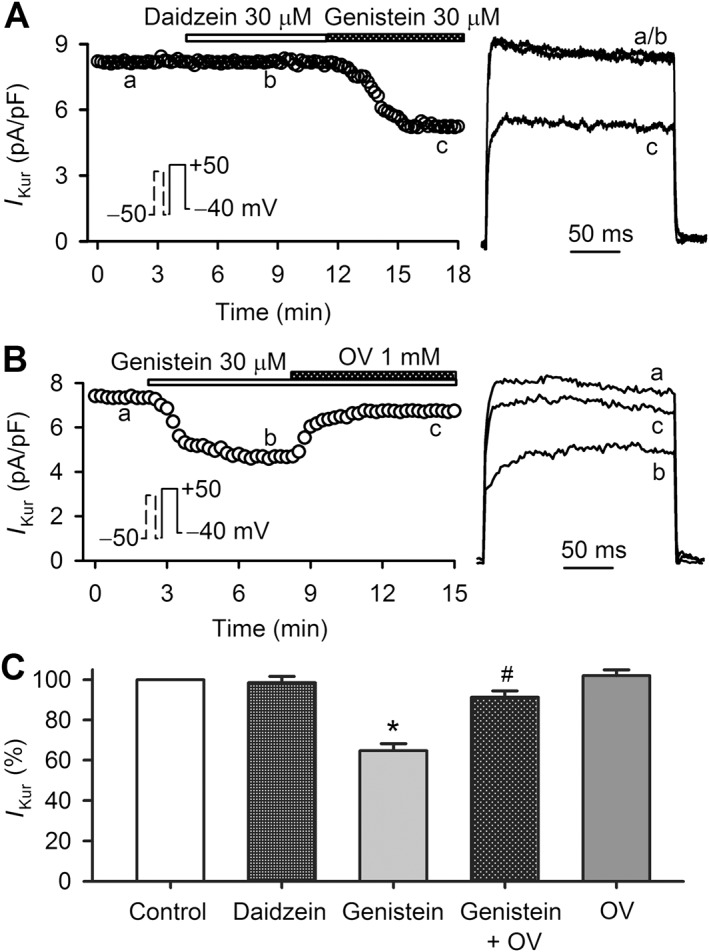
Effect of orthovanadate (OV) on genistein in human atrial myocytes. (A) Time course of I Kur recorded with the voltage protocol as shown in the inset in a typical experiment in the absence and presence of 30 μM daidzein or 30 μM genistein. I Kur traces at corresponding time points are shown in right side of the panel. (B) Time course of I Kur recorded in an atrial myocyte in the absence and presence of 30 μM genistein, and genistein plus 1 mM OV. I Kur traces at corresponding time points are shown in right side of the panel. (C) Histogram showing the mean percent values of I Kur during control, in the presence of 30 μM daidzein (n = 5), 30 μM genistein, genistein plus 1 mM OV (n = 7). *P < 0.05, significantly different from control; #P < 0.05, significantly different from genistein alone or 1 mM OV alone (n = 5).
Orthovanadate (a protein tyrosine phosphatase inhibitor) was then used to determine whether I Kur inhibition induced by genistein could be reversed by this compound. Orthovanadate at 1 mM significantly reversed the I Kur reduction induced by 30 μM genistein (Figure 2B). Figure 2C illustrates the percentage values of current amplitude (+50 mV) with daidzein, genistein and genistein plus orthovanadate or orthovanadate alone. No significant inhibition of I Kur was observed with 30 μM daidzein (n = 5, P > 0.05). Orthovanadate (1 mM) alone did not affect I Kur significantly (n = 6, P > 0.05 vs. control), while it reversed I Kur inhibition by 30 μM genistein (n = 7, P < 0.05 vs. genistein alone). These results suggest that the I Kur reduction by 30 μM genistein is a result of both PTK‐dependent and ‐independent inhibition.
Effect of AG556 on human atrial I Kur
The human atrial Ito (Zhang et al., 2012) is inhibited by the highly selective EGFR kinase inhibitor AG556. Here we determined the effects of AG566 on I Kur in human atrial myocytes. Figure 3A shows the time course of I Kur recorded in a representative cell with the voltage protocol shown in the inset before and after application of 10 μM AG556. The current was gradually decreased by AG556, and the inhibition was fully reversed on washout. Figure 3B displays the family of voltage‐dependent I Kur recorded in a typical experiment with the voltage protocol shown in the inset in the absence (control) and presence of 3, 10 and 30 μM AG556. AG556 decreased human atrial I Kur in a concentration‐dependent manner. Figure 3C illustrates the current inhibition (+50 mV) in cells treated with 1, 3, 10 and 30 μM AG556. Significant current reduction was observed with 3–30 μM AG556 (n = 6, P < 0.05 vs. control).
Figure 3.
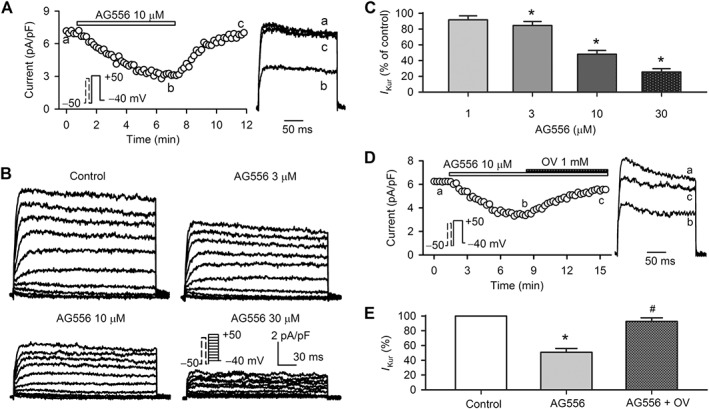
Effect of AG556 on human atrial I Kur. (A) Time course of I Kur recorded in a typical human atrial myocyte in the absence and presence of 10 μM AG556. I Kur traces at corresponding time points are shown in right of the panel. (B) Family of I Kur recorded in a representative cell using the voltage protocol shown in the inset during control and application of 3, 10 and 30 μM AG556. (C) Percent values of I Kur at +50 mV in cells treated with 1, 3, 10 and 30 μM AG556 (n = 6). *P < 0.05 , significantly different from control. (D) Time course of I Kur recorded in an atrial myocyte in the absence and presence of 10 μM AG556, and AG556 plus 1 mM orthovanadate (OV). I Kur traces at corresponding time points are shown in right side of the panel. (E) Percent values of I Kur (at +50 mV) in the absence and presence of 10 μM AG556 or AG556 plus OV (n = 7). *P < 0.05, significantly different from control; #P < 0.05, significantly different from AG556 alone.
