Abstract
AIM
To evaluate the incidence of contrast-induced acute kidney injury (CIAKI) in kidney transplant recipients.
METHODS
A literature search was performed using MEDLINE, EMBASE, and the Cochrane Database of Systematic Reviews from the inception of the databases through July 2016. Studies assessing the incidence of CIAKI in kidney transplant recipients were included. We applied a random-effects model to estimate the incidence of CIAKI.
RESULTS
Six studies of 431 kidney transplant recipients were included in the analyses to assess the incidence of CIAKI in kidney transplant recipients. The estimated incidence of CIAKI and CIAKI-requiring dialysis were 9.6% (95%CI: 4.5%-16.3%) and 0.4% (95%CI: 0.0%-1.2%), respectively. A sensitivity analysis limited only to the studies that used low-osmolar or iso-osmolar contrast showed the estimated incidence of CIAKI was 8.0% (95%CI: 3.5%-14.2%). The estimated incidences of CIAKI in recipients who received contrast media with cardiac catheterization, other types of angiogram, and CT scan were 16.1% (95%CI: 6.6%-28.4%), 10.1% (95%CI: 4.2%-18.0%), and 6.1% (95%CI: 1.8%-12.4%), respectively. No graft losses were reported within 30 d post-contrast media administration. However, data on the effects of CIAKI on long-term graft function were limited.
CONCLUSION
The estimated incidence of CIAKI in kidney transplant recipients is 9.6%. The risk stratification should be considered based on allograft function, indication, and type of procedure.
Keywords: Acute kidney injury, Kidney transplantation, Contrast-induced nephropathy, Contrast-induced acute kidney injury, Transplantation
Core tip: We conducted this meta-analysis to assess the incidence of contrast-induced acute kidney injury (CIAKI) in kidney transplant recipients. The estimated incidence of CIAKI is 9.6%. The estimated incidence of CIAKI in recipients who received contrast media is highest at 16% with cardiac catheterization, followed by 10% with other types of angiogram, and 6% with computed tomography scan. The findings from this study may impact the risk stratification for administration of contrast media and CIAKI prevention in kidney transplant recipients.
INTRODUCTION
Contrast-induced acute kidney injury (CIAKI), or contrast-induced nephropathy (CIN), is associated with a significant increase in mortality and morbidity in patients with native kidneys[1-7]. The incidence of CIAKI has been reported from 2% in the general population without risk factors, to more than 20% in high-risk patients[1,8-12]. The overall frequency of CIAKI is approximately 150000 patients each year worldwide[13]. The number of diagnostic studies and procedures with iodinated contrast media including computed tomography (CT) imaging, coronary angiography, and other types of angiograms have increased for the past decade[14].
Renal transplant recipients are at an at increased risk for developing post-contrast AKI[15] since they have a lower average estimated glomerular filtration rate (GFR) and higher prevalence of diabetes and cardiovascular disease when compared to the general populations[16]. Furthermore, the majority of kidney transplant recipients are receiving calcineurin inhibitors, which are known to cause renal afferent vasoconstriction[17-20]. For these reasons, the American College of Radiology (ACR) Committee on Drugs and Contrast Media 2015 manual consider renal transplant recipients as a potentially higher risk population for CIAKI[21], and thus clinicians may be reluctant to administer iodinated contrast to renal transplant patients[22]. However, unlike the general population, the incidence and risk factors for CIAKI in kidney transplant recipients are not well studied.
The aim of this meta-analysis was to assess the incidence and risk factors of CIAKI in kidney transplant recipients.
MATERIALS AND METHODS
Cheungpasitporn W and Thongprayoon C individually examined published studies and conference abstracts indexed in MEDLINE, EMBASE, and the Cochrane Database from the inception of the databases through July 2016. The search strategy used is detailed in the supplementary material (Supplementary material 1). Further pertinent studies were retrieved by conducting a manual search using references from the articles that were identified from the search strategy noted above. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for systematic reviews and meta-analyses[23] and previously published guidelines[24,25].
The inclusion criteria were as follows: (1) randomized controlled trials or observational studies; (2) patient population age ≥ 18 years old; and (3) and additional data on kidney transplant recipients were provided. The search was limited to English-language studies. Both published studies and conference abstracts were incorporated. Study eligibility was independently determined by the two investigators mentioned above. Differing decisions were settled by joint agreement.
