Abstract
Purpose
Pericardial adipose tissue had been shown to exert local effects on adjacent cardiac structures. Data regarding the mechanistic link between such measures and left atrial (LA) structural/functional remodeling, a clinical hallmark of early stage heart failure (HF) and atrial fibrillation (AF) incidence, in asymptomatic population remain largely unexplored.
Methods
This retrospective analysis includes 356 subjects free from significant valvular disorders, atrial fibrillation, or clinical HF. Regional adipose tissue including pericardial and periaortic fat volumes, interatrial septal (IAS), and left atrioventricular groove (AVG) fat thickness were all measured by multidetector computed tomography (MDCT) (Aquarius 3D Workstation, TeraRecon, San Mateo, CA, USA). We measured LA volumes, booster performance, reservoir capacity as well as conduit function, and analyzed their association with adiposity measures.
Results
All four adiposity measures were positively associated with greater LA volumes (all P < 0.05), while IAS and AVG fat were also related to larger LA kinetic energy and worse reservoir capacity (both P < 0.01). In multivariate models, IAS fat thickness remained independently associated with larger LA volumes, increased LA kinetic energy and ejection force (β-coef: 0.17 & 0.15, both P < 0.05), and impaired LA reservoir and conduit function (β-coef: −0.20 & −0.12, both P < 0.05) after adjusting for clinical variables.
Conclusion
Accumulated visceral adiposity, especially interatrial fat depots, was associated with certain LA structural/functional remodeling characterized by impaired LA reservoir and conduit function though augmented kinetic energy and ejection performance. Our data suggested that interatrial fat burden may be associated with certain detrimental LA functions with compensatory LA adaptation in an asymptomatic population.
Keywords: interatrial fat, pericardial fat, left atrial function, MDCT
Introduction
Over the past decade, assessment of left atrial (LA) function has been shown to be crucial in maintaining adequate cardiac mechanistic performance and pump function in subjects with preserved left ventricular (LV) systolic function with sinus rhythm (1). Throughout the cardiac cycle, LA serves multiple roles. First as a reservoir receiving pulmonary venous return during ventricular systole, then as an open conduit directing the influx of blood flow into LV during early diastole, and finally as an active booster pump during late diastole (2). It is well-established that the reservoir, conduit, and booster functions of LA are key factors to ventricular filling performance, and may serve as clinical surrogate of LA dysfunction at an earlier stage (2, 3).
Several clinical risk factors, including obesity, metabolic derangements, and LV hypertrophy secondary to chronic pressure overload, had all been shown to affect LA structure and function negatively (4). Numerous studies had demonstrated that the pathogenesis between central obesity and cardiovascular diseases (5, 6) may be partially mediated by the pericardial adipose tissue, a hypothetical source of inflammatory and atherogenic cytokines with active biological effects (7, 8). As a result of its anatomic proximity and shared blood supply with the myocardium, pericardial fat had been reported to influence various aspects of cardiac function, including LA remodeling (9) and the development of atrial fibrillation (AF) (10, 11). On the other hand, far less effort had been invested in the possible effects that atrial septal fat accumulation (also known as lipomatous septal hypertrophy) may impose on specific LA mechanics.
On the basis of this hypothesis, we sought to examine the link among different sites of visceral adiposity with LA structural remodeling and its related mechanistic performances in an asymptomatic population. We also speculated that visceral fat accumulation surrounding interatrial location is associated with some components of LA functional impairment, and will further investigate these issues.
Subjects and methods
Subjects
We consecutively studied subjects who received cardiovascular health examinations from 2010 to 2013 at a tertiary medical center in Taipei, Taiwan. The clinical setting was for clinical cardiovascular risk stratification at cardiovascular outpatient service, which was similar to our previously published data (12, 13). All study subjects underwent detailed review of medical histories, along with physical examination performed in all study subjects. Clinical symptoms including angina, chest discomfort, or heart failure (e.g. exercise intolerance) were all obtained, with subspecialty transferred if appropriate. Transthoracic echocardiography, electrocardiography (ECG), and cardiac computed tomography (CT) for coronary calcium scoring were performed in all subjects. Patients with acute decompensated heart failure (HF), unstable angina, atrial flutter/fibrillation (either persistent or paroxysmal), severe valvular disease (either stenosis or regurgitation), or end-stage renal disease were excluded. A total of 356 subjects after exclusion during study period were finally enrolled. The presence of CAD was defined as a history of previous myocardial infarction, previous angioplasty, or more than 50 % luminal narrowing diagnosed by coronary angiography. Hypertension was defined as systolic blood pressure above 140 mmHg, diastolic blood pressure above 90 mmHg or previously diagnosed hypertension under regular medication control. Diabetes was defined as a fasting glucose level above 126 mg/dL or previously diagnosed diabetes with medication use. Hyperlipidemia was defined as a history and/or use of lipid-lowering drugs such as statins or fibrates on a daily basis. Standardized sphygmomanometer cuff-defined resting blood pressures were measured at rest by medical staff blinded to the other test results. All baseline characteristics and information regarding anthropometric measures were collected, which included body size in terms of body mass index (BMI) and waist circumference.
This study has been approved by Mackay Memorial Hospital Institutional Review Board with adherence to research ethics (IRB number: 14MMHIS161).
Echocardiography and LA mechanics measures
Each subject underwent two-dimensional (2D) and color Doppler transthoracic echocardiography using a commercially available ultrasound system (Vivid 7, GE Medical System, Vingmed, Norway) equipped with 2–4 MHz transducer at the left decubitus position. All recorded images were measured and analyzed with clinical information blinded using a computerized off-line software (TomTec Image Arena, Munich, Germany). All measurements were averaged from three consecutive cardiac cycles. LV wall thickness and mass were determined from M-mode method. LV volumes and ejection fraction were obtained via the modified biplane Simpson method from the apical four- and two-chamber views according to American Society of Echocardiography recommendations (14). Diastolic functional parameters including early mitral inflow velocity (E), late mitral inflow velocity (A), deceleration time (DT), iso-volumic relaxation time (IVRT), and tissue Doppler imaging (TDI)-derived mitral annular early-diastolic velocity (e′) were all obtained. The average e′ velocity between LV basal septal and lateral segments was used for our statistical analysis. Average E/e′ ratio was used to predict LV filling pressures (15).
