Abstract
This paper provides the biochemical evidence for physical interactions between the outer membrane component, TolC, and the membrane fusion protein component, AcrA, of the major antibiotic efflux pump of Escherichia coli. Cross-linking between TolC and AcrA was independent of the presence of any externally added substrate of the efflux pump or of the pump protein, AcrB. The biochemical demonstration of a TolC-AcrA interaction is consistent with genetic studies in which extragenic suppressors of a mutant TolC strain were found in the acrA gene.
The major antibiotic efflux activity in Escherichia coli is mediated by a tripartite system consisting of the TolC, AcrA, and AcrB proteins (14, 15, 20, 22). TolC is a multifunctional outer membrane protein which, besides facilitating antibiotic efflux, facilitates alpha-hemolysin secretion (18) and the import of colicin E1 (13) and TolC- and lipopolysaccharide-specific bacteriophage TLS (8). AcrB is the pump protein that is localized in the inner membrane and belongs to the resistance-nodulation-division (RND) family of proteins (16). AcrA is a member of the membrane fusion protein (MFP) superfamily (5). Its lipid-modified amino-terminal end is anchored to the inner membrane, while the bulk of its carboxyl end extends into the periplasm (21). Null mutations in genes encoding any of these three proteins lead to a hypersensitivity phenotype. The crystal structures of TolC (11), AcrB (12, 19), and MexA (1), an AcrA homologue from Pseudomonas aeruginosa, have been resolved. Large portions of TolC and AcrB have been shown to stretch out beyond their respective membranes and into the periplasm, thus raising a possibility of direct interaction between them. AcrA is speculated to enclose the periplasmic domains of TolC and AcrB, thus sealing a contiguous protein path through which antibiotics are expelled from the cell into the external medium.
Biochemical data exist for an interaction between AcrA and AcrB (10, 23). Based on genetic (6, 7) and structural (1, 11, 12, 19) data, TolC and AcrAB must make direct physical contact, even if transiently, to carry out the efflux function. In the present paper, we carry out in vivo cross-linking studies and demonstrate TolC-AcrA interactions in the absence of AcrB or an externally added substrate of the AcrAB-TolC efflux pump.
Construction of the recombinant acrA and tolC genes.
To demonstrate a direct physical interaction between AcrA and TolC, in vivo cross-linking experiments in which AcrA or TolC acted as bait for copurification of the other protein were designed. To accomplish this task, we constructed recombinant acrA and tolC genes carrying six histidine codons at their 3′ ends and expressed under the control of the isopropyl-β-d-thiogalactopyranoside (IPTG) (acrA)- or arabinose (tolC)-inducible promoters of pTrc99A (Pharmacia) and pBAD24 (9), respectively. To clone acrA, we amplified it from the chromosome by PCR. The forward primer used (5′-GGTTTACTCATGAACAAAACAGAGGG-3′) created a unique BspHI site (underlined) encompassing the ATG start codon of acrA (shown in bold). The reverse primer (5′-GCTCTAGAAGCTTAGTGATGGTGATGGTGATGAGACTTGGACTGTTCAGGCTGAGC-3′) contained six consecutive histidine codons (italicized) and a unique HindIII site (underlined). The cloning of tolC also involved its amplification from the chromosome by PCR using a forward primer (5′-CAGGAAACAGATCATGAGGAAATTGCTCCC-3′) containing a unique restriction site for BspHI (underlined), which encompassed the ATG start codon of tolC (shown in bold). The reverse primer (5′-GCTCTAGAAGCTTAGTGATGGTGATGGTGATGGTTACGGAAAGGGTTATGACC-3′) contained six consecutive histidine codons (italicized) and a unique HindIII site (underlined). PCR-amplified DNA products were cut with appropriate restriction enzymes and ligated into properly restricted pTrc99A (acrA) or pBAD24 (tolC).