Figure 3D shows the time course of I Kur recorded in another typical experiment before and after application of 10 μM AG556, and AG556 plus 1 mM orthovanadate. AG556 gradually reduced I Kur, and the current reduction was significantly reversed by 1 mM orthovanadate. Figure 3E illustrates the I Kur at +50 mV (as % control) before and after application of 10 μM AG556 and AG556 plus 1 mM orthovanadate. AG556 decreased the current (n = 6, P < 0.05 vs. control), and this inhibition was reversed by orthovanadate (n = 6, P < 0.05 vs. AG556 alone). These results reveal that the decrease in I Kur caused by 10 μM AG556 is mostly a result of EGFR kinase inhibition with a much smaller PTK‐independent inhibition.
Inhibition of hKv1.5 current by genistein and AG556
The human cardiac I Kur is carried by Kv1.5 channels, encoded by the KCNA5 gene (Fedida et al., 1993; Feng et al., 1997). Thus, we determined whether hKv1.5 channel current is affected by genistein or AG556 and whether their effects can be reversed by orthovanadate in HEK 293 cells stably expressing hKv1.5 channels. Figure 4A shows that the family of voltage‐dependent hKv1.5 current was reversibly inhibited by 30 μM genistein and the inhibition was reversed by 1 mM orthovanadate (Figure 4B).
Figure 4.
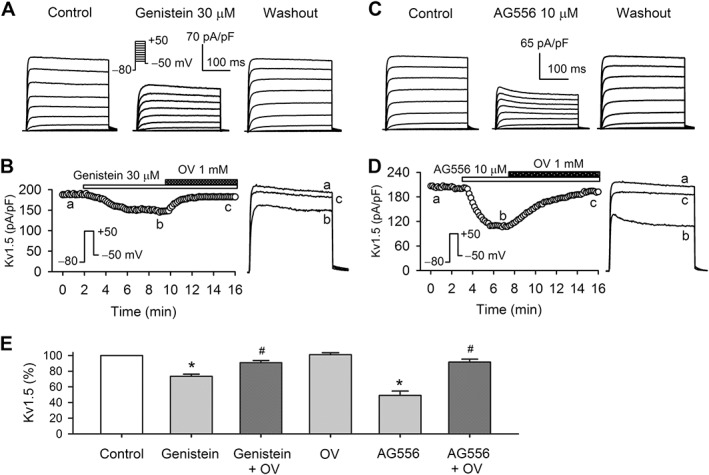
Effects of PTK inhibitors on hKv1.5 channels stably expressed in HEK 293 cells. (A) Voltage‐dependent hKv1.5 current was recorded with voltage protocol as shown in the inset in the absence (control) and presence of 30 μM genistein, and on washout. (B) Time course of hKv1.5 current (left) and original current traces (right) in the absence and presence of 30 μM genistein, and genistein plus 1 mM orthovanadate (OV). The hKv1.5 current traces at corresponding time points are shown in right side of the panel. (C) Voltage‐dependent hKv1.5 current was recorded in the absence and presence of 10 μM AG556, and on washout. (D) Time course of hKv1.5 current (left) and original current traces (right) in the absence and presence of 10 μM AG556, and AG556 plus 1 mM OV. The hKv1.5 current traces at corresponding time points are shown in right side of the panel. (E) Percentage values of hKv1.5 current (at +50 mV) in the absence (control) and presence of 30 μM genistein or genistein plus OV (n = 9). *P < 0.05, significantly different from control; #P < 0.05, significantly different from genistein alone, OV alone (n = 5), 10 μM AG556 and AG556 plus 1 mM OV (n = 8). *P < 0.05, significantly different from control; #P < 0.05, significantly different from AG556 alone).
Voltage‐dependent hKv1.5 current was also reversibly inhibited by 10 μM AG556 (Figure 4C). Figure 4D displays the time course of hKv1.5 current in a representative cell. AG556 at 10 μM gradually inhibited hKv1.5 current, and the inhibition was reversed by orthovanadate (1 mM). The result was similar to I Kur in human atrial myocytes (Figure 3D).
Figure 4E shows the hKv1.5 current (+50 mV), as %control, in cells treated with 30 μM genistein, genistein plus 1 mM orthovanadate, orthovanadate alone, 10 μM AG556 or AG556 plus orthovanadate. Orthovanadate, as in human atrial I Kur, had no effect on hKv1.5 channel current. Genistein inhibited hKv1.5 current at +50 mV (n = 9, P < 0.05 vs. control), and this inhibition was reversed by orthovanadate (n = 9, P < 0.05 vs. genistein alone). The orthovanadate‐insensitive fraction of genistein‐induced current reduction indicates a PTK‐independent inhibition of hKv1.5 channels by 30 μM genistein, as observed in human atrial I Kur.
AG556 at 10 μM decreased the current (n = 8, P < 0.05 vs. control), and the inhibitory effect was reversed by 1 mM orthovanadate (n = 8, P < 0.05 vs. AG556 alone), showing a small PTK‐independent reduction. These results indicate that hKv1.5 channels expressed in HEK 293 cells, similar to I Kur, are inhibited by genistein or AG556 via PTK‐dependent and PTK‐independent mechanisms. The PTK‐independent effect of genistein on I Kur and hKv1.5 channels is also reflected by a delayed time of the current to peak (closed channel blocking, Figures 1A and B and 4A and B), while the direct inhibition of hKv1.5 channels by AG556 is reflected by an increased current inactivation (open channel blocking, Figure 4C and D).
Effects of PP2 on I Kur and hKv1.5 channels
Whether PP2 (a Src family kinase inhibitor) could also affect I Kur/hKv1.5 current was determined in human atrial myocytes and HEK 293 cells expressing hKv1.5channels. Figure 5A shows the family of voltage‐dependent I Kur recorded in a typical experiment in the absence and presence of 1 μM PP2 [a concentration 200 times higher (Hanke et al., 1996) than the EC50 for inhibiting Src‐related kinases]. A high concentration of PP2 is usually required for Src family kinase inhibition in experiments at cellular level (Du et al., 2004; Zhang et al., 2008; Zhang et al., 2012), perhaps because PP2 does not easily cross the cell membrane. It is interesting to note that I Kur was slightly increased by PP2 (7 min superfusion), and the enhancement was also reversed by co‐application of 1 mM orthovanadate. Similar results were observed in hKv1.5 channels in HEK 293 cells (Figure 5B). Figure 5C shows the time course of hKv1.5 current recorded in an HEK 293 cell expressing hKv1.5 channels with the voltage step shown in the inset before and after application of 1 μM PP2, and PP2 plus 1 mM orthovanadate. PP2 gradually increased hKv1.5 current, and the effect was gradually reversed by orthovanadate. The inhibition of I Kur and hKv1.5 current at +50 mV are shown in Figure 5D. Human atrial I Kur was increased by PP2 (n = 6, P < 0.05), and the effect was reversed by orthovanadate, while hKv1.5 current was similarly increased by PP2 (n = 7, P < 0.05) and was reversed by orthovanadate. These results indicate that PP2 slightly, but significantly, increased I Kur/hKv1.5 current in a PTK‐dependent manner.
