Abstract
Loss of the Salmonella MsbB enzyme, which catalyzes the incorporation of myristate destined for lipopolysaccharide in the outer membrane, results in a strong phenotype of sensitivity to salt and chelators such as EGTA and greatly diminished endotoxic activity. MsbB− salmonellae mutate extragenically to EGTA-tolerant derivatives at a frequency of 10−4 per division. One of these derivatives arose from inactivation of somA, which suppresses sensitivity to salt and EGTA. Here we show that a second mode of MsbB− suppression is a RecA-dependent deletion between two IS200 insertion elements present in Salmonella enterica serovar Typhimurium strain ATCC 14028 but not in two other wild-type strains, LT2 and SL1344, which lack one of the IS200 elements. This deletion occurs spontaneously in wild-type and MsbB− strain 14028 salmonellae and accounts for about one-third of all of the spontaneous suppressors of MsbB− in strain 14028. It spans the region corresponding to 17.7 to 19.9 centisomes, which includes somA, on the sequenced map of Salmonella LT2 (136 ORFs in that strain; ATCC 14028 and other strains showed variability in this region). In addition to conferring EGTA resistance correlated with somA, the deletion confers a MacConkey galactose resistance phenotype on MsbB− Salmonella, indicating that at least one additional gene (distinct from somA) within the deletion is responsible for this phenotype. In the wild type, the deletion mutant grows with normal exponential growth rate in Luria broth but is chlorate resistant and does not grow on citrate agar. The deletion strains have lost hydrogen sulfide production, nitrate reductase activity, and gas production from glucose fermentation.
The MsbB enzyme in Salmonella, encoded by the msbB gene (= lpxM, waaN, mlt), catalyzes the addition of one of the acyl chains (myristate) to lipid IV-A, which is destined for the outer cell membrane (19). It is believed that the myristate, as one of the acyl groups, contributes to membrane integrity and function (20). As previously described (18), msbB mutant salmonellae are unusually sensitive to certain growth conditions, such as deprivation of Ca2+ and Mg2+, exhibit slow growth or autolysis, and mutate extragenically at high frequency to faster-growing, stabilized derivatives. One such suppressor mutation was found to be the result of the insertion of an IS10 element within somA, an open reading frame (ORF) whose function is unknown. Because of the high frequency of mutations that suppress the MsbB phenotype, it was conjectured that loss-of-function mutations in many genetic elements might contribute to this high frequency. The characterization of MsbB− and its suppressors is emerging as a powerful new window on interacting components that are important for membrane and virulence functions.
In an effort to determine if other suppressor strains had mutations in somA that would alter the size of the somA PCR product, we used PCR to amplify somA from other spontaneously derived suppressor strains. A high percentage (about one-third) of our strains failed to produce any somA PCR product, which suggested that, in these strains, at least one of the primer sites next to somA had mutated. We show here that these strains contain uniform large deletions that correspond to approximately 100 ORFs in size on the basis of the annotated DNA sequence of strain LT2.
As described below, this deletion results from a crossover between two IS200 elements that flank the deletion-containing area. IS200 is a small (∼700 bp) insertion sequence that is prevalent among Salmonella species. Although IS200 is rarely transposed in the laboratory, IS200 profiles from various strains suggest that it is transposed frequently in nature (22). Different Salmonella species can carry from none to more than 18 copies of IS200 (13). LT2, the best-studied (and sequenced) Salmonella enterica serovar Typhimurium strain, has six IS200 elements, whereas ATCC 14028 (the wild-type strain used for these studies) has seven (22).
Although recombination between large direct repeats occurs at a low frequency within populations, it is often disadvantageous for growth and selection usually maintains the wild-type genomic structure. Since the deletion we describe here (which we refer to as the Suwwan deletion) suppresses growth defects conferred by the msbB mutation in Salmonella, there is a strong selection for mutants carrying the deletion in an msbB background. We found that about one-third of the spontaneous msbB suppressor strains isolated have the Suwwan deletion. In addition to altering msbB-related phenotypes, several other phenotypic changes are conferred by the deletion, which occurs in both the msbB mutant and wild-type backgrounds.
MATERIALS AND METHODS
Bacterial strains, phage, and media.
The bacterial strains used in this study are listed in Table 1. The Salmonella msbB1 allele is a partial-deletion mutation containing a tetracycline resistance (Tetr) determinant (14). The ΔmsbB2 deletion is a partial-deletion mutation similar to msbB1 except that it lacks the Tetr determinant and was introduced into the chromosome with a sacB suicide vector (15) by the method of Donnenberg and Kaper (9). The two msbB deletion alleles used in these studies, msbB1 and msbB2, produced identical phenotypes. P22 mutant HT105/1int201 (obtained from the Salmonella Genetic Stock Center, Calgary, Alberta, Canada) was used for Salmonella transductions. S. enterica serovar Typhimurium strains were grown on LB (Luria-Bertani) medium (17); LB-0 medium, which is LB medium with no NaCl; or MSB medium, which consists of LB-0 medium supplemented with 2 mM MgSO4 and 2 mM CaCl2. MSB broth and agar were used for the growth of strains under nonselective conditions. LB-0 agar was used when using selective antibiotics in transductions and transformations; addition of Mg2+ and Ca2+ was found to increase phage contamination in transductions (6) and to decrease the effectiveness of certain antibiotics, such as ampicillin and tetracycline. Plates were solidified with 1.5% agar. LB-0 agar or MSB broth was supplemented with tetracycline (3, 5, or 20 μg/ml), kanamycin (5 μg/ml), or EGTA free acid (6 or 6.5 mM; Sigma, St. Louis, Mo.) as needed. A 350 mM stock of EGTA at pH 8.0 (adjusted with NaOH) was dissolved and then autoclaved. Antibiotics were added to LB-0 agar after cooling to 45°C. MacConkey agar base (Difco) was used to prepare MacConkey galactose agar.