A standardized information collection form was utilized to derive the following data: The first author of each study, year of publication, study design, country where the study was conducted, number of kidney transplant recipients studied, definition of CIAKI, number of CIAKI patients, and age and gender of CIAKI patients.
Statistical analysis
MetaXL software (EpiGear International Pty Ltd)[26] was utilized for data analysis. The incidence rates (IRs) and 95%CIs of adverse effects were reported using a DerSimonian-Laird random-effects model[27]. A random-effects model was implemented due to the high likelihood of inter-study variances. The Cochran Q test was completed to assess statistical heterogeneity. The I2 statistic was added to evaluate the degree of variation across studies related to heterogeneity instead of chance. An I2 of 0%-25% represents insignificant heterogeneity, 26%-50% low heterogeneity, 51%-75% moderate heterogeneity and > 75% high heterogeneity[28]. Bias funnel plots to assess for publication were used[29].
RESULTS
Our search strategy yielded 1664 articles. Of these, 1495 articles were excluded following the review of title and abstract based on relevance and the eligibility criteria. The remaining 169 articles underwent full-length review, and an additional 161 were excluded for failing to meet the eligibility criteria. Eight articles[19,30-36] that met all inclusion criteria were identified for our study of CIAKI in kidney transplant recipients (Table 1). Our search methodology and selection process were outlined in Figure 1.
Table 1.
| Characteristics | Light et al[30] | Peters et al[31] | Ahuja et al[32] | Agarwal et al[33] |
| Country | United States | United States | United States | United States |
| Year | 1975 | 1983 | 2000 | 2009 |
| Total number | 34 (very early post-transplant (2-24 d) | 93 | 33 | 57 |
| Male sex | NR | NR | NR | 74% |
| Mean age (yr) | NR | NR | 42 ± 2.1 | 58.2 ± 10.1 |
| Baseline creatinine (mg/dL) | NR | NR | 2.3 ± 0.25 | 1.7 ± 0.8 |
| Immunosuppression | Azathioprine, methylprednisolone with/without anti-thymocyte globulin | Azathioprine, prednisone with/without anti-thymocyte globulin | Cyclosporine (94%) | Mycophenolate (52.6%), tacrolimus (33.3%), azathioprine (26.3%), sirolimus (1.8%), cyclosporine (52.6%) |
| Procedure | Drip infusion urogram from 2-24 d post-transplantation | Intravenous pyelogram (87), allograft angiogram (6) during 2 mo post-transplantation | Coronary angiogram (6), CT scan (11), peripheral vascular angiogram (11), allograft angiogram with angioplasty (5), pulmonary angiogram (1), intravenous pyelogram (1) | Cardiac catheterization |
| Contrast used | 30% meglumine diatrizoate | NR | High osmolar contrast (The volume of contrast used was not reported) | Low-osmolar contrast (36), iso-osmolar contrast (21) |
| Hydration | NR | NR | 78.7% of patients received IV hydration | All patients received pre-procedural intravenous hydration with bicarbonate prophylaxis used in 14 patients |
| CIAKI definition | An increase of SCr > 0.4 mg/dL within 4 d after contrast | Oliguria or increase in creatinine within 12 d after contrast | An increase of SCr > 25% from baseline | An increase in SCr of ≥ 25% or 0.5 mg/dL within 3 d post-catheterization |
| CIAKI (%) | 11 (32.4%) | 45 (48.4%) | 7 (21.2%) Coronary angiogram 3/6 (50%) Angiogram 2/17 (11.8%) CT 1/11 (9.1%) IVP 1/1 (100%) | 9 (15.8%) 13.2% in eGFR < 60% and 21.1% in eGFR > 60% |
| Dialysis (%) | 2 (5.9%) | NR | 0 (0%) | 1 (1.8%) (temporary dialysis) |
| Risk factor for CIAKI | CIAKI was more common and more severe in those with impaired kidney function. Kidneys from older donors were at higher risk for CIAKI | CIAKI was common in the early post-transplant period, but no increased risk was found > 120 d post-transplant | IV hydration prior to contrast exposure was protective against CIAKI; 15% of patients who received IV hydration had CIAKI vs 49% in non-IV hydration group | Low osmolar contrast OR 7.75 (1.10-infinity) Use of NAC OR 0.29 (95%CI: 0.04-1.78) |
| Outcomes | NR | NR | NR | One patient received temporary dialysis |
AKI: Acute kidney injury; CIAKI: Contrast-induced acute kidney injury; GFR: Glomerular filtration rate; NAC: N-acetylcysteine; NR: Not reported; SCr: Serum creatinine.