The recombinant plasmids were introduced into strains with deletions of the chromosomal acrA or tolC gene. Deletions of the chromosomal tolC, acrA, and acrB genes were made by the method of Datsenko and Wanner (4) and have been described previously (2). The expression of His-tagged AcrA (AcrA-His) fully complemented the chromosomal null allele. Plasmid-borne TolC-His also complemented the hypersensitivity phenotype of the chromosomal null allele, although the complemented strain displayed some residual antibiotic sensitivity. This sensitivity is possibly due to the overexpression of TolC-His, since arabinose, the inducer of TolC expression from the plasmid, was present not only in the liquid medium used for overnight bacterial growth but also in the Luria-Bertani agar plates used for the disk sensitivity assays. Western blot analyses confirmed that the plasmids synthesized the expected recombinant proteins (data not shown). Under the experimental growth conditions under which the in vivo cross-linking was carried out (see below), the levels of the recombinant proteins in plasmid-carrying strains were approximately 5-fold (AcrA) and 10-fold (TolC) greater than in the strains expressing the wild-type forms of these proteins from the corresponding chromosomal genes. The recombinant AcrA and TolC proteins were used in cross-linking studies. Cloning of the wild-type acrA and tolC gene (without six consecutive histidine codons) has been described previously (2, 7). All bacterial strains used were derived from MC4100 [F− araD139 Δ(argF-lac)U139 rpsL150 flbB5301 ptsF25 deoC1 thi-1 rbsR relA] (3).
Whole-cell cross-linking analysis using AcrA-His.
The first cross-linking experiments were carried out with strains in which AcrA-His expression was controlled by an IPTG-inducible promoter and that of TolC (without the His tag) was controlled by an arabinose-inducible promoter. Cells were grown to exponential phase in the presence of IPTG (0.4 mM) and arabinose (0.2%) to induce the expression of AcrA and TolC, respectively. Cell pellets were washed twice in a buffer containing 20 mM NaPO4 (pH 7.2) and 150 mM NaCl. Prior to the addition of a cleavable homobifunctional cross-linker, dithiobis(succinimidylpropionate) (DSP), cells were incubated for 20 min in the presence of erythromycin (50 μg/ml). This step was done with an expectation that the presence of a substrate of the TolC-AcrAB efflux pump might elevate the number of complexes in which all three proteins are present. DSP was added to a final concentration of 0.5 mM, and cells were incubated at 37°C for 30 min. Control reactions lacking DSP were carried out in parallel. Cross-linking reactions were stopped by adding 40 mM Tris; cells were pelleted, washed with a buffer containing 10 mM Tris-HCl (pH 7.5), 0.2 mM phenylmethylsulfonyl fluoride, and 1 mM MgCl2, and lysed by passage through a French press cell; and envelopes were pelleted by centrifugation for 1 h at 105,000 × g. Envelopes were solubilized in PUTTS buffer (100 mM NaH2PO4, 8 M urea, 10 mM Tris-HCl [pH 7.5], 1% Triton X-100, 0.2% Sarkosyl) containing 5 mM imidazole by gentle rocking at room temperature for 1 h. Insoluble material was removed by centrifugation for 30 min at 105,000 × g. PUTTS buffer has been previously used in the in vivo cross-linking analysis of TolC and proteins of the hemolysin secretion system (17).
PUTTS buffer-solubilized envelope proteins were subjected to affinity chromatography with HiTrap chelating high-performance columns (Amersham BioSciences) in a low-pressure chromatography system (Bio-Rad). Proteins were allowed to bind to the column under low-stringency conditions (PUTTS buffer containing 5 mM imidazole), after which the column was washed with high-stringency PUTTS buffer containing 50 mM imidazole. This step removed proteins that nonspecifically bound to the column. Proteins that remained bound to the column were then eluted with PUTTS buffer containing 500 mM imidazole. Samples from flowthrough and elution fractions were subjected to Western blot analysis. Three identical sets of samples were analyzed on separate sodium dodecyl sulfate-polyacrylamide (11%) gels, and after transfer, membranes were probed with AcrA, TolC, or OmpF, OmpC, and OmpA antibodies.
