Abstract
Background
Plectin, a large linker protein found in many tissues, acts to connect components of the cytoskeleton to each other. In the epidermis, plectin binds keratin intermediate filaments to hemidesmosomes. A deficiency of plectin in the skin leads to blister formation in the basal layer and the disease epidermolysis bullosa simplex (EBS).
Hypothesis/Objectives
To describe a novel blistering disease that arose spontaneously in a litter of puppies.
Animals
Two female and one male 20-day-old Eurasier puppies, from a litter of six, were presented for evaluation of failure to thrive and then euthanized due to poor prognosis. The puppies had ulcers on the lips, tongue, nasal planum, paw pads and abdomen. Results – Immunolabelling on frozen skin for basement membrane proteins revealed patchy and weak to absent staining for plectin as compared with strong linear staining in normal dogs. Ultrastructurally, hemidesmosomes were irregularly shaped and had loss of distinction between inner and outer plaques. Pedigree analysis supported an autosomal recessive mode of inheritance. A premature stop codon was discovered in exon 27 of PLEC that resulted in the production of a severely truncated protein.
Conclusion
The study describes the first documented spontaneous EBS associated with a PLEC variant in domestic animals.
Résumé
Contexte
La plectine, une large protéine de liaison trouvée dans de nombreux tissus, agit en connectant entre eux les composants du cytosquelette. Dans l’épiderme, la plectine porte les filaments intermédiaires de kératine aux hémidesmosones. Une déficience en plectine dans la peau entraine un clivage dans la formation de la couche basale et la maladie EBS (epidermolysis bullosa simplex).
Hypothéses/Objectifs
Décrire une nouvelle maladie de clivage qui apparait spontanément dans une portée de chiots.
Sujets
Deux femelles et un m^ale chiots de 20 jours Eurasier, issus d’une portée de six, ont été présentés pour évaluation de défaut de croissance puis euthanasie liée au pronostic réservé. Les chiots présentaient des ulcéres sur les lévres, la langue, le planum nasal, les coussinets et l’abdomen.
Résultats
L’immunomarquage sur tissus congelés des protéines de la membrane basale a révélé une coloration faible á absente, en patch pour la plectine comparé avec une forte coloration linéaire pour les chiens normaux. Ultrastructurellement, les hémidesmosomes étaient irréguliers et avaient perdus leur distinction entre les plaques internes et externes. L’analyse du pédigrée a supporté le mode de transmission autosomal récessif. Un codon stop prématuré a été découvert dans l’exon 27 de PLEC qui résultait de la production de la protéine sévérement tronquée.
Conclusion
L’étude décrit le premier EBS spontané documenté avec un variant de PLEC chez les animaux domestiques.
Resumen
Introducción
la plectina, una proteína de gran tamaño de enlace encontrada en muchos tejidos, actúa para conectar los componentes del citoesqueleto entre ellos. En la epidermis, plectina une filamentos intermedios de queratina a hemidesmosomas. Una deficiencia de plectina en la piel conduce a la formación de ampollas en la capa basal y causa la enfermedad epidermolisis bullosa simplex (EBS).
Hipótesis / Objetivos
Describir una nueva enfermedad vesicular que surgió espontáneamente en una camada de cachorros.
Animales
Dos hembras y un macho de 20 días de edad de raza Eurasier, de una camada de seis, se presentaron para evaluación por falta de crecimiento y luego fueron sacrificados debido a un mal pronóstico. Los cachorros tenían úlceras en los labios, la lengua, plano nasal, almohadillas de las patas y el abdomen.
Resultados
la inmunodetección en piel congelada de las proteínas de la membrana basal reveló tinción irregular y débil a ausente para plectina en comparación con una fuerte tinción lineal en perros normales. Ultraestructuralmente, los hemidesmosomas eran de forma irregular con una pérdida de la diferenciación entre las placas interior y exterior. El análisis genealógico implica un modo de herencia autosómico recesivo. Un codón de parada prematuro fue descubierto en el exón 27 de PLEC que resultó en la producción de una proteína severamente truncada.
Conclusión
El estudio describe la primera EBS espontánea documentada asociado con una variante de PLEC defectuosa en animales domésticos.
