Abstract
A meta-analysis to compare ocular biometry measured by optical low-coherence reflectometry (Lenstar LS900; Haag Streit) and partial coherence interferometry (the IOLMaster optical biometer; Carl Zeiss Meditec). A systematic literature search was conducted for articles published up to August 6th 2015 in the Cochrane Library, PubMed, Medline, Embase, China Knowledge Resource Integrated Database and Wanfang Data. A total of 18 studies involving 1921 eyes were included. There were no statistically significant differences in axial length (mean difference [MD] 0 mm; 95% confidence interval (CI) −0.08 to 0.08 mm; p = 0.92), anterior chamber depth (MD 0.02 mm; 95% CI −0.07 to 0.10 mm; p = 0.67), flat keratometry (MD −0.05 D; 95% CI −0.16 to 0.06 D; p = 0.39), steep keratometry (MD −0.09 D; 95% CI −0.20 to 0.03 D; p = 0.13), and mean keratometry (MD −0.15 D; 95% CI −0.30 to 0.00 D; p = 0.05). The white to white distance showed a statistically significant difference (MD −0.14 mm; 95% CI −0.25 to −0.02 mm; p = 0.02). In conclusion, there was no difference in the comparison of AL, ACD and keratometry readings between the Lenstar and IOLMaster. However the WTW distance indicated a statistically significant difference between the two devices. Apart from the WTW distance, measurements for AL, ACD and keratometry readings may be used interchangeability with both devices.
Accurate ocular biometry is imperative for the management of cataract1,2,3,4. The parameters of axial length (AL), anterior chamber depth (ACD), keratometry (K) and white to white (WTW) distance are required in intraocular lens (IOL) power calculation, and for the implantation of phakic IOLs. Inaccurate measurement of AL, ACD, and corneal power contribute to 36%, 42%, and 22%, respectively, of the error in predicted refraction of an IOL using optical biometry1,5.
The IOLMaster (Carl Zeiss Meditec, Jena, Germany) was introduced in 1999 as the first optical biometer6,7. This non-contact technology made it possible to evaluate all the required parameters with a single device8,9. It utilities partial coherence interferometry (PCI) to measure AL with a 780 nm laser diode infrared light. ACD measurement is based on an optical section through the anterior chamber by means of a slit-illumination system with subsequent image assessment. The measurement of K readings in the flat and steep meridian is achieved by analyzing a pattern of light-emitting diodes (LEDs) imaged by the corneal front surface5. An image analysis system is used to measure the WTW distance. Each of the IOLMaster’s assessments requires realignment of the device with the visual axis of the eye. This device has been shown to provide highly repeatable measurements regardless of the examiner’s training and accurate measures in both long and short eyes8,9,10,11,12. In addition, it is also non-invasive and acquisition time is short which has led to the IOLMaster becoming the most popular biometry device used in the clinical setting around the world13.
The Lenstar (Haag-Streit, Koeniz, Switzerland), a more recent biometer to the market, has been the first competitor of the IOLMaster. It uses optical low-coherence reflectometry (OLCR) with a 820 nm laser diode infrared light to measure AL and ACD. Similar technology to the IOLMaster is used to measure K and WTW. In comparison to the IOLMaster, the Lenstar is capable of acquiring more parameters, such as corneal thickness and crystalline or intraocular lens thickness7,14,15. Unlike the IOLMaster, the Lenstar is capable of capturing all measurements simultaneously without the need for realignment14,15.
Many comparative studies have been conducted assessing the differences between common measurement parameters with both these devices. However, there have been some conflicting and inconsistent findings5,9,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31. This may in part be caused by small study sample sizes, the age or nationality of the patients, use of eyes with or without eye diseases. The aim the present meta-analysis is to combine the results of all comparative studies (IOLMaster versus Lenstar) to determine which parameters are interchangeable.
Results
Trial selection
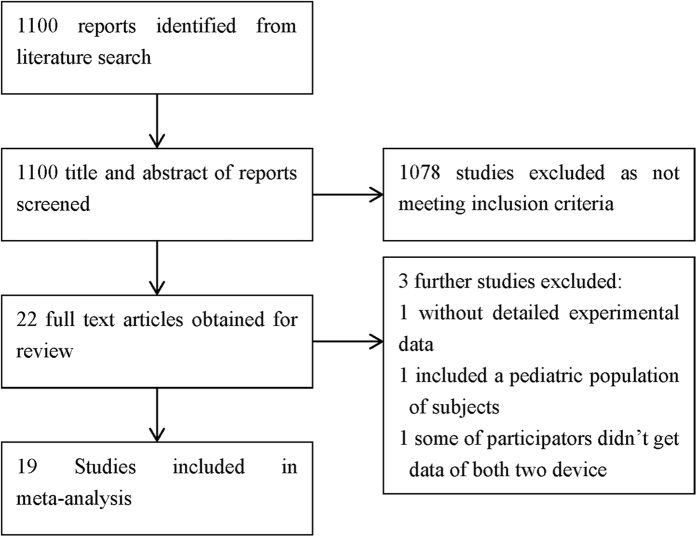
The selection flowchart is displayed in Fig. 1. The literature search identified 1100 articles. These 1100 articles and their associated abstracts were screened and 1078 were excluded. The full-text of the remaining 22 articles were obtained and a further 4excluded. The first was excluded due to the lack of the detailed experimental data32, second one because patients were children33, the third one was some of participators did not obtain data from both devices26, and the last one was a duplicate study30. Thus, the final 18 trials that met our inclusion criteria were included in the meta-analysis5,9,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,33.
