Abstract
The pks gene cluster encodes enzymes responsible for the synthesis of colibactin, a genotoxin that has been shown to induce DNA damage and contribute to increased virulence. The present study investigated the prevalence of pks in clinical K. pneumoniae isolates from a national surveillance program in Taiwan, and identified microbiological and molecular factors associated with pks-carriage. The pks gene cluster was detected in 67 (16.7%) of 400 isolates from various specimen types. Multivariate analysis revealed that isolates of K1, K2, K20, and K62 capsular types (p < 0.001), and those more susceptible to antimicrobial agents (p = 0.001) were independent factors strongly associated with pks-carriage. Phylogenetic studies on the sequence type (ST) and pulsed-field gel electrophoresis patterns indicated that the pks-positive isolates belong to a clonal group of ST23 in K1, a locally expanding ST65 clone in K2, a ST268-related K20 group, and a highly clonal ST36:K62 group. Carriage of rmpA, iutC, and ybtA, the genes associated with hypervirulence, was significantly higher in the pks-positive isolates than the pks-negative isolates (95.5% vs. 13.2%, p < 0.001). Further studies to determine the presence of hypervirulent pks-bearing bacterial populations in the flora of community residents and their association with different disease entities may be warranted.
Klebsiella pneumoniae is not only a major nosocomial pathogen but also an important cause of community-acquired infections1,2,3. Some geographic differences in disease caused by K. pneumoniae infection exist. One example is Klebsiella liver abscess (KLA), an invasive disease with severe complications3,4. KLA is more prevalent in Asia, particularly in Taiwan3,4,5,6,7, but is less common in other continents7,8,9. The differences in K. pneumoniae disease prevalence have been associated with the ethnic background or genetic predisposition of the host and differences in virulence potentials of the organism types in each geographic region3,10.
Among the 79 distinct capsular types of K. pneumoniae11, the hypervirulent strain of K1 is highly associated with KLA, and K2 is the second most commonly associated type12,13. In contrast, K1 and K2 K. pneumoniae strains are still relatively rare in other parts of the world14,15. A few other capsular types, K5, K20, K54 and K57, have also been associated with KLA invasive diseases16. These virulent types (K1, K2, K5, K20, K54, and K57) are also prevalent in community-onset K. pneumoniae pneumonia17. The presence of rmpA, iutC, ybtA, and pks, the genes responsible for hypermucoviscosity, production of iron-acquisition factors aerobactin and yersiniabactin, and production of colibactin genotoxin, respectively, have been associated with hypervirulence in K. pneumoniae7,18,19,20,21,22.
Colibactin, a hybrid peptide-polyketide genotoxin, was first identified in extraintestinal pathogenic E. coli strain IHE303423. Subsequently, it was shown that colibactin could induce double strand DNA breaks in lymphocytes to result in cell cycle arrest and cell death24. The genes responsible for colibactin synthesis were found on a gene cluster, referred to as pks colibactin gene cluster, which is located at the asnW tRNA locus in the E. coli genome23. According to a study in Europe, the pks colibactin gene cluster was detected in 34% of E. coli strains of phylogenic lineage B2, but only in 3.5% of K. pneumoniae clinical isolates25. However, data on the prevalence of the pks gene cluster in different capsular types of K. pneumoniae from different sources are limited.
Recently, sequencing of K. pneumoniae strain 1084 from Taiwan revealed the presence of a pks gene cluster that is identical to the E. coli IHE3034 version26. The pks gene cluster was found in a 208-kb high pathogenicity island (HPI), named pKPHPI208, at the asn tRNA loci of the K. pneumoniae 1084 chromosome (GenBank Accession No. NC_018522.1). The KPHPI208-like genomic island has been linked to the emerging hypervirulent K. pneumoniae lineage and may also be involved in increased virulence27. Using the clbA mutants constructed from K. pneumoniae 1084, Lai et al., showed that the pks colibactin genes were involved in DNA damage in the host28. In the same study, a high prevalence (25.6%) of the pks colibactin gene cluster was found among K. pneumoniae clinical isolates, a rate significantly higher than the 3.5% found in a recent European survey25. However, the isolates in the study by Lai et al. were from a single hospital in Taiwan in 2002, thus may not represent the current national trend. The present study was carried out to investigate the prevalence of the pks colibactin gene cluster in K. pneumoniae clinical isolates from a multicenter national surveillance program29. Microbiological and molecular characterizations were also performed to identify factors associated with pks gene cluster carriage.
Results
Prevalence of pks gene cluster and capsular types among K. pneumoniae clinical strains
A total of 400 K. pneumoniae clinical isolates comprising 100 each of blood, respiratory, urine, and other specimen sources from 2012 were randomly selected for this study. Among them, 67 (16.8%) isolates were pks-positive including 17, 26, 9, and 15 isolates from blood, respiratory, urine, and other specimen groups, respectively. The capsular type distribution and pks positivity stratified by specimen origin are listed in Table 1. Among these 400 isolates, 33 (8.3%) were K1 and 45 (11.3%) were K2, 12 (3.0%) were K5, 23 (5.8%) were K20, 20 (5.0%) were K54, 8 (2.0%) were K57, and 25 (6.3%) were K62. The pks gene cluster was detected in 26 (78.8%), 19 (42.2%), 11 (47.8%), 1 (12.5%), and 9 (36.0%) of the K1, K2, K20, K57, K62 capsular type isolates, respectively. The capsular type of the remaining 1 pks-positive isolate could not be identified and the capsular types of the 234 non-K1, K2, K5, K20, K57, K62 pks-negative isolates were not determined.
