Abstract
Objective
Glucose-dependent insulinotropic polypeptide (GIP) is released during meals and promotes nutrient uptake and storage. GIP receptor knockout mice are protected from diet induced weight gain and thus GIP antagonists have been proposed as a treatment for obesity. In this study, we assessed the role of GIP in hyperphagia induced obesity and metabolic abnormalities in leptin deficient (Lepob/ob) mice.
Methods
We crossbred GIP-GFP knock-in homozygous mice (GIPgfp/gfp) that have complete GIP knockout, and mice heterozygous for the ob mutation (Lepob/+) mice to generate Lepob/+/GIP+/+, Lepob/ob/GIP+/+, and Lepob/ob/GIPgfp/gfp mice. Male animals were weighed weekly and both oral glucose and insulin tolerance testing were performed to assess glucose homeostasis and circulating profiles of GIP and insulin. Body composition was evaluated by computerized tomography (CT) scan and analyses of indirect calorimetry and locomotor activity were performed.
Results
Postprandial GIP levels were markedly elevated in Lepob/ob/GIP+/+ mice compared to Lepob/+/GIP+/+ controls and were undetectable in Lepob/ob/GIPgfp/gfp mice. Insulin levels were equivalently elevated in both Lepob/ob/GIP+/+ and Lepob/ob/GIPgfp/gfp mice compared to controls at 8 weeks of age but the hyperinsulinemia was marginally reduced in Lepob/ob/GIPgfp/gfp by 21 weeks, in association with amelioration of glucose intolerance. Both Lepob/ob/GIP+/+ and Lepob/ob/GIPgfp/gfp mice remained equivalently insulin resistant. Body weight gain and subcutaneous and visceral fat volume of both Lepob/ob/GIP+/+ and Lepob/ob/GIPgfp/gfp mice were significantly higher than that of Lepob/+/GIP+/+ mice, while no significant differences were seen between Lepob/ob/GIP+/+ and Lepob/ob/GIPgfp/gfp mice. Locomotor activity and energy expenditure were decreased in both Lepob/ob/GIP+/+ and Lepob/ob/GIPgfp/gfp mice compared to control Lepob/+/GIP+/+ mice, while no significant differences were seen between Lepob/ob/GIP+/+ and Lepob/ob/GIPgfp/gfp mice. There was no significant difference in fat oxidation among the three groups. Fat content in liver was significantly lower in Lepob/ob/GIPgfp/gfp compared to Lepob/ob/GIP+/+ mice, while that of control Lepob/+/GIP+/+ mice was the lowest.
Conclusions
Our results indicate that GIP knockout does not prevent excess weight gain and metabolic derangement in hyperphagic leptin deficient mice.
Keywords: GIP, Hyperphagia, Obesity, Insulin resistance, ob/ob
Abbreviations: GIPR, GIP receptor; GFP, green fluorescent protein; OGTT, oral glucose tolerance test; ITT, insulin tolerance test; CT, computerized tomography
Highlights
-
•
Complete absence of GIP does not prevent hyperinsulinemia and insulin resistance from developing in Lepob/ob mice.
-
•
GIP deficiency does not alleviate excess weight gain and fat accumulation in Lepob/ob mice.
-
•
Endogenous GIP is not involved in the development of obesity in mice with complete absence of leptin.
1. Introduction
Obesity is a significant global problem that considerably increases risk of cardiovascular disease, type 2 diabetes, insulin resistance and hyperlipidemia [1]. High caloric intake, whether from consumption of foods containing more fat or overeating, and inactive lifestyle, disrupt the balance between energy intake and output and worsen obesity [2], [3], [4]. Despite considerable efforts by both academia and industry, there are presently few effective treatment options for obesity. Given the role of the gut in the absorption of nutrients and production of potent regulatory peptides that coordinate nutrient disposal and satiety, it represents an important target organ for therapeutic strategies to combat both diabetes and obesity, particularly with members of the glucagon receptor family [5].
Ingested nutrients are sensed and absorbed by the intestine, triggering the release of hormones from enteroendocrine cells lining the gut epithelium. Two such hormones are the incretins gastric inhibitory polypeptide/glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1). Mice with knockout of either the GIP receptor or GLP-1 receptor display glucose intolerance associated with blunted insulin secretion [6], [7]. The ability of these hormones to act directly on β-cells to augment insulin secretion during meals in a glucose-dependent manner is the basis for the treatment of diabetes by incretin mimics and inhibitors of the enzyme dipeptidyl-peptidase 4 (DPP-4) that rapidly degrades GIP and GLP-1 [8].
GIP is secreted by K-cells during meals, particularly when the cells come in contact with fat or glucose [9]. In addition to stimulating brisk release of insulin [10], GIP promotes adipogenesis and lipid accumulation in adipocytes [11], [12]. Therefore, there has been some debate over whether GIP agonists used to improve glucose homeostasis may increase adiposity while GIP antagonists could promote weight loss [13]. Several studies have convincingly demonstrated glucose lowering effects following long-term administration of DPP-4 resistant GIP analogs in rodents [14], [15], [16], [17]. Moreover, somewhat surprisingly, chronic overexpression of GIP in mice reduced diet-induced obesity and steatosis, in addition to improving glucose homeostasis [18]. Yet a seminal study revealed that mice with knockout of the GIP receptor are protected from high fat diet induced obesity and the development of insulin resistance [12]. Moreover, inhibition of GIP signaling in this model increases fat oxidation in peripheral tissues in associated with increased adiponectin levels [19]. These findings are bolstered by studies demonstrating that reducing circulating GIP levels with K-cell ablation [20], disruption of GIP gene expression [21], immunoneutralization [22], or vaccination against GIP [23] also reduced weight gain in mice following high fat diet, without impairing glucose homeostasis.