Figure 1. Selection flowchart of the studies included in the present meta-analysis.
Trial characteristics
The characteristics of the eligible studies are summarized in Table 15,9,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,33. Some articles lack certain information, such as the proportion of male to female subjects. In such cases, the parameter is marked as unavailable.
Table 1. Baseline characteristics.
| Author and year | Country | Number of subjects | Number of eyes | Male:Female ratio | Mean age (±SD) | Eye disease | Parameters |
|---|---|---|---|---|---|---|---|
| Olga 201531 | Israel | 48 | 73 | 21:27 | 63.2 ± 8.75 | IOL eyes | AL, ACD, Km, WTW |
| Lin 201429 | China | 76 | 76 | 35:41 | 68.1 ± 7.5 | Cataract | AL, ACD, Kf, Ks, WTW |
| Liu 201328 | China | 205 | 205 | 122:83 | 44.3 ± 5.0 | Normal eyes | AL, ACD, Rf, Rs, WTW |
| Huang 201225 | China | 98 | 157 | 43:55 | 71.6 ± 7.5 | Cataract | AL, ACD, Kf, Ks, Km |
| Wang 201227 | China | 40 | 80 | 26:14 | 68.2 ± 7.7 | Cataract | AL ACD |
| Sheng 201230 | China | 72 | 122 | 38:34 | 64.6 ± 13.4 | Cataract | AL, ACD, Kf, Ks, Km |
| Huang 2012(2)23 | China | 43 | 43 | 23:20 | 22.1 ± 4.7 | Normal | AL, ACD, Kf, Ks, Km, WTW |
| Ye 201122 | China | 30 | 60 | 10:20 | 62.5 ± 7.7 | Cataract | AL, ACD, Km |
| Hildebrandt 201120 | Germany | 140 | 137 | IOL eyes | AL | ||
| Georgios 201121 | Austria | 51 | 51 | 29:22 | 68 ± 11 | Cataract | AL, ACD, Km |
| Chen 201119 | Austria | 59 | 72.6 | Cataract | AL, ACD, Km, WTW | ||
| Kenneth 201018 | USA | 50 | 50 | 21:29 | 74.13 ± 7.12 | Cataract | AL, ACD Km |
| 50 | 50 | 24:26 | 66.22 ± 6.42 | Normal | |||
| Rabsilber 20109 | Germany | 100 | 100 | 70 ± 10.6 | Cataract | AL, ACD, Rf, Rs | |
| Buckhurst 200914 | UK | 112 | 112 | 36:76 | 76.4 ± 9.1 | Cataract | AL, ACD, Kf, Ks, WTW |
| Holzer 200918 | Germany | 100 | 200 | 27.25 ± 10.32 | Normal | AL, ACD, Kf, Ks | |
| Liampa 200917 | USA | 145 | 145 | 73.5 ± 9.5 | Cataract | AL, ACD | |
| Kaspar 20095 | America | 80 | 144 | 34:46 | 66.9 | Mixed | AL, ACD, Rf, Rs |
| Cruysberg 200915 | Netherlands | 38 | 76 | 25:23 | 25.9 ± 8.5 | Normal | AL, ACD, Rf, Rs |
AL = Axial length, ACD = anterior chamber depth, Kf = keratometry along the flattest meridian, Ks = keratometry along the steepest meridian, Km = mean keratometry, Rf = radius of curvature in the flattest meridian, Rs = radius of curvature in the steepest meridian, WTW = white to white, SD = standard deviation.
Trial Quality
Quality assessment of the included studies is shown in Fig. 2. Two independent researchers conducted the evaluation. In general, the quality of included studies was high.
Figure 2. Quality assessment of the included studies.
Analysis of AL
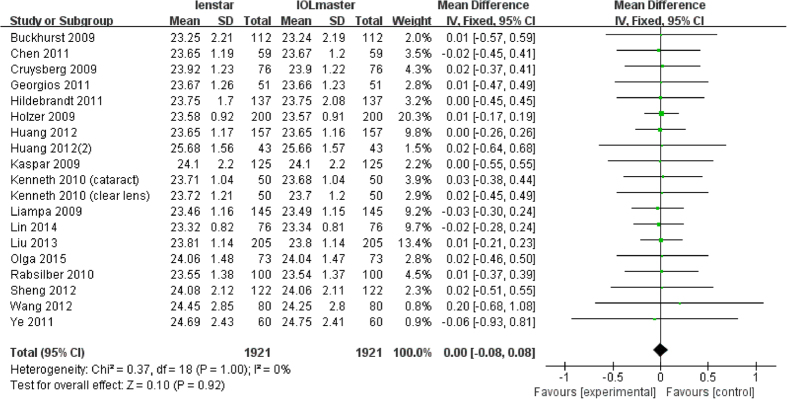
All 18 articles measured AL with both devices with a total number of 1921 eyes. The results of the meta-analysis showed that there were no statistically significant differences in the final analysis of AL (MD 0.00 mm; 95% CI −0.08 to 0.08 mm; p = 0.92). The forest plot is shown in Fig. 3.