TABLE 1.
Bacterial strains used in this study
| Salmonella enterica serovar Typhi- murium strain | Parental strain | Genotype or phenotype | Derivation or source (reference) |
|---|---|---|---|
| ATTC 14028 | 14028 | Wild type | ATTC |
| KR1657 | recA1 zfi-1623::Tn10d-Camr | Salmonella Genetic Stock Centre, Calgary, Alberta, Canada | |
| TT14282 | srl-1203::Tn10d-CamrrecA1 | Salmonella Genetic Stock Centre, Calgary, Alberta, Canada | |
| YS1 | 14028 | msbB1::ΩTetr | P22 · YS8211 × 14028 → Tet5r, where YS8211 = donor and 14028 = recipient in P22 transduction (18) |
| YS871 | 14028 | ΔmsbB2 Δpur13252 somA+zbj-10::Tn10 | P22 · 14028 Tn10 pool × YS1456 → Tet20r (EGTAs) |
| YS1124 | 14028 | ΔmsbB2 ΔpurI3252 Suwwan deletion | Spontaneous EGTAr derivative of msbB1::ΩTet ΔpurI3252 derivative of 14028; replacement of msbB1::ΩTetr with ΔmsbB2 by homologous recombination (9) |
| YS1170 | 14028 | msbB1::ΩTetr Suwwan deletion | YS8211; spontaneous selection on EGTA medium |
| YS1317 | 14028 | ΔmsbB2 ΔpurI3252 somA+zbj-10::Tn10 | P22 · YS871 × YS1456 → Tet20r (EGTAs) |
| YS1456 | 14028 | ΔmsbB2 ΔpurI3252 somA1 | Spontaneous EGTAr derivative of msbB1::ΩTetr ΔpurI3252 derivative of 14028; replacement of msbB1::ΩTetr with ΔmsbB2 by homologous recombination (9, 18) |
| YS1770 | 14028 | somA2::Kanr | Lambda red system used to integrate somA2::Kanr PCR product (5) |
| YS1920 | 14028 | msbB1::ΩTetr Suwwan deletion | YS1; spontaneous selection on EGTA medium |
| YS1925 | 14028 | msbB1::ΩTetr Suwwan deletion | YS1; spontaneous selection on EGTA medium |
| YS2049 | 14028 | somA2::Kanrzbj-10::Tn10 | P22 · YS871 × YS1770 → Kan5r |
| YS2062 | 14028 | Suwwan deletion | Bochner selection (3) of YS2049 |
| YS2200 | 14028 | ΔmsbB2 ΔpurI3252 somA+zbj-10::Tn10 srl-203::Tn10d-CamrrecA1 | P22 · TT14282 × YS1317 → Cam20r |
| YS2201 | 14028 | ΔmsbB2 Δpur13252 somA+zbj-10::Tn10 recA1 zfi-1623::Tn10d-Camr | P22 · KR1657 × YS1317 → Cam20r |
| YS2202 | 14028 | somA2::Kanrzbj-10::Tn10 recA1 srl::Tn10d-Camr | P22 · TT14282 × YS2049 → Cam20r |
| YS8211 | 14028 | msbB1::ΩTetr | Low et al. (14) |
| TT16812 | LT2 | recD541::Tn10dCmr | Salmonella Genetic Stock Center, Calgary, Alberta, Canada; used as a representative Salmonella LT2 strain |
| YS2 | LT2 | msbB1::ΩTetrrecD541::Tn10dCmr | P22 · YS8211 × TT16812 → Tet5r (18) |
| YS2050 | LT2 | ΔmsbB2 zbj-10::Tn10 recD541::Tn10dCmr | P22 · YS871 × YS2 → Tet20r |
| YS2057 | LT2 | recD541::Tn10dCmrsomA2::Kanr | P22 · YS1770 × TT16182 → Kan5r |
| YS2087 | LT2 | recD541::Tn10dCmrsomA2::Kanrzbj-10::Tn10 | P22 · YS871 × YS2057 → Tet20r |
| YS2107 | LT2 | recD thy somA2::Kanrzbj-10::Tn10 | Trimethoprim selection (8) of YS2087 |
| YS2111 | LT2 | recD somA2::Kanrzbj-10::Tn10 | P22 · 14028 × YS2107 → Thy+ |
| SL1344 | SL1344 | his Strr; mouse virulent | Sunshine et al. (24) |
| YS3 | SL1344 | msbB1::ΩTetr | P22 · YS8211 × SL1344 → Tet5r (18) |
| YS2051 | SL1344 | msbB1::ΩTetrzbj-10::Tn10 | P22 · YS871 × YS3 → Tet20r |
| YS2058 | SL1344 | somA2::Kanr | P22 · YS1770 × SL1344 → Kan5r |
| YS2096 | SL1344 | somA2::Kanrzbj-10::Tn10 | P22 · YS1770 × YS2058 → Kan5r |
Transduction.
Salmonella P22 transductions were performed by the method of Davis et al. (6), except that LB-0 medium plates supplemented with the appropriate antibiotic were used. EGTA was not added to the antibiotic-containing plates for transductions.
Growth analysis.
Phenotypes of strains were confirmed by replica plating. Replica plating was performed by the double-velvet technique (12). Plates were incubated for 10 h at 37°C. To generate growth curves, MSB broth was inoculated from patches with verified phenotypes, which were grown on slants overnight at 37°C. Tubes (2.5-cm diameter) with 10 ml of broth were then inoculated with cells to achieve an optical density at 600 nm (OD600) of 0.05. Cells were held on ice until all inoculations were completed. The cultures were then placed in a 37°C or room temperature (21 to 23°C) water bath on a 30° angle with translational movement at 100 rpm. OD600 was measured every 30 min for 420 min. BioMérieux API 20E test kits were used in accordance with the manufacturer's suggested protocol to assay various biochemical phenotypes.