Here, we examined whether complete ablation of GIP production could reduce weight gain in the absence of the adipocyte hormone leptin, a condition that results in extreme hyperphagia, obesity, hyperinsulinemia, and insulin resistance, in both mice [24] and humans [25]. We found that Lepob/ob mice became equally obese and insulin resistant whether or not GIP was present, suggesting that GIP antagonism is unlikely to be effective at improving metabolism in extreme obesity associated with defective leptin action.
2. Materials and methods
2.1. Animals
The insertion of a sequence encoding green fluorescent protein (GFP) into preproGIP gene disrupts the expression of GIP, resulting in complete GIP peptide knockout in homozygous animals; GIP-GFP knock-in (GIP-GFP) mice were generated as described previously [21]. Leptin-knockout heterozygous (Lepob/+) mice were purchased from Charles River Laboratories, Inc., Kanagawa, Japan. We first crossbred Lepob/+ mice and GIP-GFP homozygous (GIPgfp/gfp) mice and generated Lepob/+/GIPgfp/+ mice. Next, we crossbred Lepob/+/GIPgfp/+ mice to each other to produce Lepob/+/GIP+/+, Lepob/ob/GIP+/+, and Lepob/ob/GIPgfp/gfp mice. All experiments were conducted with two cohorts of male mice; oral glucose tolerance tests (OGTTs) and insulin tolerance tests (ITTs) were performed on cohort 1, and computerized tomography (CT) analysis, indirect calorimetry and locomotor activity were performed with cohort 2. The mice were housed in groups (n = 4 for Lepob/ob/GIP+/+ and Lepob/ob/GIPgfp/gfp, n = 6 for Lepob/+GIP+/+) at 25 °C with a 10:14-h dark–light cycle under free access to water and food. Diet was purchased from Funabashi Farm Co., Ltd., Chiba, Japan (F2, containing 11.6% fat, 22.4% protein, 66.0% carbohydrate, 3.73 kcal/g). Mice were weighed weekly during experiments. Food intake was measured in mice at 32–33 weeks of age (cohort 1). Animal care and procedures were approved by the Kyoto University Animal Care Committee.
2.2. Oral glucose tolerance tests (OGTTs) and insulin tolerance tests (ITTs)
OGTTs were performed with 8 and 21 week old mice after overnight fasting. Glucose (1 g/kg body weight) was administered by oral gavage and blood glucose levels were measured by glucometer (Sanwa Kagaku Kenkyusho, Nagoya, Japan). Plasma insulin and total GIP levels were measured at 0, 15, 30, 60, and 120 min after glucose administration. Plasma insulin levels were measured using a mouse insulin ELISA kit (Shibayagi, Gunma, Japan) and plasma total GIP levels were measured using a GIP ELISA kit (EMD Millipore Corporation, Billerica, MA, USA). ITTs were performed on mice at 10 and 30 weeks old using 1.0 U/kg and 3.0 U/kg human insulin (100 IU/ml, Eli Lilly, Hyogo, Japan), respectively, by intraperitoneal injection after 4–5 h fasting. Blood glucose levels were measured at 0, 30, 60, and 90 min after insulin administration.
2.3. Computerized tomography (CT) analysis
For CT analysis of body fat composition, mice were anesthetized at 36 weeks of age and scanned using a Latheta experimental animal CT system (LCT-100M, Aloka, Tokyo, Japan). Contiguous 2 mm slice images from shoulder to caudal region were used for quantitative analysis by Latheta software (version 3.00).
2.4. Indirect calorimetry and locomotor activity
Indirect calorimetry and locomotor activity of mice were measured at 34–35 weeks of age (ARCO 2000, ARCO System, Chiba, Japan). The mice were housed individually with free access to water and food. Energy expenditure (kcal/min/kg), fat oxidation (mg/min/kg), and locomotor activity (count/min) were measured every 5 min over 24 h.
2.5. Statistical analysis
Data represent mean ± SEM. Statistical differences between groups were assessed using one-way ANOVA with Tukey–Kramer Multiple–Comparison Test. P < 0.05 was considered statistically significant.
3. Results
3.1. Body weight change, locomotor activity, energy expenditure, and fat oxidation of Lepob/+/GIP+/+, Lepob/ob/GIP+/+, and Lepob/ob/GIPgfp/gfp mice
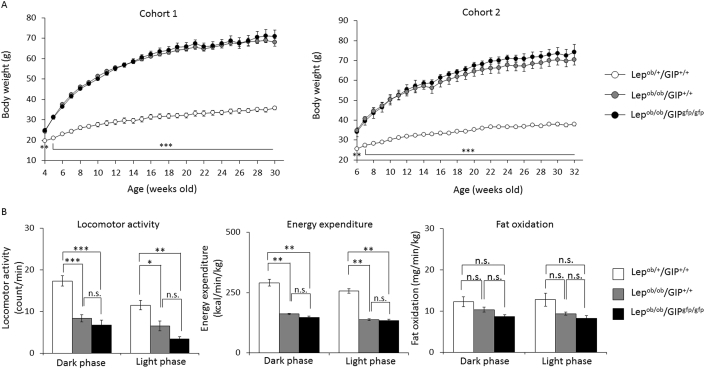
Body weight of Lepob/+/GIP+/+, Lepob/ob/GIP+/+, and Lepob/ob/GIPgfp/gfp mice was tracked in cohort 1 and cohort 2 (Figure 1A). Body weight gain of Lepob/ob/GIP+/+ and Lepob/ob/GIPgfp/gfp mice was similar and significantly higher than that of Lepob/+/GIP+/+ mice (maximum weight; 68.0 ± 1.8 g, 70.9 ± 3.0 g, 35.8 ± 0.8 g, respectively). Locomotor activity was similarly decreased in both the dark and light phases in Lepob/ob/GIP+/+ mice (52%, 44%) and Lepob/ob/GIPgfp/gfp mice (61%, 70%) compared to control Lepob/+/GIP+/+ mice (Figure 1B). Likewise, energy expenditure was decreased in Lepob/ob/GIP+/+ mice (44%, 46%) and Lepob/ob/GIPgfp/gfp mice (49%, 48%) compared to control Lepob/+/GIP+/+ mice in dark and light phases, respectively, but did not differ between Lepob/ob mice with or without GIP (Figure 1B). There was no significant difference in fat oxidation among the three groups. There were no significant differences in food intake among the three groups (data not shown), and aside from the metabolic features indicated below, GIP knockout did not result in any other obvious phenotype or behavioral changes.