Zusammenfassung
Hintergrund
Plectin, ein großes Verbindungsprotein, welches in vielen Geweben gefunden werden kann, dient dazu, verschiedene Komponenten des Zytoskelettes miteinander zu verbinden. In der Epidermis verbindet Plektin Intermediärfilamente von Keratin mit Hemidesmosomen. Ein Defizit an Plektin in der Haut führt zu Blasenbildung in der Basalschicht und der Krankheit namens Epidermolysis bullosa simplex (EBS).
Hypothese/Ziele
Die Beschreibung einer neuen blasenbildenden Erkrankung, die spontan bei einem Wurf von Hundewelpen auftrat.
Tiere
Zwei weibliche und ein männlicher 20 Tage alter Eurasierwelpen aus einem Wurf von sechs Wurfgeschwistern wurde vorgestellt, da sie nicht ausreichend wuchsen. Die Welpen wurden letztlich wegen einer schlechten Prognose eingeschläfert. Die Welpen hatten Ulzera an den Lippen, der Zunge, dem Nasenspiegel, den Zehenballen und am Bauch.
Ergebnisse
Die Immunmarkierung in Gefrierschnitten der Basalmembran der Haut zeigte eine fleckige und schwache bis fehlende Pektinfärbung im Vergleich zu einer starken linearen Färbung bei normalen Hunden. Ultrastrukturell zeigten sich die Hemidesmosomen in unregelmäßiger Form und hatten die Abgrenzung zwischen inneren und äußeren Plaques verloren. Eine Pedigree Analyse unterstützte einen autosomal rezessiven Modus der Vererbung. Ein frühreifes Stopcodon wurde in Exon 27 des PLEC entdeckt, welches in der Produktion eines stark abgekürzten Proteins resultierte.
Schlussfolgerung
Diese Studie beschreibt die erste dokumentierte spontane EBS im Zusammenhang mit einer PLEC Variante bei Haustieren.
Resumo
Contexto
A plectina, grande proteína de ligação encontrada em diversos tecidos, atua na ligação de componentes do citoesqueleto entre si. Na epiderme, a plectina liga a filamentos intermediários de queratina a hemidesmossomos. A deficiência de plectina na pele leva á formação de vesículas na camada basal e á doencá epidermólise bolhosa simples (EBS).
Hipótese/objetivos
Descrever uma nova doencá vesicular que surgiu espontaneamente em uma ninhada de filhotes de cães.
Animais
Duas fêmeas e um macho da racá Eurasier com 20 dias de vida, oriundos de uma ninhada de seis filhotes, foram apresentados para avaliação de deficiência de desenvolvimento e, posteriormente, eutanasiados devido ao mau prognóstico. Os filhotes apresentavam úlceras nos lábios, língua, plano nasal, coxins e abdômen.
Resultados
Imunomarcação de proteínas da membrana basal de fragmentos de pele congelados, revelaram marcação irregular e fraca a ausente para plectina, quando comparada com a marcação forte e linear em cães normais. Ultraestruturalmente, os hemidesmossomos tiveram formato irregular e perda da distinção entre placas internas e externas. Análise do pedigree corroborou com a existência de herancá autoss^ omica recessiva. Um códon de parada prematura foi descoberto no exon 17 de PLEC que resultou na produção de uma proteína intensamente truncada.
Conclusão
O estudo descreveu o primeiro relato de EBS associada á variante PLEC em animais domésticos.