For volumetric assessment of LA reservoir, conduit, and booster functions, maximum LA volume (LAV max, at end-systole, just before mitral valve opening), pre-A LA volume (LAV pre-A, immediately before atrial contraction, marked by the electrocardiographic P-wave), and minimum LA volume (LAV min, at end-diastole, when the mitral valve closes) were measured by biplane method and indexed to body-surface area (14, 16) (Fig. 1). LA reservoir function was estimated by expansion index, computed as [(LAV max – LAV min)/LAV min] × 100%. LA booster function was estimated by active emptying fraction, computed as [(LAV pre-A – LAV min)/LAV pre-A] × 100%. LA conduit function was estimated by passive emptying fraction, computed as [(LAV max – LAV pre-A)/LAV max] × 100% (2). Advanced markers of atrial systolic function such as LA ejection force and kinetic energy were calculated according to the modified Manning method. LA kinetic energy (kdynes·cm/m2) was defined as 0.5 × ρ × LA active emptying volume × A2, whereρwas blood density (1.06 g/cm3) and A was late-diastolic mitral inflow velocity (cm/s) (17). LA ejection force (kdynes/m2) was defined as (0.5 × ρ × LA active emptying volume × A2)/late-diastolic transmitral flow velocity time integral (VTIA, cm) (17). Finally, a subset sample of 50 cases was selected for comparing continuous speckle tracking analysis and manually traced method for echo-defined LA volume data in our lab, which showed high correlation (r = 0.96, P < 0.05). To further validate the accuracy of our echo-based LA volumes, we randomly selected 59 cases who received contrast-enhanced computed tomography (CECT) using multidetector computed tomography (MDCT) coronary artery imaging protocol during the study conduction time. LA volumes were then quantified and compared with echo-based measures which had been approved by research ethics committee.
Figure 1.
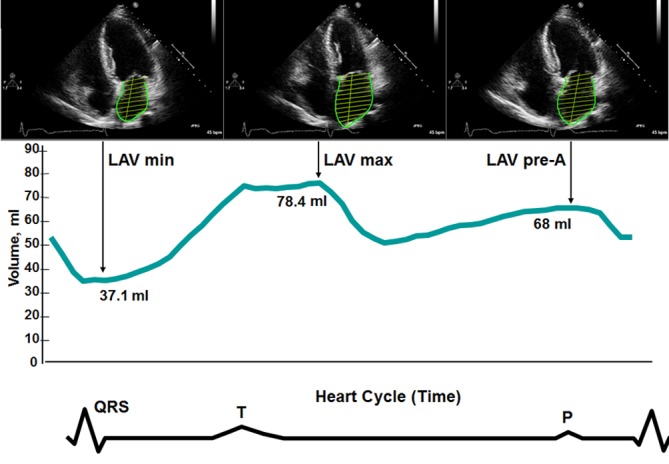
LA volumes measured by biplane area-length method. Volume curve was obtained by continuous speckle tracking. Minimum volume (LAV min) is measured at the closure of the mitral valve in end-diastole. Maximum volume (LAV max) is measured just before the opening of the mitral valve in end-systole. Pre-A volume (LAV pre-A) is measured at the onset of the P-wave on electrocardiogram.
Quantification of regional pericardial and periaortic fat
All participants had undergone MDCT originally for coronary calcium score measurement, and we reconstructed the images for visceral fat assessment. MDCT was performed by using a 16-slice scanner (Sensation 16, Siemens Medical Solutions, Forchheim, Germany) with 16 mm × 0.75 mm collimation, rotation time 420 ms and tube voltage of 120 kV. In one breath-hold, images were acquired from above the level of tracheal bifurcation to below the base of heart using prospectively ECG triggering with the centre of the acquisition at 70% of the R-R interval. From the raw data, the images were reconstructed with standard kernel in 3 mm thick axial, non-overlapping slices and 25 cm field of view.
Visceral adipose tissue measurements were carried out offline (Aquarius iNtuition Cloud, TeraRecon, SanMateo, CA, USA) on a single workstation using methods described previously (18). Total pericardial adipose tissue volumes were quantified using a semi-automatic segmentation technique, in which the region of interest was defined by manually tracing the pericardial borders. Fat tissue was defined as pixels within a window of −195 to −45 HU, and pericardial fat was then selected as adipose tissue within the pericardial sac (Fig. 2A). Thoracic periaortic adipose tissue was defined as all adipose tissue surrounding the thoracic aorta extending 67.5 mm caudally from the level of the bifurcation of the pulmonary arteries (Fig. 2B). For regional fat thickness measurements, we performed multiplanar reconstructions to obtain the standardized horizontal long-axis plane from axial and longitudinal two-chamber views for subsequent measurement of the interatrial septum (IAS) and left atrioventricular groove (AVG) based on methods validated by a previous study (19) (Fig. 2C). For reproducibility assessment, intra-observer and inter-observer coefficients of variation were determined by repeated measurement on 50 randomized cases by two experienced readers blinded to the subjects’ clinical data. The intra-observer and inter-observer coefficients of variation for pericardial fat and interatrial fat were 4.27%, 4.87% and 6.58%, 6.81%, respectively.
Figure 2.
(A) 3D-reconstruction of total pericardial fat volume from each axial image (left panel). Pericardial fat (orange shadow) was selected as all adipose tissue within the pericardial sac and subtracted from the adjacent cardiac structures (right panel). (B) 3D-reconstruction of thoracic periaortic fat from each axial image (left panel). Thoracic periaortic fat (orange shadow) was defined as all adipose tissue surrounding the thoracic aorta extending 67.5 mm caudally from the level of the bifurcation of the pulmonary arteries (right panel). (C) Multiplanar reconstructions (red line for each plane) for regional fat thickness measurements over the interatrial septum (IAS) and left atrioventricular (AV) groove.
Statistical analysis
Continuous data were shown as mean with standard deviation. Categorical information was expressed as the frequency and proportion of prevalence, and then compared using a χ2-test or Fisher’s exact test if appropriate. Baseline continuous data were compared by a Wilcoxon nonparametric test for significant trend across regional adipose tissue (IAS) strata based on tertiles. The trend between LA/LV structural/functional parameters and increasing IAS fat volume was examined across IAS strata, with uni- and multi-variable regression models used to determine the associations between various regional adipose tissue burden and LA mechanical/functional indices (including volumes, booster performance, reservoir capacity as well as conduit function).