Figure 1.
Body weight, locomotor activity, energy expenditure, and fat oxidation of Lepob/+/GIP+/+, Lepob/ob/GIP+/+, and Lepob/ob/GIPgfp/gfp male mice. (A) Body weight tracking of Lepob/+/GIP+/+ (white circles), Lepob/ob/GIP+/+ (gray circles), and Lepob/ob/GIPgfp/gfp mice (black circles) in cohort 1 (n = 5–8) and cohort 2 (n = 4–6). (B) Locomotor activity, energy expenditure and fat oxidation of Lepob/+/GIP+/+ (white bars), Lepob/ob/GIP+/+ (gray bars), and Lepob/ob/GIPgfp/gfp mice (black bars) at 34–35 weeks of age in cohort 2 (n = 3–6). *P < 0.05, **P < 0.01, ***P < 0.001 compared to Lepob/ob/GIP+/+. n.s; not significantly different. Data are mean ± SEM.
3.2. CT scan analysis of fat and liver in Lepob/+/GIP+/+, Lepob/ob/GIP+/+, and Lepob/ob/GIPgfp/gfp mice
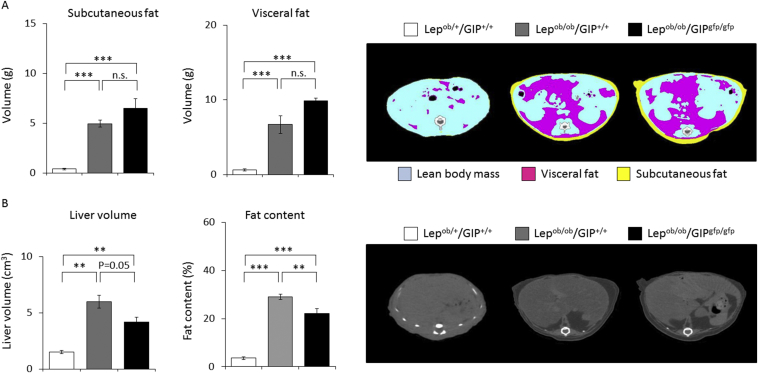
Subcutaneous and visceral fat volumes were similarly increased in Lepob/ob/GIP+/+ mice (∼13-fold, ∼10-fold) and in Lepob/ob/GIPgfp/gfp mice (∼17-fold, ∼15-fold) relative to Lepob/+/GIP+/+ mice (Figure 2A). Fat volumes were not significant different between Lepob/ob/GIP+/+ and Lepob/ob/GIPgfp/gfp animals. Liver volume and fat content in liver were significantly increased in Lepob/ob/GIP+/+ and Lepob/ob/GIPgfp/gfp mice compared to Lepob/+/GIP+/+ mice (Figure 2B). Fat content in liver was significantly lower in Lepob/ob/GIPgfp/gfp mice compared to Lepob/ob/GIP+/+ mice, although liver volume was not significantly different between the two groups (P = 0.05).
Figure 2.
CT scan analysis of fat and liver in Lepob/+/GIP+/+, Lepob/ob/GIP+/+, and Lepob/ob/GIPgfp/gfp male mice. (A) Subcutaneous and visceral fat volume of Lepob/+/GIP+/+ (white bars), Lepob/ob/GIP+/+ (gray bars), and Lepob/ob/GIPgfp/gfp mice (black bars) at 36 weeks of age in cohort 2 (n = 3–6). Pink, yellow, and blue areas represent visceral fat, subcutaneous fat, and lean body mass, respectively. (B) Liver volume and liver fat contents of Lepob/+/GIP+/+ (white bars), Lepob/ob/GIP+/+ (gray bars), and Lepob/ob/GIPgfp/gfp mice (black bars) at 36 weeks of age in cohort 2 (n = 3–6). n.s; not significantly different. Data are mean ± SEM.