Table 1. Prevalence and capsular type distribution of pks colibactin gene cluster in clinical Klebsiella pneumoniae isolates stratified by specimen type.
| Capsular typea (n) | No. of isolates in each specimen group (no. of isolates pks positive) | Capsular type prevalenceb | pks prevalencec | |||
|---|---|---|---|---|---|---|
| Blood (n = 100) | Respiratory (n = 100) | Urine (n = 100) | Others (n = 100) | |||
| K1 (33) | 9 (6) | 10 (8) | 4 (4) | 10 (8) | 8.3 (33/400) | 78.8 (26/33) |
| K2 (45) | 12 (7) | 16 (6) | 7 (3) | 10 (3) | 11.3 (45/400) | 42.2 (19/45) |
| K5 (12) | 4 (0) | 6 (0) | 0 | 2 (0) | 3.0 (12/400) | 0 (0/12) |
| K20 (23) | 4 (3) | 10 (5) | 4 (1) | 5 (2) | 5.8 (23/400) | 47.8 (11/23) |
| K54 (20) | 5 (0) | 2 (0) | 8 (0) | 5 (0) | 5.0 (20/400) | 0 (0/20) |
| K57 (8) | 1 (0) | 6 (1) | 0 | 1 (0) | 2.0 (8/400) | 12.5 (1/8) |
| K62 (25) | 3 (0) | 7 (6) | 6 (1) | 9 (2) | 6.3 (25/400) | 36.0 (9/25) |
| Unknown (234)a | 64 (1) | 58 (0) | 43 (0) | 68 (0) | 0.4 (1/234) | |
aIn the 234 non-K1, K2, K5, K20, K54, K57, K62 isolates, capsular type analysis was carried out on the 1 pks-positive isolate but its capsular type could not be identified. bNumber of isolates with the capsular type/400 isolates studied. cNumber of isolates positive for pks/number of isolates within each capsular type.
Cocarriage of pks and hypervirulence genes
To test for co-carriage of pks and hypervirulence factors, we performed PCR to determine the presence of iucA (aerobactin), rmpA (hypermucositity), and ybtA (yersiniabactin), the genes associated with hypervirulence, Among the 67 pks-positive isolates, 64 (95.5%) were positive for rmpA, iutC, and ybtA, The remaining 3 isolates (all K2) were ybtA-positive and iutC-negative, and 2 of which were also rmpA-negative. In the 333 pks-negative isolates, only 13.2% (44) were iutC/rmpA/ybtA-positive. Thus the pks-positive isolates had significantly higher carriage rate of rmpA, iutC, and ybtA, than the pks-negative isolates (95.5% vs. 13.2%, p < 0.001).
Antimicrobial susceptibility of pks-positive and pks-negative isolates
The pks-positive K. pneumoniae isolates were significantly more susceptible to 13 of the 14 antimicrobial agents tested (Table 2), including aminoglycosides, β-lactams, β-lactam/β-lactamase inhibitors, fluoroquinolones, and folate pathway inhibitors. For example, susceptibility to cefazolin, ciprofloxacin, gentamicin, and trimethoprim/sulfamethoxazole was 94.0%, 100%, 100%, and 98.5% vs. 66.4%, 69.7%, 71.8%, and 64%, respectively, in the pks-positive vs. pks-negative isolates (all P < 0.001). Ertapenem was the only agent to which there was no significant difference in rates of susceptibility between the pks-positive and pks-negative isolates (100% vs. 97.3%, P = 0.36).
Table 2. Antimicrobial susceptibility of pks-positive and pks-negative Klebsiella pneumoniae isolates.
| Antimicrobial agenta | N (%) of pks-positive isolates (n = 67) | N (%) of pks-negative isolates (n = 333) | p | ||
|---|---|---|---|---|---|
| Resistantb | Susceptible | Resistantb | Susceptible | ||
| Amikacin | 0 | 67 (100.0) | 25 (7.5) | 306 (91.9) | 0.013 |
| Aztreonam | 0 | 67 (100.0) | 68 (20.4) | 260 (78.1) | <0.001 |
| Ceftazidime | 0 | 67 (100.0) | 68 (20.4) | 245 (73.6) | <0.001 |
| Cefazolin | 4 (6.0) | 63 (94.0) | 109 (32.7) | 221 (66.4) | <0.001 |
| Ciprofloxacin | 0 | 67 (100.0) | 92 (27.6) | 232 (69.7) | <0.001 |
| Cefepime | 0 | 66 (98.5) | 40 (12.0) | 283 (85.0) | 0.005 |
| Cefoxitin | 3 (4.5) | 63 (94.0) | 82 (24.6) | 236 (70.9) | <0.001 |
| Cefuroxime | 5 (7.5) | 62 (92.5) | 104 (31.2) | 221 (66.4) | <0.001 |
| Cefotaxime | 1 (1.5) | 66 (98.5) | 58 (17.4) | 259 (77.8) | <0.001 |
| Ertapenem | 0 | 67 (100.0) | 8 (2.4) | 324 (97.3) | 0.36 |
| Gentamicin | 0 | 67 (100.0) | 84 (25.2) | 239 (71.8) | <0.001 |
| Piperacillin/Tazobactam | 0 | 67 (100.0) | 61 (18.3) | 256 (76.9) | <0.001 |
| Ticarcillin/Clavulanate | 2 (3.0) | 62 (92. 5) | 100 (30.0) | 215 (64.6) | <0.001 |
| Trimethoprim/Sulfamethoxazole | 1 (1.5) | 66 (98.5) | 120 (36.0) | 213 (64.0) | <0.001 |
aAmpicillin was also tested but not included in analysis because isolates of K. pneumoniae have intrinsic resistance to this agent. bResistant data do not include intermediate results.
Microbiological and molecular factors associated with pks-positive isolates
Univariate analysis revealed that isolates of K1, K2, K20, and K62 capsular types, isolates from respiratory tract origin, and isolates less resistant were significantly associated with pks-carriage (Table 3). Multivariate analysis revealed that isolates of K1 [Odds ratio (OR) 1112.7, 95% confidence interval (95% CI) 103.535–11957], K2 (OR 187.456, 95% CI 19.813–1773.6,), K20 (OR 425.418, 95% CI 38.925–4649.5), and K62 (OR 508.24, 95% CI 42.06–6141.5) types (all P < 0.001), and being resistant to fewer than 1 or none of the antimicrobial agents tested (OR 18.281, CI 3.288–101.658, P = 0.001) remained independent factors strongly associated with pks-positivity.