All data were analyzed using a commercial software STATA 8.2 package (Stata Corp, College Station, TX, USA). The significance of p level (α-value) for all analysis was two-sided, with a value less than 0.05 considered to be statistically significant.
Results
Basic information, biomarkers and adiposity measures
The associations of IAS fat with baseline demographics, biochemical, or inflammatory markers and other adiposity measures were summarized in Table 1. Among all 356 participants, 245 (68.8%) were men, with an average age of 51.8 ± 8.8 years. With increasing IAS fat measure, there were stepwise increases in BMI and waist circumference (trend P < 0.001). Furthermore, increasing fat were significantly associated with graded increase in fasting glucose, triglyceride, hs-CRP, insulin and insulin resistance (HOMA-IR), as well as decreased HDL (all trend P < 0.005). The mean total pericardial fat volume and volume index were 80.39 ± 26.05 mL, 42.97 ± 13.68 mL/m2, which were within the normal limit (125 mL or 68 mL/m2) as suggested by a previous literature (20). Mean IAS thickness was 5.7 ± 1.71 mm, which was comparable to the control group data of a previous study (20). Both pericardial and periaortic fat volumes indexed to body-surface area had significant increase along with increased IAS thickness (all P < 0.001). For those subjects without any known hypertension, diabetes, hyperlipidemia or coronary artery disease, the mean IAS thickness was 5.48 ± 1.7 mm (n = 219). A search of our hospital database found that 329 (92.4% available) of the participants were followed at least once in outpatient clinics. The median follow-up period was 3.4 years. Four patients had documented atrial fibrillation (AF); two were hospitalized for heart failure. Five of those six patients were clustered in the third IAS tertile group; the remaining one came from the second tertile group (P = 0.025).
Table 1.
Relationship of baseline demographics, biochemical and inflammatory markers, medical histories, and other adiposity measures with interatrial fat.
| Interatrial fat thickness (mm) | P (trend) | |||
|---|---|---|---|---|
| 1st tertile group | 2nd tertile group | 3rd tertile group | ||
| 2.00–2.48 | 4.83–6.36 | 6.37–10.8 | ||
| (n = 119) | (n = 119) | (n = 118) | ||
| Baseline demographics | ||||
| Age (years) | 50.25 ± 7.90 | 50.77 ± 9.05 | 54.09 ± 9.24 | 0.003 |
| Male (%) | 71 (60.17%) | 81 (69.23%) | 93 (78.15%) | 0.007 |
| SBP (mmHg) | 119.16 ± 16.76 | 121.34 ± 16.37 | 125.15 ± 16.63 | 0.048 |
| DBP (mmHg) | 75.90 ± 10.42 | 74.91 ± 10.79 | 77.92 ± 10.47 | 0.113 |
| BMI (kg/m2) | 23.40 ± 2.77 | 24.49 ± 2.64 | 25.86 ± 3.00 | <0.001 |
| WC (cm) | 80.72 ± 8.43 | 83.42 ± 8.19 | 89.15 ± 8.06 | <0.001 |
| Body fat (%) | 25.69 ± 6.70 | 25.28 ± 6.17 | 27.86 ± 7.15 | 0.051 |
| Biochemical data | ||||
| Fasting glucose (mg/dL) | 95.26 ± 11.15 | 99.81 ± 17.57 | 104.90 ± 26.21 | <0.001 |
| HbA1c (%) | 5.84 ± 0.54 | 5.93 ± 0.59 | 6.15 ± 1.05 | 0.079 |
| Cholesterol (mg/dL) | 201.04 ± 36.34 | 198.30 ± 35.07 | 198.26 ± 36.87 | 0.786 |
| TG (mg/dL) | 122.47 ± 56.54 | 139.09 ± 88.32 | 160.07 ± 92.94 | 0.004 |
| LDL (mg/dL) | 133.48 ± 31.30 | 128.48 ± 33.20 | 129.93 ± 30.09 | 0.444 |
| HDL (mg/dL) | 51.97 ± 12.89 | 51.96 ± 14.35 | 45.69 ± 10.61 | 0.001 |
| eGFR (mL/min/m2) | 85.76 ± 13.70 | 86.88 ± 16.07 | 83.22 ± 19.95 | 0.173 |
| hs-CRP (mg/dL) | 0.18 ± 0.16 | 0.17 ± 0.16 | 0.33 ± 0.34 | <0.001 |
| Insulin (μIU/mL) | 5.35 ± 2.87 | 5.88 ± 3.17 | 7.17 ± 3.87 | 0.004 |
| HOMA-IR (IU/mL) | 1.24 ± 0.83 | 1.44 ± 0.92 | 1.86 ± 1.33 | 0.001 |
| CCS | 145.9 ± 38.51 | 105.4 ± 21.49 | 123.52 ± 52.56 | 0.117 |
| Medical histories | ||||
| Hyperlipidemia (%) | 0.09 ± 0.29 | 0.14 ± 0.35 | 0.14 ± 0.35 | 0.534 |
| Hypertension (%) | 0.22 ± 0.42 | 0.27 ± 0.45 | 0.34 ± 0.47 | 0.138 |
| Diabetes (%) | 0.08 ± 0.27 | 0.14 ± 0.35 | 0.22 ± 0.42 | 0.008 |
| CAD history (%) | 0.01 ± 0.11 | 0.05 ± 0.22 | 0.04 ± 0.19 | 0.337 |
| Adiposity measures | ||||
| Pericardial fat (mL) | 79.25 ± 21.96 | 83.06 ± 27.96 | 80.72 ± 29.13 | 0.539 |
| Pericardial fat index (mL/m2) | 28.46 ± 5.91 | 42.04 ± 5.73 | 57.69 ± 9.7 | <0.001 |
| Periaortic fat (mL) | 7.64 ± 3.09 | 8.49 ± 3.91 | 8.19 ± 4.14 | 0.208 |
| Periaortic fat index (mL/m2) | 3.19 ± 1.53 | 4.02 ± 1.51 | 5.63 ± 2.07 | <0.001 |
| Left AVG fat (mm) | 3.99 ± 1.02 | 5.37 ± 0.67 | 7.42 ± 1.77 | <0.001 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; WC, waist circumference; TG, triglyceride; LDL, low-density lipoprotein; HDL, high-density lipoprotein; eGFR, estimated glomerular filtration rate; Hs-CRP, high sensitivity C-reactive protein; HOMA-IR, homeostatic model assessment-estimated insulin resistance; CCS, coronary calcium score; CAD, coronary artery disease; AVG, atrioventricular groove.
