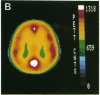
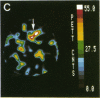
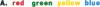Abstract
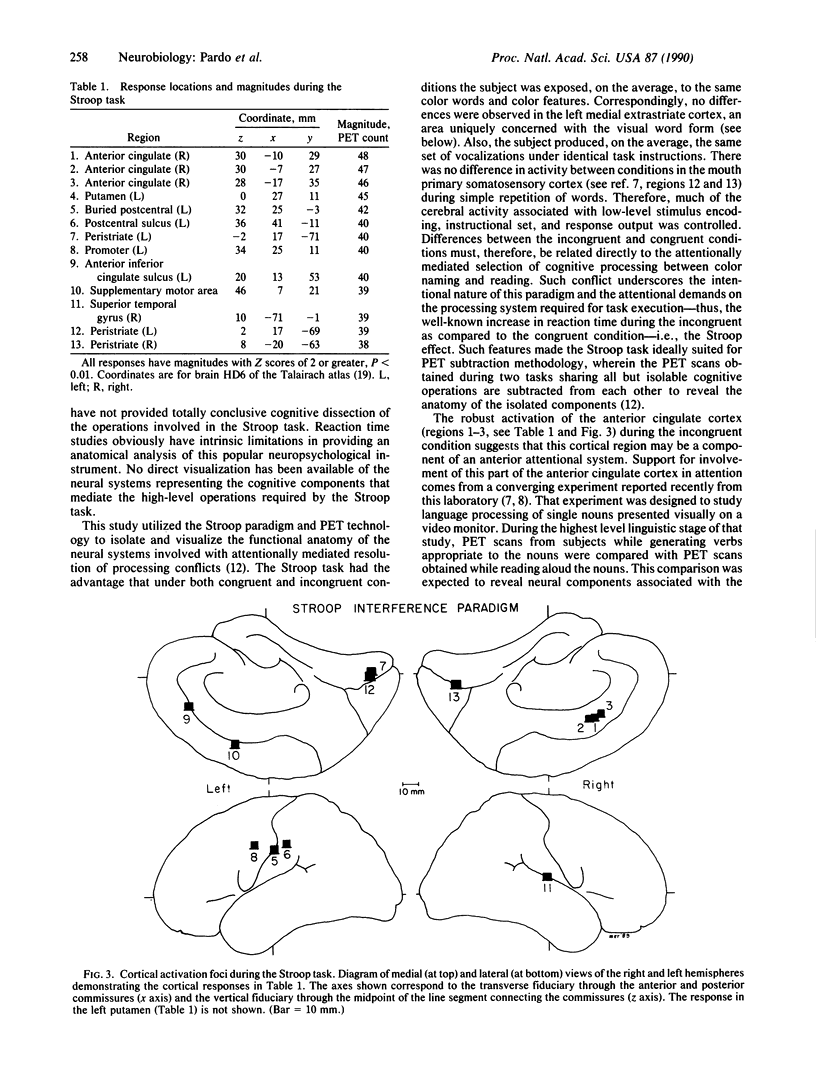
Regional cerebral blood flow, an index of local neuronal activity, was measured using positron emission tomography (PET) during the performance of the classic Stroop color/word task in eight healthy right-handed subjects. In the first condition of this paradigm, subjects name the color of the words presented on a video monitor. All the words are the color names congruent to the color presented (e.g., the noun "red" displayed in red color). In the second condition, subjects also name the color of the words presented on the monitor. However, during these trials all words are color names incongruent to the color presented (e.g., the noun "red" displayed in green color). The difference in brain activity between these two conditions (i.e., incongruent minus congruent) could reveal brain systems involved in the attentionally mediated resolution of the conflict between the habitual response of reading words vs. the task demands of naming the color of the words--i.e., the Stroop interference effect. The most robust responses occurred in the anterior cingulate cortex. Other responses noted were in the left premotor cortex, left postcentral cortex, left putamen, supplementary motor area, right superior temporal gyrus, and bilateral peristriate cortices. These data provide support for the role of the anterior cingulate cortex in attentional processing through the selection and recruitment of processing centers appropriate for task execution. Furthermore, the extensive distributed network of activated regions suggests that the Stroop interference effect cannot be explained simply in terms of stimulus encoding or response interference.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benes F. M., Bird E. D. An analysis of the arrangement of neurons in the cingulate cortex of schizophrenic patients. Arch Gen Psychiatry. 1987 Jul;44(7):608–616. doi: 10.1001/archpsyc.1987.01800190024004. [DOI] [PubMed] [Google Scholar]
- Fox P. T., Miezin F. M., Allman J. M., Van Essen D. C., Raichle M. E. Retinotopic organization of human visual cortex mapped with positron-emission tomography. J Neurosci. 1987 Mar;7(3):913–922. doi: 10.1523/JNEUROSCI.07-03-00913.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox P. T., Mintun M. A., Reiman E. M., Raichle M. E. Enhanced detection of focal brain responses using intersubject averaging and change-distribution analysis of subtracted PET images. J Cereb Blood Flow Metab. 1988 Oct;8(5):642–653. doi: 10.1038/jcbfm.1988.111. [DOI] [PubMed] [Google Scholar]