Mutation frequency determination.
Frozen stocks of strains were streaked on MSB medium and incubated overnight at 37°C to isolate individual clones. Tubes containing 10 ml of MSB broth (2.5-cm diameter) were inoculated with independent colonies. They were grown in a water bath as described above to an OD600 of 0.10. The tubes were placed on ice, and the cultures were diluted in ice-cold MSB broth. A 2 × 10−6-ml volume was plated onto MSB agar to calculate the number of CFU per milliliter. Volumes of 10−2 and 10−3 ml were plated on 6 mM EGTA and MacConkey galactose plates and incubated overnight at 37°C. Clones were purified and checked for both suppressor phenotypes and Tetr. The mutation frequency was calculated as the ratio of the number of CFU on EGTA (or MacConkey) agar to the number of CFU on MSB agar at equivalent dilutions. Most clones from colonies on EGTA (88%) or MacConkey galactose (100%) plates were found to have stable suppressed phenotypes.
Bochner selection for tetracycline-sensitive derivatives.
The Tetr determinant in Tn10 has been shown to confer sensitivity to fusaric acid. Bochner et al. (3) have reported that fusaric acid can be used to select for loss of the Tn10 marker. Frozen stocks of YS2049 (strain 14028 derivative), YS2111 (LT2 derivative), and YS2096 (SL1344 derivative) (which all contain Tn10s at 19.7 centisomes (Cs), which lies within the hot spot for deletion) were streaked onto LB medium and incubated overnight at 37°C to isolate individual clones. LB broth tubes (10 ml, 2.5-cm diameter) were inoculated with independent colonies. They were grown in a water bath as described above to an OD600 of 0.10. The tubes were then placed on ice, and the cultures were diluted in ice-cold LB broth. Dilutions of 2 × 10−6 ml were plated onto LB agar to calculate the number of CFU per milliliter. Volumes of 10−2 and 10−3 ml were plated onto LB plates containing 9.6 μg of fusaric acid per ml (3) and incubated overnight at 37°C. Clones were purified and checked for sensitivity to tetracycline and for fusaric acid resistance.
PCR.
Three colonies were resuspended in 100 μl of water, and 1 μl of this suspension was added to each PCR to serve as the template. The sequences of the primers used are available upon request. Primers were made by the Yale University Keck Facility. PCRs were performed with HotStarTaq (QIAGEN, Inc.). Thermocycler reactions ran at 95°C for 15 min, followed by 35 cycles of 95°C for 1 min, 60°C for 1 min, and 72°C for 2 min, followed by a 10-min extension at 72°C and a 4°C hold.
DNA cloning and sequencing.
DNA sequencing was performed at the Yale University Keck Facility by fluorescent-dye-terminated thermocycler sequencing. To sequence PCR products, the DNA was cloned into the TOPO TA cloning vector pCR2.1-TOPO (Invitrogen, Inc.) and sequenced with M13 forward and M13 reverse primers.
RESULTS
Detection and mapping of a deletion in msbB suppressor strain YS1170.
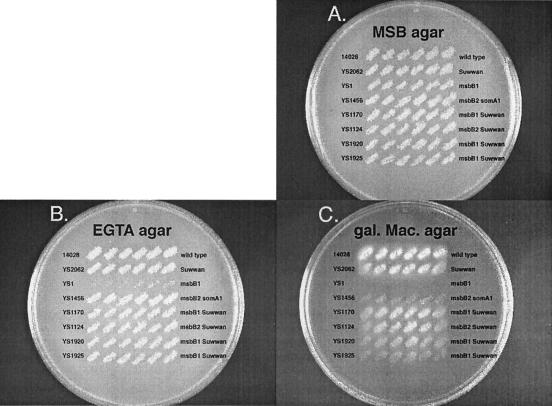
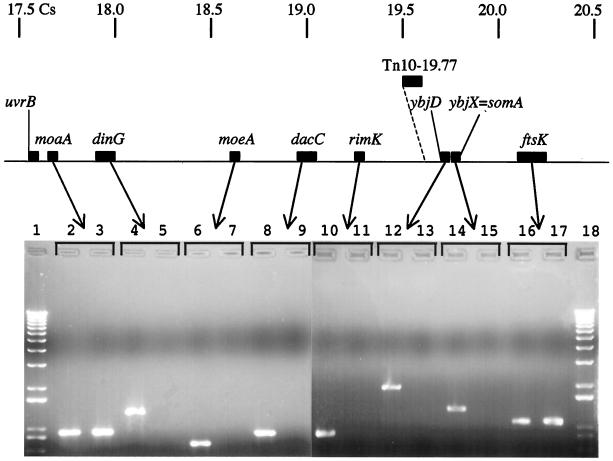
Figure 1 depicts the phenotypes of wild-type strain 14028, unsuppressed msbB derivative YS1, and several suppressor strains. YS1456 (Fig. 1) has a suppressor phenotype caused by an IS10 insertion in somA (somA1) that is identifiable by PCR (16). Suppressor strains YS1170, YS1124, YS1920, and YS1925 (Fig. 1) are more resistant to MacConkey galactose medium than is YS1456. During the course of studying somA, it was amplified by PCR from various suppressor strains to see if any of the other suppressor strains contained a mutated allele of somA that could be detected by PCR. As shown in Fig. 2, no somA PCR product was produced from suppressor strain YS1170 (lane 15) whereas a clear somA band was produced from the wild type (lane 14). The absence of a somA PCR product in YS1170 suggested a possible chromosomal rearrangement in this strain, and so we designed primers to amplify other genes lying near the somA (19.8 Cs) region of the chromosome. As shown in Fig. 2, the primers used to amplify dinG (17.9 Cs), moeA (18.6 Cs), dacC (19.0 Cs), rimK (19.2 Cs), ybjD (19.7 Cs), and somA (= ybjX) (19.8 Cs) produce products from the wild-type, but not YS1170, cells. Likewise, the primers used to amplify ybhM (17.7 Cs), ybhR (17.8 Cs), ompX (18.3 Cs), and clpA (19.9 Cs) produced products in the wild type but not in strain YS1170 (data not shown). In contrast, the amplification of moaA (17.6 Cs), moaCD (17.6 Cs), STM0951 (19.9 Cs), cydC (20.0 Cs), and ftsK (20.1 Cs) produces PCR products from both strains (moaA and ftsK are shown in Fig. 2; others are not shown). The location of the PCR products demonstrates that at least part of the region between moaA and dinG (left side) and that between somA and ftsK (right side) were deleted in YS1170. The same pattern of marker deletion was found in several other suppressor strains having a phenotype similar to that of YS1170, namely, YS1124, YS1920, and YS1925.