Figure 1.
Search strategy. CIAKI: Contrast-induced acute kidney injury.
CIAKI definition
All included studies[19,30-36] identified CIAKI occurrence by either change in serum creatinine (SCr), GFR, or the need for dialysis after administration of contrast media, as shown in Table 1. All included studies, except by Light et al[30] and Peters et al[31], defined CIAKI as an increase in SCr of > 25% from baseline and/or ≥ 0.5 mg/dL after 48 to 72 h. This definition is also widely used for the diagnosis of CIAKI in general patient population[37].
incidence of CIAKI in kidney transplant recipients
The incidence of AKI and severe AKI requiring dialysis after contrast exposures in kidney transplant recipients within the eight individual studies ranged from 1.8% to 48.4% and 0% to 5.9%, respectively.
Two early studies by Light et al[30] and Peters et al[31] included patients who had contrast exposure in the early post-transplant period (within 1-2 mo) and reported incidences of CIAKI of 32.4% and 48.4%, respectively. Since AKI is common in the early post-transplant period, and it is difficult to differentiate CIAKI from other causes such as calcineurin inhibitor toxicity, dehydration, acute tubular necrosis, acute allograft rejection and surgical related etiologies[32], we omitted the aforementioned two studies and performed a meta-analysis of CIAKI incidence utilizing the remaining six studies[19,32-36] with 431 kidney transplant recipients. These studies were conducted in the era of calcineurin inhibitor-based immunosuppression in kidney transplant patients with stable baseline serum creatinine before contrast administration. The estimated incidence of CIAKI was 9.6% (95%CI: 4.5%-16.3%) with evidence of a high level of heterogeneity (I2 = 75%, P < 0.001; Figure 2). The estimated incidence of CIAKI requiring dialysis was 0.4% (95%CI: 0.0%-1.2%, I2 = 0%). We performed a sensitivity analysis limited only to studies[19,33-36] that used low-osmolar or iso-osmolar contrast; this estimated incidence of CIAKI was 8.0% (95%CI: 3.5%-14.2%, I2 = 72%).
Figure 2.
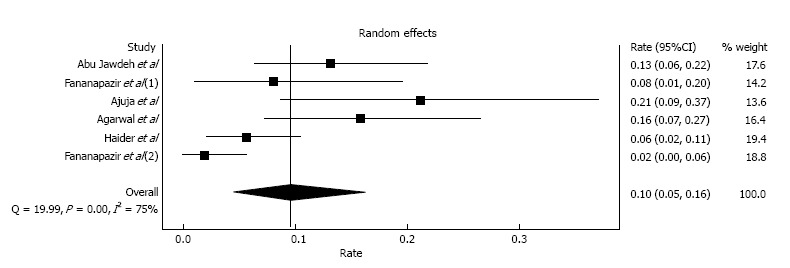
Forest plot of incidence of contrast-induced acute kidney injury in kidney transplant populations.
Types of procedure or intervention with contrast media
The types of procedure or intervention with systemic contrast media administration in our meta-analysis of CIAKI incidence included CT scan (59.1%), coronary angiogram (23.1%), other types of angiogram (17.6%), and intravenous pyelogram (IVP) (0.2%).
Subgroup analyses by types of procedure were also performed. The estimated incidences of CIAKI in kidney transplant recipients who received contrast media with cardiac catheterization, other types of angiogram, and CT scan were 16.1% (95%CI: 6.6%-28.4%, I2 = 40%), 10.1% (95%CI: 4.2%-18.0%, I2 = 0%), and 6.1% (95%CI: 1.8%-12.4%, I2 = 60%), respectively. Fananapazir et al[35] specifically studied the CIAKI in kidney transplant recipients who underwent allograft angiogram and reported the incidence of CIAKI of 8.1%. Data on the incidence of CIAKI in kidney transplant recipients, who underwent IVP, were limited as shown in Table 1. The incidence of CIAKI in patients who received IVP during early post-transplant period ranged from 32.4% to 100%[30-32].