Introduction
Epidermolysis bullosa (EB) is the name given to a group of blistering diseases that manifest as loss of epidermal–dermal integrity.1,2 The phenotypic changes are due to abnormalities in specific structural proteins within the epidermis and basement membrane zone. Characterization of EB is further subdivided into four major types based on the location of the subcellular defect. EB simplex (EBS) is the most superficial form and involves proteins in the cytoskeleton of basal or suprabasal keratinocytes. In junctional EB (JEB), blisters arise in the lamina lucida, whereas in dystrophic EB (DEB) the defect occurs in the superficial dermis at the level of anchoring fibrils. Kindler syndrome is a mixed pattern that has not been described in domestic animals.1,2 Light microscopy is generally unable to differentiate the subtypes of EB and some types of EB have undergone extensive reclassification. The current approach to classification of EB in humans is an “onion skinning” method based on (i) subcellular location of the blister, (ii) clinical features, (iii) heritability and (iv) identification of the gene involved by immunohistochemical and mutational analysis.2
In EBS, the cleavage plane lies within the epidermis and may involve basal keratinocytes (basal EBS) or keratinocytes within the middle to upper layers of the epidermis (suprabasal EBS). Proteins involved in basal EBS include keratins 5 and 14, plectin, BPAG1e (BP230), exophilin 5 and kindling 1. Suprabasal EBS may involve transglutaminase 5 in the upper epidermis, or plakoglobin, plakophilin 1 and desmoplakin in the middle epidermis. The clinical subtypes relate to extent of lesions (e.g. generalized severe EBS versus localized EBS) or the presence of concurrent conditions (e.g. EBS with mottled pigmentation, EBS with muscular dystrophy).2,4–6
In humans, three subsets of plectin-associated EBS have been identified: EBS with muscular dystrophy (EBS-MD OMIM # 226670), EBS with pyloric atresia (EBS-PA OMIM #612138) and EBS-Ogna. OMIM #131950). EBS-MD and EBS-PA are autosomal recessive and have skin lesions with abnormalities in other organs. EBS-Ogna is autosomal dominant and characterized by relatively mild blistering without lesions in other organs.4–6
Plectin, which is encoded by PLEC, is a large protein found in many tissues (skin, bone, muscle and nervous system); it links components of the cytoskeleton (e.g. actin microfilaments, microtubules, intermediate filaments) to the cell membrane.5,7,8 In basal keratinocytes, plectin and BPAG1e are the main components of the inner plaque of hemidesmosomes. Although all major forms of EB have been identified in domestic animals,9 this study documents the first spontaneous EBS associated with deleterious variant of plectin.
Materials and methods
Two female and one male 20-day-old Eurasier puppies, from a litter of six with an asymptomatic dam and sire, presented to the Matthew J. Ryan Veterinary Hospital of the University of Pennsylvania for failure to thrive. The puppies were underweight and approximately 50% smaller than the normal littermates. Physical examination revealed widespread ulcers on the lips, tongue, nasal planum, paw pads and abdomen with a positive Nikolskiy sign (Figure 1). The puppies were diagnosed with probable JEB and were humanely euthanized due to poor prognosis. At the time of death, peripheral blood was obtained for DNA analysis and skin samples were fixed in modified Karnosky’s fixative for ultrastructural analysis and snap frozen in liquid nitrogen for immunostaining and RNA analysis.
Figure 1.
Clinical images; Male and female 20-day-old Eurasier puppies with epidermolysis bullosa simplex. (a) Large ulcers on the paw pads, (b) inguinum and vulva, (c) prepuce and peripreputial skin, and (d) sloughing of the oral mucosa on the tongue.
Skin samples for conventional transmission EM (TEM) were processed by standard techniques to evaluate the basal layer and basement membrane zone (BMZ).10
Immunostaining for basement membrane proteins was performed to identify a defect in protein expression. Briefly, five micrometer frozen sections were cut from perilesional skin of the oral cavity (tongue or lip), pawpad and haired skin of the three EBaffected dogs. Sections were stained with a panel of antibodies specific for basement membrane proteins (BPAG1e, integrin alpha6 and beta4, collagen XVII, laminin 332 and collagen VII) as described previously11 with the addition of a rabbit polyclonal plectin antibody at dilution 1:100 (Table S1). All samples were stained for each individual protein on the same day to avoid any variability between assays. The pattern and intensity of staining were compared to those of normal controls.
Genomic DNA was extracted from EDTA blood obtained from all members of the family. Because there is no curated canine PLEC transcript or gene reference sequence available (RefSeq data from the National Center for Biotechnology Information, US National Library of Medicine, Bethesda, MD, USA), exons were determined by examination of the CanFam 3.1 reference genome assembly [NCBI assembly/GCF_000002285.3 (Broad CanFam3.1)] using the UCSC Genome Browser (http://genome.ucsc.edu/), including the Broad Institute CanFam3 Improved Annotation Data v1, which contains additional SNP and RNAseq data, including RNAseq from skin. Exons and the intron–exon boundaries of the canine PLEC gene were sequenced.
The human PLEC gene encodes several isoforms that differ primarily by the use of different first exons, which are followed by the same 31 additional exons to encode a protein of 4,684 amino acids for the most common isoform (RefSeq NM_201380). The canine orthologue of this transcript is encoded by 32 exons and codes for a protein of 4,686 amino acids that is 92.6% identical to the human protein. Primers (Table S2) for amplification of the canine exons were designed using commercially available software (DNAStar Inc.; Madison, WI, USA). Sequence data were analysed using Lasergene software (DNAStar Inc.) and the sequences compared to the respective canine PLEC region from the CanFam 3.1 reference sequence.