3.3. OGTTs and ITTs in Lepob/+/GIP+/+, Lepob/ob/GIP+/+, and Lepob/ob/GIPgfp/gfp mice
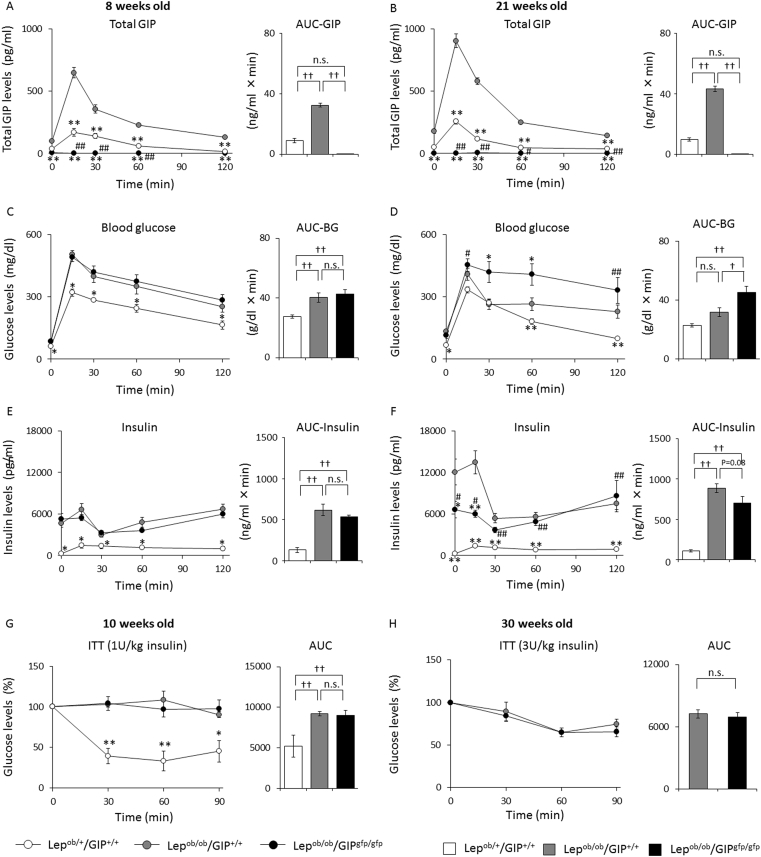
During the OGTTs, plasma GIP levels and area under the curve (AUC) of GIP were significantly higher in Lepob/ob/GIP+/+ mice than in Lepob/+/GIP+/+ mice at both 8 weeks of age (peak values: 647 ± 42 vs. 168 ± 32 pg/ml) and 21 weeks of age (peak values: 902 ± 55 vs. 254 ± 19 pg/ml) (Figure 3A and B). As expected, GIP levels were below detection in Lepob/ob/GIPgfp/gfp mice. Blood glucose levels and glucose AUC were similar in Lepob/ob/GIPgfp/gfp and Lepob/ob/GIP+/+ mice at 8 weeks of age and both were significantly higher than in control Lepob/+/GIP+/+ mice (Figure 3C). These findings paralleled plasma insulin levels, which were similarly increased in Lepob/ob/GIP+/+ and Lepob/ob/GIPgfp/gfp mice relative to control Lepob/+/GIP+/+ mice (Figure 3E). Blood glucose levels were significantly elevated in Lepob/ob/GIPgfp/gfp mice relative to the others at 21 weeks old (Figure 3D), and this was associated with significantly lower plasma insulin levels at 0 and 15 min during the OGTT in Lepob/ob/GIPgfp/gfp compared to Lepob/ob/GIP+/+ animals (Figure 3F). The AUC-insulin tended to be lower in Lepob/ob/GIPgfp/gfp mice than in Lepob/ob/GIP+/+, but it did not reach statistical significance (P = 0.08); insulin levels in Lepob/ob/GIPgfp/gfp and Lepob/ob/GIP+/+ mice were markedly elevated compared to levels in Lepob/+/GIP+/+ mice (AUC-insulin: 43-fold, 89-fold increase, respectively). During ITTs in 10 week old mice, blood glucose levels were not decreased in Lepob/ob/GIP+/+ and Lepob/ob/GIPgfp/gfp animals, while blood glucose levels were reduced by 67% in Lepob/+/GIP+/+ given the same 1 U/kg dose of insulin (Figure 3G). When given a higher dose of insulin (3 U/kg) at 30 weeks of age, blood glucose was maximally reduced by 35% similarly in both Lepob/ob/GIPgfp/gfp and Lepob/ob/GIP+/+ mice (Figure 3H).
Figure 3.
OGTT and ITT in Lepob/+/GIP+/+, Lepob/ob/GIP+/+, Lepob/ob/GIPgfp/gfp male mice. OGTTs and ITTs were performed with Lepob/+/GIP+/+ (white circles and bars), Lepob/ob/GIP+/+ (gray circles and bars), and Lepob/ob/GIPgfp/gfp mice (black circles and bars) in cohort 1 (n = 5–6). OGTTs were performed with 8 week (A, B, C) and 21 week (B, D, E) old mice using 1 g/kg glucose. ITTs were performed with 10 week (G) and 30 week (H) old mice using 1 U/kg and 3 U/kg regular insulin, respectively. The glucose levels during ITTs represent the percentage change from fasting glucose levels. Three Lepob/+/GIP+/+ mice (10 weeks old) exhibited symptoms of severe hypoglycemia 60 min after insulin injection and were rescued by oral glucose administration. Thus, the data of these mice at 90 min during the ITT were excluded. Glucose levels during ITTs were not evaluated in 30 week old Lepob/+/GIP+/+ mice because of severe hypoglycemia. (A, B) Plasma total GIP levels, (C, D) blood glucose levels, and (E, F) plasma insulin levels during the OGTT. (G, H) Glucose levels (%) during ITT. #P < 0.05, ##P < 0.01 vs. Lepob/+/GIP+/+. †P < 0.05, ††P < 0.01, ††P < 0.01. n.s; not significantly different. Data are mean ± SEM.