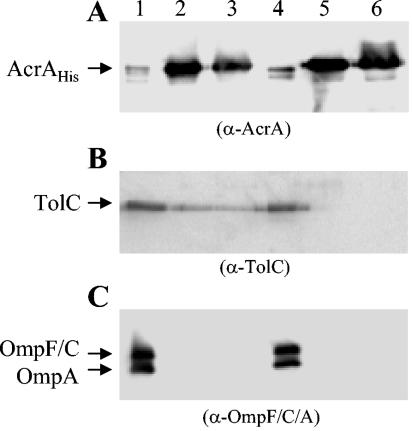
As expected, large amounts of AcrA-His were detected in the elution fractions, although some was also detected in the flowthrough fraction where the majority of unbound proteins was present (Fig. 1A). TolC was detected in the same two elution fractions in which the AcrA-His levels peaked (Fig. 1B). The absence of DSP cross-linker did not affect the binding or elution of AcrA-His, but TolC was no longer detectable in the elution fractions (Fig. 1B). We tested the specificity of these cross-linking reactions by probing for the presence of the major outer membrane proteins (OmpF, OmpC, and OmpA) in fractions where AcrA-His was eluted. None were detected in the elution fractions; instead, they were present in the flowthrough fraction (Fig. 1C). Thus, the elution of TolC with AcrA-His was highly specific and dependent on the presence of DSP cross-linker, showing the existence of complexes between these two proteins in the cell.
FIG. 1.
DSP-mediated cross-linking of AcrA-His and TolC. Cells were treated with (lanes 1 to 3) and without (lanes 4 to 6) DSP, after which envelopes were isolated and treated with a detergent- and denaturant-containing buffer. Solubilized envelope proteins were subjected to affinity chromatography, as described in the text. All flowthrough and elution samples were first analyzed by Western blotting to determine AcrA peaks. Subsequently, one flowthrough fraction (lanes 1 and 4) and at least two elution fractions (lanes 2 and 3 and lanes 5 and 6), where AcrA peaked, were reanalyzed by Western blotting with AcrA, TolC, or OmpF, OmpC, and OmpA antibodies. Three separate membranes (A, B, and C) carrying identical protein samples were probed with three different antibodies (shown in parentheses). Positions of AcrA-His, TolC, OmpF, OmpC, and OmpA are shown. α, anti.
Whole-cell cross-linking analysis using TolC-His.
Similar cross-linking experiments to those described above were carried out, except that in this case, the TolC-His protein was used as a bait to copurify AcrA. After chromatography, fractions were analyzed by Western blotting to detect TolC-His and AcrA (data not shown). The peak of TolC-His eluted in similar fractions regardless of whether the protein extracts were prepared from DSP-treated or untreated cells. AcrA coeluted with the TolC-His peak and was distinctly absent when DSP was not added; instead, AcrA eluted in the flowthrough fraction (data not shown). The cross-linking of AcrA with TolC-His confirmed the existence of complexes between these two proteins in vivo.
Is the presence of an externally added efflux pump substrate required to detect AcrA-TolC cross-linking?
In the cross-linking experiments described above, we incubated cells in the presence of erythromycin, a substrate of the TolC-AcrAB efflux pump, prior to the addition of DSP cross-linker. This step was considered necessary because of the possibility that the presence of a known efflux pump substrate would increase the number of efflux protein complexes in the cell, thus increasing the likelihood of detecting TolC-AcrA cross-linking.
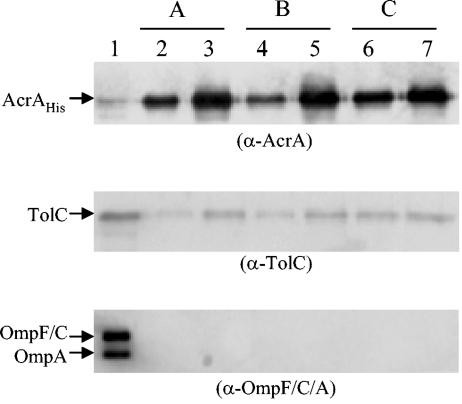
We repeated the cross-linking experiments under the optimized conditions described above (Fig. 1) and included two additional control cultures, one of which was incubated with just ethanol in which erythromycin was dissolved while the other received neither. The final concentration of ethanol was 0.1%. After these treatments, DSP was added and chromatography was carried out with protein extracts as described in the legend to Fig. 1. The results presented in Fig. 2 show that TolC cross-linked with AcrA-His regardless of whether the cultures were preincubated with the antibiotic (Fig. 2A), the solvent alone (Fig. 2B), or neither (Fig. 2C) prior to the addition of the cross-linker. Again, the absence of the major outer membrane proteins from the elution peaks illustrated the specificity of AcrA-His and TolC interactions. These experiments showed that TolC-AcrA complexes exist even when cells are not challenged by the addition of a substrate of the TolC-AcrAB efflux pump.