Results
Postmortem examination abnormalities were confined to the haired and nonhaired skin and oral cavity; significant lesions were not apparent in other organs including the gastrointestinal tract and skeletal muscle. Microscopic examination of lesions skin revealed clefts, vesicles and broad areas of epithelial detachment in both the haired skin and mucous membranes (lips, tongue, oral cavity, genitalia) with ulcers and fibrinosuppurative inflammation (Figure 2a). Periodic acid Schiff staining revealed weakly positive stain uptake on the floor of the blisters (Figure 2b).
Figure 2.
Photomicrographs of haired skin from Eurasier dog with epidermolysis bullosa simplex. (a) Note the large subepidermal vesicle. Haematoxylin and eosin 4×. (b) Weakly positive periodic acid Schiff staining (arrow) on the floor of the blister. 10×.
Transmission EM showed the lamina densa on the floor of the cleavage. Hemidesmosomes were located on the roof of the cleavage. The hemidesmosomes had an electron dense conical shape that attached to thickened keratin intermediate filaments. A loss of distinction was seen between inner and outer plaques (Figure 3).
Figure 3.
Electron microscopy of skin from Eurasier dog with epidermolysis bullosa simplex. (a) Eurasier puppy with separation in the basement membrane zone. Note the electron-dense hemidesmosomes (arrowheads) as compared with hemidesmosome in an aged matched control dog (b). Bar = 2 µm. (c) At higher magnification the hemidesmosomes are cone shaped (arrowheads) with loss of distinction between inner and outer plaques. (d) Normal hemidesmosomes from an age-matched control dog showing distinct inner and outer plaques (arrowheads). Bar = 0.5 µm
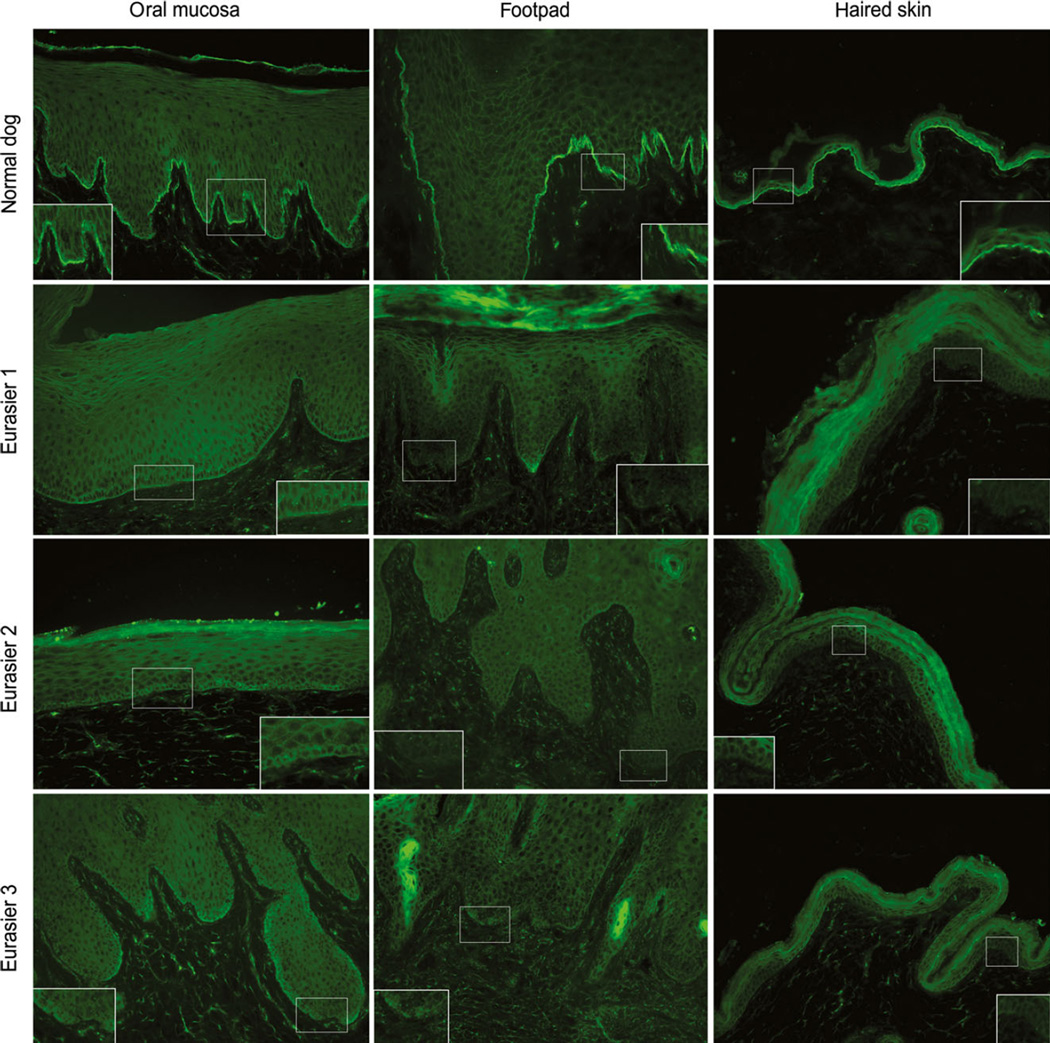
Indirect immunofluorescence for basement membrane proteins revealed patchy and weak to absent plectin staining as compared with strong linear staining in normal dogs (Figure 4); other stains were unremarkable. A microscopic cleft was seen in biopsies from two of the three Eurasier puppies and, when present, cleavage was located above the stains for laminin 332 and collagen VII (not shown). These anomalies, which were restricted to sections stained for plectin, suggested that the PLEC gene encoding plectin might harbour a DNA variant leading to a defective expression of this protein.