4. Discussion
Currently, the only therapy leading to substantial and sustained body weight is bariatric surgery. These procedures can also produce a remarkable resolution of type 2 diabetes within days after surgery, long before any significant weight loss takes place, leading some to perform bariatric surgery to treat diabetes even in non-obese individuals [26], [27], [28]. The altered flow of nutrients in the gut following bariatric surgery may be associated with adaptive changes in the enteroendocrine cell populations [29], [30], [31] and altered production of gastrointestinal hormones, including increases in plasma GIP and GLP-1 levels post surgery [28], [32], [33]. Changes in basal and/or postprandial release of gut hormones are among the potential mechanisms of improved glucose homeostasis and weight loss following bariatric surgery [34], [35]. Therefore, it may be possible to mimic the effects of surgery by gut hormone delivery and single-molecule peptides integrating the complementary actions of multiple hormones have demonstrated promising results [5]. A unimolecular dual incretin derived from intermixed sequences of GLP-1 and GIP demonstrated enhanced anti-hyperglycemic efficacy relative to selective GLP-1 agonists in rodents, monkeys, and humans [36]. Furthermore, while a selective GIP agonist did not alter body weight in high fat fed mice, the co-agonist treatment produced significant weight loss [36]. Even greater efficacy was obtained in high fat fed mice with a tritagonist incorporating a glucagon sequence for the synergistic action of glucagon to increase energy expenditure [37]. Therefore, activation of GIP receptors could be part of an effective strategy to treat diabetes and obesity.
Shortly after its discovery, endogenous GIP was implicated in linking over-nutrition to the development of obesity [38], [39], in part because the expression of GIP appears to be coordinated with nutritional status. Oral fat is a potent stimulator of GIP release that is augmented by bile, and mediated through direct actions on K-cells via fatty acid-binding protein 5 and G protein-coupled receptor 120 [40], [41]. Diets rich in fat increase intestinal K-cell number [42], GIP expression and circulating GIP levels [43], and the GIP response to oral glucose is enhanced by prior exposure to a high-fat diet [44]. Obese individuals have elevated plasma GIP levels that are associated with reduced post-prandial plasma triglycerides, suggesting a role for GIP in triglyceride uptake [39], [45]. GIP reduces plasma triglyceride increments following meals [46], an effect that could be mediated in part by increasing the activity of lipoprotein lipase, by direct actions of GIP on adipocytes [47], [48]. In some rodent models of obesity, the insulinotropic action of GIP is no longer restrained at basal glucose levels and thus can contribute to hyperinsulinemia [44]. We observed marked elevations of both GIP and insulin in Lepob/ob mice and perhaps their combined anabolic activity contributed to the excessive fat mass.
Consistent with a role of GIP in fat accumulation, GIP receptor knockout mice were protected from weight gain and hepatic steatosis when placed on a high fat diet [12], [49]. Moreover, when crossed onto mice with the ob mutation, the severity of obesity in homozygous offspring was reduced by 23%, although these mice remained almost twice the weight of control mice [12]. These findings contrast our observations in which complete ablation of GIP in homozygous ob/ob mice had no impact on weight gain, while hepatic fat content was modestly reduced. It is difficult to reconcile these differences resulting from knockout of GIP versus its receptor, particularly as alternate endogenous ligands for the GIP receptor have not been reported. Perhaps variations in diets, housing conditions or mouse microbiomes contributed to the differences. In our studies, Lepob/ob/GIPgfp/gfp mice had insulin levels equivalent to Lepob/ob/GIP+/+ mice at 8 weeks of age and lower insulin levels at 21 weeks, yet they still remained severely hyperinsulinemic. In contrast, we previously observed a complete normalization of insulin levels in GIP knockout mice on high fat diet, associated with a significant reduction in weight gain relative to wild type controls [21]. A reduction in insulin production has been demonstrated to dramatically reduce weight gain in both ob/ob mice [50] and mice on a high fat diet [51]. We speculate that the reduction in insulin achieved in the Lepob/ob/GIPgfp/gfp animals in our current study was insufficient to promote weight loss.
It is possible that regulation of adiposity and glucose homeostasis by GIP are in part mediated by altering leptin levels and/or leptin signaling. However, we are unaware of reports that support this mechanism of action of GIP. In addition, leptin levels in GIP receptor knockout mice [12], [52] and mice with ablation of K-cells [20] remained proportional to fat mass, suggesting that GIP action does not directly regulate leptin production. The concept of an adipo–enteroendocrine axis has been proposed, based upon observations that leptin directly stimulates GLP-1 secretion from rodent and human intestinal L cells [53], but whether leptin regulates GIP secretion from K-cells is unknown. Our mouse model enabled us to investigate the impact of GIP deficiency independent of leptin signaling. Collectively, our findings suggest that endogenous GIP is not involved in the development of obesity in mice with complete absence of leptin.
Disclosure statement
This study was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan Society for the Promotion of Science (JSPS), Ministry of Health, Labour, and Welfare, Ministry of Agriculture, Forestry and Fisheries, Japan Diabetes Foundation, Japan Association for Diabetes Education and Care, Merck Sharp & Dohme (MSD) Life Science Foundation, and Public Interest Incorporated Foundation, Japan Diabetes Foundation, Suzuken Memorial Foundation.
Acknowledgments
The authors thank Mr. Shoichi Asano and Dr. Daniela Nasteska from the Department of Diabetes, Endocrinology and Nutrition, Graduate School of medicine, Kyoto University, for technical support regarding the study. T.J. Kieffer gratefully acknowledges fellowship support from JSPS while on sabbatical at Kyoto University.