FIG. 1.
Replica plate series. Replica plates were incubated for 10 h at 37°C. gal. Mac., MacConkey galactose.
FIG. 2.
Agarose gel electrophoresis of PCR products from wild-type strain ATCC 14028 and suppressor strain YS1170. The centisome coordinates correspond to the equivalent genes in E. coli K-12, whose centisome positions are available on the E. coli website [http://cgsc.biology.yale.edu/cgibin/sybgw/cgsc/Map?!Name = CGSC(Mary%20Berlyn)]. Lanes: 1, Kb Plus Ladder (Gibco-BRL); 2, moaA (17.6 Cs) of strain 14028; 3, moaA of strain YS1170; 4, dinG (17.9 Cs) of strain 14028; 5, dinG of strain YS1170; 6, moeA (18.6 Cs) of strain 14028; 7, moeA of strain YS1170; 8, dacC (19.0 Cs) of strain 14028; 9, dacC of strain YS1170; 10, rimK (19.2 Cs) of strain 14028; 11, rimK of strain YS1170; 12, ybjD (19.7 Cs) of strain 14028; 13, ybjD of strain YS1170; 14, somA (19.8 Cs) of strain 14028; 15, somA of strain YS1170; 16, ftsK (20.1 Cs) of strain 14028; 17, ftsK of strain YS1170; 18, Kb Plus Ladder (Gibco-BRL).
Cloning and sequencing of the deletion endpoints.
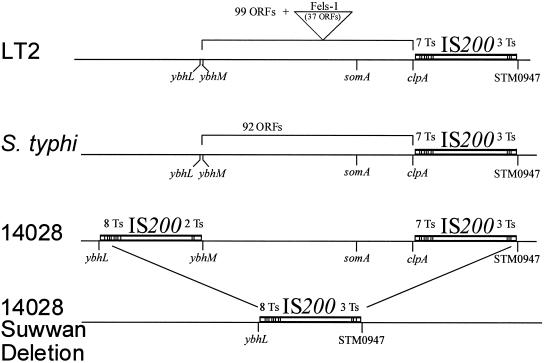
On the basis of the putative sites of the deletion endpoints, primers were designed to amplify the new junction formed in YS1170. These primers were used to amplify this region, forming an ∼3.6-kb fragment that was cloned into the TOPO TA cloning vector pCR2.1-TOPO (Invitrogen, Inc.) and sequenced. Sequence analysis revealed that the new joint in the deleted genomic structure is ybhL (17.7 Cs)-IS200-STM0947 (19.9 Cs). On the basis of the published LT2 sequence (GenBank accession numbers AE008733 to AE008740) and the results shown in Fig. 2, we would predict that 136 ORFs (16) would be deleted between 17.7 and 19.9 Cs on the Salmonella chromosome in these suppressor strains if strains LT2 and 14028 were homologous throughout (see below). The structure of the deletion joint suggests that IS200 played a role in the deletion recombination event.
To analyze the genomic structure at the two regions of the chromosome involved in the deletion, for comparison with the genomic structure of LT2 (16), we PCR amplified the two recombinogenic regions from wild-type Salmonella ATCC 14028. The 19.9-Cs sequence was nearly identical to that in the LT2 sequence database; IS200 lies between clpA and STM0947. However, LT2 and 14028 were found to differ in the 17.7-Cs sequence. Strain 14028 contains an IS200 element between ybhL and ybhM; LT2 and SL1344, do not. We cloned and sequenced both regions from LT2 and SL1344. As expected from the LT2 database, both have the 19.9-Cs region IS200 element but lack the 17.7-Cs region IS200 element. The lack of the 17.7-Cs IS200 element, as shown in Fig. 3, suggests that strains LT2 and SL1344 would not undergo the same IS200-specific deletion (which we designate the Suwwan deletion) observed in YS1170 and other strain 14028 derivatives.
FIG. 3.
The Suwwan deletion. In LT2, somA and the Fels-1 prophage both lie within the 17.7- to 19.9-Cs region of LT2, which contains 136 ORFs (99 plus 37 in Fels-1). The corresponding region in S. enterica serovar Typhi contains 92 ORFs and lacks Fels-1. The corresponding region in strain 14028 has an IS200 element similar to that in LT2 and S. enterica serovar Typhi that contains seven T's on the left and three T's on the right and a second IS200 element inserted between ybhL and ybhM containing eight T's on the left and two T's on the right. Fels-1 is lacking in the corresponding region of ATCC 14028. In strain 14028 with the Suwwan deletion, the corresponding region is deleted and the recombinant IS200 element contains eight T's on the left and two T's on the right. The figure is not drawn to scale.