Figure 3. Comparison of AL measurement with the Lenstar and IOLMaster.
df = degree of freedom; I2 = extent of inconsistency; Z = overall effect.
Analysis of ACD
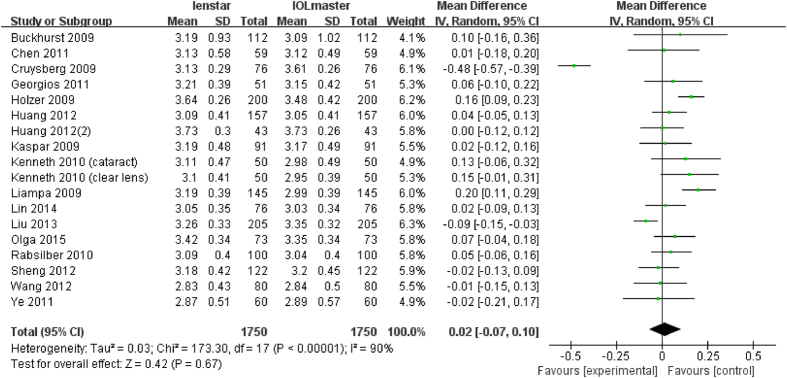
There were 18 articles which measured ACD with a total of 1750 eyes. Liu et al. found a statistically significant lower ACD measurement with the Lenstar whereas Holzer et al. and Liampa et al. found the opposite16,17,28. The meta-analysis showed no statistically significant differences in the final analysis of ACD (MD 0.02 mm; 95% CI −0.07 to 0.10 mm; p = 0.67) between the Lenstar and IOLMaster (Fig. 4).
Figure 4. Comparison of ACD measurement with the Lenstar and IOLMaster.
df = degree of freedom; I2 = extent of inconsistency; Z = overall effect.
Analysis of Kf
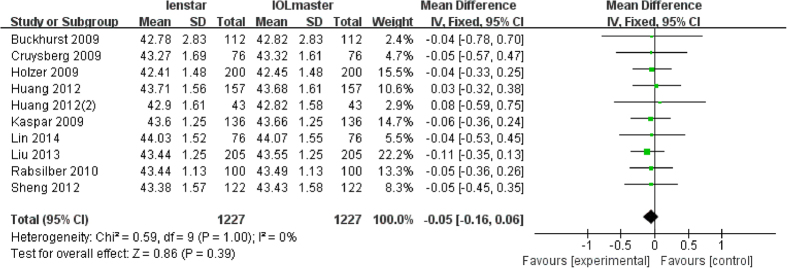
There were 10 articles which measured Kf with a total of 1227 eyes. None of these studies reported a statistically significant difference in the measurement of Kf with both devices. Further, the meta-analysis indicated no statistically significant difference (MD −0.05 D; 95% CI −0.16 to 0.06 D; p = 0.39) (Fig. 5).
Figure 5. Comparison of Kf measurement with the Lenstar and IOLMaster.
df = degree of freedom; I2 = extent of inconsistency; Z = overall effect.
Analysis of Ks
There were 10 articles which measured Ks with a total of 1227 eyes. None of these studies reported a statistically significant difference in the measurement of Ks with both devices. Combining the results, the meta-analysis also showed there was no statistically significant difference (MD −0.09 D; 95% CI −0.20 to 0.03 D; p = 0.13) (Fig. 6), i.e. the Lenstar provides a flatter Ks reading than the IOLMaster.
Figure 6. Comparison of Ks measurement with the Lenstar and IOLMaster.
df = degree of freedom; I2 = extent of inconsistency; Z = overall effect.
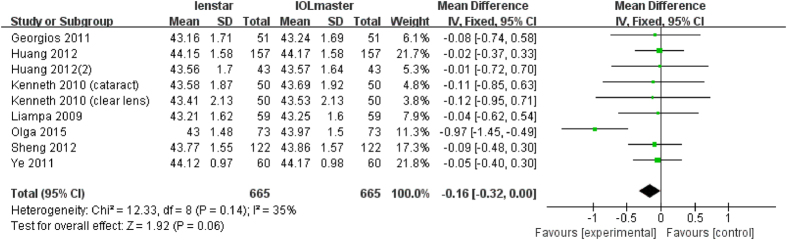
Analysis of Km
There were 10 articles which measured Km with a total of 790 eyes. The study by Olga et al. found a statistically significant flatter Km reading with the Lenstar31. The remaining 9 studies found no difference. The meta-analysis found no statistically significant difference (MD −0.15 D; 95% CI −0.30 to 0.00 D; p = 0.05) (Fig. 7).