- Herscovitch P., Markham J., Raichle M. E. Brain blood flow measured with intravenous H2(15)O. I. Theory and error analysis. J Nucl Med. 1983 Sep;24(9):782–789. [PubMed] [Google Scholar]
- Hock H. S., Egeth H. Verbal interference with encoding in a perceptual classification task. J Exp Psychol. 1970 Feb;83(2):299–303. doi: 10.1037/h0028512. [DOI] [PubMed] [Google Scholar]
- Lueck C. J., Zeki S., Friston K. J., Deiber M. P., Cope P., Cunningham V. J., Lammertsma A. A., Kennard C., Frackowiak R. S. The colour centre in the cerebral cortex of man. Nature. 1989 Aug 3;340(6232):386–389. doi: 10.1038/340386a0. [DOI] [PubMed] [Google Scholar]
- Mintun M. A., Fox P. T., Raichle M. E. A highly accurate method of localizing regions of neuronal activation in the human brain with positron emission tomography. J Cereb Blood Flow Metab. 1989 Feb;9(1):96–103. doi: 10.1038/jcbfm.1989.13. [DOI] [PubMed] [Google Scholar]
- Perret E. The left frontal lobe of man and the suppression of habitual responses in verbal categorical behaviour. Neuropsychologia. 1974 Jul;12(3):323–330. doi: 10.1016/0028-3932(74)90047-5. [DOI] [PubMed] [Google Scholar]
- Petersen S. E., Fox P. T., Posner M. I., Mintun M., Raichle M. E. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988 Feb 18;331(6157):585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- Posner M. I., Early T. S., Reiman E., Pardo P. J., Dhawan M. Asymmetries in hemispheric control of attention in schizophrenia. Arch Gen Psychiatry. 1988 Sep;45(9):814–821. doi: 10.1001/archpsyc.1988.01800330038004. [DOI] [PubMed] [Google Scholar]
- Posner M. I., Petersen S. E., Fox P. T., Raichle M. E. Localization of cognitive operations in the human brain. Science. 1988 Jun 17;240(4859):1627–1631. doi: 10.1126/science.3289116. [DOI] [PubMed] [Google Scholar]
- Proctor R. W. Sources of color-word interference in the Stroop color-naming task. Percept Psychophys. 1978 May;23(5):413–419. doi: 10.3758/bf03204145. [DOI] [PubMed] [Google Scholar]
- Seymour P. H. Conceptual encoding and locus of the Stroop effect. Q J Exp Psychol. 1977 May;29(2):245–265. doi: 10.1080/14640747708400601. [DOI] [PubMed] [Google Scholar]
- Weinberger D. R., Berman K. F., Zec R. F. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986 Feb;43(2):114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]