Echocardiographic parameters and multivariate analysis
The relationship between IAS fat thickness and various LV structural and functional parameters is presented in Table 2. Mean LAV max index, LAV pre-A index and LAV min index were 18.34 ± 6.04 mL/m2, 10.79 ± 3.75 mL/m2, 7.5 ± 3.02 mL/m2, respectively. We observed a high correlation between echo and CT-based LA maximum volume among 59 random cases in our lab (Echo: 40.6 ± 12.8 ml vs CT: 48.6 ±11.5 ml, r = 0.94, P < 0.001), with a mean difference of 7.91 ± 4.25 mL (95% CI: −9.02 to −6.81).
Table 2.
Relationship of LV structural/functional parameters with interatrial fat.
| Interatrial fat thickness (mm) | P (trend) | |||
|---|---|---|---|---|
| 1st tertile group | 2nd tertile group | 3rd tertile group | ||
| 2.00–2.48 | 4.83–6.36 | 6.37–10.8 | ||
| (n = 119) | (n = 119) | (n = 118) | ||
| IVS (mm) | 9.33 ± 1.22 | 9.60 ± 1.15 | 10.14 ± 1.15 | <0.001 |
| PWT (mm) | 9.03 ± 1.20 | 9.50 ± 1.22 | 9.99 ± 1.10 | <0.001 |
| LVEDV (mL) | 96.58 ± 14.87 | 102.68 ± 15.22 | 109.32 ± 16.68 | <0.001 |
| LVESV (mL) | 30.93 ± 7.35 | 32.89 ± 6.94 | 34.87 ± 7.85 | <0.001 |
| LVM (gm) | 141.90 ± 33.70 | 156.82 ± 34.22 | 175.31 ± 39.15 | <0.001 |
| LVMI (gm/m2) | 77.83 ± 15.89 | 83.23 ± 14.88 | 89.21 ± 16.60 | <0.001 |
| LVEF (%) | 68.95 ± 2.76 | 68.56 ± 3.74 | 68.29 ± 3.46 | 0.463 |
| E (cm/s) | 61.51 ± 14.91 | 61.57 ± 13.48 | 60.51 ± 16.76 | 0.637 |
| A (cm/s) | 60.56 ± 12.28 | 62.59 ± 12.69 | 66.18 ± 15.82 | 0.018 |
| DT (ms) | 204.36 ± 45.60 | 206.94 ± 51.67 | 211.69 ± 48.49 | 0.383 |
| IVRT (ms) | 91.44 ± 15.92 | 93.34 ± 14.32 | 94.89 ± 17.36 | 0.121 |
| e′ (cm/s) | 10.2 ± 2.66 | 8.76 ± 3.02 | 7.8 ± 2.04 | 0.014 |
| E/e′ | 7.17 ± 2.21 | 7.9 ± 4.03 | 8.49 ± 3.19 | 0.001 |
IVS, interventricular septum; PWT, posterior wall thickness; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; LVM, left ventricular mass; LVMI, left ventricular mass index; LVEF, left ventricular ejection fraction; E, early mitral inflow velocity; A, late mitral inflow velocity; DT, deceleration time; IVRT, isovolumetric relaxation time; e′, early-diastolic tissue velocity.
Increasing IAS tertiles were associated with graded increases in LV wall thickness, chamber size as well as total LV mass and LV mass index (all trend P < 0.001), whereas global LV systolic function remained relatively unaffected. There were no remarkable differences in mitral inflow Doppler-derived diastolic functional parameters across IAS tertiles, except for a trend towards higher A velocity with greater IAS (trend P: 0.018). As for TDI-derived parameters, there were significant reductions in average e’ velocity (trend P: 0.014) and increases in E/e’ ratio (trend P: 0.001).
We showed that greater IAS fat was strongly related to all three volume indices (1st vs 3rd tertiles: 16.85 vs 19.87 mL/m2 for LAV max index, 9.73 vs 12.03 mL/m2 for LAV pre-A index, 6.68 vs 8.61 mL/m2 for LAV min index, all P < 0.05) (Table 3). With each increasing IAS tertile, there was a significant decrease in all three functional parameters (1st vs 3rd tertiles: 159.44 vs 142.4% for reservoir function/expansion index, 31.94 vs 29.72% for booster function/active emptying fraction, 41.84 vs 39.97% for conduit function/passive emptying faction, all P < 0.05). However, both LA kinetic energy and ejection force had graded increases (1st vs 3rd tertiles: 5.84 vs 7.75 kdynes·cm/m2 for LA kinetic energy, 1.12 vs 1.29 kdynes/m2 for LA ejection force, both P < 0.05). Figure 3 shows the linear regression analysis and scatter plots for the afore-mentioned parameters in relation to IAS fat thickness. The fitted lines point to a gradual decline in all three functional indices and increases in both LA kinetic energy and ejection force (all P < 0.001).
Table 3.
Relationship of LA structural/functional parameters with interatrial fat.
| Interatrial fat thickness (mm) | P (trend) | |||
|---|---|---|---|---|
| 1st tertile group | 2nd tertile group | 3rd tertile group | ||
| 2.00–2.48 | 4.83–6.36 | 6.37–10.8 | ||
| (n = 119) | (n = 119) | (n = 118) | ||
| LAV max index (mL/m2) | 16.85 ± 5.53 | 18.37 ± 6.03 | 19.87 ± 6.23¥ | 0.001 |
| LAV pre-A index (mL/m2) | 9.73 ± 3.20 | 10.65 ± 3.55 | 12.03 ± 4.17¥※ | <0.001 |
| LAV min index (mL/m2) | 6.68 ± 2.55 | 7.27 ± 2.65 | 8.61 ± 3.51¥※ | <0.001 |
| LA reservoir function/expansion index (%) | 159.44 ± 46.64 | 157.94 ± 45.65 | 142.4 ± 40.8¥※ | 0.004 |
| LA booster function/active emptying fraction (%) | 31.94 ± 7.37 | 31.92 ± 6.71 | 29.72 ± 6.52¥※ | 0.022 |
| LA conduit function/passive emptying fraction (%) | 41.84 ± 5.77 | 41.59 ± 6.03 | 39.97 ± 5.58¥ | 0.009 |
| LA kinetic energy (kdynes·cm/m2) | 5.84 ± 2.56 | 7.13 ± 3.55¥ | 7.75 ± 3.84¥ | <0.001 |
| LA ejection force (kdynes/m2) | 1.12 ± 0.41 | 1. 26 ± 0.46¥ | 1.29 ± 0.43¥ | 0.001 |
LAV max, LA maximum volume; LAV pre-A, LA pre-A volume; LAV min, LA minimum volume.