Table 3. Factors associated with carriage of pks gene cluster in Klebsiella pneumoniae clinical isolates.
| Variable (n) | No. (%) of isolates | Pa | ORb | 95%CIb | Pb | |
|---|---|---|---|---|---|---|
| pks-Positive | pks-Negative | |||||
| Total (400) | 67 | 333 | ||||
| Age | 0.719 | |||||
| < = 15 y.o. (18) | 2 (3.0) | 16 (4.8) | 0.749 | |||
| 16–64 y.o. (163) | 26 (38.8) | 137 (41.1) | 0.723 | |||
| > = 65 y.o. (219) | 39 (58.2) | 180 (54.1) | 0.533 | |||
| Specimen source | 0.014 | |||||
| Blood (100) | 17 (25.4) | 83 (24.9) | 0.938 | 2.525 | 0.644–9.902 | 0.184 |
| Respiratory (100) | 26 (38.8) | 74 (22.2) | 0.004 | 1.501 | 0.387–5.828 | 0.557 |
| Urine (100) | 9 (13.4) | 91 (27.3) | 0.017 | Reference | ||
| Abscess & Others (100) | 15 (22.4) | 85 (25.5) | 0.588 | 0.809 | 0.203–3.222 | 0.763 |
| Capsular type | <0.001 | |||||
| K1 (33) | 26 (38.8) | 7 (2.1) | <0.001 | 1112.7 | 103.535–11957 | <0.001 |
| K2 (45) | 19 (28.4) | 26 (7.8) | <0.001 | 187.456 | 19.813–1773.6 | <0.001 |
| K20 (23) | 11 (16.4) | 12 (3.6) | <0.001 | 425.418 | 38.925–4649.5 | <0.001 |
| K57 (8) | 1 (1.5) | 7 (2.1) | 1 | 25.798 | 1.16–573.73 | 0.04 |
| K62 (25) | 9 (13.4) | 16 (4.8) | 0.022 | 508.24 | 42.06–6141.5 | <0.001 |
| Non-K1, K2, K20, K57, K62 (266) | 1 (1.5) | 265 (79.6) | <0.001 | Reference | ||
| Antimicrobial resistance | <0.001 | |||||
| Resistant to <= 1 agent (272) | 65 (97.0) | 207 (62.2) | 18.281 | 3.288–101.658 | 0.001 | |
| Resistant to >= 2 agents (128) | 2 (3.0) | 126 (37.8) | Reference | |||
aP value by univariate analysis. Only variables having statistical significance by univariate analysis were subject to multivariate analysis. bP value by multivariate analysis; OR, odds ratio; CI, confidence interval.
Clonality of pks-positive isolates and that of K1 and K2 isolates
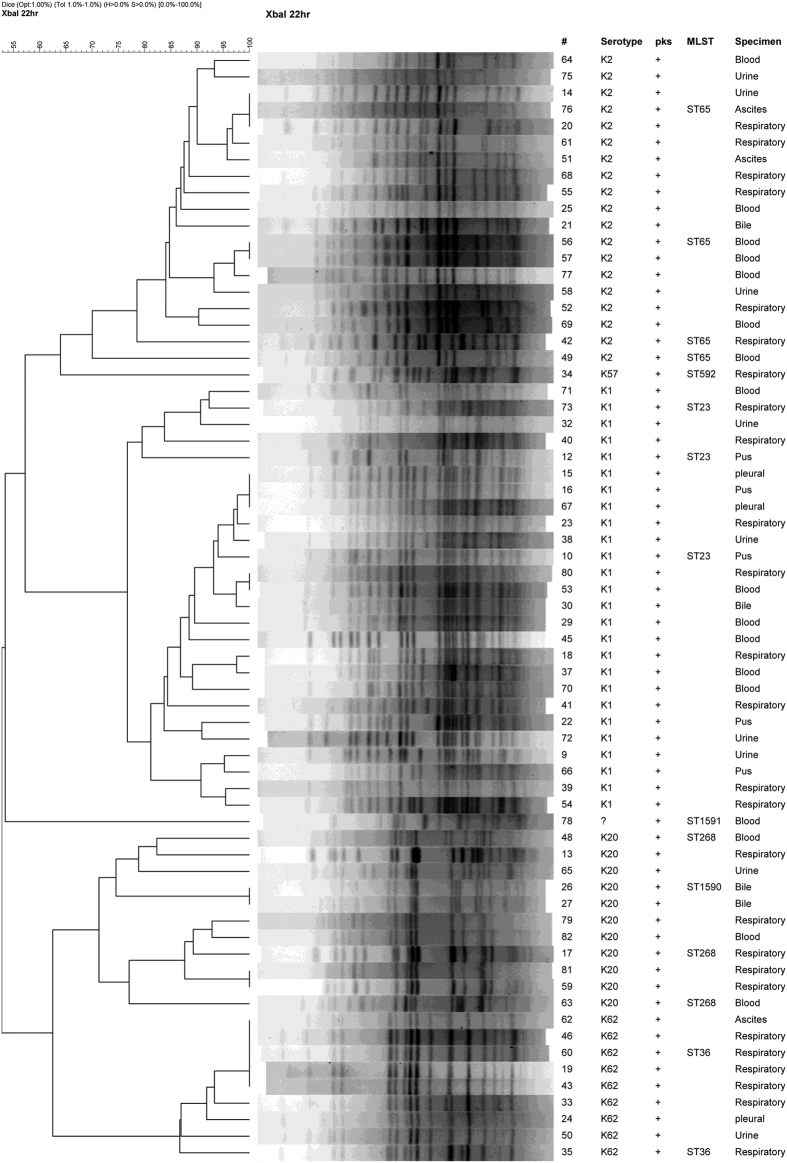
Based on the pulsed-field gel electrophoresis (PFGE) pattern, the pks-positive isolates fall into 4 major clusters, with K1, K2, K20, and K62 isolates each belonging to its own distinct cluster (Fig. 1). Within the K1 isolates, two sub-clusters sharing >=80% similarity were seen but both sub-clusters belonged to sequence type (ST) 23. All but 2 of the pks-positive K2 isolates shared >=84% similarity in PFGE patterns and all were ST65 (including the 2 having <80% similarity in PFGE pattern). The K20 isolates were slightly more diverse in PFGE pattern, sharing 70% similarity, but had ST268 and a previously unreported new sequence type, ST1590, which is a single locus variant (SLV) of ST268. The pks-positive K62 (ST36) isolates were highly clonal with >=80% similarity in PFGE pattern including several isolates whose patterns were indistinguishable.