Figure 4.
Indirect immunofluorescence staining for plectin in epidermolysis bullosa (EB). Samples from the oral cavity, pawpad and haired skin from a normal dog and three EB-affected Eurasier puppies were immunostained for plectin on the same day. Whereas the staining for plectin was strong, linear and continuous at the basement membrane zone, that of the three Eurasier puppies was very faint and often patchy or absent. 20×
A homozygous non-sense variant was identified in the affected puppies. The variant was located in exon 27 and predicted to truncate more than 70% of the open reading frame. A restriction fragment length polymorphism (RFLP) assay was developed to screen for the variant. DNA was amplified and digested with BmtI restriction enzyme (Table S3), which digests DNA when the disease-associated allele is present. The parents of the affected puppies were heterozygous for this non-sense variant and a clinically normal littermate was homozygous for the wild-type sequence (Figure 5a).
Figure 5.
Pedigree and restriction fragment length polymorphism (RFLP) analysis. A PCR product of 606 bp was generated covering portions of exon 26 and 27 that contain the G>A non-sense polymorphism. The product is cleaved by BtmI in the presence of the variant (177 and 429 bp, respectively) but not in the wild-type sequence. Squares are males and circles females. Filled in symbols are affected animals (puppies 2, 3 and 4); half-filled symbols represent heterozygotes (sire 1 and dam 8; puppies 5 and 6); and empty symbols are homozygote normal animals (puppy 7). The gel depicts a homozygous wild-type, uncut band (7), homozygous affected, cut bands (2, 3 and 4), and heterozygotes, cut and uncut bands (1, 5, 6 and 8). The 177 bp fragment is not shown. M is100 bp ladder.
In order to verify that the variant found was not a polymorphism found in the general dog population, DNA was sampled from five different breeds (German shorthair pointer, American bulldog, Irish wolfhound, bull terrier and mixed breed; 25 dogs each). All 125 dogs were homozygous for the reference allele (G/G). Several silent and mis-sense variants in PLEC were also detected in DNA from the affected animal; many have been previously identified (Table S3).