FIG. 2.
Cross-linking of AcrA-His and TolC in the presence of erythromycin (A), ethanol (B), or neither (C). DSP cross-linking, affinity chromatography, and sample analysis by Western blotting are described in the text and the Fig. 1 legend. Lane 1, flowthrough fractions; lanes 2 to 7, elution fractions. Three separate membranes containing identical protein samples were probed with different antibodies, as shown in parentheses. Positions of AcrA-His, TolC, OmpF and OmpC, and OmpA are shown. α, anti.
Is the presence of AcrB required to detect TolC-AcrA interactions?
Through reversion analysis of a hypersensitive TolC mutant, we found extragenic suppressor mutations that either alter the mature AcrA sequence or elevate wild-type AcrA expression (7). Besides antibiotic hypersensitivity, these suppressors reversed the bacteriophage and colicin resistance phenotypes of the TolC mutant. Interestingly, AcrA-mediated suppression of the latter two phenotypes was dependent on AcrB, indicating that AcrB, either directly or through AcrA, interacts with mutant TolC to influence its function. This finding provided strong genetic evidence for the functional interaction of, and the existence of a complex between, the three proteins. The absence of AcrA or AcrB, however, does not influence wild-type TolC-mediated phage and colicin sensitivities. Thus, interactions between wild-type TolC and AcrA or AcrB are not obligatory for TolC's cell surface-associated activities. In this context, we asked whether wild-type TolC interacts with AcrA in the absence of AcrB. To address this question, cross-linking reactions were carried out as described in Fig. 1, in which AcrA-His was used as a bait to copurify wild-type TolC. The strains used had a chromosomal deletion removing either just acrA (this strain expressed acrB normally) or both the acrA and acrB genes.
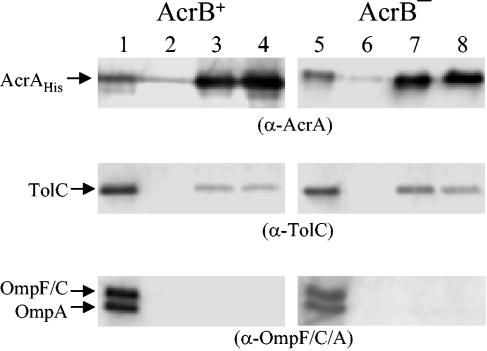
AcrA-His was eluted from Ni2+-chelating columns, and elution and flowthrough fractions were analyzed by Western blotting. Membranes were probed with antibodies to detect AcrA, TolC, and OmpF, OmpC, and OmpA (Fig. 3). TolC was primarily detected in fractions in which the majority of AcrA was present. No detectable amounts of OmpF, OmpC, or OmpA were present in these elution fractions. Similar protein profiles were found regardless of whether AcrB was present or absent (Fig. 3). The blotting of protein samples eluted from AcrB+ and AcrB− strains with AcrB antibodies showed the presence and absence of AcrB, respectively (data not shown). These results showed that TolC-AcrA complexes are present in the cell independent of the AcrB protein.
FIG. 3.
Cross-linking of AcrA-His and TolC in the presence or absence of AcrB. DSP cross-linking, affinity chromatography, and sample analysis by Western blotting are described in the text and the Fig. 1 legend. Lanes 1 and 5, flowthrough fractions; lanes 2 to 4 and 6 to 8, elution fractions. As done in earlier experiments, three separate blots containing identical protein samples were probed with different antibodies. Positions of AcrA-His, TolC, OmpF and OmpC, and OmpA are shown. α, anti.