Risk factors and prevention measures for CIAKI
Studies have identified early post-transplant period, older donor kidney, impaired baseline GFR, and lack of prophylactic volume hydration as potential important risk factors for CIAKI in kidney transplant recipients[30,31,38]. Ahuja et al[32] reported a CIAKI incidence of 15% in kidney transplant recipients with intravenous (IV) hydration before contrast exposure vs 49% in the non-IV hydration group. Despite limited data on the use of sodium bicarbonate and N-acetylcysteine (NAC), these studies did not find associated significant protective effects on the incidence of CIAKI[19,33,34,36].
Regarding the type of radiocontrast, high-osmolar contrast was associated with a higher incidence of CIAKI[32]. Compared to iso-osmolar contrast, Agarwal et al[33] found that low osmolar contrast was associated with increased CIAKI risk in kidney transplant recipients with an OR of 7.75 (1.10-infinity). In the setting of allograft angiogram, there was an increased incidence of CIAKI in recipients undergoing allograft angiogram alone (25%) compared to those who had allograft angiogram with stenting (0%).
Data on patients’ comorbidities and the risk of CI AKI were limited. Abu Jawdeh et al[36] reported an association between low hemoglobin and increased risk of CIAKI[36]. Recently, Haider et al[34] found no significant effects of diabetes mellitus, age, race, gender, baseline SCr, ACE inhibitor, angiotensin receptor blocker, or diuretics use on the incidence of CIAKI. In addition, studies did not find a significant association between calcineurin inhibitor use and CIAKI[33,36].
Effects of CIAKI on renal allograft function and/or allograft failure
Although there were reported cases of severe CIAKI requiring dialysis[30,33], no studies reported persistent renal allograft failure requiring dialysis. Fananapazir et al[19,35] reported no graft loss at 30 d post contrast media administration with CT scan and renal allograft angiogram. Haider et al[34] reported that kidney allograft function returned to baseline in five of the seven patients who developed CIAKI within three weeks[34]. In two patients, SCr continued to be elevated due to recurrent AKI episodes from other causes. Data on the effects of CIAKI on long-term graft function or survival were limited.
Evaluation for publication bias
Funnel plots evaluating publication bias for the incidence of CIAKI in kidney transplant recipients demonstrated slight asymmetry of the graph and thus suggested the presence of publication bias for positive studies regarding the incidence of CIAKI.
DISCUSSION
In this meta-analysis, we demonstrated that overall incidence of CIAKI and CIAKI-requiring dialysis in kidney transplant recipients were 9.6% and 0.4%, respectively. The type of procedure with contrast media affected the CIAKI incidences, with estimated incidences undergoing cardiac catheterization, other types of angiogram, and CT scan of 16.1%, 10.1% and 6.1%, respectively. While no graft losses were reported within 30 d post-contrast media administration, data on the effects of CIAKI on long-term graft function were limited.
The incidence of CIAKI has been ranged from 1% in the general population without risk factors to 10%-20% among high-risk patients (especially those with diabetes and CKD)[1,2,8-12]. Not surprisingly, the incidence of CIAKI in kidney transplant recipients from our meta-analysis is relatively similar with those reported in the general adult high-risk populations since transplant recipients also have lower GFR and greater prevalence of diabetes and hypertension than the overall general population[17-20].
Our meta-analysis demonstrated higher rates of CIAKI in kidney transplant recipients who underwent cardiac catheterization and other angiograms than in those who had CT scans. These differences are likely due to intra-arterial contrast administration which may expose the kidney to higher contrast concentrations[39]. In addition, catheter manipulation may provoke atherosclerotic microemboli to the kidney[19]. Despite the higher rate of AKI and the requirement of temporary dialysis after cardiac catheterization[33], our study found no allograft failure noted at 30 d. After a CIAKI event, renal allograft function usually returns to baseline unless the patients develop recurrent AKI episodes from other causes[34]. Thus, our study supports findings from previous studies that coronary angiography is safe with respect to allograft function[40,41].