IS200 elements typically target AT-rich regions (2). As shown in Fig. 3, sequence analysis of our strain 14028 clones showed that the 17.7-Cs IS200 element is flanked by eight T's on the left and two T's on the right. The 19.9-Cs IS200 element is flanked by seven T's on the left and three T's on the right. The T's serve as molecular markers, and the outermost T's are conserved in the Suwwan deletion recombinant: it has eight T's on the left and three T's on the right, which flank the single recombined IS200 element.
A prophage known as Fels-1 (16) (Fig. 3) is known to lie in the Suwwan deletion interval of the map of sequenced strain LT2. However, we found that this prophage was not present at that site in strain 14028 or SL1344. This was tested by PCR amplification of the Fels-1 endpoint junction regions and also the putative joint that would result from deletion of Fels-1, from all of the strains tested. The resulting presence or absence of PCR products was totally consistent with Fels-1 lying within LT2 but absent at that site from strains 14028 and SL1344 (data not shown). Fels-1 is also absent from this region in S. enterica serovar Typhi strains CT18 (7, 21) and TY2 (7).
The ATCC 14028 17.7- to 19.9-Cs genomic structure is not conserved in other Salmonella strains.
Since ATCC 14028, but not LT2 or SL1344, has a genomic structure that allows the Suwwan deletion to occur, other Salmonella strains were obtained to test if they have the 17.7- and 19.9-Cs IS200 elements found in ATCC 14028. We tested isolates of S. enterica serovars Dublin, Montevideo, Derby, Enteritidis, Typhi, Gallinarum, Paratyphi (A, B, and C), St. Paul, and Infantis, and none, other than ATCC 14028, has a 17.7-Cs PCR product suggestive of an IS200 insertion at this location. In addition, the 19.9-Cs PCRs produce products of various sizes, and none of these seem to have the same sequence (on the basis of PCR product size) as strain 14028, suggesting that these strains may lack the IS200 element (and/or other DNA) that is present in ATCC 14028. The variation in PCR product size suggests that the 19.9-Cs region may be prone to genetic alteration.
Chromosome structure of ATCC 14028, but not LT2 or SL1344, carries a hot spot for deletion.
Since several of our spontaneously selected suppressors contained the Suwwan deletion, we investigated the frequency and strain dependence of this genomic rearrangement by first moving a Tn10 transposon lying at 19.77 Cs into YS1456 (14028 msbB), YS2 (LT2 msbB), and YS3 (SL1344 msbB) (resulting in strains YS1317, YS2050, and YS2051, respectively) and then determining the frequencies of suppressor formation. Our results, as shown in Table 2, indicate that while the mutation frequencies of suppressors are similar among the three strains, only YS1317 can undergo the Suwwan deletion identified by loss of the Tn10 (Tetr) marker (see Materials and Methods). The presence of the Suwwan deletion in tetracycline-sensitive suppressor colonies was confirmed by colony PCR. In addition to having the same genomic structure as YS1170 (and other Suwwan deletion strains), they also had identical phenotypes when replica plated onto EGTA and MacConkey galactose media (data not shown). As shown in Table 2, ∼37% of the suppressor mutants (44% of the EGTA suppressors and 29% of the MacConkey galactose suppressors) in a YS1317 culture carry the Suwwan deletion. The msbB LT2 derivative YS2050 had a significantly lower suppressor mutation frequency for MacConkey galactose resistance because the recD mutation makes the strain more sensitive to MacConkey medium (data not shown). YS2201 had a jackpot mutation for an EGTA suppressor in one of the clones.
TABLE 2.
Frequencies of suppressor formation
| Strain | Avg no. of CFU/ml on MSB agar | Suppressor mutation frequency | Suwwan deletion mutation frequency | % of suppressed strains with Suwwan deletion |
|---|---|---|---|---|
| YS1317 (msbB 14028) EGTA selection | 7.9 × 106 | 3.1 × 10−4 | 1.0 × 10−4 | 44 |
| YS1317 (msbB 14028) Mac-Conkey galactose selection | 7.9 × 106 | 5.4 × 10−4 | 9.2 × 10−5 | 29 |
| YS2050 (msbB recD LT2) EGTA selection | 2.3 × 107 | 1.9 × 10−4 | 0 | 0 |
| YS2050 (msbB recD LT2) Mac-Conkey galactose selection | 2.3 × 107 | 4.3 × 10−6 | 0 | 0 |
| YS2051 (msbB SL1344) EGTA selection | 3.6 × 107 | 1.1 × 10−4 | 0 | 0 |
| YS2051 (msbB SL1344) Mac-Conkey galactose selection | 3.6 × 107 | 6.9 × 10−5 | 0 | 0 |
| YS2200 (recA1 msbB 14028) EGTA selection | 8.3 × 106 | 2.5 × 10−4 | 0 | 0 |
| YS2200 (recA1 msbB 14028) Mac-Conkey galactose selection | 8.3 × 106 | 1.3 × 10−4 | 0 | 0 |
| YS2201 (recA1 msbB 14028) EGTA selection | 1.3 × 107 | 9.8 × 10−3 | 0 | 0 |
| YS2201 (recA1 msbB 14028) Mac-Conkey galactose selection | 1.3 × 107 | 6.1 × 10−4 | 0 | 0 |
Thus, the 17.7- to 19.9-Cs region of the chromosome is a hot spot for recombination in the ATCC 14028, but not in the LT2 or the SL1344, genetic background. Furthermore, we found that the frequency of the Suwwan deletion among suppressed msbB clones is relatively constant during growth between OD600s of 0.1 and 1.1 in MSB broth (data not shown).