Figure 7. Comparison of Km measurement with the Lenstar and IOLMaster.
df = degree of freedom; I2 = extent of inconsistency; Z = overall effect.
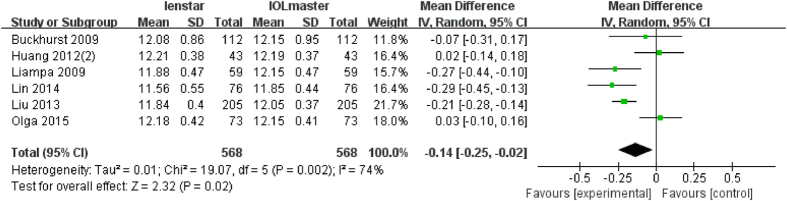
Analysis of WTW
There were 6 articles which measured WTW with a total of 568 eyes. Of these, 3 articles found a statically significant lower WTW distance with the Lenstar17,28,29. The meta-analysis also found a statistically significant lower WTW distance with the Lenstar compared to the IOLMaster (MD −0.14 mm; 95% CI −0.25 to −0.02 mm; p = 0.02) (Fig. 8).
Figure 8. Comparison of WTW measurement with the Lenstar and IOLMaster.
df = degree of freedom; I2 = extent of inconsistency; Z = overall effect.
Sensitivity analysis
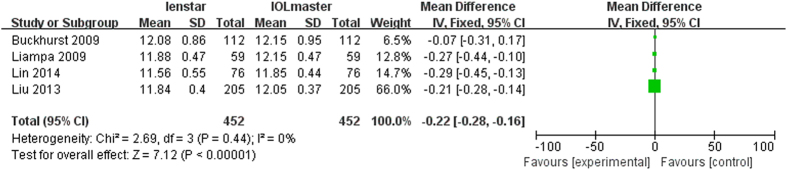
For each parameter investigated the effect of statistical modeling (fixed-effect model or random-effect model) was evaluated with the results shown in Table 2. The primary heterogeneity of ACD was high, so the sensitivity analysis was performed by excluded Crusberg et al.15, and the heterogeneity decreased from 90% to 65%, the subjects in the study were found to be younger (all under 50) than in other studies. Another three studies16,23,28 were also excluded since the mean age was younger than 50. The heterogeneity decreased to 20% after those studies (Fig. 9) were excluded. With these exclusions, the overall results did change to become statistically significantly different (p = 0.0003). The same method was performed for WTW. With the removal of two studies: Olga et al.31 and Huang et al.23, the heterogeneity decreased from 74% to 0% (Fig. 10). The difference between these two trials and others is that the eyes included had clear lenses whilst the other studies included eyes with lens opacities. The overall results did not change with their removal (p < 0.001).
Table 2. Sensitivity analysis performed by evaluating the effect of the statistical model.
| Parameters | Fixed-effect model |
Random-effect model |
||
|---|---|---|---|---|
| MD (95% CI) | P | MD (95% CI) | P | |
| AL (mm) | 0.00 (−0.08, 0.08) | 0.92 | 0.00 (−0.08, 0.08) | 0.92 |
| ACD (mm) | 0.00 (−0.02, 0.03) | 0.91 | 0.02 (−0.07, 0.10) | 0.67 |
| Kf (D) | −0.05 (−0.16, 0.06) | 0.39 | −0.05 (−0.16, 0.06) | 0.39 |
| Ks (D) | −0.09 (−0.20, 0.03) | 0.13 | −0.09 (−0.20, 0.03) | 0.13 |
| Km (D) | −0.15 (−0.30, 0.00) | 0.05 | −0.16 (−0.34, 0.03) | 0.09 |
| WTW (mm) | −0.16 (−0.21, −0.10) | <0.001 | −0.14 (−0.25, −0.02) | 0.02 |
AL = Axial length, ACD = anterior chamber depth, Kf = keratometry in the flattest meridian, Ks = keratometry in the steepest meridian, Km = mean keratometry, WTW = white to white, MD = Mean Difference, CI = confidence interval.
Figure 9. Comparison of ACD measurement with the Lenstar and IOLMaster (mean age > 50).
df = degree of freedom; I2 = extent of inconsistency; Z = overall effect.
Figure 10. Comparison of WTW measurement with the Lenstar and IOLMaster (exclude Olga2015 and Huang2012(2)).
df = degree of freedom; I2 = extent of inconsistency; Z = overall effect.
Subgroup analysis
Subgroup analyses were performed based on the types of eye diseases. Studies were grouped as either eyes with cataract9,14,17,19,20,21,22,24,25,26,27,29,30,31,33 or other eyes5,15,16,18,23,28. An analysis of the ACD in eyes with cataract indicated a statistically significant (p = 0.001) lower measurement with the IOLMaster. On the contrary, there was a statistically significant (p = 0.006) lower measurement with the Lenstar in other eyes. An analysis of Km indicated a statistically significant (p = 0.04) lower/flatter measurement with the Lenstar in the cataract eyes subgroup (MD −0.16 D) but no statistically significant difference in the other eyes group (p = 0.84). The Lenstar was found to measure a lower WTW distance in the both the cataract and other eyes group (p < 0.0001). Full results of the subgroup analysis is shown in Table 3.