Renal allograft angiogram is performed for assessment and treatment of allograft renal artery stenosis, pseudoaneurysms, and arteriovenous fistulas[35]. Renal angiogram, which requires contrast media to be directly administered into the graft renal artery, correlates with a CIAKI risk of only 8.1% and is unassociated with any reported cases of dialysis or renal allograft failure[35]. Interestingly, allograft angiogram alone was associated with a higher incidence of CIAKI than allograft angiogram with stenting[35]. It is possible that improved renal allograft function from treating graft renal artery stenosis with stenting ameliorated the nephrotoxicity of iodinated contrast media[35].
Although renin-angiotensin-aldosterone system inhibitors/blockers and calcineurin inhibitors were studied as potential nephrotoxic medications that were commonly discontinued perioperatively, or before systemic contrast exposure due to concern for their afferent arteriolar vasoconstriction effect[42], the evidence from our study does not currently support withholding these medications prior to contrast studies. In addition, reduction of immunosuppression may put the recipients at risk of allograft rejection. Data on preventative measures for CIAKI in renal transplant recipients is limited. As in general patient populations, optimization of volume status with adequate hydration before contrast exposure may help prevent CIAKI. There was also no supported data on the use of sodium bicarbonate and NAC to prevent CIAKI in kidney transplant recipients.
There are several limitations to our study. First, there were statistical heterogeneities in the analysis of the incidence of CIAKI. The potential sources of this heterogeneity included differences in baseline characteristics, types of procedure, and contrast media. Thus, we performed a sensitivity analysis of studies which only used low-osmolar or iso-osmolar contrast and a subgroup analysis of different procedure types, which yielded lower levels of heterogeneity. Second, selection bias may occur as contrast administration could have been avoided in patients with significantly reduced GFR. This effect may be due to the observation that most patients in the included studies had reasonable renal allograft function (eGFR > 30 mL/min per 1.73 m2). In addition, most included studies assessed the incidence of CIAKI in a relatively low risk kidney transplant population. Although several studies have suggested safety of contrast administration in patients with significantly reduced GFR[35,43,44], more studies involving high risk patients are needed to make more definitive conclusions. Finally, data on the effect of CIAKI on long-term graft function and allograft survival are lacking. Further studies elucidating the impact of the incidence and severity of CIAKI on long-term allograft outcomes will influence clinical management.
In summary, our meta-analysis demonstrates that the estimated incidence of CIAKI in kidney transplant recipients is 9.6%. Risk stratification for the administration of contrast media in kidney transplant patients include GFR estimation or measurement, clinical indication, and type of procedure. Future studies are needed to further evaluate preventive strategies to reduce CIAKI and the effect of CIAKI on long-term graft function in kidney transplant recipients.
COMMENTS
Background
Renal transplant recipients have been considered at an increased risk for developing post-contrast acute kidney injury (AKI) because they have lower glomerular filtration rate (GFR), GFR and higher prevalence of diabetes and cardiovascular disease. In addition, the majority of kidney transplant recipients are currently on calcineurin inhibitors, which are known to cause renal afferent vasoconstriction. However, unlike the general population, the incidence and risk factors for contrast-induced acute kidney injury (CIAKI) in kidney transplant recipients are not well studied.
Research frontiers
It is necessary to assess the incidence of CIAKI and risk factors for CIAKI in kidney transplant recipients.
Innovations and breakthroughs
In this study, the authors demonstrated that an overall incidence of CIAKI and CIAKI-requiring dialysis in kidney transplant recipients was 9.6% and 0.4%, respectively. The estimated incidences of CIAKI in kidney transplant recipients undergoing cardiac catheterization, other types of angiogram, and computed tomography scan were 16.1%, 10.1% and 6.1%, respectively. No graft losses were reported within 30 d post contrast media administration.
Applications
The data in this study demonstrates an estimated incidence of CIAKI in kidney transplant recipients of 9.6%. Risk stratification for administration of contrast media in kidney transplant patients includes GFR, clinical indication, and type of procedure. While adequate hydration prior to contrast exposure may help to reduce CIAKI risk, there is currently no evidence for withholding renin-angiotensin system and calcineurin inhibitors prior to contrast studies. In addition, there is no supportive data on the use of sodium bicarbonate and N-acetylcysteine to prevent CIAKI in kidney transplant recipients.