¥P<0.05 versus 1st tertile group by ANOVA.
※P<0.05 versus 2nd tertile group by ANOVA.
Figure 3.
Linear regression plots of (A) left atrial reservoir function, (B) booster function, (C) conduit function, (D) kinetic energy (E) and ejection force in relation to IAS thickness. The fitted lines point to a gradual decline in all three functional indices and increases in both LA kinetic energy and ejection force (all P < 0.001).
Table 4 presents both univariate and multivariate models regarding the differential association between LA structural/functional parameters and regional fat measures. In univariate analysis, both IAS and left AVG fat thickness were significantly related to increased LA volumes, impaired LA reservoir function (β-coef: −0.15, P < 0.01 for AVG fat; β-coef: −0.19, P < 0.001 for IAS fat), and increased kinetic energy (β-coef: 0.17, P < 0.01 for AVG fat; β-coef: 0.18, P < 0.001 for IAS fat). In addition, thicker IAS fat was also associated with decreased LA booster function (β-coef: −0.13, P < 0.05), conduit function (β-coef: −0.15, P < 0.01), and increased ejection force (β-coef: 0.14, P < 0.001). In the multivariate analysis, IAS fat thickness remained significantly associated with increased LA volumes (β-coef: 0.19, 0.3, 0.29, respectively, all P < 0.01), increased LA kinetic energy and ejection force (β-coef: 0.17 & 0.15, respectively, both P < 0.05), impaired LA reservoir and conduit function (β-coef: −0.2 & −0.12, respectively, both P < 0.05) after adjusting for age, gender, eGFR, LV mass, and history of hypertension, diabetes, hyperlipidemia or CAD.
Table 4.
Univariate and multivariate models regarding the differential association between LA structural/functional parameters and regional fat measures (standardized β-coefficients shown in table).
| LAV max index | LAV min index | LAV pre-A index | LA reservoir function | LA booster function | LA conduit function | LA kinetic energy | LA ejection force | |
|---|---|---|---|---|---|---|---|---|
| Model 1 | ||||||||
| Pericardial fat | 0.11* | 0.14** | 0.14* | −0.07 | 0.07 | 0.13* | 0.07 | 0.05 |
| Periaortic fat | 0.12* | 0.13* | 0.13* | −0.06 | 0.08 | 0.18*** | 0.06 | 0.05 |
| Left AVG fat | 0.15** | 0.19*** | 0.18** | −0.15** | 0.10 | 0.10 | 0.17** | 0.10 |
| IAS fat | 0.22*** | 0.32*** | 0.30*** | −0.19*** | −0.13* | −0.15** | 0.18*** | 0.14*** |
| Model 2 | ||||||||
| Pericardial fat | 0.09 | 0.12* | 0.12* | −0.05 | 0.08 | 0.12* | 0.06 | 0.05 |
| Periaortic fat | 0.11* | 0.11* | 0.11* | −0.03 | 0.08 | 0.17** | 0.04 | 0.04 |
| Left AVG fat | 0.14*** | 0.16** | 0.15** | −0.11* | 0.11 | 0.09 | 0.14* | 0.08 |
| IAS fat | 0.21 | 0.29*** | 0.29*** | −0.16** | 0.14** | −0.13* | 0.17** | 0.13* |
| Model 3 | ||||||||
| Pericardial fat | 0.05 | 0.09 | 0.09 | −0.05 | 0.04 | 0.07 | 0.05 | 0.04 |
| Periaortic fat | 0.09 | 0.07 | 0.08 | 0.01 | 0.07 | 0.15* | 0.02 | 0.02 |
| Left AVG fat | 0.09 | 0.10 | 0.11 | −0.11 | 0.08 | 0.08 | 0.17* | 0.10 |
| IAS fat | 0.19** | 0.30*** | 0.29*** | −0.20* | −0.13 | −0.12* | 0.17* | 0.15* |
LAV max, LA maximum volume; LAV pre-A, LA pre-A volume; LAV min, LA minimum volume; AVG, atrioventricular groove; IAS, interatrial septum.
Model 1: Univariate.
Model 2: Multivariate: adjusted for age, gender.
Model 3: Multivariate: adjusted for age, gender, eGFR, LV mass, history of hypertension, diabetes, hyperlipidemia or CAD.
*P<0.05; **P<0.01; ***P<0.001.
Discussion
In this study, we observed that regional adiposity in terms of interatrial fat was partially associated with several LV geometric alterations and diastolic dysfunction. Furthermore, greater IAS was associated with LA structural and functional adaptations including LA enlargement, attenuated reservoir, conduit and booster performance, yet globally enhanced kinetic energy and ejection force. After control of multiple variables, these associations remained mostly significant except for booster function.
Potential mechanisms for the association of interatrial fat and atrial function
Lipomatous septal hypertrophy is a well-documented clinical condition associated with sick sinus syndrome (21) and atrial arrhythmias (22, 23, 24). Previous studies based on both necropsy (25) and image data (19, 26) had confirmed that these fatty infiltrates in the interatrial septum are proportionately correlated with total pericardial fat volume and one-dimensional fat thickness over grooved segments. In a previous study by Shin et al. [19], after dividing 160 patients into persistent AF, paroxysmal AF and control groups, the authors demonstrated that increased interatrial fat thickness was associated with AF severity. However, to the best of our knowledge, no investigation had yet been aimed specifically at the mechanistic link between interatrial fat and LA structural remodeling and functional alterations.
It has been previously reported that pericardial fat was independently associated with metabolic derangements, fatty liver disease, insulin resistance and systemic inflammation, by utilizing CT measures (13, 27). Histological studies of lipomatous septal hypertrophy have shown an abundance of inflammatory cells and fibrosis permeating throughout the fatty infiltrates (25). It has also been suggested that certain cytokines and inflammation process from accumulated adiposity may have direct adverse effects on LA mechanics or mediated arrhythmogenesis (19, 26, 28), leading to consequent structural remodeling, conduction disturbances and AF incidence (29). This study compared the associations among LA regional adiposity, pericardial, periaortic fat, and several LA structural/functional measures respectively. Since only interatrial fat had significant correlations with LA functions after multivariate adjustment, this may imply that interatrial fat should be viewed as a separate entity from pericardial fat. In contrast, AV groove fat may be regarded an extension of pericardial fat by sharing similar characteristics. While the enveloping pericardial fat may affect global cardiac function by both mechanical compression and cytokine secretion, the different anatomic nature of interatrial septal fat suggests a more biochemically oriented pathway.