Table 3. Subgroup analysis (cataract and other eyes).
| Parameters | Overall |
Cataract subgroup |
Other eyes subgroup |
|||
|---|---|---|---|---|---|---|
| MD (95% CI) | P-value | MD (95% CI) | P-value | MD (95% CI) | P-value | |
| AL (mm) | 0.00 (−0.08,0.08) | 0.92 | −0.00 (−0.11,0.11) | 0.97 | 0.01 (−0.11,0.13) | 0.85 |
| ACD (mm) | 0.02 (−0.07,0.10) | 0.67 | 0.06 (0.02,0.10) | 0.001 | −0.05 (−0.08,−0.01) | 0.006 |
| Kf (D) | −0.05 (−0.16,0.06) | 0.39 | −0.03 (−0.21,0.15) | 0.77 | −0.07 (−0.21,0.08) | 0.38 |
| Ks (D) | −0.09 (−0.02,0.03) | 0.13 | −0.09 (−0.27,0.09) | 0.32 | −0.08 (−0.22,0.06) | 0.26 |
| Km (D) | −0.15 (−0.30,0.00) | 0.05 | −0.16 (−0.32,0.00) | 0.04 | −0.06 (−0.59, 0.48) | 0.84 |
| WTW (mm) | −0.16 (−0.21,−0.10) | <0.001 | −0.14 (−0.22,−0.06) | <0.001 | −0.17 (−0.24, −0.10) | <0.001 |
AL = Axial length, ACD = anterior chamber depth, Kf = keratometry in the flattest meridian, Ks = keratometry in the steepest meridian, Km = mean keratometry, WTW = white to white, MD = Mean Difference, CI = confidence interval.
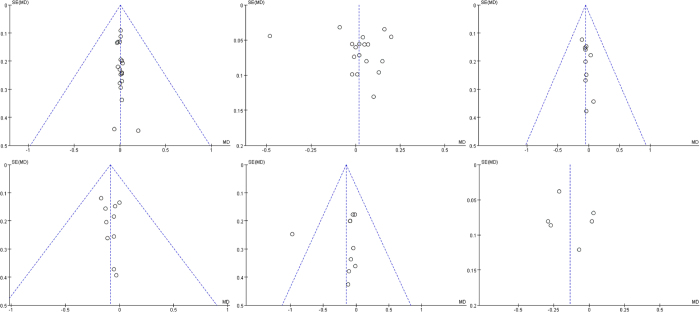
Publication Bias
The publication bias test was performed separately for each parameter. The funnel plot for all parameters is shown in Fig. 11. The funnel plot for the parameters AL, Kf and Ks are symmetry, while other three parameters (ACD, Km and WTW) are not which suggests possible publication bias.
Figure 11. The forest figures of every parameter.
The horizontal axis was MD (Mean Difference), ordinates axis was SE (Standard Error).
Dicussion
In the 1990s, ultrasound derivation of AL and manual keratometry measurements were the gold standard in ophthalmology to determine the power of IOL during cataract surgery. The introduction of PCI biometry and automated keratometry measurements was an important step in the field of cataract and refractive surgery11,16. The IOLMaster was the first device to achieve this in 19997. In more recent years, there have been a number of new non-invasive, non-contact biometers developed, one such is the Lenstar which uses OLCR34.
Our meta-analysis evaluates optical biometry measurements between the Lenstar and IOLMaster by comparing the six common measurement parameters. There was no significant difference in AL measurements between the Lenstar and the IOLMaster, and the results indicate excellent agreement between the two devices. Eibschitz-Tsimhoni et al. found that a 0.1 mm error value in AL measurement can produce 0.2 to 0.35 D of refractive error35. Rabsilber et al.9, Holzer et al.16, Hoffer et al.18 and Buckhurst et al.14 have all reported a longer AL measurement with the OLCR unit but none of these reached statistical significance. The 95% LoA confirmed the very high level of agreement between the 2 devices9,14,16,18. Compared to PCI (laser diode infrared light of wavelength 700 nm) used with the IOLMaster, the OLCR (superluminescent diode infrared light of wavelength 830 nm) used with the Lenstar has a stronger penetrating power and a higher signal-to-noise ratio. In this study, the difference between the two devices machines is small and not of clinical significance.