Discussion
Plectin is an ubiquitous linker protein that serves to connect components of the cellular cytoskeleton to proteins in the skin, nervous system and skeletal muscle. The protein comprises four major domains: the amino-terminal or calponin homology domain, the plakin domain, the rod domain and the carboxy terminal domain;4–6,8,12 the different isoforms (i.e. 1a–g) correlate with tissue specificity (e.g. 1a and c for epidermis; 1d for skeletal muscle).4–6 Plectin 1a is the only isoform known to bind to the beta unit of the integrin α6;24 via the plakin domain, adjacent to the amino terminus, and to keratin intermediate filaments via its carboxy terminal domain. Therefore, plectin serves as a vital structural component of the BMZ; as a bridge between the inner cytoskeleton to the basal lamina.5,6
The importance of plectin in the BMZ was illustrated in the three Eurasier dogs in which a homozygous G to A variant in the PLEC gene resulted in the conversion of a tryptophan to a premature stop codon in exon 27. This resulted in a truncated 1,315 amino acid protein (normal 4,686 amino acids) and thus the loss of a large portion of the rod domain and, more importantly, the C terminus that would bind to the keratin intermediate filaments. In this family of dogs, review of the pedigree, together with the mutational analysis, confirmed an autosomal recessive mode of inheritance.
Autosomal recessive, basal forms of EBS in humans include EBS with muscular dystrophy (EBS-MD) and EBS with pyloric atresia (EBS-PA). KRT5, KRT14, BPAG1 and PLEC are the genes most commonly affected in cases of basal EBS in humans; variants in PLEC only account for about 8% of the cases.13 A number of variants in human PLEC have been deemed responsible for EBS and the location of the variant will dictate the severity of disease.14,15 EBS-MD is characterized by mild to subtle blistering and nail dystrophy early in life with variable onset muscular weakness (i.e. congenital to as late as the 4th decade). EBS-PA has a more severe congenital phenotype (often lethal) with severe skin blistering and pyloric or duodenal atresia. It has been hypothesized that the pyloric stenosis is related to localized blistering and chronic inflammation.12 Like the Eurasier puppies, the severe EBS-PA phenotype may result from plectin variants that affect the rod and carboxy domain and thereby impair binding to keratin and possibly to integrin β4 as an alternative binding site.4,5
In the affected dogs, the hemidesmosomes were abnormally electron dense and globular to ovoid with thickening of the connecting tonofilaments. The thickening of keratin may represent a compensatory change due to the truncated protein and failure to cross-link. Furthermore, immunofluorescence demonstrated a marked decrease to absence in plectin staining. This finding led to the discovery of the candidate gene. However, based on the C-terminal location of the epitope in the polyclonal antibody, one would predict a complete lack of plectin staining. The light patchy staining is most likely explained by cross-reacting amino acid sequence homology with other basement membrane proteins.
Based on light microscopy and ultrastructural clefting, EB in this group of Eurasier puppies was initially classified as JEB, and the more severe form, Herlitz (now termed JEB generalized severe). Identification of the PLEC variant led to the appropriate categorization as basal EBS: (i) the subcellular location of the defect lies in the basal keratinocyte; (ii) clinical features were generalized; (iii) the disorder was shown to have an autosomal recessive mode of inheritance; and (iv) the variant was discovered in PLEC and resulted in perturbation of the target protein plectin. This form of EBS is not characterized by cytolysis of basal keratinocytes as seen in EBS due to variants of keratin 5 and 14.2 Light microscopy is unable to distinguish severe JEB from basal plectin-associated EBS.
The Eurasier puppies had severe lesions confined to the skin and oral cavity as documented on complete post-mortem examination. Weakening of the dermo-epidermal integrity lead to blistering and skin sloughing in areas prone to frictional trauma (e.g. oral cavity, genitalia, paw pads). Sloughing of the oral mucosa may have led to nutritional deprivation as evidenced by the small size as compared to the littermates. It is unknown if the dogs eventually would have developed either PA or MD, as they were humanely euthanized at 20 days of age.
Treatment of the various forms of EB is similar: wound care, preventing and treating infection, as well as supportive care including pain management and nutritional support. A few clinical trials have been performed or are underway in humans.3 This is the first canine model of EBS in which a variant in PLEC has been demonstrated. Studies herein may advance the understanding of PLEC and EBS pathogenesis and provide an avenue to explore new therapies such as topical protein replacement, viral vector gene therapy, small interfering (si)RNAs or gene editing by Clustered Regularly Interspaced Short Palindromic Repeats (referred to as CRISPR).
Supplementary Material
Acknowledgments
Source of Funding: US Department of Health and Human Services; National Institutes of Health – RR02152 (P40 OD010939).
The authors would like to thank Tosso Leeb (University of Bern) for his generous review of this manuscript.
Footnotes
This article is based on a Supporting Original Study presented at the 8th World Congress of Veterinary Dermatology held May 2016 in Bordeaux, France.