Peer-review
Very well-written review article, the authors were investigating the incidence and risk factors for AKI in renal transplant recipients by reviewing what were published in this field.
Footnotes
Conflict-of-interest statement: All authors report no conflicts-of-interest.
Data sharing statement: No additional data are available.
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: August 26, 2016
First decision: September 27, 2016
Article in press: December 29, 2016
P- Reviewer: Hilmi I, Salvadori M, Sureshkumar KK S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
References
- 1.Marenzi G, Lauri G, Assanelli E, Campodonico J, De Metrio M, Marana I, Grazi M, Veglia F, Bartorelli AL. Contrast-induced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction. J Am Coll Cardiol. 2004;44:1780–1785. doi: 10.1016/j.jacc.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 2.Solomon RJ, Mehran R, Natarajan MK, Doucet S, Katholi RE, Staniloae CS, Sharma SK, Labinaz M, Gelormini JL, Barrett BJ. Contrast-induced nephropathy and long-term adverse events: cause and effect? Clin J Am Soc Nephrol. 2009;4:1162–1169. doi: 10.2215/CJN.00550109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheungpasitporn W, Thongprayoon C, Brabec BA, Edmonds PJ, O’Corragain OA, Erickson SB. Oral hydration for prevention of contrast-induced acute kidney injury in elective radiological procedures: a systematic review and meta-analysis of randomized controlled trials. N Am J Med Sci. 2014;6:618–624. doi: 10.4103/1947-2714.147977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thongprayoon C, Cheungpasitporn W, Podboy AJ, Gillaspie EA, Greason KL, Kashani KB. The effects of contrast media volume on acute kidney injury after transcatheter aortic valve replacement: a systematic review and meta-analysis. J Evid Based Med. 2016 doi: 10.1111/jebm.12208. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 5.Cheungpasitporn W, Thongprayoon C, Kashani K. Transcatheter Aortic Valve Replacement: a Kidney’s Perspective. J Renal Inj Prev. 2016;5:1–7. doi: 10.15171/jrip.2016.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thamcharoen N, Thongprayoon C, Edmonds PJ, Cheungpasitporn W. Periprocedural Nebivolol for the Prevention of Contrast-Induced Acute Kidney Injury: A Systematic Review and Meta-analysis. N Am J Med Sci. 2015;7:446–451. doi: 10.4103/1947-2714.168670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, Edmonds PJ, O’Corragain OA, Srivali N, Ungprasert P, Erickson SB. Periprocedural effects of statins on the incidence of contrast-induced acute kidney injury: a systematic review and meta-analysis of randomized controlled trials. Ren Fail. 2015;37:664–671. doi: 10.3109/0886022X.2015.1010939. [DOI] [PubMed] [Google Scholar]
- 8.Briguori C, Airoldi F, D’Andrea D, Bonizzoni E, Morici N, Focaccio A, Michev I, Montorfano M, Carlino M, Cosgrave J, et al. Renal Insufficiency Following Contrast Media Administration Trial (REMEDIAL): a randomized comparison of 3 preventive strategies. Circulation. 2007;115:1211–1217. doi: 10.1161/CIRCULATIONAHA.106.687152. [DOI] [PubMed] [Google Scholar]
- 9.Marenzi G, Assanelli E, Marana I, Lauri G, Campodonico J, Grazi M, De Metrio M, Galli S, Fabbiocchi F, Montorsi P, et al. N-acetylcysteine and contrast-induced nephropathy in primary angioplasty. N Engl J Med. 2006;354:2773–2782. doi: 10.1056/NEJMoa054209. [DOI] [PubMed] [Google Scholar]