Figure 5.
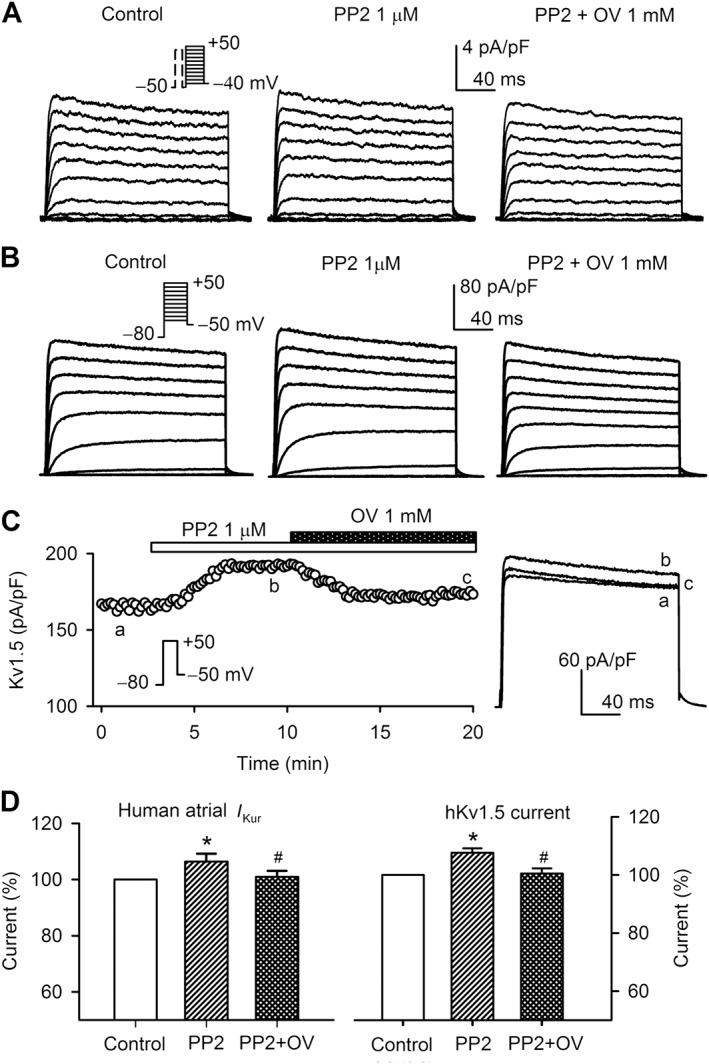
Effects of PP2 on I Kur/hKv1.5current. (A) Voltage‐dependent I Kur recorded in a human atrial myocyte with the voltage protocol as shown in the inset in the absence and presence of 1 μM PP2, and PP2 plus 1 mM orthovanadate (OV). (B) Voltage‐dependent hKv1.5current recorded in HEK 293 cells expressing KCNA5 with the voltage protocol as shown in the inset in the absence and presence of 1 μM PP2, and PP2 plus 1 mM OV. (C) Time course of hKv1.5 current recorded in a typical experiment in the absence and presence of 1 μM PP2 and PP2 plus 1 mM OV. The hKv1.5 current traces at corresponding time points are shown in right side of the panel with expanded Y‐axis. (D) Percentage values of I Kur (left panel, n = 7) and hKv1.5 current (right panel, n = 7) during control, in the presence of 1 μM PP2, and PP2 plus 1 mM OV (n = 7). *P < 0.05, significantly different from control; #P < 0.05, significantly different from PP2 alone.
Tyrosine phosphorylation of hKv1.5 channels
If the suppression of I Kur/hKv1.5 channels by genistein and AG556 or the increase of I Kur/hKv1.5 channels by PP2 is mediated by EGFR kinase inhibition or Src family kinases reduction, tyrosine phosphorylation of the channel would be reduced by these PTK inhibitors. The tyrosine phosphorylation of hKv1.5 protein was therefore determined in HEK 293 cells stably expressing hKv1.5 channels, but not in human atrial myocytes due to the limited cells isolated from human atrial specimens. Figure 6A displays the tyrosine phosphorylation images of hKv1.5 channels in the HEK 293 cells treated with 1 mM orthovanadate, 30 μM genistein, genistein plus orthovanadate, 10 μM AG556, AG556 plus orthovanadate, 1 μM PP2 or PP2 plus orthovanadate (30 min). Genistein, AG556 and PP2 significantly decreased the phosphorylation level of hKv1.5 channel protein, and the reduction in phosphorylation was reversed by pretreatment (30 min) with 1 mM orthovanadate. Orthovanadate itself had no effect on phosphorylation levels of the hKv1.5 protein. This indicates that the phosphorylation level of hKv1.5 channels, like hERG channels (Zhang et al., 2008), Kir2.1 channels (Zhang et al., 2011a) and hKv4.3 channels (Zhang et al., 2012), is saturated under basal physiological conditions.
Figure 6.
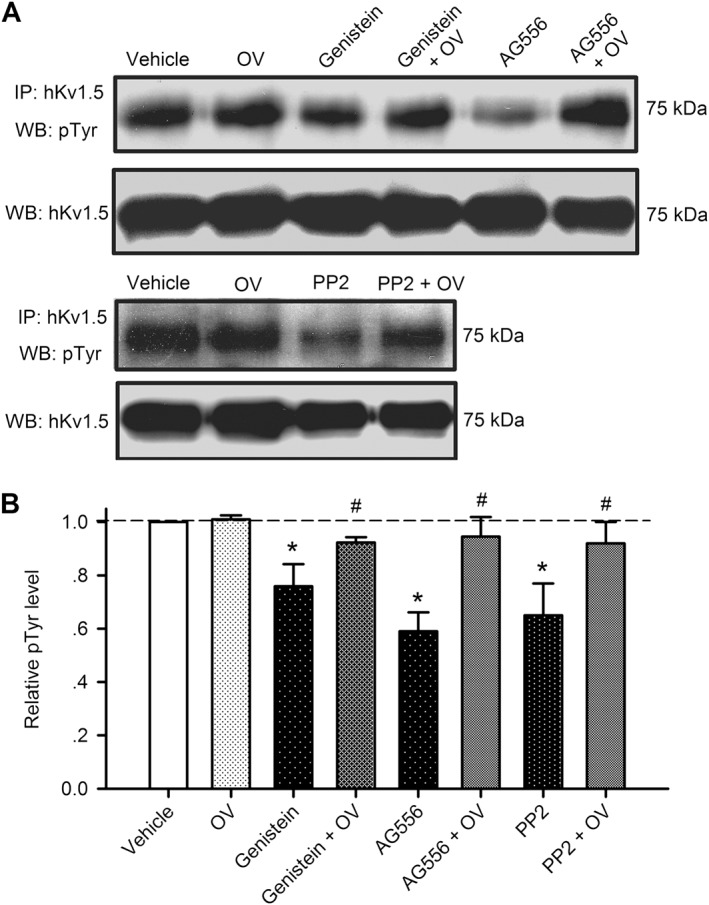
Tyrosine phosphorylation levels of hKv1.5 channels. (A) Images of immunoprecipitation (IP) and western blot (WB) in cells treated with vehicle (control), 1 mM orthovanadate (OV), 30 μM genistein, genistein plus 1 mM OV, 10 μM AG556, AG556 plus 1 mM OV, 1 μM PP2 and PP2 plus OV. (B) Relative phosphorylated hKv1.5 levels were determined by dividing pTyr‐ Kv1.5 density by total hKv1.5 protein density in cells treated with OV, genistein, AG556 or PP2 as described in (A) and then normalizing to vehicle control (n = 5). *P < 0.05, significantly different from vehicle control; #P < 0.05, significantly different from genistein, AG556 or PP2 alone.