The Suwwan deletion occurs in wild-type ATCC 14028.
To determine whether the Suwwan deletion is an msbB-related phenomenon, we moved the 19.8-Cs Tn10 transposon and somA2::Kanr into ATCC 14028, LT2, and SL1344, creating strains YS2049 (ATCC 14028 derivative), YS2111 (LT2 derivative), and YS2096 (SL1344 derivative). By the technique of Bochner et al. (3), we used fusaric acid to select spontaneous mutants that had lost the Tetr function carried by Tn10 (see Materials and Methods). As shown in Table 3, the mutation frequencies for obtaining fusaric acid-resistant (i.e., tetracycline-sensitive) colonies are similar for YS2049, YS2111, and YS2096. However, loss of the tetracycline marker in YS2111 and YS2096 never concurred with loss of the somA2::Kanr marker, which lies ∼3.0 kb away from the 19.8-Cs Tn10 transposon (18). In contrast, all of the 18 tetracycline-sensitive, fusaric acid-resistant YS2049 spontaneous mutants lost the somA2::Kanr marker as a result of the Suwwan deletion (confirmed by PCR; data not shown). The measured frequency of the Suwwan deletion under fusaric acid selection was ∼3.7 × 10−5.
TABLE 3.
Mutation frequencies for obtaining fusaric acid-resistant colonies
| Strain | Avg no. of CFU/ml on LB agar | Avg no. of fusaric acidr (i.e., Tets) colonies/ 10−3 ml | Avg no. of fusaric acidr Tets Kans colonies/ 10−3 ml | Frequency of fusaric acidr (i.e., Tets) clones | % of Tets colonies with Suwwan deletion |
|---|---|---|---|---|---|
| YS2049 (somA2::Kanrzbj-10::Tn10 14028) | 1.7 × 108 | 6.3 | 6.3 | 3.7 × 10−5 | 100 |
| YS2111 (somA2::Kanrzbj-10::Tn10 LT2) | 1.1 × 108 | 2.3 | 0 | 2.1 × 10−5 | 0 |
| YS2096 (somA2::Kanrzbj-10::Tn10 SL1344) | 1.2 × 108 | 1.6 | 0 | 1.3 × 10−5 | 0 |
| YS2202 (recA1 somA2::Kanrzbj-10::Tn10 14028) | 2.7 × 107 | 0 (2.8/10−2 ml) | 0 | 1.2 × 10−5 | 0 |
RecA function is necessary for this deletion hot spot.
Recombination between large direct repeats is strongly dependent on RecA function (1). Since our sequence analysis of wild-type and Suwwan deletion strains suggested that the deletion is a result of a recombination event between two IS200 elements, we hypothesized that a loss-of-function mutation in recA would prevent the deletion from occurring at high frequency. As shown in Table 2, suppressors containing the Suwwan deletion were not detected in the recA genetic backgrounds (YS2200 and YS2201). Thus, they either did not occur or were nonviable.
To further investigate this question, we constructed an ATCC 14028 19.8-Cs Tn10 somA2::Kanr recA1 strain (designated YS2202), performed a Bochner selection, and selected fusaric acid-resistant colonies. As shown in Table 3, none of the fusaric acid-resistant (i.e., tetracycline-sensitive) clones derived from the Bochner selection contain the Suwwan deletion, although the frequency of fusaric acid-resistant clones remained constant between recA+ and recA1 mutant strains.
To test the possibility that a recA mutation and the Suwwan deletion are incompatible, the recA1 allele from strains TT14282 and KR1657 (used to make strains YS2200 to YS2202) was transduced, with the nearby Camr marker, into wild-type strain 14028 and also into a Suwwan deletion derivative of strain 14028. The frequencies of cotransduction and stable incorporation of the recA1 allele, scored as mitomycin sensitive, were similar in all cases (in the 40 to 70% range) (data not shown). Thus, the observed absence of Suwwan deletions in a RecA− background cannot be explained by incompatibility of recA and Suwwan deletion alleles, and in light of the above results this indicates that the Suwwan deletion events require RecA function.
Phenotypic analysis of strains containing the Suwwan deletion.
To investigate Suwwan deletion strains for changes in a representative spectrum of biochemical phenotypes, API 20E metabolic test strips from BioMérieux, as well as media indicative of outer membrane barrier function, were used for phenotypic analysis of wild-type (msbB+) strains carrying the Suwwan deletion. The results revealed that Suwwan deletion strains are H2S−, citrate utilization−, nitrate reductase−, formate dehydrogenase− (i.e., they do not produce gas [CO2 and H2] from glucose), and chlorate resistant (data not shown). The H2S−, nitrate reductase−, formate dehydrogenase−, and chlorate reductase− phenotypes are likely due to the loss of the moeAB genes, which are necessary for the production of molybdopterin from molybdenum. Molybdopterin is a cofactor that is necessary for the function of the enzymes nitrate reductase, formate dehydrogenase, thiosulfate reductase, and chlorate reductase (10). However, we could not find any report of genes within the deletion that are known to affect citrate utilization. In rich media, growth curve analysis showed no obvious growth defects in Suwwan deletion strains (data not shown), and they maintain the ability to grow on minimal medium (data not shown).