Conflict of Interest: No conflicts of interest have been declared.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Table S1. Antibodies used for indirect immunofluorescence for basement membrane proteins.
Table S2. Primers and restriction fragment lengths for detection of disease-associated PLEC non-sense variant.
Table S3. Single nucleotide polymorphisms identified in PLEC exons by sequencing DNA from an affected Eurasier dog.
References
- 1.Natsuga K, Shinkuma S, Nishie W, et al. Animal models of epidermolysis bullosa. Dermatol Clin. 2010;28:137–142. doi: 10.1016/j.det.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Fine JD, Bruckner-Tuderman L, Eady RA, et al. Inherited epidermolysis bullosa: updated recommendations on diagnosis and classification. J Am Acad Dermatol. 2014;70:1103–1126. doi: 10.1016/j.jaad.2014.01.903. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Doval I, Davila-Seijo P, Langan SM. Updated systematic review of randomized controlled trials of treatments for inherited forms of epidermolysis bullosa. Clin Exp Dermatol. 2013;38:92–94. doi: 10.1111/j.1365-2230.2012.04419.x. [DOI] [PubMed] [Google Scholar]
- 4.Natsuga K. Plectin-related skin diseases. J Dermatol Sci. 2015;77:139–145. doi: 10.1016/j.jdermsci.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Castanon MJ, Walko G, Winter L, et al. Plectin-intermediate filament partnership in skin, skeletal muscle, and peripheral nerve. Histochem Cell Biol. 2013;140:33–53. doi: 10.1007/s00418-013-1102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winter L, Wiche G. The many faces of plectin and plectinopathies: pathology and mechanisms. Acta Neuropathol. 2013;125:77–93. doi: 10.1007/s00401-012-1026-0. [DOI] [PubMed] [Google Scholar]
- 7.Ortega E, Buey RM, Sonnenberg A, et al. The structure of the plakin domain of plectin reveals a non-canonical SH3 domain interacting with its fourth spectrin repeat. J Biol Chem. 2011;286:12,429–12,438. doi: 10.1074/jbc.M110.197467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiche G. Role of plectin in cytoskeleton organization and dynamics. J Cell Sci. 1998;111:2477–2486. doi: 10.1242/jcs.111.17.2477. [DOI] [PubMed] [Google Scholar]
- 9.Medeiros GX, Riet-Correa F. Epidermolysis bullosa in animals: a review. Vet Dermatol. 2015;26:3–13. e1–e2. doi: 10.1111/vde.12176. [DOI] [PubMed] [Google Scholar]
- 10.Andziak B, Buffenstein R. Disparate patterns of age-related changes in lipid peroxidation in long-lived naked mole-rats and shorter-lived mice. Aging Cell. 2006;5:525–532. doi: 10.1111/j.1474-9726.2006.00246.x. [DOI] [PubMed] [Google Scholar]
- 11.Olivry T, Poujade-Delverdier A, Dunston SM, et al. Absent expression of collagen XVII (BPAG2, BP180) in canine familial localized junctional epidermolysis bullosa. Vet Dermatol. 1997;8:203–212. doi: 10.1046/j.1365-3164.1997.d01-17.x. [DOI] [PubMed] [Google Scholar]
- 12.Maman E, Maor E, Kachko L, et al. Epidermolysis bullosa, pyloric atresia, aplasia cutis congenita: histopathological delineation of an autosomal recessive disease. Am J Med Genet. 1998;78:127–133. [PubMed] [Google Scholar]
- 13.Bolling MC, Jongbloed JD, Boven LG, et al. Plectin mutations underlie epidermolysis bullosa simplex in 8% of patients. J Invest Dermatol. 2014;134:273–276. doi: 10.1038/jid.2013.277. [DOI] [PubMed] [Google Scholar]
- 14.Pfendner E, Rouan F, Uitto J. Progress in epidermolysis bullosa: the phenotypic spectrum of plectin mutations. Exp Dermatol. 2005;14:241–249. doi: 10.1111/j.0906-6705.2005.00324.x. [DOI] [PubMed] [Google Scholar]
- 15.Pfendner E, Uitto J. Plectin gene mutations can cause epidermolysis bullosa with pyloric atresia. J Invest Dermatol. 2005;124:111–115. doi: 10.1111/j.0022-202X.2004.23564.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.