Figure 1. Dendrogram of the pks-positive Klebsiella pneumoniae clinical isolates.
#Isolate number; MLST, Multilocus sequence typing; ST, sequence type;?, capsular type unidentified.
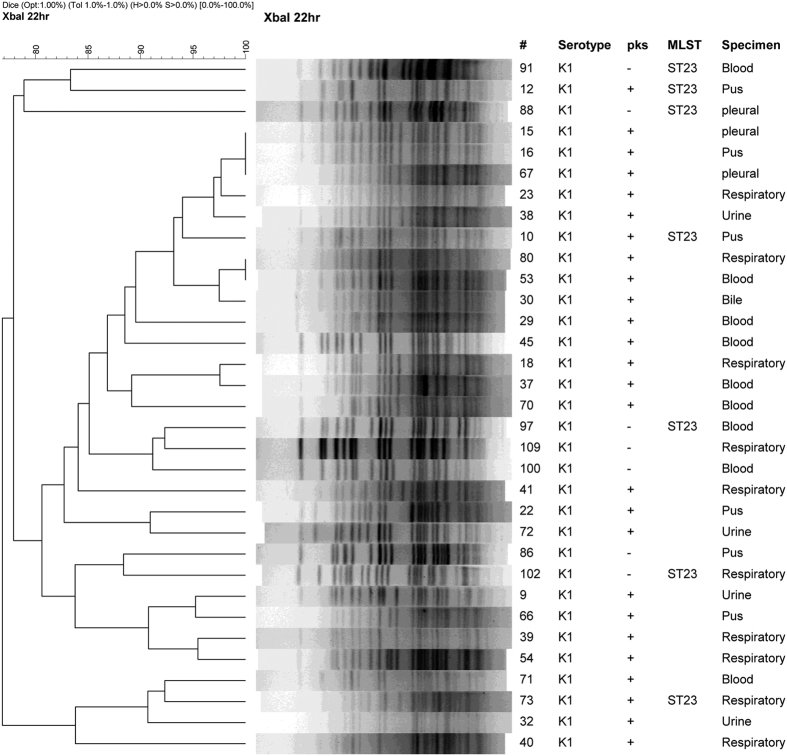
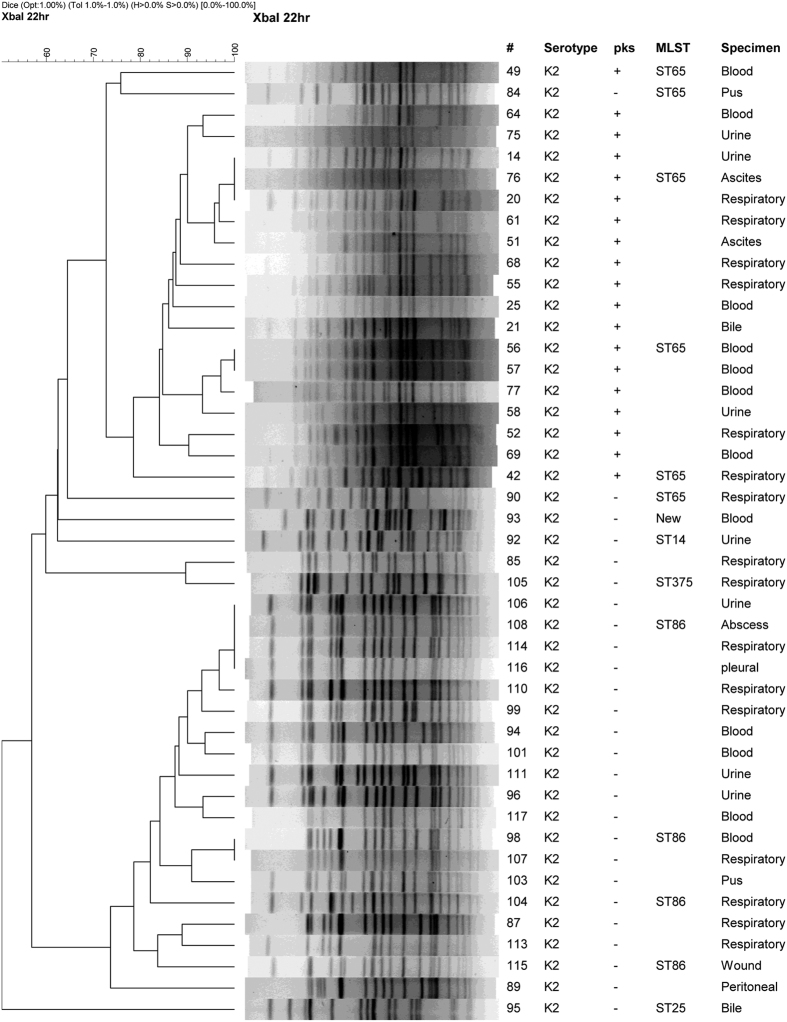
Because K1 and K2 capsular types comprised the largest proportions of pks-positive isolates, and are independent factors associated with pks-positivity, we also investigated the clonality of all K1 and K2 isolates regardless of their pks status. PFGE clustering for isolates belonging to each of the two capsular types were performed (Figs 2 and 3). For K1 isolates, the pks-negative isolates are scattered in-between the pks-positive isolates in the tree. MLST analysis suggests that the pks-negative K1 isolates, like the pks-positive ones, are also all ST23 (Fig. 2). For K2 isolates, a distinct clade having <65% similarity to the rest of the K2 isolates were found to contain all of the pks-positive strains. The ST of the predominant pks-negative K2 isolates is ST86, which is distinct from the ST65 pks-positive K2 isolates. These 2 STs differ by 5 out of the 7 alleles in the MLST profile. The ST of the remaining pks-negative K2 isolates also differed from ST65 and ST86, and included ST14, ST25, ST375, and a new ST that is a SLV of ST110 (Fig. 3)
Figure 2. Dendrogram of pks-positive and pks-negative K1 capsular type Klebsiella pneumoniae clinical isolates.