DISCUSSION
The msbB suppressor strain YS1170 (as well as YS1124, YS1920, and YS1925) has a deletion of approximately 100 ORFs that suppresses msbB growth defects. somA, a suppressor gene that we previously reported to confer an EGTA resistance phenotype on msbB salmonellae (18), lies within this deletion. As shown in Fig. 1, MsbB− Suwwan deletion strains (e.g., YS1170) in which somA and the other genes in the region are deleted were resistant to both EGTA and MacConkey galactose media, which is a different phenotype from that of a simple msbB somA mutant strain. Therefore, we propose that there are at least two msbB suppressor genes within the deletion. The loss of somA function alone results in an EGTA-resistant but MacConkey galactose-sensitive phenotype. We designated the gene (or set of genes) responsible for the MacConkey galactose suppressor phenotype somB (suppressor of msbB B). We have ruled out a loss-of-function mutation in ompX, which is an outer membrane protein lying within the deletion, as a candidate for somB by constructing an msbB somA ompX mutant strain that has an EGTA and MacConkey galactose phenotype similar to that of msbB somA but not msbB Suwwan deletion strains (S. R. Murray, unpublished results). Furthermore, thin-layer chromatography of radiolabeled lipid A from msbB somA and msbB somA somB Suwwan deletion strains shows thin-layer chromatography spots identical to those of unsuppressed msbB, suggesting that these suppressors do not significantly change lipid A structure (S. R. Murray and K. B. Low, unpublished results). Silver-stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels of lipopolysaccharide (LPS) from msbB somA and msbB Suwwan deletion and unsuppressed msbB mutant strains show similar banding patterns, suggesting that there are no gross changes in the overall LPS structure, other than the changes conferred by the msbB mutation (Murray and Low, unpublished). Thus, suppression of msbB growth defects (at least in the cases of somA and somA somB) appears to involve non-LPS-associated changes in the outer membrane.
The Suwwan deletion occurs at a high frequency, which was measured as ∼10−4 in msbB mutant salmonellae. This represents a hot spot in terms of msbB suppression, since about one-third of the suppressors in the ATCC 14028 background contain this mutation. Furthermore, the deletion is not an msbB-specific phenomenon since we found that the mutation frequency was ∼3.7 × 10−5 in an msbB+ genetic background. In addition, under selection for chlorate resistance (23), we found that the Suwwan deletion frequency was ∼4.7 × 10−5 in an msbB genetic background (data not shown).
Sequence analysis and the dependence of this genomic rearrangement on RecA function indicate that the rearrangement is the result of homologous recombination between two IS200 elements. Several examples of recombination between direct repeats have been reported. In addition to insertion sequence elements, Escherichia coli and Salmonella have seven rrn operons that provide large direct repeats (1). The two closest, the rrnB and rrnE operons, lie 39.5 kb apart. Spontaneous recombination between these two loci occurs at a frequency of 1 × 10−4 to 2 × 10−4 (11), which is similar to the Suwwan deletion frequency. Chumley and Roth (4) placed two Tn10 elements around the histidine operon and measured the frequency of loss of this 9.3-kb fragment. Deletion of this region occurred at a frequency of 0.5%. In a recA1 background, the frequency was reduced 100-fold.
Although the Suwwan deletion did not confer any generalized growth defects in rich media, it conferred an inability to produce H2S, produce gas from glucose, reduce nitrogen, or utilize citrate as a carbon source and produced chlorate resistance. Four of these phenotypes, namely, H2S production, chlorate reduction (reduction of chlorate produces a toxic product), gas production from glucose, and nitrate reductase activity, require enzymes that need molybdopterin as a cofactor (10). Two of the molybdopterin biosynthetic genes, moeAB, lie within the Suwwan deletion. To our knowledge, no genes within the deletion have been linked to citrate utilization.
We have investigated the 17.7- and 19.9-Cs regions in various Salmonella strains to see if the ATCC 14028 genomic structure is conserved among various Salmonella species. Our results suggest that none of the strains we tested, other than ATCC 14028, contains the 17.7-Cs IS200 element. All of the strains tested have a 17.7-Cs genomic structure similar to that observed in LT2 and SL1344. However, a great amount of genetic diversity was seen in the 19.9-Cs region. ATCC 14028, LT2, and SL1344 all contain the 19.9-Cs IS200 element, but none of the other strains tested have similar-sized PCR products for this locus, suggesting that this region of the chromosome may be subject to high levels of genetic alteration. It is interesting that none of the strains produce a PCR product with a size suggestive of lack of only the IS200 element.
We have shown that deletion of this area of the chromosome is advantageous under at least two conditions: (i) exposure to chlorate in the wild-type background and (ii) exposure to certain growth conditions in the msbB genetic background. Since the 17.7-Cs and 19.9-Cs IS200 elements have evolved with the ATCC 14028 strain, it would be of interest to know if this genomic structure could confer an evolutionary advantage on the wild-type bacteria under other conditions.
We have found that the published strain VNP20009 (=YS1646) (15) carries a Suwwan deletion, which explains its MsbB-suppressed phenotype.
Acknowledgments
We are grateful to the editor and the reviewers for pointing out uncertainties in the possible chromosome structures, including Fels-1, that we had overlooked and for other improvements in the manuscript. We thank Hiroshi Nikaido for critical reading of the manuscript. We thank R. Lannigan for stocks of S. enterica serovars Infantis and St. Paul. We thank Igor Brodsky for unpublished information on Fels-1 genes.
This research was supported by a grant from Vion Pharmaceuticals, Inc., and by SBIR grant 1 R43 CA97595-01 from the National Institutes of Health. S.R.M. was supported by a National Institutes of Health predoctoral training grant in genetics (5 TM32 GM07499) and a Yale University fellowship. P.L.O. was supported by a National Institutes of Health predoctoral training grant in cellular and molecular biology (T32 GM07223).
REFERENCES
- 1.Bachellier, S., E. Gilson, M. Hofnung, and C. W. Hill. 1996. Repeated sequences, p. 2012-2040. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 2. ASM Press, Washington, D.C.