In this study the results of the meta-analysis indicated that there were no statistically significant differences in the analysis of the ACD measurement. The IOLMaster measures ACD using lateral-slit illumination at approximately 38 degrees to the optical axis. The calculation of ACD is by image analysis of the distance between the corneal epithelium and the anterior surface of the crystalline lens. Further, ACD image analysis as performed with the IOLMaster is also influenced by measurements of the keratometry5,29. The Lenstar uses OLCR technology superluminescent LED (830 nm) as light. It scans 16 times in one measurement to detect the corneal thickness from epithelium to endothelium and the distance from the endothelium to the anterior surface of the crystalline lens which represents the anatomical ACD25. A subgroup analysis of the ACD in eyes with cataract indicated a statistically significant (p = 0.003) lower measurement with the IOLMaster but the opposite was found in eyes without cataract. However the mean difference is of magnitude 0.05 mm which is perhaps not clinically significant. The different results of two subgroups may come from the opacities from eyes with cataract compared to the clear lens in normal eyes.
As for K, the meta-analysis result indicated no statistical significant difference between the two devices. In previous study, Olga et al.31 found a significant difference in K values (p = 0.031) between the Lenstar and the IOLMaster, but they did not consider the difference to be clinically relevant. The Lenstar takes readings in two circles, 16 points in each circle. The diameter of the inner circle is 1.65 mm, and the outer circle is 2.3 mm. However, the IOLMaster just has one circle which has a diameter of 2.3 mm, and takes readings from 6 points5,29. Because of more superluminescent EDs imaged on a camera, the Lenstar may have a more repetitive and accurate measurement. In IOL calculation, a 1.0 D measuring error in K-reading may cause an error between 0.9 to 1.4 D in IOL power22. A subgroup analysis indicated that the Lenstar measured Km lower/flatter than the IOLMaster in the cataract group. The magnitude of the mean difference was 0.16 D which is perhaps not clinically significant.
The comparison of the WTW distance between the two instruments showed a statistically significant lower measurement with the Lenstar compared to the IOLMaster. Both devices use image analysis systems to measure WTW, but the resolution of the two image analysis systems is different and hence may explain a possible reason for the differences found in this study. The IOLMaster uses slit illumination whereas the Lenstar uses OLCR and a high-definition image sensor. Based on these results, the WTW measurements with the Lenstar and the IOLMaster cannot be used interchangeably. Previous studies have found a similar trend17,28,29.
There are some limitations to this study. The patient groups included those with cataract and normal eyes, although a subanalysis was performed. The performance of these devices in other eye disease such as corneal disease and retinal disease has not been assessed. The study was also limited to adult patients and hence is not applicable to pediatric populations.
In summary, this meta-analysis shows that the Lenstar provides similar results to the gold standard IOLMaster for AL, ACD and K-readings. Hence, both devices can be used interchangeably for these parameters. The WTW distance was found to be different and cannot be used interchangeably.
Patients and Methods
Search strategy
A literature search of the Cochrane Library, PubMed, Medline, Embase, China Knowledge Resource Integrated Database and Wanfang Data was performed by J.H.H. and Y.Y.H. The keywords and Medical Subject Headings were “Lenstar” or “IOLMaster” or “optical low-coherence reflectometry” or “optical low-coherence reflectometer” or “partial coherence interferometry” or “partial coherence interferometer”, the time limit was up to August 6th 2015, and the language was not limited.
Trials Selection
Studies fulfilling the following inclusion criteria were included in the present meta-analysis: (1) Adults; (2) Measurements acquired with both the Lenstar and IOLMaster with the same operator with a short time interval between the two devices; (3) Original data provided in the results section. The full-text of articles with ambiguous titles or abstracts were reviewed for eligibility.
Parameter Extraction
A customized form for parameter extraction was used to record the study authors, publication year, country of origin, number of patients and eyes, proportion of male to female, average patient age, eye disease and other pertinent parameters to the study recorded.
Qualitative Assessment
The Quality Assessment of Diagnostic Accuracy Studies (QUADAS) tool was used to assess methodological quality36,37. The tool is structured as a list of 14 questions which should each be answered “yes”, “no”, or “unclear”. The majority of items included in QUADAS relate to bias (items 3, 4, 5, 6, 7, 10, 11, 12 and 14), with two items each relating to variability (items 1 and 2) and three relating to reporting (items 8, 9 and 13)36,37. Questions 1, 2, 12, 13 and 14 were assessed to be unsuitable for this study, hence the remaining 9 items were chosen to assess study quality.
Outcomes
The following common parameters to both the Lenstar and IOLMaster were assessed in this review: AL (mm), ACD (mm), keratometry in the flattest meridian (Kf in diopters [D]), keratometry in the steepest meridian (Ks in D), mean keratometry (Km = (Kf + Ks)/2 in D) and WTW (mm). Original parameters were obtained from the articles as far as possible and parameters that could not be obtained were calculated if possible. Corneal curvatures were analyzed using the refractive index 1.3375 in all but one study which reported a refractive index of 1.33214. In this study, the data were recalculated using a refractive index of 1.3375. One article obtained measurements with both devices in a group of 50 subjects with cataract and 50 without cataract18. These two groups were assessed separately.