Figure 6B summarises the mean levels of hKv1.5 tyrosine phosphorylation. Orthovanadate itself had no effect on the saturated tyrosine phosphorylation of hKv1.5 channels. Genistein (30 μM) decreased the tyrosine phosphorylation of hKv1.5 channel protein (n = 5, P < 0.05 vs. vehicle control), and the reduction was countered by 1 mM orthovanadate (P < 0.05 vs. genistein alone). AG556 (10 μM) decreased the tyrosine phosphorylation (n = 5, P < 0.05 vs. control) and this effect was reversed by 1 mM orthovanadate (P < 0.05 vs. AG556 alone). PP2 (1 μM) decreased the tyrosine phosphorylation level (n = 5, P < 0.05 vs. control) and the inhibition was reversed by co‐application of orthovanadate (P < 0.05 vs. PP2 alone). These results indicate that the inhibition of hKv1.5 current by genistein or AG556 and the increase of hKv1.5current by PP2 are mediated by reducing the tyrosine phosphorylation of the channel by EGFR TK or Src family kinases.
Potential tyrosine phosphorylation sites of hKv1.5channels
To determine the potential EGFR tyrosine phosphorylation sites of hKv1.5 channels, we initially generated three mutants (Y155F, Y521F and Y601F) of predicted tyrosine phosphorylation sites and tested the inhibitory response of these mutants to the selective EGFR kinase inhibitor AG556. The wild‐type (WT) hKv1.5 and the mutant currents recorded in HEK 293 cells transiently expressing the corresponding hKv1.5 channel mutants are displayed in Figure 7A–D in the absence and presence of 10 μM AG556. It appears that current density is greater in WT hKv1.5 channels than in hKv1.5 mutants (Table 1, n = 7–12, P < 0.05). The sensitivity of Y155F, Y521F and Y601F to AG556 was reduced, which suggests that Y155, Y521 and Y601 may be the EGFR kinase phosphorylation sites. However, the triple mutant Y155F–Y521F–Y601F of hKv1.5 channels still showed a significant inhibitory response to 10 μM AG556, though it was more sensitive to AG556 (P < 0.05 vs. other mutants). This differs from the hKv10.1 channels, in which triple tyrosine phosphorylation site mutation abolishes the inhibitory response to AG556 (Wu et al., 2012). These results suggest that the tyrosine phosphorylation sites of hKv1.5 channels are not limited to Y155, Y521 and Y601.
Figure 7.
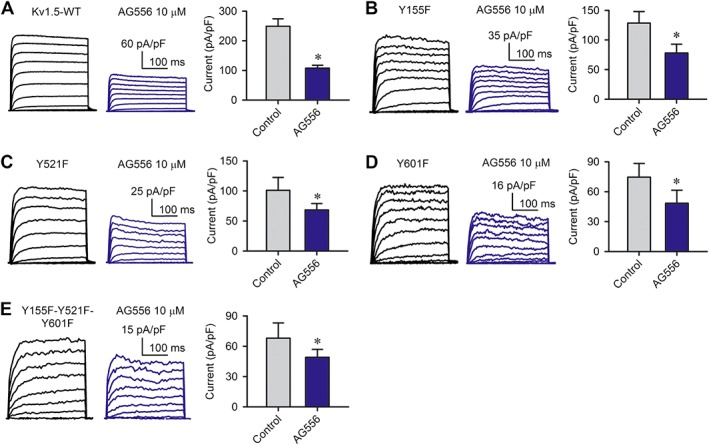
Effects of AG556 on mutant hKv1.5 channels. (A) WT hKv1.5 current recorded with the voltage protocol as shown in Figure 4A in a representative cell treated with 10 μM AG556; bar graph shows WT hKv1.5 current densities at +50 mV (n = 12). *P < 0.05, significant effect of AG556). (B) Y155F current recorded in a representative cell treated with 10 μM AG556; bar graph shows Y155F current densities at +50 mV (n = 9). *P < 0.05, significant effect of AG556). (C) Y521F current recorded in a representative cell treated with 10 μM AG556; bar graph shows Y521F current densities at +50 mV (n = 8). *P < 0.05, significant effect of AG556). (D) F601F current recorded in a representative cell treated with 10 μM AG556; bar graph shows Y601F current densities at +50 mV (n = 8). *P < 0.05, significant effect of AG556). (E) Y155F–Y521F–Y601F current recorded in a representative cell treated with 10 μM AG556; bar graph shows the triple mutant current densities at +50 mV (n = 7). *P < 0.05, significant effect of AG556).
Table 1.
Current inhibition by AG556 (10 μM) in HEK 293 cells expressing WT or various mutants of hKv1.5 channels
| hKv1.5 | n | Control | AG556 | Inhibition % |
|---|---|---|---|---|
| WT | 12 | 249.1 ± 25.2* | 107.5 ± 9.9# | 54.8† |
| Y155F | 9 | 128.5 ± 19.2 | 77.9 ± 14.8# | 39.3 |
| Y521F | 8 | 101.1 ± 21.5 | 60.3 ± 10.6# | 38.5 |
| Y601F | 8 | 76.4 ± 13.7 | 48.4 ± 13.1# | 37.7 |
| Triple mutant | 7 | 68.1 ± 14.9 | 49.1 ± 7.8# | 29.4Ψ |
P < 0.05, significantly different from mutants.
P < 0.05, significantly different from control (before AG556).
P < 0.05, significantly different from hKv1.5 mutants.
P < 0.05, significantly different from Y155F, Y521F or Y601F.