Previous studies on patients with mild-to-moderate heart failure symptoms have shown that higher LA contractile or ejection force and greater kinetic energy may exist in these patients compared with healthy people (17, 30), possibly as compensation for maintaining resting cardiac output (31, 32). Our study showed that greater fat depots surrounding LA, especially interatrial fat, was accompanied by decreased reservoir function though augmented LA kinetic energy and ejection force. This supports the concept that such regional adipose tissues can be beneficial by preserving myocardial energy supply under certain circumstances, yet simultaneously impose certain negative effects generated by inflammatory process. Similar paradoxical effects of central obesity have been widely explored in previous literature (33, 34, 35). Investigators had previously observed an early reduction in LA reservoir and conduit functions along with compensatory increase in contractile function among patients with either hypertension (36, 37) or heart failure with preserved ejection fraction (32), possibly at the expense of LA enlargement. Another reason for LA volume expansion was believed to result from compensatory increased preload as a result of the optimal use of Frank–Starling mechanism to provoke atrial muscle shortening and contractility for same reasons (38). Taken together, our findings can be interpreted as subclinical LA functional disturbances possibly driven by the detrimental effects of interatrial fat, leading to impaired LA reservoir and conduit functions. On the meanwhile, cardiac performance was preserved by LA enlargement and augmented LA ejection force and kinetic energy in this process.
Potential clinical implications
A previous study showed that individuals without AF history who had increased atrial fat infiltration may be exposed to higher risk of developing AF, according to the ARIC risk score. They also found that IAS width strongly correlated with the extent of atrial fat infiltration (39). So far, impaired LA reservoir and conduit functions in individuals with central obesity or metabolic derangements had been speculated to be associated with AF substrate formation (10, 11), which may be independent of LA size (40). While different “weight” of various sites adiposity on cardiac structure/functions or electrical properties may exist (8, 9, 28), we and others demonstrated that IAS fat infiltrate may be of potential clinical significance (19, 38). In particular, as reported before, higher prevalence of atrial arrhythmias (for instance, AF) and overt LA remodeling had been observed with lipomatous septal hypertrophy (24). Compared with other fat depots, interatrial fat burden was more strongly linked to deteriorated LA mechanics of several aspects in our work after accounting for clinical covariates. An in-depth understanding of such findings may therefore help to identify a specific population at higher risk of AF development in daily practice. Thus, we speculate that early prevention of such fat infiltration or aggressive control of underlying clinical risks might prevent AF development, which may require further confirmation in future research.
Limitations
There are several limitations in our study. The first, our study has a male gender predominance, which may be somewhat biased. Secondly, this survey is mainly retrospective and cross-sectional, without an elaborate longitudinal follow-up of clinical outcomes. The actual number of patients who developed AF or heart failure was very low. This could be partially explained by the practice of national health insurance, which mandates nearly full population coverage in general disease management. However, the lack of ambulatory ECG monitoring rendered it impossible to determine whether silent paroxysmal arrhythmias might be missed. Thirdly, we primarily assessed LA volume by 2D echocardiography at specific time points, which is limited in accuracy due to the left atrium’s complex geometry and surrounding structures. Data of advanced imaging techniques such as speckle tracking and 3D-based echocardiography were also limited. However, we also performed CT measurements on nearly one-sixths of the study population, and the high correlation suggested that the echocardiographic method can be validated. Finally, although most data from our analysis had high statistical significance, the actual correlation was not strong, indicating that interatrial fat is only a minority in the wide range of multiple factors accounting for variations in LA mechanics.
Conclusions
This study showed that in an asymptomatic population, regional adipose tissue may have a negative impact on the LV geometry and LA remodeling process, which primarily manifested as LA enlargement accompanied by functional decline of several LA mechanics, including conduit and reservoir functions. These remodeling processes were associated with increased LA global kinetic energy and ejection force at the hemodynamic level, which may serve to compensate for those functional deteriorations of LA in asymptomatic population. More studies may be required to determine whether this association with LA function was mainly a causative of interatrial fat deposition, or vice versa.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This study was in part supported by funds NSC 101-2314-B-195-020, NSC 103-2314-B-010-005-MY3, NSC 103-2314-B-195-001-MY3 and funds from Medical Research Department, Mackay Memorial Hospital.
Authors’ contributions
YH Lai performed the echocardiographic measurements, participated in study design, and drafted the manuscript. CH Yun performed the CT measurements. CH Su, HI Yeh, and CJ Hou participated in study design and provided assistance in literature review. FS Yang, TS Wu, RC Cury, and HG Bezerra provided assistance in CT measurement and method standardization. CL Hung conceived this study concept, performed statistical analysis, and revised the manuscript. All authors have read and approved the final manuscript.