#Isolate number; MLST, Multilocus sequence typing; ST, sequence type.
Figure 3. Dendrogram of pks-positive and pks-negative K2 capsular type Klebsiella pneumoniae clinical isolates.
Sequence type (ST) 65 and ST86 differ by 5 out of the 7 alleles in the MLST (multilocus sequence typing) profile. The STs of the remaining pks-negative isolates are also distinct from ST65 and ST86. The new ST is a single locus variant of ST110.
Discussion
The pks gene cluster encodes enzymes that are responsible for the synthesis of colibactin, a genotoxin that has been shown to induce host DNA damage, thus may contribute to other disease entities and increased virulence in E. coli and K. pneumoniae27,30. However, data on the prevalence and microbiological factors associated with pks-positive K. pneumoniae isolates are limited. The present study showed that the prevalence of the pks colibactin gene cluster among clinical K. pneumoniae isolates from multiple hospitals in different regions of Taiwan was 16.7%. Our result corroborated the finding of high pks prevalence among K. pneumoniae by Lai et al.28, who studied isolates from a single hospital in Taiwan. Since the bacterial strains in our study were from 2012 and the study of Lai et al. used isolates from 2002, our results indicated persistence of the pks gene cluster in the clinical K. pneumoniae isolates in Taiwan.
While originally identified as a genotoxin targeting the host cells, the role of colibactin in the aspect of an emerging virulence pathogen lineage is just beginning to unfold. In E. coli, it was reported that the presence of the pks genes is strongly associated with bacteremia isolates, such as E. coli group B231. Recently, it was shown that mice infected with colibactin-producing E. coli also had significantly lower survival rate than those infected with the isogenic colibactin-negative mutant24. It has been further demonstrated in a neonatal sepsis rat model, that colibactin contributes to the virulence of pks-positive E. coli in influencing its ability to colonize the gut of the neonate and to cause lethal invasive disease32. Our study revealed that K. pneumoniae isolates of K1, K2, K20, and K62 capsular types are independent factors strongly associated with pks carriage. K1, K2, K20 types have been associated with Klebsiella liver abscess (KLA) invasive disease and community-onset pneumonia, especially K1 and K2 isolates3,16,17. The pks-positive isolates in the present study were detected in different specimen types including blood, respiratory, urine, and abscess plus other specimen origins. Therefore, the pks gene cluster in K. pneumoniae may also contribute to invasive infections in different anatomical sites.
The virulence of the pks-positive K. pneumoniae isolates is further supported by our finding that 95.5% of them were also positive for rmpA, ybt, and iutA, the genes involved in hypermucoviscosity, yersiniabactin, and aerobactin production, respectively7,18,19,20,21,22. These virulence genes have been detected in large plasmid pLVPK homologs or genomic islands associated with hypervirulent K. pneumoniae clinical isolates27,33. Since nearly all of the genotoxic K. pneumoniae clinical isolates were positive for all three virulence factors (rmpA, ybtA, and iutA), it is plausible that the emerging pks genotoxic trait is associated with increased hypervirulent K. pneumoniae strains.
In K. pneumoniae, K1 and K2 strains have been regarded as the most virulent among its 79 capsular types34. In a recent study of 69 K. pneumoniae strains (including 32 K1 and 5 K2 type) from different regions of the world, genomic island variants carrying both pks colibactin and yersiniabactin siderophore-producing modules were found in nearly all hypervirulent K1 strains27. The genomic islands with both pks colibactin and yersiniabactin gene modules have also been identified in K2 strains but at much lower frequency27,35. It has been reported that the hypervirulent K1 strains mostly belong to ST23 or its SLV, whereas the hypervirulent K2 strains are more genetically diverse with different STs (ST65, ST86, and others)10,27,36,37. The present study also found that the K1:ST23 strains (33 isolates) were clonal regardless of their pks status based on PFGE. In contrast, the pks-positive (19 isolates) and pks-negative (26 isolates) K2 strains were segregated into two major clades, ST65 and ST86, respectively. The remaining pks-negative K2 isolates had different PFGE patterns and STs, including ST14, ST25, ST375, and a new ST that is a SLV of ST110. Our results suggest that the pks-positive lineage may have expanded locally among K. pneumoniae in Taiwan, especially a K2:ST65 clone, perhaps after acquisition of pks and associated genomic island modules that confer increased virulence or environmental fitness.
Of interest also was the pks-positive K62:ST36 isolates. These isolates came from 9 hospitals and are either indistinguishable or highly similar by PFGE. Reports on K62 capsular type K. pneumoniae are scarce. In a study of 592 K. pneumoniae isolates colonizing the intestinal tract of healthy Chinese and overseas Chinese adults in eight Asian countries, only 6 (1%) K62 were found38. The higher overall prevalence of K62 (25, 6.3%) in our 400 randomly selected clinical K. pneumoniae isolates might be due to differences in the study population. In addition, K62 comprised 13.4% of the pks-positive isolates in the present study. Therefore, more studies are needed to understand the epidemiology this capsular type in different disease entities in Taiwan.
We also found that isolates carrying the pks colibactin gene cluster are highly associated with low antimicrobial resistance. This is likely due in part to the fact that most of these isolates belong to K1 and K2 capsular types, as isolates of these capsular types recovered from KLA cases are usually less resistant to other antimicrobial agents3,16. However, multidrug-resistant hypervirulent K. pneumoniae isolates with extended spectrum β-lactamase (ESBL) or KPC carbapenemase have also been detected in different parts of the world39,40,41. The emergence of multidrug-resistance combined with genotoxicity in hypervirulent K. pneumoniae strains is worrisome. Careful monitoring of isolates with genotoxic colibactin pks gene cluster for acquired antimicrobial resistance is warranted.
In conclusion, the prevalence of pks colibactin gene cluster is high among clinical K. pneumoniae isolates in Taiwan. The finding of pks-positive hypervirulent isolates in different specimen types raised questions on the contribution of the genotoxic K. pneumoniae strains to the development of different disease entities. Since the isolates used in the present study were recovered from inpatients and outpatients from hospitals, thus may comprise more strains with resistance phenotypes, it is possible that the prevalence of pks colibactin gene cluster among K. pneumoniae strains in the general population in Taiwan may be even higher. Whether the genotoxic K. pneumoniae is associated with different disease entities in the locality requires extensive investigations. Further studies to determine the presence of pks gene bearing bacterial populations in the flora of community residents may also be warranted.