- 10.Tepel M, van der Giet M, Schwarzfeld C, Laufer U, Liermann D, Zidek W. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med. 2000;343:180–184. doi: 10.1056/NEJM200007203430304. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell AM, Jones AE, Tumlin JA, Kline JA. Incidence of contrast-induced nephropathy after contrast-enhanced computed tomography in the outpatient setting. Clin J Am Soc Nephrol. 2010;5:4–9. doi: 10.2215/CJN.05200709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huber W, Eckel F, Hennig M, Rosenbrock H, Wacker A, Saur D, Sennefelder A, Hennico R, Schenk C, Meining A, et al. Prophylaxis of contrast material-induced nephropathy in patients in intensive care: acetylcysteine, theophylline, or both? A randomized study. Radiology. 2006;239:793–804. doi: 10.1148/radiol.2393041456. [DOI] [PubMed] [Google Scholar]
- 13.Feldkamp T, Kribben A. Contrast media induced nephropathy: definition, incidence, outcome, pathophysiology, risk factors and prevention. Minerva Med. 2008;99:177–196. [PubMed] [Google Scholar]
- 14.Katzberg RW, Haller C. Contrast-induced nephrotoxicity: clinical landscape. Kidney Int Suppl. 2006;(100):S3–S7. doi: 10.1038/sj.ki.5000366. [DOI] [PubMed] [Google Scholar]
- 15.Becker CR, Davidson C, Lameire N, McCullough PA, Stacul F, Tumlin J, Adam A. High-risk situations and procedures. Am J Cardiol. 2006;98:37K–41K. doi: 10.1016/j.amjcard.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 16.Cheungpasitporn W, Thongprayoon C, Mao MA, Kittanamongkolchai W, Sathick IJ, Erickson SB. The Effect of Renin-angiotensin System Inhibitors on Kidney Allograft Survival: A Systematic Review and Meta-analysis. N Am J Med Sci. 2016;8:291–296. doi: 10.4103/1947-2714.187141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karthikeyan V, Karpinski J, Nair RC, Knoll G. The burden of chronic kidney disease in renal transplant recipients. Am J Transplant. 2004;4:262–269. doi: 10.1046/j.1600-6143.2003.00315.x. [DOI] [PubMed] [Google Scholar]
- 18.Ekberg H, Tedesco-Silva H, Demirbas A, Vítko S, Nashan B, Gürkan A, Margreiter R, Hugo C, Grinyó JM, Frei U, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007;357:2562–2575. doi: 10.1056/NEJMoa067411. [DOI] [PubMed] [Google Scholar]
- 19.Fananapazir G, Troppmann C, Corwin MT, Nikpour AM, Naderi S, Lamba R. Incidences of acute kidney injury, dialysis, and graft loss following intravenous administration of low-osmolality iodinated contrast in patients with kidney transplants. Abdom Radiol (NY) 2016;41:2182–2186. doi: 10.1007/s00261-016-0827-3. [DOI] [PubMed] [Google Scholar]
- 20.Morales JM, Andres A, Rengel M, Rodicio JL. Influence of cyclosporin, tacrolimus and rapamycin on renal function and arterial hypertension after renal transplantation. Nephrol Dial Transplant. 2001;16 Suppl 1:121–124. doi: 10.1093/ndt/16.suppl_1.121. [DOI] [PubMed] [Google Scholar]
- 21.Radiology ACo. Reston (VA): ACR Committee on Drugs and Contrast Media of the ACR Commission on Quality and Safety; 2015. Manual on Contrast Media. Version 10.1. 2015. [Google Scholar]
- 22.Elicker BM, Cypel YS, Weinreb JC. IV contrast administration for CT: a survey of practices for the screening and prevention of contrast nephropathy. AJR Am J Roentgenol. 2006;186:1651–1658. doi: 10.2214/AJR.05.0407. [DOI] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 25.STROBE statement--checklist of items that should be included in reports of observational studies (STROBE initiative) Int J Public Health. 2008;53:3–4. doi: 10.1007/s00038-007-0239-9. [DOI] [PubMed] [Google Scholar]
- 26.Barendregt J, Doi S. Wilston, Australia: EpiGear International Pty Ltd; 2010. MetaXL User Guide: Version 1.0. [Google Scholar]
- 27.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28:105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337:867–872. doi: 10.1016/0140-6736(91)90201-y. [DOI] [PubMed] [Google Scholar]