References
- 1.Kurt M, Wang J, Torre-Amione G, Nagueh SF. 2009. Left atrial function in diastolic heart failure. Circulation: Cardiovascular Imaging 2 10–15. ( 10.1161/CIRCIMAGING.108.813071) [DOI] [PubMed] [Google Scholar]
- 2.Hoit BD. 2014. Left atrial size and function: role in prognosis. Journal of the American College of Cardiology 63 493–505. ( 10.1016/j.jacc.2013.10.055) [DOI] [PubMed] [Google Scholar]
- 3.Todaro MC, Choudhuri I, Belohlavek M, Jahangir A, Carerj S, Oreto L, Khandheria BK. 2012. New echocardiographic techniques for evaluation of left atrial mechanics. European Heart Journal: Cardiovascular Imaging 13 973–984. ( 10.1093/ehjci/jes174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattioli AV, Bonatti S, Monopoli D, Zennaro M, Mattioli G. 2005. Influence of regression of left ventricular hypertrophy on left atrial size and function in patients with moderate hypertension. Blood Pressure 14 273–278. ( 10.1080/08037050500235523) [DOI] [PubMed] [Google Scholar]
- 5.Janssen I, Heymsfield SB, Allison DB, Kotler DP, Ross R. 2002. Body mass index and waist circumference independently contribute to the prediction of nonabdominal, abdominal subcutaneous, and visceral fat. American Journal of Clinical Nutrition 75 683–688. [DOI] [PubMed] [Google Scholar]
- 6.Després JP. 2012. Abdominal obesity and cardiovascular disease: is inflammation the missing link? Canadian Journal of Cardiology 28 642–652. ( 10.1016/j.cjca.2012.06.004) [DOI] [PubMed] [Google Scholar]
- 7.Iacobellis G, Ribaudo MC, Assael F, Vecci E, Tiberti C, Zappaterreno A, Di Mario U, Leonetti F. 2003. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. Journal of Clinical Endocrinology & Metabolism 88 5163–5168. ( 10.1210/jc.2003-030698) [DOI] [PubMed] [Google Scholar]
- 8.Iacobellis G, Pond CM, Sharma AM. 2006. Different “weight” of cardiac and general adiposity in predicting left ventricle morphology. Obesity 14 1679–1684. ( 10.1038/oby.2006.192) [DOI] [PubMed] [Google Scholar]
- 9.Fox CS, Gona P, Hoffmann U, Porter SA, Salton CJ, Massaro JM, Levy D, Larson MG, D’Agostino RB Sr, O’Donnell CJ, et al. 2009. Pericardial fat, intrathoracic fat, and measures of left ventricular structure and function: the Framingham Heart Study. Circulation 119 1586–1591. ( 10.1161/CIRCULATIONAHA.108.828970) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thanassoulis G, Massaro JM, O’Donnell CJ, Hoffmann U, Levy D, Ellinor PT, Wang TJ, Schnabel RB, Vasan RS, Fox CS, et al. 2010. Pericardial fat is associated with prevalent atrial fibrillation: the Framingham Heart Study. Circulation Arrhythmia and Electrophysiology 3 345–350. ( 10.1161/CIRCEP.109.912055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al Chekakie MO, Welles CC, Metoyer R, Ibrahim A, Shapira AR, Cytron J, Santucci P, Wilber DJ, Akar JG. 2010. Pericardial fat is independently associated with human atrial fibrillation. Journal of the American College of Cardiology 56 784–788. ( 10.1016/j.jacc.2010.03.071) [DOI] [PubMed] [Google Scholar]
- 12.Liao ZY, Peng MC, Yun CH, Lai YH, Po HL, Hou CJ, Kuo JY, Hung CL, Wu YJ, Bulwer BE, et al. 2012. Relation of carotid artery diameter with cardiac geometry and mechanics in heart failure with preserved ejection fraction. Journal of the American Heart Association 1 e003053 ( 10.1161/JAHA.112.003053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai YH, Yun CH, Yang FS, Liu CC, Wu YJ, Kuo JY, Yeh HI, Lin TY, Bezerra HG, Shih SC, et al. 2012. Epicardial adipose tissue relating to anthropometrics, metabolic derangements and fatty liver disease independently contributes to serum high-sensitivity C-reactive protein beyond body fat composition: a study validated with computed tomography. Journal of the American Society of Echocardiography 25 234–241. ( 10.1016/j.echo.2011.09.018) [DOI] [PubMed] [Google Scholar]
- 14.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. 2015. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography 28 1–39.e14. ( 10.1016/j.echo.2014.10.003) [DOI] [PubMed] [Google Scholar]
- 15.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. 2009. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Journal of the American Society of Echocardiography 22 107–133. ( 10.1016/j.echo.2008.11.023) [DOI] [PubMed] [Google Scholar]
- 16.To AC, Flamm SD, Marwick TH, Klein AL. 2011. Clinical utility of multimodality LA imaging: assessment of size, function, and structure. Journal of the American College of Cardiology Imaging 4 788–798. ( 10.1016/j.jcmg.2011.02.018) [DOI] [PubMed] [Google Scholar]
- 17.Triposkiadis F, Harbas C, Sitafidis G, Skoularigis J, Demopoulos V, Kelepeshis G. 2008. Echocardiographic assessment of left atrial ejection force and kinetic energy in chronic heart failure. International Journal of Cardiovascular Imaging 24 15–22. [DOI] [PubMed] [Google Scholar]
- 18.Yun CH, Lin TY, Wu YJ, Liu CC, Kuo JY, Yeh HI, Yang FS, Chen SC, Hou CJ, Bezerra HG, et al. 2012. Pericardial and thoracic peri-aortic adipose tissues contribute to systemic inflammation and calcified coronary atherosclerosis independent of body fat composition, anthropometric measures and traditional cardiovascular risks. European Journal of Radiology 81 749–756. ( 10.1016/j.ejrad.2011.01.035) [DOI] [PubMed] [Google Scholar]
- 19.Shin SY, Yong HS, Lim HE, Na JO, Choi CU, Choi JI, Kim SH, Kim JW, Kim EJ, Park SW, et al. 2011. Total and interatrial epicardial adipose tissues are independently associated with left atrial remodeling in patients with atrial fibrillation. Journal of Cardiovascular Electrophysiology 22 647–655. ( 10.1111/j.1540-8167.2010.01993.x) [DOI] [PubMed] [Google Scholar]
- 20.Shmilovich H, Dey D, Cheng VY, Rajani R, Nakazato R, Otaki Y, Nakanishi R, Slomka PJ, Thomson LE, Hayes SW, et al. 2011. Threshold for the upper normal limit of indexed epicardial fat volume: derivation in a healthy population and validation in an outcome-based study. American Journal of Cardiology 108 1680–1685. ( 10.1016/j.amjcard.2011.07.031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato Y, Matsuo S, Kusama J, Kunimasa T, Yoda S, Matsumoto N, Tani S, Saito S. 2007. Lipomatous hypertrophy of the interatrial septum presenting as sick sinus syndrome. International Journal of Cardiology 119 280–281. ( 10.1016/j.ijcard.2006.07.161) [DOI] [PubMed] [Google Scholar]