Methods
Isolates
Klebsiella pneumoniae isolates used in the present study were selected from the Taiwan Surveillance of Antimicrobial Resistance (TSAR) program conducted at the National Health Research Institutes (NHRI). The collection process for TSAR has been described previously and the 2012 isolates were collected from 27 hospitals located in different regions of Taiwan29. All isolates were stored at −80 °C at NHRI for subsequent testing. The species identification was confirmed using a combination of Triple Sugar Iron, indole test, and Vitek II GN card (bioMérieux, Marcy l’Etoile, France).
For determination of pks prevalence in the contemporary clinical K. pneumoniae isolate pool, the 400 isolates were randomly selected from the 831 K. pneumoniae isolates in the 2012 TSAR collection to include 100 each of 4 specimen categories: blood, respiratory, urine, and others. The others category included a mixture of isolates with a specimen source of ascites, abscess, bile, pleural, pus, tissue, wound, and miscellaneous sources.
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing was performed using broth microdilution method following the guidelines of the Clinical and Laboratory Standards Institute (CLSI) and the results were interpreted based on the CLSI breakpoints42.
Detection of the pks gene cluster and hypervirulence genes in clinical K. pneumoniae isolates
The presence of the pks colibactin genes among 400 clinical K. pneumoniae isolates was determined by PCR using published primers23. Genomic DNA of each isolate was prepared by picking 3 to 5 colonies lightly from fresh overnight culture plate in 150 μl AE buffer [50 mM sodium acetate (pH 5.2) and 10 mM EDTA (pH 8.0)]. The suspension was heated at 95 °C for 15 min then centrifuged at 1000 g for 10 min to remove cellular debris, after which 100 μl of the supernatant was transferred to a new vial. The DNA preparation was stored at −20 °C and used as template for subsequent amplifications. Primer sets for the 3′ and 5′ regions of the pks colibactin gene cluster and its two internal loci were used for PCR following previously published protocols28. K. pneumoniae 1084 and NTUH K2044 were used as pks-positive and pks-negative controls, respectively.
To investigate the association of pks and hypervirulence, the presence of three hypervirulence genes, iucA (aerobactin), rmpA (hypermucositity), and ybtA (yersiniabactin) were determined by PCR following previously published protocols43,44. The primers used in the present study for pks and hypervirulence detection are listed in Supplemental Table 1 (Table S1).
Determination of capsular types
All 400 isolates were first subject to capsular typing for K1, K2, K5, K20, K54, K57 using primers and protocols previously published45. For pks-positive isolates that were negative for K1, K2, K5, K20, K54, and K57, their capsular types were determined by PCR followed by sequencing of the wzi gene using previously published protocols46. Because 9 of the pks-positive isolates were K62, the pks-negative isolates were then also checked for K62 capsular type47. The primers are listed in Supplemental Table 1 (Table S1).
Pulsed field gel electrophoresis (PFGE)
All pks-positive isolates and all K1 & K2 isolates (regardless of pks status) were subject to PFGE to determine strain relatedness. Plug preparation, restriction enzyme digestion, and electrophoresis conditions were performed as previously described48. Briefly, genomic DNAs of isolates were prepared and digested with 50 U XbaI (NEB, New England Biolabs Inc., Ipswich, MA, USA) at 37 °C for 16 to 18 h. The enzyme-digested DNA fragments were separated in 0.5 × Tris-Borate-EDTA buffer (pH 7.5) at 14 °C for 22 h with a voltage of 6 V/cm at a fixed angle of 120° and pulse times ranging from 2.16 to 54.17 s by CHEF MAPPER (Bio-Rad Laboratories, Richmond, CA). Salmonella Choleraesuis Brenderup H9812 (ATCC BAA664) was used as standard for DNA patterns normalization. PFGE patterns were analyzed using BioNumerics software (Applied Maths NV, Sint-Martens-Latem, Belgium).
Multilocus sequence typing (MLST)
MLST was performed on isolates chosen from PFGE clusters and included each capsular type. The MLST procedure followed previously published protocols49 and information from the Institut Pasteur MLST website (http://www.pasteur.fr/mlst). Sequence types (STs) were assigned using the Klebsiella MLST database. New ST information was deposited at the Institut Pasteur MLST web site.
Ethics statement
The bacterial isolates were recovered from clinical samples taken as part of standard care. The TSAR project was approved by the Research Ethics Committee of National Health Research Institutes (EC1010602-E). The study methods were performed in accordance with the relevant guidelines and regulations.
Data analysis
Susceptibility interpretation analysis was made using the WHONET software50. Significance of differences in frequencies and proportions was tested by the χ2 test or Fisher’s exact test (if the number was less than 10). Predictor variables associated with pks-carriage investigated included capsular type (K1, K2, K20, K57, K62 vs. other), age (<=18 y.o.; 19–64 y.o., >=65 y.o.), specimen source (blood, respiratory, urine, and others), and antimicrobial susceptibility (being resistant to one or none of the antimicrobial agents tested). Multivariable logistic regression analysis was performed to assess the relationship between predictor variables among pks-positive and pks-negative isolates. All analyses were performed with the Statistical Package for the Social Sciences version 18.0 (SPSS, Chicago, IL, USA). A P < 0.05 was considered to be statistically significant.
Additional Information
How to cite this article: Chen, Y.-T. et al. Prevalence and characteristics of pks genotoxin gene cluster-positive clinical Klebsiella pneumoniae isolates in Taiwan. Sci. Rep. 7, 43120; doi: 10.1038/srep43120 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
We express our sincere appreciation to the hospitals that participated in the Taiwan Surveillance of Antimicrobial Resistance (TSAR) project. We also thank the team of the curators of the Institut Pasteur MLST system (Paris, France) for importing novel alleles, profiles and/or isolates at http:/ www.pasteur.fr/mlst. This work was supported in part by the Ministry of Science and Technology of Taiwan (104-2622-B-005-003 and 104-2311-B-005-011) to Ying-Tsong Chen, plus intramural grants of the National Health Research Institutes to Ying-Tsong Chen (MG-104-PP-17) and Tsai-Ling Lauderdale (IV-103-PP01 and IV-104-SP04).