Conflict of interest
N. Inagaki served as a medical advisor for Takeda, Taisho Pharmaceutical, GlaxoSmithKline, and Mitsubishi Tanabe Pharma, and lectured for MSD, Sanofi, Novartis Pharma, Dainippon Sumitomo Pharma, Kyowa Kirin, and Mitsubishi Tanabe Pharma and received payment for services. No other potential conflicts of interest relevant to this article are reported.
References
- 1.Després J.P., Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 2.Hill J.O., Melanson E.L., Wyatt H.T. Dietary fat intake and regulation of energy balance: implications for obesity. The Journal of Nutrition. 2000;130(2S Suppl):284S–288S. [PubMed] [Google Scholar]
- 3.Bray G.A., Nielsen S.J., Popkin B.M. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. The American Journal of Clinical Nutrition. 2004;79(4):537–543. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- 4.Bray G.A., Popkin B.M. Dietary fat intake does affect obesity! The American Journal of Clinical Nutrition. 1998;68(6):1157–1173. doi: 10.1093/ajcn/68.6.1157. [DOI] [PubMed] [Google Scholar]
- 5.Cho Y.M., Merchant C.E., Kieffer T.J. Targeting the glucagon receptor family for diabetes and obesity therapy. Pharmacology & Therapeutics. 2012;135(3):247–278. doi: 10.1016/j.pharmthera.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Scrocchi L.A., Brown T.J., MaClusky N., Brubaker P.L., Auerbach A.B., Joyner A.L. Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nature Medicine. 1996;2(11):1254–1258. doi: 10.1038/nm1196-1254. [DOI] [PubMed] [Google Scholar]
- 7.Miyawaki K., Yamada Y., Yano H., Niwa H., Ban N., Ihara Y. Glucose intolerance caused by a defect in the entero-insular axis: a study in gastric inhibitory polypeptide receptor knockout mice. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(26):14843–14847. doi: 10.1073/pnas.96.26.14843. https://www.ncbi.nlm.nih.gov/pubmed/?term=Miyawaki%2C+GIP%2C+PNAS [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho Y.M., Fujita Y., Kieffer T.J. Glucagon-like peptide-1: glucose homeostasis and beyond. Annual Review of Physiology. 2014;76:535–559. doi: 10.1146/annurev-physiol-021113-170315. [DOI] [PubMed] [Google Scholar]
- 9.Cho Y.M., Kieffer T.J. K-cells and glucose-dependent insulinotropic polypeptide in health and disease. Vitamines and Hormones. 2010;84:111–150. doi: 10.1016/B978-0-12-381517-0.00004-7. [DOI] [PubMed] [Google Scholar]
- 10.Dupre J., Ross S.A., Watson D., Brown J.C. Stimulation of insulin secretion by gastric inhibitory polypeptide in man. The Journal of Clinical Endocrinology & Metabolism. 1973;37(5):826–828. doi: 10.1210/jcem-37-5-826. [DOI] [PubMed] [Google Scholar]
- 11.Kim S.J., Nian C., McIntosh C.H. Activation of lipoprotein lipase by glucose-dependent insulinotropic polypeptide in adipocytes. A role for a protein kinase B, LKB1, and AMP-activated protein kinase cascade. The Journal of Biological Chemistry. 2007;282(12):8557–8567. doi: 10.1074/jbc.M609088200. [DOI] [PubMed] [Google Scholar]
- 12.Miyawaki K., Yamada Y., Ban N., Ihara Y., Tsukiyama K., Zhou H. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nature Medicine. 2002;8(7):738–742. doi: 10.1038/nm727. [DOI] [PubMed] [Google Scholar]
- 13.Kieffer T.J. GIP or not GIP? That is the question. Trends in Pharmacological Sciences. 2003;24(3):110–112. doi: 10.1016/S0165-6147(03)00031-2. [DOI] [PubMed] [Google Scholar]
- 14.McIntosh C.H., Widenmaier S., Kim S.J. Glucose-dependent insulinotropic polypeptide (gastric inhibitory polypeptide; GIP) Vitamines and Hormones. 2009;80:409–471. doi: 10.1016/S0083-6729(08)00615-8. [DOI] [PubMed] [Google Scholar]
- 15.Hinke S.A., Gelling R.W., Pederson R.A., Manhart S., Nian C., Demuth H.U. Dipeptidyl peptidase IV-resistant [D-Ala2]glucose-dependent insulinotropic polypeptide (GIP) improves glucose tolerance in normal and obese diabetic rats. Diabetes. 2002;51(3):652–661. doi: 10.2337/diabetes.51.3.652. [DOI] [PubMed] [Google Scholar]
- 16.Gault V.A., Porter D.W., Irwin N., Flatt P.R. Comparison of sub-chronic metabolic effects of stable forms of naturally occurring GIP(1-30) and GIP(1-42) in high-fat fed mice. Journal of Endocrinology. 2011;208(3):265–271. doi: 10.1530/JOE-10-0419. [DOI] [PubMed] [Google Scholar]
- 17.Widenmaier S.B., Kim S.J., Yang G.K., De Los Reyes T., Nian C., Asadi A. A GIP receptor agonist exhibits beta-cell anti-apoptotic actions in rat models of diabetes resulting in improved beta-cell function and glycemic control. PLoS One. 2010;5(3):e9590. doi: 10.1371/journal.pone.0009590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim S.J., Nian C., Karunakaran S., Clee S.M., Isales C.M., McIntosh C.H. GIP-overexpressing mice demonstrate reduced diet-induced obesity and steatosis, and improved glucose homeostasis. PLoS One. 2012;7(7):e40156. doi: 10.1371/journal.pone.0040156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naitoh R., Miyawaki K., Harada N., Mizunoya W., Toyoda K., Fushiki T. Inhibition of GIP signaling modulates adiponectin levels under high-fat diet in mice. Biochemical and Biophysical Research Communications. 2008;376(1):21–25. doi: 10.1016/j.bbrc.2008.08.052. [DOI] [PubMed] [Google Scholar]