Discussion
It is well known that PTKs not only modulate cell growth and differentiation (Hubbard and Till, 2000) but also regulate ion channels (Davis et al., 2001). Earlier studies have used the inhibitors of receptor TKs, for example, the isoflavone genistein and the tyrphostin compounds (Levitzki and Mishani, 2006), or the non‐receptor TK (e.g. Src family kinases) inhibitor PP2, and/or a protein tyrosine phosphatase inhibitor (e.g. orthovanadate) in different types of cells and investigated the regulation of a number of ion channels and currents by TKs, including L‐type Ca2+ current (ICa.L) in cardiac myocytes (Ogura et al., 1999), volume‐sensitive chloride current (ICl.vol) in dog and human atrial cardiac myocytes (Sorota, 1995; Du et al., 2004), cardiac voltage‐gated Na+ current (INa) (Liu et al., 2007) and several types of K+ channels in different types of cells (Gao et al., 2004; Zhang et al., 2008, 2011a,b, 2012; Dong et al., 2010; Wu et al., 2012, 2013).
In the present study, the Src family kinase inhibitor PP2 slightly increased human atrial I Kur and hKv1.5 current expressed in HEK 293 cells and significantly inhibited tyrosine phosphorylation of hKv1.5 channels and the effects were countered by the protein tyrosine phosphatase inhibitor orthovanadate, suggesting that endogenous tyrosine phosphorylation of hKv1.5 channels by Src family kinases would inhibit I Kur/hKv1.5 current. This notion is supported by the early report of a direct association of Src TK with native hKv1.5 channels in human myocardium and cloned hKv1.5 channels. This occurred at a proline‐rich motif of the channel and the SH3 domain of Src, and tyrosine phosphorylation of hKv1.5 channels suppressed the channel current in cells coexpressing v‐Src (Holmes et al., 1996). A recent study found that removing this proline‐rich region resulted a mutant hKv1.5 channel with reduced current and lack of response to v‐Src‐induced current reduction, which suggests the abnormal atrial repolarization control due to variable Src family TK signalling as a mechanism in familial atrial fibrillation (Yang et al., 2010). A more recent report demonstrated that activation of the TK JAK3 down‐regulated Kv1.5 channels with an effect similar to Src family kinases (Warsi et al., 2015).
The present study provided new evidence that I Kur/hKv1.5 current may also be regulated by EGFR kinase. Genistein is a broad‐spectrum PTK inhibitor. It strongly inhibits EGFR kinase and also the Src family kinases (Akiyama and Ogawara, 1991), and is widely utilized for investigating ion channel regulation by PTKs (Ogura et al., 1999; Gao et al., 2004). The present study showed that genistein did not increase but inhibited I Kur/hKv1.5 current. PTK‐independent suppression by genistein has been previously observed in cardiac IKs (Washizuka et al., 1998), IKr (Missan et al., 2006), neuronal INa (Liu et al., 2004), Kir2.1 and Kir2.3 channels (Zhao et al., 2008) and Kv4.3 channels expressed in CHO cells (Kim et al., 2011). However, PTK‐dependent inhibition of human cardiac IKs and hERG channels and hKir2.1, hKir2.3 and hKv4.3 channels by genistein was revealed by the application of the protein tyrosine phosphatase inhibitor orthovanadate and the determination of tyrosine phosphorylation levels of these channel in our earlier reports (Zhang et al., 2008; Dong et al., 2010; Zhang et al., 2011a,b, 2012). In this study, we found that genistein (3–100 μM) inhibited human atrial I Kur in a concentration‐dependent manner. The reduction of I Kur/hKv1.5 current by 30 μM genistein was significantly reversed by orthovanadate. A small fraction of PTK‐independent inhibitory action of genistein is also involved in the overall inhibition of I Kur/hKv1.5 current. This suggests that the effects of 30 μM genistein on I Kur/hKv1.5 current is mainly due to inhibition of EGFR kinase, which was confirmed with the selective EGFR kinase inhibitor AG556.
AG556 is a highly selective inhibitor of EGFR TK (Levitzki and Mishani, 2006). Earlier studies demonstrated that AG556 can inhibit EGFR kinase activation and thereby improve rat spinal cord injury (Usul et al., 2004) and cardiac arrhythmias (Feng et al., 2012) induced by ischaemia/reperfusion injury accompanied with EGFR activation. Also, AG556 reversed the EGF‐induced enhancement of cardiac INa (Liu et al., 2007) and Kir2.3 current (Zhang et al., 2011b). In the present study, we demonstrated that AG556 inhibited human atrial I Kur in a concentration‐dependent manner. The decrease of I Kur/hKv1.5 current by 10 μM AG556 is mainly due to inhibiting EGFR TK, though a small fraction of PTK‐independent effect cannot be excluded. The EGFR TK inhibition is supported by the evidence that the reduced current and tyrosine phosphorylation level by AG556 are reversed by the protein tyrosine phosphatase inhibitor orthovanadate. Tyrosine phosphorylation of hKv1.5 channels by EGFR kinase activates the channel and may thereby enhance the current. However, orthovanadate did not affect I Kur/hKv1.5 current or the channel phosphorylation level, which suggests the basal tyrosine phosphorylation of hKv1.5 channels is saturated, as observed in rat cardiac Kv currentS (Gao et al., 2004), human cardiac Ito (Zhang et al., 2012), hERG (Zhang et al., 2008), IKs (Dong et al., 2010), Kir2.1 (Zhang et al., 2011a) and also hEAG1 (Wu et al., 2012) and hSKCa1(Wu et al., 2013).
I Kur is a rapidly activating, delayed rectifier current in response to depolarization, is predominantly present in human atria (Li et al., 1996b) and is responsible for human atrial repolarization (Feng et al., 1997; Wettwer et al., 2004; Li et al., 2008). Previous studies demonstrate that hKv1.5 channels can be down‐regulated by Src family kinases (Holmes et al., 1996; Yang et al., 2010). In this study, we showed that in addition to Src family kinase regulation, EGFR TK also regulates I Kur/hKv1.5 current. EGFR TK and Src kinases regulate I Kur/ hKv1.5 current with opposite actions. Under physiological conditions, control by EGFR kinase is clearly dominant (increasing the current). The basal full‐stoichiometric phosphorylation of hKv1.5 channels with other K+ channels (Kir2.1, Kir2.3, IKr, IKs) is responsible for maintaining normal human atrial repolarization. So inhibition of EGFR tyrosine phosphorylation of these K+ channels by genistein and/or AG556 would decrease the current amplitude and therefore delay human atrial repolarization. On the other hand, under pathophysiological conditions, control by Src kinases may be important. Recent studies have demonstrated that loss‐of‐function and/or gain‐of function mutations of hKv1.5 channels increase susceptibility to atrial fibrillation (Olson et al., 2006; Christophersen et al., 2013). Abnormal Src kinase phosphorylation of hKv1.5 channels is implicated in familial atrial fibrillation (Yang et al., 2010).