Footnotes
The authors declare no competing financial interests.
Author Contributions Y.-T.C. and T.-L.L. designed the project and wrote the paper. Y.-C.L. and J.-T.W. assisted in the project design, reviewed the paper and provided recommendations. M.-C.T. and A.-C.L. performed the molecular characterizations of the isolates. L.-Y.H. and J.-T.W. performed the statistical analysis. Y.-R.S., H.-Y.W., J.-F.L., and I.-W.H. performed the antimicrobial susceptibility testing and analysis on the antimicrobial susceptibility results. Y.-T.C. and T.-L.L. coordinated and oversaw the project.
References
- Paczosa M. K. & Mecsas J. Klebsiella pneumoniae: Going on the offense with a strong defense. MMBR 80, 629–661, doi: 10.1128/mmbr.00078-15 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podschun R. & Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev 11, 589–603 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu L. K., Yeh K. M., Lin J. C., Fung C. P. & Chang F. Y. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis 12, 881–887, doi: 10.1016/s1473-3099(12)70205-0 (2012). [DOI] [PubMed] [Google Scholar]
- Wang J. H. et al. Primary liver abscess due to Klebsiella pneumoniae in Taiwan. Clin Infect Dis 26, 1434–1438 (1998). [DOI] [PubMed] [Google Scholar]
- Fang C. T., Chuang Y. P., Shun C. T., Chang S. C. & Wang J. T. A novel virulence gene in Klebsiella pneumoniae strains causing primary liver abscess and septic metastatic complications. J Exp Med 199, 697–705, doi: 10.1084/jem.20030857 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F. C., Sandler N. & Libby S. J. Liver abscess caused by magA+Klebsiella pneumoniae in North America. J Clin Microbiol 43, 991–992, doi: 10.1128/jcm.43.2.991-992.2005 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shon A. S., Bajwa R. P. & Russo T. A. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: a new and dangerous breed. Virulence 4, 107–118, doi: 10.4161/viru.22718 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimian J., Wilson T., Oram V. & Holzman R. S. Pyogenic liver abscess: recent trends in etiology and mortality. Clin Infect Dis 39, 1654–1659, doi: 10.1086/425616 (2004). [DOI] [PubMed] [Google Scholar]
- Turton J. F. et al. Genetically similar isolates of Klebsiella pneumoniae serotype K1 causing liver abscesses in three continents. J Med Microbiol 56, 593–597, doi: 10.1099/jmm.0.46964-0 (2007). [DOI] [PubMed] [Google Scholar]
- Lee I. R. et al. Differential host susceptibility and bacterial virulence factors driving Klebsiella liver abscess in an ethnically diverse population. Sci Rep 6, 29316, doi: 10.1038/srep29316 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y. J. et al. Genetic analysis of capsular polysaccharide synthesis gene clusters in 79 capsular types of Klebsiella spp. Sci Rep 5, 15573, doi: 10.1038/srep15573 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung C. P. et al. A global emerging disease of Klebsiella pneumoniae liver abscess: is serotype K1 an important factor for complicated endophthalmitis? Gut 50, 420–424 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Wang Y., Ye L. & Yang J. Molecular epidemiology and virulence factors of pyogenic liver abscess causing Klebsiella pneumoniae in China. Clin Microbiol Infect, doi: 10.1111/1469-0691.12664 (2014). [DOI] [PubMed] [Google Scholar]
- Decre D. et al. Emerging severe and fatal infections due to Klebsiella pneumoniae in two university hospitals in France. J Clin Microbiol 49, 3012–3014, doi: 10.1128/jcm.00676-11 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu V. L. et al. Virulence characteristics of Klebsiella and clinical manifestations of K. pneumoniae bloodstream infections. Emerg Infect Dis 13, 986–993, doi: 10.3201/eid1307.070187 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang C. T. et al. Klebsiella pneumoniae genotype K1: an emerging pathogen that causes septic ocular or central nervous system complications from pyogenic liver abscess. Clin Infect Dis 45, 284–293, doi: 10.1086/519262 (2007). [DOI] [PubMed] [Google Scholar]
- Lin Y.-T., Wang W.-P., Wang F.-D. & Fung C.-P. Community-onset Klebsiella pneumoniae pneumonia in Taiwan: clinical features of the disease and associated microbiological characteristics of isolates from pneumonia and nasopharynx. Front Microbiol 6, doi: 10.3389/fmicb.2015.00122 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carniel E. The Yersinia high-pathogenicity island: an iron-uptake island. Microbes Infect 3, 561–569 (2001). [DOI] [PubMed] [Google Scholar]
- Cheng H. Y. et al. RmpA regulation of capsular polysaccharide biosynthesis in Klebsiella pneumoniae CG43. J Bacteriol 192, 3144–3158, doi: 10.1128/jb.00031-10 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt K. E. et al. Genomic analysis of diversity, population structure, virulence, and antimicrobial resistance in Klebsiella pneumoniae, an urgent threat to public health. Proc Natl Acad Sci USA 112, E3574–3581, doi: 10.1073/pnas.1501049112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y. C., Peng H. L. & Chang H. Y. RmpA2, an activator of capsule biosynthesis in Klebsiella pneumoniae CG43, regulates K2 cps gene expression at the transcriptional level. J Bacteriol 185, 788–800 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassif X. & Sansonetti P. J. Correlation of the virulence of Klebsiella pneumoniae K1 and K2 with the presence of a plasmid encoding aerobactin. Infect Immun 54, 603–608 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nougayrede J. P. et al. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science 313, 848–851, doi: 10.1126/science.1127059 (2006). [DOI] [PubMed] [Google Scholar]
- Marcq I. et al. The genotoxin colibactin exacerbates lymphopenia and decreases survival rate in mice infected with septicemic Escherichia coli. J Infect Dis, doi: 10.1093/infdis/jiu071 (2014). [DOI] [PubMed] [Google Scholar]