- 30.Light JA, Perloff LJ, Etheredge EE, Hill G, Spees EK. Adverse effects of meglumine diatrizoate on renal function in the early post-transplant period. Transplantation. 1975;20:404–409. doi: 10.1097/00007890-197511000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Peters C, Delmonico FL, Cosimi AB, Rubin RH, Tolkoff-Rubin N, Baker G, Russell PS. Risks versus benefits of contrast medium exposure in renal allograft recipients. Surg Gynecol Obstet. 1983;156:467–472. [PubMed] [Google Scholar]
- 32.Ahuja TS, Niaz N, Agraharkar M. Contrast-induced nephrotoxicity in renal allograft recipients. Clin Nephrol. 2000;54:11–14. [PubMed] [Google Scholar]
- 33.Agrawal V, Swami A, Kosuri R, Alsabbagh M, Agarwal M, Samarapungavan D, Rocher LL, McCullough PA. Contrast-induced acute kidney injury in renal transplant recipients after cardiac catheterization. Clin Nephrol. 2009;71:687–696. doi: 10.5414/cnp71687. [DOI] [PubMed] [Google Scholar]
- 34.Haider M, Yessayan L, Venkat KK, Goggins M, Patel A, Karthikeyan V. Incidence of contrast-induced nephropathy in kidney transplant recipients. Transplant Proc. 2015;47:379–383. doi: 10.1016/j.transproceed.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 35.Fananapazir G, Troppmann C, Corwin MT, Bent CK, Vu CT, Lamba R. Incidence of Contrast-Induced Nephropathy After Renal Graft Catheter Arteriography Using Iodine-Based Contrast Medium. AJR Am J Roentgenol. 2016;206:783–786. doi: 10.2214/AJR.15.15501. [DOI] [PubMed] [Google Scholar]
- 36.Abu Jawdeh B, Sharma Y, Katipally S, Leonard A, Alloway R, Woodle E, Thakar C. Incidence and risk factors of contrast-induced nephropathy in renal allograft recipients. 15th American Transplant Congress, ATC;; Proceedings of the American Journal of Transplantation Conference. 2015. [Google Scholar]
- 37.Lameire N, Kellum JA. Contrast-induced acute kidney injury and renal support for acute kidney injury: a KDIGO summary (Part 2) Crit Care. 2013;17:205. doi: 10.1186/cc11455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moreau JF, Kreis H, Barbanel Cl, Michel JR. [Effects of iodine contrast medias on the function of transplanted kidneys] Nouv Presse Med. 1975;4:2643–2646. [PubMed] [Google Scholar]
- 39.Katzberg RW, Lamba R. Contrast-induced nephropathy after intravenous administration: fact or fiction? Radiol Clin North Am. 2009;47:789–800, v. doi: 10.1016/j.rcl.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Pirat B, Müderrisoglu H, Korkmaz ME, Ozin B. Characteristics of coronary heart disease in renal transplant recipients. Transplant Proc. 2004;36:152–155. doi: 10.1016/j.transproceed.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 41.Ferguson ER, Hudson SL, Diethelm AG, Pacifico AD, Dean LS, Holman WL. Outcome after myocardial revascularization and renal transplantation: a 25-year single-institution experience. Ann Surg. 1999;230:232–241. doi: 10.1097/00000658-199908000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheungpasitporn W, Thongprayoon C, Srivali N, O’Corragain OA, Edmonds PJ, Ungprasert P, Kittanamongkolchai W, Erickson SB. Preoperative renin-angiotensin system inhibitors use linked to reduced acute kidney injury: a systematic review and meta-analysis. Nephrol Dial Transplant. 2015;30:978–988. doi: 10.1093/ndt/gfv023. [DOI] [PubMed] [Google Scholar]
- 43.McDonald RJ, McDonald JS, Bida JP, Carter RE, Fleming CJ, Misra S, Williamson EE, Kallmes DF. Intravenous contrast material-induced nephropathy: causal or coincident phenomenon? Radiology. 2013;267:106–118. doi: 10.1148/radiol.12121823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McDonald JS, Katzberg RW, McDonald RJ, Williamson EE, Kallmes DF. Is the Presence of a Solitary Kidney an Independent Risk Factor for Acute Kidney Injury after Contrast-enhanced CT? Radiology. 2016;278:74–81. doi: 10.1148/radiol.2015142676. [DOI] [PubMed] [Google Scholar]