In addition, Kv1.5 current plays a role in the maintenance of the cell membrane potential in a wide variety of cells/tissues, such as pancreatic beta cells (MacDonald and Wheeler, 2003), brain tissue (Tipparaju et al., 2012), macrophages (Vicente et al., 2006) and skeletal muscle (Kang et al., 2009), as well as smooth muscles in vessels (Overturf et al., 1994) and airways (Adda et al., 1996). Therefore, tyrosine phosphorylation of hKv1.5 channels could also be important in maintaining normal cellular function of these types of cells. Moreover, Kv1.5 channels are also expressed in several tumour cells and participate in the modulation of cell adhesion, proliferation and apoptosis (Bonnet et al., 2007; Ousingsawat et al., 2007; Arvind et al., 2012). Thus, Kv1.5 channels has been considered to be a potential target to regulate tumour growth (Felipe et al., 2012). Inhibition of Kv1.5 channel activity via EGFR inhibitors is a possible pathway for the clinical treatment of cancers (Tan et al., 2015).
A major limitation of this study was that we did not identify all of the tyrosine phosphorylation sites involved in the EGFR kinase phosphorylation of hKv1.5 channels. The mutants Y155F, Y521F and Y601F showed a reduced response to AG556 inhibition. However, the triple mutant Y155F–Y521F–Y601F did not completely eliminate the inhibitory response to 10 μM AG556 (−29.4%). The small fraction (8.5%) of PTK‐independent inhibition of hKv1.5 channels by 10 μM AG556 cannot account for the significant inhibition of the triple mutant, which suggests that in addition to Y155, Y521 and Y601, other tyrosine sites may also be involved in EGFR kinase phosphorylation.
The tyrosine phosphorylation of hKv1.5 channels by EGFR TKs is clearly not simple as EGFR kinase phosphorylation of cardiac Kir2.1, Kir2.3 and SKCa1, in which only one tyrosine site is involved in EGFR kinase phosphorylation (Zhang et al., 2011a,b; Wu et al., 2013). A similar phenomenon was also observed in cardiac IKs in which several tyrosine sites are involved in EGFR kinase phosphorylation of KCNQ1 (Missan et al., 2009). Another limitation was that we were unable to obtain data on the changes in tyrosine phosphorylation in mutant hKv1.5 channels because our very low transfection rate (typically <10%) precluded biochemical analysis by immunoprecipitation and Western blotting. Although mass spectrometry can be used to accurately determine the specific levels of protein phosphorylation in tiny amounts of sample (Mann et al., 2002), we were not able to employ such approaches in the present study. The levels of tyrosine phosphorylation associated with the data reported in this paper remain to be determined.
Collectively, the present study provides the first indication that PTKs show dual regulating effects on I Kur/hKv1.5 current. Src family kinases decrease but EGFR kinase increases the activity of I Kur/hKv1.5 channels. This information is important not only for understanding cardiac electrophysiological regulation by PTKs but also for interpreting the therapeutic potential of PTK inhibitors in humans, especially EGFR inhibitors.
Author contributions
G.S.X., Y.W. and G.R.L. conceived and designed the experiments; G.S.X., Y.H.Z., W.W. and H.Y.S. performed the experiments; G.S.X., H.Y.S. and G.R.L. analysed the data; G.S.X. and G.R.L. wrote the paper; G.S.X., Y.H.Z., W.W., H.Y.S., Y.W. and G.R.L. approved the submission.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Acknowledgements
The work was supported in part by a grant from Sun Chieh Yeh Heart Foundation of Hong Kong, Hong Kong, China, a Collaborative Grant for Study on Prevention and Control of Major Chronic Non‐infectious Diseases of the 13th Five‐Year National Research Plan from Ministry of Science and Technology, China, and a Key Cardiovascular Laboratory Fund (3502Z20150050) from Department of Xiamen Science and Technology, Xiamen, China. Zhang Y.H. and Wu W. were supported by a postgraduate studentship from the University of Hong Kong, Hong Kong, China.
Xiao, G.‐S. , Zhang, Y.‐H. , Wu, W. , Sun, H.‐Y. , Wang, Y. , and Li, G.‐R. (2017) Genistein and tyrphostin AG556 decrease ultra‐rapidly activating delayed rectifier K+ current of human atria by inhibiting EGF receptor tyrosine kinase. British Journal of Pharmacology, 174: 454–467. doi: 10.1111/bph.13710.
References
- Adda S, Fleischmann BK, Freedman BD, Yu M, Hay DW, Kotlikoff MI (1996). Expression and function of voltage‐dependent potassium channel genes in human airway smooth muscle. J Biol Chem 271: 13239–13243. [DOI] [PubMed] [Google Scholar]
- Akiyama T, Ogawara H (1991). Use and specificity of genistein as inhibitor of protein‐tyrosine kinases. Methods Enzymol 201: 362–370. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Catterall WA, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: Voltage‐gated ion channels. Br J Pharmacol 172: 5904–5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015c). The concise guide to PHARMACOLOGY 2015/16: Catalytic receptors. Br J Pharmacol 172: 5979–6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvind S, Arivazhagan A, Santosh V, Chandramouli BA (2012). Differential expression of a novel voltage gated potassium channel–Kv1.5 in astrocytomas and its impact on prognosis in glioblastoma. Br J Neurosurg 26: 16–20. [DOI] [PubMed] [Google Scholar]
- Bonnet S, Archer SL, Allalunis‐Turner J, Haromy A, Beaulieu C, Thompson R et al. (2007). A mitochondria‐K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell 11: 37–51. [DOI] [PubMed] [Google Scholar]
- Christophersen IE, Olesen MS, Liang B, Andersen MN, Larsen AP, Nielsen JB et al. (2013). Genetic variation in KCNA5: impact on the atrial‐specific potassium current IKur in patients with lone atrial fibrillation. Eur Heart J 34: 1517–1525. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al. (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MJ, Wu X, Nurkiewicz TR, Kawasaki J, Gui P, Hill MA et al. (2001). Regulation of ion channels by protein tyrosine phosphorylation. Am J Physiol Heart Circ Physiol 281: H1835–H1862. [DOI] [PubMed] [Google Scholar]