- Putze J. et al. Genetic structure and distribution of the colibactin genomic island among members of the family Enterobacteriaceae. Infect Immun 77, 4696–4703, doi: 10.1128/iai.00522-09 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A. C. et al. Complete genome sequence of Klebsiella pneumoniae 1084, a hypermucoviscosity-negative K1 clinical strain. J Bacteriol 194, 6316, doi: 10.1128/jb.01548-12 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struve C. et al. Mapping the evolution of hypervirulent Klebsiella pneumoniae. mBio 6, doi: 10.1128/mBio.00630-15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y. C. et al. Genotoxic Klebsiella pneumoniae in Taiwan. PLoS One 9, e96292, doi: 10.1371/journal.pone.0096292 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. T. et al. Antimicrobial non-susceptibility of Escherichia coli from outpatients and patients visiting emergency rooms in Taiwan. PLoS One 10, e0144103, doi: 10.1371/journal.pone.0144103 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas-Ramos G. et al. Escherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cells. Proc Natl Acad Sci USA 107, 11537–11542, doi: 10.1073/pnas.1001261107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. R., Johnston B., Kuskowski M. A., Nougayrede J. P. & Oswald E. Molecular epidemiology and phylogenetic distribution of the Escherichia coli pks genomic island. J Clin Microbiol 46, 3906–3911, doi: 10.1128/jcm.00949-08 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy A. J. et al. The genotoxin colibactin Is a determinant of virulence in Escherichia coli K1 experimental neonatal systemic infection. Infect Immun 83, 3704–3711, doi: 10.1128/iai.00716-15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. T. et al. Sequencing and analysis of the large virulence plasmid pLVPK of Klebsiella pneumoniae CG43. Gene 337, 189–198, doi: 10.1016/j.gene.2004.05.008 (2004). [DOI] [PubMed] [Google Scholar]
- Yeh K. M. et al. Capsular serotype K1 or K2, rather than magA and rmpA, is a major virulence determinant for Klebsiella pneumoniae liver abscess in Singapore and Taiwan. J Clin Microbiol 45, 466–471, doi: 10.1128/jcm.01150-06 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lery L. M. et al. Comparative analysis of Klebsiella pneumoniae genomes identifies a phospholipase D family protein as a novel virulence factor. BMC Biol 12, 41, doi: 10.1186/1741-7007-12-41 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C. H., Huang Y. T., Chang C. Y., Hsu H. S. & Hsueh P. R. Capsular serotypes and multilocus sequence types of bacteremic Klebsiella pneumoniae isolates associated with different types of infections. Eur J Clin Microbiol Infect Dis 33, 365–369, doi: 10.1007/s10096-013-1964-z (2014). [DOI] [PubMed] [Google Scholar]
- Lin J. C. et al. Genotypes and virulence in serotype K2 Klebsiella pneumoniae from liver abscess and non-infectious carriers in Hong Kong, Singapore and Taiwan. Gut Pathog 6, 21, doi: 10.1186/1757-4749-6-21 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. T. et al. Seroepidemiology of Klebsiella pneumoniae colonizing the intestinal tract of healthy Chinese and overseas Chinese adults in Asian countries. BMC Microbiol 12, 13, doi: 10.1186/1471-2180-12-13 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialek-Davenet S. et al. Genomic definition of hypervirulent and multidrug-resistant Klebsiella pneumoniae clonal groups. Emerg Infect Dis 20, 1812–1820, doi: 10.3201/eid2011.140206 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cejas D. et al. First isolate of KPC-2-producing Klebsiella pneumoniae sequence type 23 from the Americas. J Clin Microbiol 52, 3483–3485, doi: 10.1128/jcm.00726-14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. et al. Increasing occurrence of antimicrobial-resistant hypervirulent (hypermucoviscous) Klebsiella pneumoniae isolates in China. Clin Infect Dis 58, 225–232, doi: 10.1093/cid/cit675 (2014). [DOI] [PubMed] [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing: 22nd Informational Supplement M100-S22. Clinical and Laboratory Standards Institute, Wayne, PA. (2012).
- Hsieh P. F., Lin T. L., Lee C. Z., Tsai S. F. & Wang J. T. Serum-induced iron-acquisition systems and TonB contribute to virulence in Klebsiella pneumoniae causing primary pyogenic liver abscess. J Infect Dis 197, 1717–1727, doi: 10.1086/588383 (2008). [DOI] [PubMed] [Google Scholar]
- Tang H. L. et al. Correlation between Klebsiella pneumoniae carrying pLVPK-derived loci and abscess formation. Eur J Clin Microbiol Infect Dis 29, 689–698, doi: 10.1007/s10096-010-0915-1 (2010). [DOI] [PubMed] [Google Scholar]
- Turton J. F., Perry C., Elgohari S. & Hampton C. V. PCR characterization and typing of Klebsiella pneumoniae using capsular type-specific, variable number tandem repeat and virulence gene targets. J Med Microbiol 59, 541–547, doi: 10.1099/jmm.0.015198-0 (2010). [DOI] [PubMed] [Google Scholar]
- Brisse S. et al. wzi Gene sequencing, a rapid method for determination of capsular type for Klebsiella strains. J Clin Microbiol 51, 4073–4078, doi: 10.1128/jcm.01924-13 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y. J. et al. Capsular types of Klebsiella pneumoniae revisited by wzc sequencing. PloS one 8, e80670, doi: 10.1371/journal.pone.0080670 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S. B. et al. Establishment of a universal size standard strain for use with the PulseNet standardized pulsed-field gel electrophoresis protocols: converting the national databases to the new size standard. J Clin Microbiol 43, 1045–1050, doi: 10.1128/jcm.43.3.1045-1050.2005 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diancourt L., Passet V., Verhoef J., Grimont P. A. & Brisse S. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J Clin Microbiol 43, 4178–4182, doi: 10.1128/jcm.43.8.4178-4182.2005 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien T. F. & Stelling J. Integrated multilevel surveillance of the world’s infecting microbes and their resistance to antimicrobial agents. Clin Microbiol Rev 24, 281–295, doi: 10.1128/CMR.00021-10 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.