Two important conclusions can be drawn from this study: (i) TolC and AcrA complexes can form independently of an externally added TolC-AcrAB efflux pump substrate, and (ii) AcrB is not required for AcrA to physically interact with TolC. Recently, we obtained genetic verification for TolC-AcrA interactions by isolating extragenic acrA suppressors of a TolC mutant (7). The majority of suppressors affected residues between positions 201 and 266 of the mature AcrA protein, which likely defines an AcrA domain that interacts with TolC. The AcrB dependence of this AcrA-mediated suppression of the mutant TolC phenotypes (colicin E1 and phage TLS resistance) indicated that all three proteins must interact in vivo. Unlike the mutant TolC protein, wild-type TolC can facilitate colicin E1 import and TLS phage infection independent of AcrA and AcrB. We believe that the AcrA and AcrB dependence of the mutant TolC protein stems from the fact that it is a highly unstable protein, which is stabilized in an AcrB-dependent manner by either the suppressor forms of AcrA or wild-type AcrA when the protein is overexpressed (7). The intrinsic stability of the wild-type TolC protein renders it independent of AcrAB for its surface-associated functions.
Binding of antibiotics to AcrB is shown to slightly alter its conformation (19). However, the AcrB independence of the TolC-AcrA interaction suggests that a switch from the uninduced state to the antibiotic-induced state of AcrB may not be essential in catalyzing the assembly of the tripartite efflux pump complex. In contrast to these findings, it has been shown that the presence of HlyB, the traffic ATPase, which is analogous to AcrB, is essential to detect DSP-mediated cross-linking of TolC with HlyD, an AcrA analogue (17). More importantly, the presence of HlyA (alpha-hemolysin), the substrate of the TolC-HlyDB type I export system, is mandatory to observe TolC-HlyD cross-linking in vivo. Since, in the absence of HlyB, HlyA cannot interact with HlyD or TolC, this finding suggests that the interaction among HlyB, HlyD, and TolC is mediated by alpha-hemolysin itself. This, however, cannot be the case with the TolC-AcrAB efflux system, because their substrates are small molecules that cannot simultaneously interact with more than one protein at a time. Therefore, the AcrB independence but HlyB dependence of TolC's cross-linking with its cognate membrane fusion protein partners reflects both structural and mechanistic differences between the two transport systems. It is likely that TolC-AcrA, AcrA-AcrB, or TolC-AcrAB complexes are formed spontaneously, with the composition of complexes modulating according to levels of the individual proteins.
ADDENDUM IN PROOF
After the submission of this paper, two separate groups also showed TolC-AcrA cross-linking (T. Touze, J. Eswaran, E. Bokma, E. Koronakis, C. Hughes, and V. Koronakis, Mol. Microbiol. 53:697-706, 2004; E. B. Tikhonova and H. I. Zgurskaya, J. Biol. Chem. 279:32116-32124, 2004).
Acknowledgments
This work was supported by a grant from the National Institutes of Health (GM-R01-066988).
We thank Leanne Misra for reading the manuscript and Rhesa Stidham for constructing a plasmid clone expressing AcrA-His.