- Dong MQ, Sun HY, Tang Q, Tse HF, Lau CP, Li GR (2010). Regulation of human cardiac KCNQ1/KCNE1 channel by epidermal growth factor receptor kinase. Biochim Biophys Acta 1798: 995–1001. [DOI] [PubMed] [Google Scholar]
- Du XL, Gao Z, Lau CP, Chiu SW, Tse HF, Baumgarten CM et al. (2004). Differential effects of tyrosine kinase inhibitors on volume‐sensitive chloride current in human atrial myocytes: evidence for dual regulation by Src and EGFR kinases. J Gen Physiol 123: 427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedida D, Wible B, Wang Z, Fermini B, Faust F, Nattel S et al. (1993). Identity of a novel delayed rectifier current from human heart with a cloned K+ channel current. Circ Res 73: 210–216. [DOI] [PubMed] [Google Scholar]
- Felipe A, Bielanska J, Comes N, Vallejo A, Roig S, Ramon YCS et al. (2012). Targeting the voltage‐dependent K(+) channels Kv1.3 and Kv1.5 as tumor biomarkers for cancer detection and prevention. Curr Med Chem 19: 661–674. [DOI] [PubMed] [Google Scholar]
- Feng J, Wible B, Li GR, Wang Z, Nattel S (1997). Antisense oligodeoxynucleotides directed against Kv1.5 mRNA specifically inhibit ultrarapid delayed rectifier K+ current in cultured adult human atrial myocytes. Circ Res 80: 572–579. [DOI] [PubMed] [Google Scholar]
- Feng M, Xiang JZ, Ming ZY, Fu Q, Ma R, Zhang QF et al. (2012). Activation of epidermal growth factor receptor mediates reperfusion arrhythmias in anaesthetized rats. Cardiovasc Res 93: 60–68. [DOI] [PubMed] [Google Scholar]
- Gao Z, Lau CP, Wong TM, Li GR (2004). Protein tyrosine kinase‐dependent modulation of voltage‐dependent potassium channels by genistein in rat cardiac ventricular myocytes. Cell Signal 16: 333–341. [DOI] [PubMed] [Google Scholar]
- Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ et al. (1996). Discovery of a novel, potent, and Src family‐selective tyrosine kinase inhibitor. Study of Lck‐ and FynT‐dependent T cell activation. J Biol Chem 271: 695–701. [DOI] [PubMed] [Google Scholar]
- Holmes TC, Fadool DA, Ren R, Levitan IB (1996). Association of Src tyrosine kinase with a human potassium channel mediated by SH3 domain. Science 274: 2089–2091. [DOI] [PubMed] [Google Scholar]
- Hubbard SR, Till JH (2000). Protein tyrosine kinase structure and function. Annu Rev Biochem 69: 373–398. [DOI] [PubMed] [Google Scholar]
- Kang LS, Kim S, Dominguez JM 2nd, Sindler AL, Dick GM, Muller‐Delp JM (2009). Aging and muscle fiber type alter K+ channel contributions to the myogenic response in skeletal muscle arterioles. J Appl Physiol 107: 389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Ahn HS, Choi BH, Hahn SJ (2011). Inhibition of Kv4.3 by genistein via a tyrosine phosphorylation‐independent mechanism. Am J Physiol Cell Physiol 300: C567–C575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak YG, Hu N, Wei J, George AL Jr, Grobaski TD, Tamkun MM et al. (1999). Protein kinase A phosphorylation alters Kvbeta1.3 subunit‐mediated inactivation of the Kv1.5 potassium channel. J Biol Chem 274: 13928–13932. [DOI] [PubMed] [Google Scholar]
- Levitan IB (1994). Modulation of ion channels by protein phosphorylation and dephosphorylation. Annu Rev Physiol 56: 193–212. [DOI] [PubMed] [Google Scholar]
- Levitzki A, Mishani E (2006). Tyrphostins and other tyrosine kinase inhibitors. Annu Rev Biochem 75: 93–109. [DOI] [PubMed] [Google Scholar]
- Li GR, Feng J, Wang Z, Fermini B, Nattel S (1996a). Adrenergic modulation of ultrarapid delayed rectifier K+ current in human atrial myocytes. Circ Res 78: 903–915. [DOI] [PubMed] [Google Scholar]
- Li GR, Feng J, Yue L, Carrier M, Nattel S (1996b). Evidence for two components of delayed rectifier K+ current in human ventricular myocytes. Circ Res 78: 689–696. [DOI] [PubMed] [Google Scholar]
- Li GR, Wang HB, Qin GW, Jin MW, Tang Q, Sun HY et al. (2008). Acacetin, a natural flavone, selectively inhibits human atrial repolarization potassium currents and prevents atrial fibrillation in dogs. Circulation 117: 2449–2457. [DOI] [PubMed] [Google Scholar]
- Liu H, Sun HY, Lau CP, Li GR (2007). Regulation of voltage‐gated cardiac sodium current by epidermal growth factor receptor kinase in guinea pig ventricular myocytes. J Mol Cell Cardiol 42: 760–768. [DOI] [PubMed] [Google Scholar]
- Liu L, Yang T, Simon SA (2004). The protein tyrosine kinase inhibitor, genistein, decreases excitability of nociceptive neurons. Pain 112: 131–141. [DOI] [PubMed] [Google Scholar]
- Loose S, Mueller J, Wettwer E, Knaut M, Ford J, Milnes J et al. (2014). Effects of IKur blocker MK‐0448 on human right atrial action potentials from patients in sinus rhythm and in permanent atrial fibrillation. Front Pharmacol 5: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald PE, Wheeler MB (2003). Voltage‐dependent K+ channels in pancreatic beta cells: role, regulation and potential as therapeutic targets. Diabetologia 46: 1046–1062. [DOI] [PubMed] [Google Scholar]
- Mann M, Ong SE, Grønborg M, Steen H, Jensen ON, Pandey A (2002). Analysis of protein phosphorylation using mass spectrometry: deciphering the phosphoproteome. Trends Biotechnol 20: 261–268. [DOI] [PubMed] [Google Scholar]
- Mia S, Munoz C, Pakladok T, Siraskar G, Voelkl J, Alesutan I et al. (2012). Downregulation of Kv1.5 K channels by the AMP‐activated protein kinase. Cell Physiol Biochem 30: 1039–1050. [DOI] [PubMed] [Google Scholar]
- Missan S, Qi J, Crack J, McDonald TF, Linsdell P (2009). Regulation of wild‐type and mutant KCNQ1/KCNE1 channels by tyrosine kinase. Pflugers Arch 458: 471–480. [DOI] [PubMed] [Google Scholar]
- Missan S, Zhabyeyev P, Linsdell P, McDonald TF (2006). Insensitivity of cardiac delayed‐rectifier IKr to tyrosine phosphorylation inhibitors and stimulators. Br J Pharmacol 148: 724–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura T, Shuba LM, McDonald TF (1999). L‐type Ca2+ current in guinea pig ventricular myocytes treated with modulators of tyrosine phosphorylation. Am J Physiol Heart Circ Physiol 276: H1724–H1733. [DOI] [PubMed] [Google Scholar]
- Olson TM, Alekseev AE, Liu XK, Park S, Zingman LV, Bienengraeber M et al. (2006). Kv1.5 channelopathy due to KCNA5 loss‐of‐function mutation causes human atrial fibrillation. Hum Mol Genet 15: 2185–2191. [DOI] [PubMed] [Google Scholar]