- 22.Shirani J, Roberts WC. 1993. Clinical, electrocardiographic and morphologic features of massive fatty deposits (“lipomatous hypertrophy”) in the atrial septum. Journal of the American College of Cardiology 22 226–238. ( 10.1016/0735-1097(93)90839-S) [DOI] [PubMed] [Google Scholar]
- 23.Isner JM, Swan CS 2nd, Mikus JP, Carter BL. 1982. Lipomatous hypertrophy of the interatrial septum: in vivo diagnosis. Circulation 66 470–473. ( 10.1161/01.CIR.66.2.470) [DOI] [PubMed] [Google Scholar]
- 24.Heyer CM, Kagel T, Lemburg SP, Bauer TT, Nicolas V. 2003. Lipomatous hypertrophy of the interatrial septum: a prospective study of incidence, imaging findings, and clinical symptoms. Chest 124 2068–2073. ( 10.1378/chest.124.6.2068) [DOI] [PubMed] [Google Scholar]
- 25.Gay JD, Guileyardo JM, Townsend-Parchman JK, Ross K. 1996. Clinical and morphologic features of lipomatous hypertrophy (“massive fatty deposits”) of the interatrial septum. American Journal of Forensic Medicine & Pathology 17 43–48. ( 10.1097/00000433-199603000-00007) [DOI] [PubMed] [Google Scholar]
- 26.Marcus GM, Whooley MA, Glidden DV, Pawlikowska L, Zaroff JG, Olgin JE. 2008. Interleukin-6 and atrial fibrillation in patients with coronary artery disease: data from the Heart and Soul Study. American Heart Journal 155 303–309. ( 10.1016/j.ahj.2007.09.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macor C, Ruggeri A, Mazzonetto P, Federspil G, Cobelli C, Vettor R. 1997. Visceral adipose tissue impairs insulin secretion and insulin sensitivity but not energy expenditure in obesity. Metabolism 46 123–129. ( 10.1016/S0026-0495(97)90288-2) [DOI] [PubMed] [Google Scholar]
- 28.Tselentakis EV, Woodford E, Chandy J, Gaudette GR, Saltman AE. 2006. Inflammation effects on the electrical properties of atrial tissue and inducibility of postoperative atrial fibrillation. Journal of Surgical Research 135 68–75. ( 10.1016/j.jss.2006.03.024) [DOI] [PubMed] [Google Scholar]
- 29.Friedman DJ, Wang N, Meigs JB, Hoffmann U, Massaro JM, Fox CS, Magnani JW. 2014. Pericardial fat is associated with atrial conduction: the Framingham Heart Study. Journal of the American Heart Association 3 e000477 ( 10.1161/JAHA.113.000477) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazzone C, Cioffi G, Faganello G, Cherubini A, Tarantini L, Di Lenarda A, Russo TE, Selmi A, Stefenelli C, Furlanello F. 2014. Left atrial work in patients with stable chronic heart failure: factors associated and prognostic role. Echocardiography 31 123–132. ( 10.1111/echo.12325) [DOI] [PubMed] [Google Scholar]
- 31.Linderer T, Chatterjee K, Parmley WW, Sievers RE, Glantz SA, Tyberg JV. 1983. Influence of atrial systole on the Frank-Starling relation and the end-diastolic pressure-diameter relation of the left ventricle. Circulation 67 1045–1053. ( 10.1161/01.CIR.67.5.1045) [DOI] [PubMed] [Google Scholar]
- 32.Morris DA, Gailani M, Vaz Pérez A, Blaschke F, Dietz R, Haverkamp W, Ozcelik C. 2011. Left atrial systolic and diastolic dysfunction in heart failure with normal left ventricular ejection fraction. Journal of the American Society of Echocardiography 24 651–662. ( 10.1016/j.echo.2011.02.004) [DOI] [PubMed] [Google Scholar]
- 33.Bechlioulis A, Vakalis K, Naka KK, Bourantas CV, Papamichael ND, Kotsia A, Tzimas T, Pappas K, Katsouras CS, Michalis LK. 2013. Paradoxical protective effect of central obesity in patients with suspected stable coronary artery disease. Obesity 21 E314–E321. ( 10.1002/oby.20074) [DOI] [PubMed] [Google Scholar]
- 34.Lavie CJ, De Schutter A, Patel DA, Romero-Corral A, Artham SM, Milani RV. 2012. Body composition and survival in stable coronary heart disease: impact of lean mass index and body fat in the “obesity paradox”. Journal of the American College of Cardiology 60 1374–1380. ( 10.1016/j.jacc.2012.05.037) [DOI] [PubMed] [Google Scholar]
- 35.Lavie CJ, Cahalin LP, Chase P, Myers J, Bensimhon D, Peberdy MA, Ashley E, West E, Forman DE, Guazzi M, et al. 2013. Impact of cardiorespiratory fitness on the obesity paradox in patients with heart failure. Mayo Clinic Proceedings 88 251–258. ( 10.1016/j.mayocp.2012.11.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kokubu N, Yuda S, Tsuchihashi K, Hashimoto A, Nakata T, Miura T, Ura N, Nagao K, Tsuzuki M, Wakabayashi C, et al. 2007. Noninvasive assessment of left atrial function by strain rate imaging in patients with hypertension: a possible beneficial effect of renin-angiotensin system inhibition on left atrial function. Hypertension Research 30 13–21. ( 10.1291/hypres.30.13) [DOI] [PubMed] [Google Scholar]
- 37.Mondillo S, Cameli M, Caputo ML, Lisi M, Palmerini E, Padeletti M, Ballo P. 2011. Early detection of left atrial strain abnormalities by speckle-tracking in hypertensive and diabetic patients with normal left atrial size. Journal of the American Society of Echocardiography 24 898–908. ( 10.1016/j.echo.2011.04.014) [DOI] [PubMed] [Google Scholar]
- 38.Qirko S, Tase M, Lushnjari V, Sinjari T. 1996. Left atrial contractility function in hypertension. Archives des maladies du coeur et des vaisseaux 89 1003–1007. [PubMed] [Google Scholar]
- 39.Tereshchenko LG, Rizzi P, Mewton N, Volpe GJ, Murthy S, Strauss DG, Liu CY, Marchlinski FE, Spooner P, Berger RD, Kellman P, Lima JA. 2014. Infiltrated atrial fat characterizes underlying atrial fibrillation substrate in patients at risk as defined by the ARIC atrial fibrillation risk score. International Journal of Cardiology 172 196–201. ( 10.1016/j.ijcard.2014.01.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fang NN, Sui DX, Yu JG, Gong HP, Zhong M, Zhang Y, Zhang W. 2015. Strain/strain rate imaging of impaired left atrial function in patients with metabolic syndrome. Hypertension Research 38 758–764. ( 10.1038/hr.2015.76) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a 