- 20.Althage M.C., Ford E.L., Wang S., Tso P., Polonsky K.S., Wice B.M. Targeted ablation of glucose-dependent insulinotropic polypeptide-producing cells in transgenic mice reduces obesity and insulin resistance induced by a high fat diet. The Journal of Biological Chemistry. 2008;283(26):18365–18376. doi: 10.1074/jbc.M710466200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nasteska D., Harada N., Suzuki K., Yamane S., Hamasaki A., Joo E. Chronic reduction of GIP secretion alleviates obesity and insulin resistance under high-fat diet conditions. Diabetes. 2014;63(7):2332–2343. doi: 10.2337/db13-1563. [DOI] [PubMed] [Google Scholar]
- 22.Boylan M.O., Glazebrook P.A., Tatalovic M., Wolfe M.M. Gastric inhibitory polypeptide immunoneutralization attenuates development of obesity in mice. American Journal of Physiology - Endocrinology and Metabolism. 2015;309(12):E1008–E1018. doi: 10.1152/ajpendo.00345.2015. [DOI] [PubMed] [Google Scholar]
- 23.Fulurija A., Lutz T.A., Sladko K., Osto M., Wielinga P.Y., Bachmann M.F. Vaccination against GIP for the treatment of obesity. PLoS One. 2008;3(9):e3163. doi: 10.1371/journal.pone.0003163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J.M. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 25.Strobel A., Issad T., Camoin L., Ozata M., Strosberg A.D. A leptin missense mutation associated with hypogonadism and morbid obesity. Nature Genetics. 1998;18(3):213–215. doi: 10.1038/ng0398-213. [DOI] [PubMed] [Google Scholar]
- 26.Rubino F., Amiel S.A. Is the gut the “sweet spot” for the treatment of diabetes? Diabetes. 2014;63(7):2225–2228. doi: 10.2337/db14-0402. [DOI] [PubMed] [Google Scholar]
- 27.Keidar A. Bariatric surgery for type 2 diabetes reversal: the risks. Diabetes Care. 2011;34(Suppl. 2):S361–S366. doi: 10.2337/dc11-s254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cui J.F., Chen T., Shi L., Yan H.T., Tang L.J. Gastric bypass surgery in non-obese patients with type 2 diabetes mellitus: a 1-year follow-up of 58 cases in Chinese. International Journal of Clinical and Experimental Medicine. 2015;8(3):4393–4398. [PMC free article] [PubMed] [Google Scholar]
- 29.Speck M., Cho Y.M., Asadi A., Rubino F., Kieffer T.J. Duodenal-jejunal bypass protects GK rats from β-cell loss and aggravation of hyperglycemia and increases enteroendocrine cells coexpressing GIP and GLP-1. American Journal of Physiology – Endocrinology and Metabolism. 2011;300(5):E923–E932. doi: 10.1152/ajpendo.00422.2010. [DOI] [PubMed] [Google Scholar]
- 30.Hansen C.F., Vassiliadis E., Vrang N., Sangild P.T., Cummings B.P., Havel P. The effect of ileal interposition surgery on enteroendocrine cell numbers in the UC Davis type 2 diabetes mellitus rat. Regulatory Peptides. 2014;189:31–39. doi: 10.1016/j.regpep.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Rhee N.A., Wahlgren C.D., Pedersen J., Mortensen B., Langholz E., Wandall E.P. Effect of Roux-en-Y gastric bypass on the distribution and hormone expression of small-intestinal enteroendocrine cells in obese patients with type 2 diabetes. Diabetologia. 2015;58(10):2254–2258. doi: 10.1007/s00125-015-3696-3. [DOI] [PubMed] [Google Scholar]
- 32.Laferrère B., Teixeira J., McGinty J., Tran H., Egger J.R., Colarusso A. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. The Journal of Clinical Endocrinology & Metabolism. 2008;93(7):2479–2485. doi: 10.1210/jc.2007-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laferrère B., Heshka S., Wang K., Khan Y., McGinty J., Teixeira J. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007;30(7):1709–1716. doi: 10.2337/dc06-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cho Y.M. A gut feeling to cure diabetes: potential mechanisms of diabetes remission after bariatric surgery. Diabetes & Metabolism Journal. 2014;38(6):406–415. doi: 10.4093/dmj.2014.38.6.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ionut V., Burch M., Youdim A., Bergman R.N. Gastrointestinal hormones and bariatric surgery-induced weight loss. Obesity (Silver Spring) 2013;21(6):1093–1103. doi: 10.1002/oby.20364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finan B., Ma T., Ottaway N., Müller T.D., Habegger K.M., Heppner K.M. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Science Translational Medicine. 2013;5(209):209ra151. doi: 10.1126/scitranslmed.3007218. [DOI] [PubMed] [Google Scholar]