- Ousingsawat J, Spitzner M, Puntheeranurak S, Terracciano L, Tornillo L, Bubendorf L et al. (2007). Expression of voltage‐gated potassium channels in human and mouse colonic carcinoma. Clin Cancer Res 13: 824–831. [DOI] [PubMed] [Google Scholar]
- Overturf KE, Russell SN, Carl A, Vogalis F, Hart PJ, Hume JR et al. (1994). Cloning and characterization of a Kv1.5 delayed rectifier K+ channel from vascular and visceral smooth muscles. Am J Physiol Cell Physiol 267: C1231–C1238. [DOI] [PubMed] [Google Scholar]
- Schumacher SM, McEwen DP, Zhang L, Arendt KL, Van Genderen KM, Martens JR (2009). Antiarrhythmic drug‐induced internalization of the atrial‐specific K+ channel Kv1.5. Circ Res 104: 1390–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyders DJ, Tamkun MM, Bennett PB (1993). A rapidly activating and slowly inactivating potassium channel cloned from human heart. Functional analysis after stable mammalian cell culture expression. J Gen Physiol 101: 513–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorota S (1995). Tyrosine protein kinase inhibitors prevent activation of cardiac swelling‐induced chloride current. Pflugers Arch 431: 178–185. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al. (2016). The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44 (Database Issue): D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamargo J, Caballero R, Gomez R, Delpon E (2009). IKur/Kv1.5 channel blockers for the treatment of atrial fibrillation. Expert Opin Investig Drugs 18: 399–416. [DOI] [PubMed] [Google Scholar]
- Tan CS, Gilligan D, Pacey S (2015). Treatment approaches for EGFR‐inhibitor‐resistant patients with non‐small‐cell lung cancer. Lancet Oncol 16: e447–e459. [DOI] [PubMed] [Google Scholar]
- Tang Q, Jin MW, Xiang JZ, Dong MQ, Sun HY, Lau CP et al. (2007). The membrane permeable calcium chelator BAPTA‐AM directly blocks human ether a‐go‐go‐related gene potassium channels stably expressed in HEK 293 cells. Biochem Pharmacol 74: 1596–1607. [DOI] [PubMed] [Google Scholar]
- Tipparaju SM, Li XP, Kilfoil PJ, Xue B, Uversky VN, Bhatnagar A et al. (2012). Interactions between the C‐terminus of Kv1.5 and Kvbeta regulate pyridine nucleotide‐dependent changes in channel gating. Pflugers Arch 463: 799–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usul H, Cakir E, Cobanoglu U, Alver A, Peksoylu B, Topbas M et al. (2004). The effects of tyrphostine Ag 556 on experimental spinal cord ischemia reperfusion injury. Surg Neurol 61: 45–54. [DOI] [PubMed] [Google Scholar]
- Vicente R, Escalada A, Villalonga N, Texido L, Roura‐Ferrer M, Martin‐Satue M et al. (2006). Association of Kv1.5 and Kv1.3 contributes to the major voltage‐dependent K+ channel in macrophages. J Biol Chem 281: 37675–37685. [DOI] [PubMed] [Google Scholar]
- Wang Z, Fermini B, Nattel S (1993). Sustained depolarization‐induced outward current in human atrial myocytes. Evidence for a novel delayed rectifier K+ current similar to Kv1.5 cloned channel currents. Circ Res 73: 1061–1076. [DOI] [PubMed] [Google Scholar]
- Warsi J, Elvira B, Bissinger R, Hosseinzadeh Z, Lang F (2015). Regulation of voltage‐gated K+ channel Kv1.5 by the Janus kinase JAK3. J Membr Biol 248: 1061–1070. [DOI] [PubMed] [Google Scholar]
- Washizuka T, Horie M, Obayashi K, Sasayama S (1998). Genistein inhibits slow component delayed‐rectifier K currents via a tyrosine kinase‐independent pathway. J Mol Cell Cardiol 30: 2577–2590. [DOI] [PubMed] [Google Scholar]
- Wettwer E, Hala O, Christ T, Heubach JF, Dobrev D, Knaut M et al. (2004). Role of IKur in controlling action potential shape and contractility in the human atrium: influence of chronic atrial fibrillation. Circulation 110: 2299–2306. [DOI] [PubMed] [Google Scholar]
- Williams CP, Hu N, Shen W, Mashburn AB, Murray KT (2002). Modulation of the human Kv1.5 channel by protein kinase C activation: role of the Kvbeta1.2 subunit. J Pharmacol Exp Ther 302: 545–550. [DOI] [PubMed] [Google Scholar]
- Wu HJ, Wu W, Sun HY, Qin GW, Wang HB, Wang P et al. (2011). Acacetin causes a frequency‐ and use‐dependent blockade of hKv1.5 channels by binding to the S6 domain. J Mol Cell Cardiol 51: 966–973. [DOI] [PubMed] [Google Scholar]
- Wu W, Dong MQ, Wu XG, Sun HY, Tse HF, Lau CP et al. (2012). Human ether‐a‐go‐go gene potassium channels are regulated by EGFR tyrosine kinase. Biochim Biophys Acta 1823: 282–289. [DOI] [PubMed] [Google Scholar]
- Wu W, Sun HY, Deng XL, Li GR (2013). EGFR tyrosine kinase regulates human small‐conductance Ca2+‐activated K+ (hSKCa1) channels expressed in HEK‐293 cells. Biochem J 452: 121–129. [DOI] [PubMed] [Google Scholar]
- Yang T, Yang P, Roden DM, Darbar D (2010). Novel KCNA5 mutation implicates tyrosine kinase signaling in human atrial fibrillation. Heart Rhythm 7: 1246–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DY, Wang Y, Lau CP, Tse HF, Li GR (2008). Both EGFR kinase and Src‐related tyrosine kinases regulate human ether‐a‐go‐go‐related gene potassium channels. Cell Signal 20: 1815–1821. [DOI] [PubMed] [Google Scholar]
- Zhang DY, Wu W, Deng XL, Lau CP, Li GR (2011a). Genistein and tyrphostin AG556 inhibit inwardly‐rectifying Kir2.1 channels expressed in HEK 293 cells via protein tyrosine kinase inhibition. Biochim Biophys Acta 1808: 1993–1999. [DOI] [PubMed] [Google Scholar]
- Zhang DY, Zhang YH, Sun HY, Lau CP, Li GR (2011b). Epidermal growth factor receptor tyrosine kinase regulates the human inward rectifier potassium KIR2.3 channel, stably expressed in HEK 293 cells. Br J Pharmacol 164: 1469–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Wu W, Sun HY, Deng XL, Cheng LC, Li X et al. (2012). Modulation of human cardiac transient outward potassium current by EGFR tyrosine kinase and Src‐family kinases. Cardiovasc Res 93: 424–433. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Liu B, Zhang G, Jia Z, Jia Q, Geng X et al. (2008). Molecular basis for genistein‐induced inhibition of Kir2.3 currents. Pflugers Arch 456: 413–423. [DOI] [PubMed] [Google Scholar]