REFERENCES
- 1.Akama, H., T. Matsuura, S. Kashiwagi, H. Yoneyama, T. Tsukihara, A. Nakagawa, and T. Nakae. 2004. Crystal structure of the membrane fusion protein, MexA, of the multidrug transporter in Pseudomonas aeruginosa. J. Biol. Chem. 279:25939-25942. [DOI] [PubMed] [Google Scholar]
- 2.Augustus, A. M., T. Celaya, F. Husain, M. Humbard, and R. Misra. 2004. Antibiotic-sensitive TolC mutants and their suppressors. J. Bacteriol. 186:1851-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casadaban, M. J. 1976. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J. Mol. Biol. 141:541-555. [DOI] [PubMed] [Google Scholar]
- 4.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinh, T., I. T. Paulsen, and M. H. Saier, Jr. 1994. A family of extracytoplasmic proteins that allow transport of large molecules across the outer membranes of gram-negative bacteria. J. Bacteriol. 176:3825-3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fralick, J. A. 1996. Evidence that TolC is required for functioning of the Mar/AcrAB efflux pump of Escherichia coli. J. Bacteriol. 178:5803-5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerken, H., and R. Misra. 2004. Genetic evidence for AcrB-dependent functional interactions between TolC and AcrA proteins of a major antibiotic efflux pump of Escherichia coli. Mol. Microbiol. 54:620-631. [DOI] [PubMed] [Google Scholar]
- 8.German, G. J., and R. Misra. 2001. The TolC protein of Escherichia coli serves as a cell-surface receptor for the newly characterized TLS bacteriophage. J. Mol. Biol. 308:579-585. [DOI] [PubMed] [Google Scholar]
- 9.Guzman, L.-M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawabe, T., E. Fujihira, and A. Yamaguchi. 2000. Molecular construction of a multidrug exporter system, AcrAB: molecular interaction between AcrA and AcrB, and cleavage of the N-terminal signal sequence of AcrA. J. Biochem. 128:195-200. [DOI] [PubMed] [Google Scholar]
- 11.Koronakis, V., A. Sharff, E. Koronakis, B. Luisi, and C. Hughes. 2000. Crystal structure of the bacterial membrane protein TolC central to multidrug efflux and protein export. Nature 405:914-919. [DOI] [PubMed] [Google Scholar]
- 12.Murakami, S., R. Nakashima, E. Yamashita, and A. Yamaguchi. 2002. Crystal structure of bacterial multidrug efflux transporter AcrB. Nature 419:587-593. [DOI] [PubMed] [Google Scholar]
- 13.Nagel de Zwaig, R., and S. E. Luria. 1967. Genetics and physiology of colicin-tolerant mutants of Escherichia coli. J. Bacteriol. 94:1112-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nikaido, H. 1994. Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science 264:382-388. [DOI] [PubMed] [Google Scholar]
- 15.Nikaido, H., and H. I. Zgurskaya. 2001. AcrAB and related multidrug efflux pumps of Escherichia coli. J. Mol. Microbiol. Biotechnol. 3:215-218. [PubMed] [Google Scholar]
- 16.Saier, M. H., Jr., R. Tam, A. Reizer, and J. Reizer. 1994. Two novel families of bacterial membrane proteins concerned with nodulation, cell division and transport. Mol. Microbiol. 11:841-847. [DOI] [PubMed] [Google Scholar]
- 17.Thanabalu, T., E. Koronakis, C. Hughes, and V. Koronakis. 1998. Substrate-induced assembly of a contiguous channel for protein export from E. coli: reversible bridging of an inner-membrane translocase to an outer membrane exit pore. EMBO J. 17:6487-6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wandersman, C., and P. Delepelaire. 1990. TolC, an Escherichia coli outer membrane protein required for hemolysin secretion. Proc. Natl. Acad. Sci. USA 87:4746-4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu, E. W., G. McDermott, H. I. Zgurskaya, H. Nikaido, and D. E. Koshland, Jr. 2003. Structural basis of multiple drug-binding capacity of the AcrB multidrug efflux pump. Science 300:976-980. [DOI] [PubMed] [Google Scholar]
- 20.Yu, E. W., J. R. Aires, and H. Nikaido. 2003. AcrB multidrug efflux pump of Escherichia coli: composite substrate-binding cavity of exceptional flexibility generates its extremely wide substrate specificity. J. Bacteriol. 185:5657-5664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zgurskaya, H. I., and H. Nikaido. 1999. AcrA is a highly asymmetric protein capable of spanning the periplasm. J. Mol. Biol. 285:409-420. [DOI] [PubMed] [Google Scholar]
- 22.Zgurskaya, H. I., and H. Nikaido. 2000. Multidrug resistance mechanisms: drug efflux across two membranes. Mol. Microbiol. 37:219-225. [DOI] [PubMed] [Google Scholar]
- 23.Zgurskaya, H. I., and H. Nikaido. 2000. Cross-linked complex between oligomeric periplasmic lipoprotein AcrA and the inner-membrane-associated multidrug efflux pump AcrB from Escherichia coli. J. Bacteriol. 182:4264-4267. [DOI] [PMC free article] [PubMed] [Google Scholar]