- 37.Finan B., Yang B., Ottaway N., Smiley D.L., Ma T., Clemmensen C. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nature Medicine. 2015;21(1):27–36. doi: 10.1038/nm.3761. [DOI] [PubMed] [Google Scholar]
- 38.Brown J.C., Dryburgh J.R., Frost J.L., Otte S.C., Pederson R.A. Physiology and pathophysiology of GIP. Advances in Experimental Medicine and Biology. 1978;106:169–171. doi: 10.1007/978-1-4684-7248-6_21. [DOI] [PubMed] [Google Scholar]
- 39.Creutzfeldt W., Ebert R., Willms B., Frerichs H., Brown J.C. Gastric inhibitory polypeptide (GIP) and insulin in obesity: increased response to stimulation and defective feedback control of serum levels. Diabetologia. 1978;14(1):15–24. doi: 10.1007/BF00429703. [DOI] [PubMed] [Google Scholar]
- 40.Shibue K., Yamane S., Harada N., Hamasaki A., Suzuki K., Joo E. Fatty acid-binding protein 5 regulates diet-induced obesity via GIP secretion from enteroendocrine K cells in response to fat ingestion. American Journal of Physiology – Endocrinology and Metabolism. 2015;308(7):E583–E591. doi: 10.1152/ajpendo.00543.2014. [DOI] [PubMed] [Google Scholar]
- 41.Iwasaki K., Harada N., Sasaki K., Yamane S., Iida K., Suzuki K. Free fatty acid receptor GPR120 is highly expressed in enteroendocrine K cells of the upper small intestine and has a critical role in GIP secretion after fat ingestion. Endocrinology. 2015;156(3):837–846. doi: 10.1210/en.2014-1653. [DOI] [PubMed] [Google Scholar]
- 42.Bailey C.J., Flatt P.R., Kwasowski P., Powell C.J., Marks V. Immunoreactive gastric inhibitory polypeptide and K cell hyperplasia in obese hyperglycaemic (ob/ob) mice fed high fat and high carbohydrate cafeteria diets. Acta Endocrinologica (Copenhagen) 1986;112(2):224–229. doi: 10.1530/acta.0.1120224. [DOI] [PubMed] [Google Scholar]
- 43.Morgan L.M., Hampton S.M., Tredger J.A., Cramb R., Marks V. Modifications of gastric inhibitory polypeptide (GIP) secretion in man by a high-fat diet. British Journal of Nutrition. 1988;59(3):373–380. doi: 10.1079/bjn19880046. [DOI] [PubMed] [Google Scholar]
- 44.Chan C.B., Pederson R.A., Buchan A.M., Tubesing K.B., Brown J.C. Gastric inhibitory polypeptide (GIP) and insulin release in the obese Zucker rat. Diabetes. 1984;33(6):536–542. doi: 10.2337/diab.33.6.536. [DOI] [PubMed] [Google Scholar]
- 45.Raben A., Andersen H.B., Christensen N.J., Madsen J., Holst J.J., Astrup A. Evidence for an abnormal postprandial response to a high-fat meal in women predisposed to obesity. American Journal of Physiology – Endocrinology and Metabolism. 1994;267(4):E549–E559. doi: 10.1152/ajpendo.1994.267.4.E549. [DOI] [PubMed] [Google Scholar]
- 46.Wasada T., McCorkle K., Harris V., Kawai K., Howard B., Unger R.H. Effect of gastric inhibitory polypeptide on plasma levels of chylomicron triglycerides in dogs. The Journal of Clinical Investigation. 1981;68(4):1106–1107. doi: 10.1172/JCI110335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim S.J., Nian C., McIntosh C.H. GIP increases human adipocyte LPL expression through CREB and TORC2-mediated trans-activation of the LPL gene. The Journal of Lipid Research. 2010;51(11):3145–3157. doi: 10.1194/jlr.M006841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eckel R.H., Fujimoto W.Y., Brunzell J.D. Gastric inhibitory polypeptide enhanced lipoprotein lipase activity in cultured preadipocytes. Diabetes. 1979;28(12):1141–1142. doi: 10.2337/diab.28.12.1141. [DOI] [PubMed] [Google Scholar]
- 49.Yamada Y., Miyawaki K., Tsukiyama K., Harada N., Chizumi Y., Seino Y. Pancreatic and extrapancreatic effects of gastric inhibitory polypeptide. Diabetes. 2006;55(Suppl. 2):S86–S91. [Google Scholar]
- 50.D'souza A.M., Johnson J.D., Clee S.M., Kieffer T.J. Suppressing hyperinsulinemia prevents obesity but causes rapid onset of diabetes in leptin-deficient Lepob/ob mice. Molecular Metabolism. 2016;5(11):1103–1112. doi: 10.1016/j.molmet.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mehran A.E., Templeman N.M., Brigidi G.S., Lim G.E., Chu K.Y., Hu X. Hyperinsulinemia drives diet-induced obesity independently of brain insulin production. Cell Metabolism. 2012;16(6):723–737. doi: 10.1016/j.cmet.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 52.Yamada C., Yamada Y., Tsukiyama K., Yamada K., Yamane S., Harada N. Genetic inactivation of GIP signaling reverses aging-associated insulin resistance through body composition changes. Biochemical and Biophysical Research Communications. 2007;364(1):175–180. doi: 10.1016/j.bbrc.2007.09.128. [DOI] [PubMed] [Google Scholar]
- 53.Anini Y., Brubaker P.L. Role of leptin in the regulation of glucagon-like peptide-1 secretion. Diabetes. 2003;52(2):252–259. doi: 10.2337/diabetes.52.2.252. [DOI] [PubMed] [Google Scholar]