- 2.Beuzen, C. R., and J. Casadesus. 1997. Conserved structure of IS200 elements in Salmonella. Nucleic Acids Res. 25:1355-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bochner, B. R., H.-C. Huang, G. L. Schieven, and B. N. Ames. 1980. Positive selection for loss of tetracycline resistance. J. Bacteriol. 143:926-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chumley, F. G., and J. R. Roth. 1979. Rearrangement of the bacterial chromosome using Tn10 as a region of homology. Genetics 94:1-14. [Google Scholar]
- 5.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis, R. W., D. Botstein, and J. R. Roth. 1980. Advanced bacterial genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 7.Deng, W., S. R. Liou, G. Plunkett III, G. F. Mayhew, D. J. Rose, V. Burland, V. Kodoyianni, D. C. Schwartz, and F. R. Blattner. 2003. Comparative genomics of Salmonella enterica serovar Typhi strains Ty2 and CT18. J. Bacteriol. 185:2330-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devriese, L. A., and J. Hommez. 1974. Thymine-requiring Escherichia coli mutants isolated from animals. An unusual type of resistance to trimethoprim. Zentbl. Veterinaermed. B 21:211-215. [DOI] [PubMed] [Google Scholar]
- 9.Donnenberg, M. S., and J. B. Kaper. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 59:4310-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gennis, R. B., and V. Stewart. 1996. Respiration, p. 217-261. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C.
- 11.Hill, C. W., and B. W. Harnish. 1982. Transposition of a chromosomal segment bounded by redundant rRNA genes into other rRNA genes in Escherichia coli. J. Bacteriol. 149:449-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolodkin, A. L., M. A. Capage, E. I. Golub, and K. B. Low. 1983. F sex factor of Escherichia coli K-12 codes for a single-stranded DNA binding protein. Proc. Natl. Acad. Sci. USA 80:4422-4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lam, S., and J. R. Roth. 1983. IS200: a Salmonella-specific insertion sequence. Cell 34:951-960. [DOI] [PubMed] [Google Scholar]
- 14.Low, K. B., M. Ittensohn, T. Le, J. Platt, S. Sodi, M. Amoss, O. Ash, E. Carmichael, A. Chakraborty, J. Fischer, S. Lin, X. Luo, S. I. Miller, L. Zheng, I. King, J. Pawelek, and D. Bermudes. 1999. Lipid A mutant Salmonella with suppressed virulence and TNF-α induction retain tumor-targeting in vivo. Nat. Biotechnol. 17:37-41. [DOI] [PubMed] [Google Scholar]
- 15.Low, K. B., M. Ittensohn, X. Luo, L.-M. Zheng, I. King, J. M. Pawelek, and D. Bermudes. 2003. Construction of VNP20009, a novel, genetically stable antibiotic-sensitive strain of tumor-targeting Salmonella for parenteral administration in humans. Methods Mol. Med. 90:47-59. [PubMed] [Google Scholar]
- 16.McClelland, M., K. E. Sanderson, J. Spieth, S. W. Clifton, P. Latreille, L. Courtney, S. Porwollik, J. Ali, M. Dante, F. Du, S. Hou, D. Layman, S. Leonard, C. Nguyen, K. Scott, A. Holmes, N. Grewal, E. Mulvaney, E. Ryan, H. Sun, L. Florea, W. Miller, T. Stoneking, M. Nhan, R. Waterson, and R. K. Wilson. 2001. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature 413:852-856. [DOI] [PubMed] [Google Scholar]
- 17.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 18.Murray, S. R., D. Bermudes D, K. Suwwan de Felipe, and K. B. Low. 2001. Extragenic suppressors of growth defects in msbB Salmonella. J. Bacteriol. 183:5554-5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nikaido, H. 1996. Outer membrane, p. 29-47. In F. C. Neidhardt, J. L. Ingraham, K. B. Low, B. Magasanik, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed, vol. 1. ASM Press, Washington, D.C.
- 20.Nikaido, H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67:593-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parkhill, J., G. Dougan, K. D. James, N. R. Thomson, D. Pickard, J. Wain, C. Churcher, K. L. Mungall, S. D. Bentley, M. T. Holden, M. Sebaihia, S. Baker, D. Basham, K. Brooks, T. Chillingworth, P. Connerton, A. Cronin, P. Davis, R. M. Davies, L. Dowd, N. White, J. Farrar, T. Feltwell, N. Hamlin, A. Haque, T. T. Hien, S. Holroyd, K. Jagels, A. Krogh, T. S. Larsen, S. Leather, S. Moule, P. O'Gaora, C. Parry, M. Quail, K. Rutherford, M. Simmonds, J. Skelton, K. Stevens, S. Whitehead, and B. G. Barrell. 2001. Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature 413:848-852. [DOI] [PubMed] [Google Scholar]
- 22.Stanley, J., N. Baquar, and E. J. Threlfall. 1993. Genotypes and phylogenetic relationships of Salmonella typhimurium are defined by molecular fingerprinting of IS200 and 16S rrn loci. J. Gen. Microbiol. 139:1133-1140. [DOI] [PubMed] [Google Scholar]
- 23.Stouthamer, A. H. 1969. A genetical and biochemical study of chlorate-resistant mutants of Salmonella typhimurium. Antonie van Leeuwenhoek 35:505-521. [DOI] [PubMed] [Google Scholar]
- 24.Sunshine, M., B. Gibson, J. Engstrom, W. Nichols, B. Jones, and M. Apicella. 1997. Mutation of the htrB gene in a virulent Salmonella typhimurium strain by intergenic transduction: strain construction and phenotype characterization. J. Bacteriol. 179:5521-5533. [DOI] [PMC free article] [PubMed] [Google Scholar]