Statistical Analysis
The data obtained was entered into the RevMan (version 5.2) statistical analysis software. Heterogeneity between studies was assessed by the I2 statistic. The 95% confidence interval (CI) estimates were calculated by taking a weighted average of individual study results using a fixed effects model. If the heterogeneity was higher than 50%, the random effects model was used to pool the data. A two-sided P-value of <0.05 was considered statistically significant. Subgroup analysis was performed based on eye diseases. Sensitivity analysis was performed by evaluating the effect of statistical model (fixed-effect model or random-effect model) and excluding the potential high heterogeneity studies. A funnel plot and a statistical test for asymmetry was used to assess for the potential for publication bias38.
Additional Information
How to cite this article: Huang, J. et al. Meta-analysis of optical low-coherence reflectometry versus partial coherence interferometry biometry. Sci. Rep. 7, 43414; doi: 10.1038/srep43414 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
Supported in part by the National Natural Science Foundation of China (81300807); Foundation of Wenzhou City Science & Technology Bureau (J20140014, Y20140619, Y20150076); Medical and Health Science and Technology Program of Zhejiang Province (2016RCB013); National Science and Technology Major Project (2014ZX09303301).
Footnotes
The authors declare no competing financial interests.
Author Contributions Concept and design J.H., C.M., G.S., R.T., Q.W.; data collection J.H., Y.H., D.W.; statistical expertise J.H., Y.H., D.W.; literature research J.H., Y.H., D.W.; administrative, technical or logistic support Y.H., D.W., Q.W.; analysis and interpretation J.H., C.M., R.T., Q.W.; writing the article J.H., C.M., R.T., Q.W.; critical revision of the article J.H., C.M., Y.H., G.S., Q.W.; final approval of the article C.M., R.T., Q.M., J.H.; All authors reviewed the manuscript.
References
- Olsen T. Calculation of intraocular lens power: a review. Acta Ophthalmol Scand 85, 472–485 (2007). [DOI] [PubMed] [Google Scholar]
- Norrby S. Sources of error in intraocular lens power calculation. J Cataract Refract Surg 34, 368–376 (2008). [DOI] [PubMed] [Google Scholar]
- Hodge C. et al. Intraocular lens power calculation following laser refractive surgery. Eye Vis (Lond) 2, 7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skiadaresi E. et al. Subjective Quality of Vision Before and After Cataract Surgery. Arch Ophthalmol-Chic 130, 1377–1382 (2012). [DOI] [PubMed] [Google Scholar]
- Rohrer K. et al. Comparison and evaluation of ocular biometry using a new noncontact optical low-coherence reflectometer. Ophthalmology 116, 2087–2092 (2009). [DOI] [PubMed] [Google Scholar]
- McAlinden C. et al. Axial Length Measurement Failure Rates with the IOLMaster and Lenstar LS 900 in Eyes with Cataract. PLoS One 10, e0128929 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigis W., Lege B., Miller N. & Schneider B. Comparison of immersion ultrasound biometry and partial coherence interferometry for intraocular lens calculation according to Haigis. Graefes Arch Clin Exp Ophthalmol 238, 765–773 (2000). [DOI] [PubMed] [Google Scholar]
- Kielhorn I., Rajan M. S., Tesha P. M., Subryan V. R. & Bell J. A. Clinical assessment of the Zeiss IOLMaster. J Cataract Refract Surg 29, 518–522 (2003). [DOI] [PubMed] [Google Scholar]
- Rabsilber T. M., Jepsen C., Auffarth G. U. & Holzer M. P. Intraocular lens power calculation: clinical comparison of 2 optical biometry devices. Journal of cataract and refractive surgery 36, 230–234 (2010). [DOI] [PubMed] [Google Scholar]
- MacLaren R. E. et al. Biometry and formula accuracy with intraocular lenses used for cataract surgery in extreme hyperopia. Am J Ophthalmol 143, 920–931 (2007). [DOI] [PubMed] [Google Scholar]
- Drexler W. et al. Partial coherence interferometry: a novel approach to biometry in cataract surgery. Am J Ophthalmol 126, 524–534 (1998). [DOI] [PubMed] [Google Scholar]
- Santodomingo-Rubido J., Mallen E. A., Gilmartin B. & Wolffsohn J. S. A new non-contact optical device for ocular biometry. Br J Ophthalmol 86, 458–462 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaswin G., Rousseau A., Mgarrech M., Barreau E. & Labetoulle M. Biometry and intraocular lens power calculation results with a new optical biometry device: comparison with the gold standard. J Cataract Refract Surg 40, 593–600 (2014). [DOI] [PubMed] [Google Scholar]
- Buckhurst P. J. et al. A new optical low coherence reflectometry device for ocular biometry in cataract patients. The British journal of ophthalmology 93, 949–953 (2009). [DOI] [PubMed] [Google Scholar]
- Cruysberg L. P. et al. Evaluation of the Lenstar LS 900 non-contact biometer. The British journal of ophthalmology 94, 106–110 (2010). [DOI] [PubMed] [Google Scholar]
- Holzer M. P., Mamusa M. & Auffarth G. U. Accuracy of a new partial coherence interferometry analyser for biometric measurements. The British journal of ophthalmology 93, 807–810 (2009). [DOI] [PubMed] [Google Scholar]
- Liampa Z., Kynigopoulos M., Pallas G. & Gerding H. Comparison of two partial coherence interferometry devices for ocular biometry. Klin Monbl Augenheilkd 227, 285–288 (2009). [DOI] [PubMed] [Google Scholar]
- Hoffer K. J., Shammas H. J. & Savini G. Comparison of 2 laser instruments for measuring axial length. Journal of cataract and refractive surgery 36, 644–648 (2010). [DOI] [PubMed] [Google Scholar]
- Chen Y. A., Hirnschall N. & Findl O. Evaluation of 2 new optical biometry devices and comparison with the current gold standard biometer. Journal of cataract and refractive surgery 37, 513–517 (2011). [DOI] [PubMed] [Google Scholar]
- Hildebrandt A. L., Auffarth G. U. & Holzer M. P. Precision of a new device for biometric measurements in pseudophakic eyes. Der Ophthalmologe: Zeitschrift der Deutschen Ophthalmologischen Gesellschaft 108, 739–744 (2011). [DOI] [PubMed] [Google Scholar]
- Mylonas G. et al. Performance of three biometry devices in patients with different grades of age-related cataract. Acta ophthalmologica 89, e237–241 (2011). [DOI] [PubMed] [Google Scholar]
- Xiang-Yu Y. et al. Comparison of anterior segment biometry and intraocular lens power measured by Lenstar LS900 and IOLMaster. Rec Adv Ophthalmol 31, 1039–1041 (2011). [Google Scholar]
- Huang J. et al. The Effect of Cycloplegia on the Lenstar and the IOLMaster Biometry. Optometry and Vision Science 89, 1691–6 (2012). [DOI] [PubMed] [Google Scholar]
- Hui S. & Yi L. Comparison of two optical biometers in intraocular lens power calculation. Chin J Ophthalmol and Otorhinolaryngol 12, 164–167 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- jin-hai H., Xin Y., Qin-mei W., Shi-ming C. & Jie C. Comparison of Lenstar and IOLMaster for intraocular lens power calculation. Chin J Ophthalmol 48, 1005–1010 (2012). [PubMed] [Google Scholar]
- Lei Y., Baichuan W. & Haike G. Application of Axial Length Measurements by IOL Master 500 and Lenstar LS 900 before Cataract Surgery. Acta Med Univ Sci Technol Huazbong 41, 1039–1041 (2012). [Google Scholar]
- Zheng W., Fang Z., Wen-lin W. & Xiang-yu Y. Comparison of intraocular lens power measured by Lenstar LS900, IOLMaster and A-scan on patients with cataracts. Fujian Med J 34, 115–117 (2012). [Google Scholar]
- Bin L., Xian-hu D., Qun-xiao H., Qin-xia Y. & Ming-guang H. Comparison of ocular biometric measurements between Lenstar and IOL Master. Chin J Ocul Traum Occupat Eye Dis 35, 98–102 (2013). [Google Scholar]
- Huayou L. et al. Effect of cycloplegia on ocular parameters and intraocular lens calculation with Lenstar on patients with cataracts. Chin J Optom Opthalmol Vis Sci 16, 301–305 (2014). [Google Scholar]
- Hui S. & Yi L. Comparison of two optical biometers in intraocular lens power calculation. Indian journal of ophthalmology 62, 931–934 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitblat O. et al. Accuracy of predicted refraction with multifocal intraocular lenses using two biometry measurement devices and multiple intraocular lens power calculation formulas. Clin Experiment Ophthalmol 43, 328–334 (2015). [DOI] [PubMed] [Google Scholar]
- Jasvinder S., Khang T. F., Sarinder K. K., Loo V. P. & Subrayan V. Agreement analysis of LENSTAR with other techniques of biometry. Eye (Lond) 25, 717–724 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei-Yang S. et al. Comparison of ocular biometry using Lentar LS 900 and IOL Master. Rec Adv Ophthalmol 34, 560–563 (2014). [Google Scholar]
- Chen W. et al. Scheimpflug-Placido topographer and optical low-coherence reflectometry biometer: repeatability and agreement. Journal of cataract and refractive surgery 38, 1626–1632 (2012). [DOI] [PubMed] [Google Scholar]
- Eibschitz-Tsimhoni M., Tsimhoni O., Archer S. M. & Del Monte M. A. Effect of axial length and keratometry measurement error on intraocular lens implant power prediction formulas in pediatric patients. J AAPOS 12, 173–176 (2008). [DOI] [PubMed] [Google Scholar]
- Whiting P., Rutjes A. W., Reitsma J. B., Bossuyt P. M. & Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 3, 25 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting P. et al. Development and validation of methods for assessing the quality of diagnostic accuracy studies. Health Technol Assess 8, iii, 1–234 (2004). [DOI] [PubMed] [Google Scholar]
- Ahmed I., Sutton A. J. & Riley R. D. Assessment of publication bias, selection bias, and unavailable data in meta-analyses using individual participant data: a database survey. BMJ 344, d7762 (2012). [DOI] [PubMed] [Google Scholar]