Abstract
Streptococcus mutans F-ATPase, the major component of the acid-adaptive response of the organism, is transcriptionally upregulated at low pH. Fusions of the F-ATPase promoter to chloramphenicol acetyltransferase indicated that pH-dependent expression is still observed with a short promoter that contains a domain conserved between streptococcal ATPase operons.
During growth at acidic pH values, Streptococcus mutans and S. sanguis both increase production of their F-ATPases (4, 5, 12). By pumping protons out of cells, the enzymes function to assist in maintaining internal pH values (6, 8, 13, 14). The ATPases from S. mutans and S. sanguis differ in their innate abilities to function in acidic conditions, as evidenced by the pH optima of the enzymes: approximately 6.0 for S. mutans and 7.0 for S. sanguis, with the maximal induction of the S. mutans enzyme occurring at pH 5. Thus, the ATPase is likely a significant reason why the mutans streptococci are able to outcompete S. sanguis during growth in low-pH environments (21) and to cause dental caries (18, 24).
The present study was undertaken to determine whether control of ATPase production in oral streptococci occurs at the transcriptional level. RNA slot blots, reporter gene fusions, and computer modeling were used to examine the pH-dependent expression of the S. mutans F-ATPase promoter in S. mutans and S. sanguis.
(The material presented here was used in partial fulfillment of the Ph.D. requirements of the University of Rochester by W. L. Kuhnert.).
RNA samples from cells grown at pH 5 have greater abundance of ATPase β-subunit transcript.
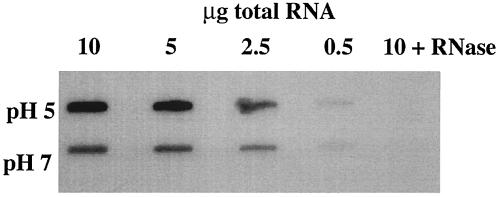
We first determined whether ATPase-specific mRNA levels differed in RNA samples prepared from cells grown at pH 5 or 7. S. mutans UA159 was grown in continuous culture to steady state at a dilution rate of 0.24 h−1, as described previously (10, 15). RNA slot blots hybridized to a β-subunit-specific DNA probe (amplified with primers β Fwd and β Rev; Table 1) showed an approximately twofold increase in the abundance of ATPase message in the cells grown at pH 5 (Fig. 1).
TABLE 1.
Strains and plasmids
| Strain, oligonucleotide, or plasmid | Source, reference, or sequencea | Relevant feature |
|---|---|---|
| Strains | ||
| S. mutans GS5 | 11 | atp promoter |
| S. mutans UA159 | 1, 22 | Test strain |
| S. sanguis 10904 | American Type Culture Collection | Test strain |
| S. mutans UR122 | This study | Single-copy fusion |
| S. mutans UR123 | This study | Single-copy fusion |
| S. mutans UR124 | This study | Single-copy fusion |
| E. coli DH10B | Invitrogen | Cloning host |
| E. coli DH5α | Invitrogen | Cloning host |
| E. coli GBE180 | 23 | Cloning host |
| Oligonucleotides | ||
| 3′ CAT-BAM | 5′ CAAGGATCCAAGCTTCGACGAATT 3′ | Amplify cat gene |
| BGL II CAT | 5′ GCGAAGGAAAGATCTATGGAGAAAAAAATCAC 3′ | Amplify cat gene |
| BGL II ATP | 5′ ATTCAACATAGATCTTTCTCCTTC 3′ | Amplify atp promoter |
| BRL P556BAM | 5′ CGACGGATCCTCTCTTTTTTGACCTAATA 3′ | Amplify atp promoter |
| BRL P680BAM | 5′ CGACGGATCCTTTCTTGTTTTCATTGAC 3′ | Amplify atp promoter |
| AMY-HIN | 5′ GGCCAAGCTTCAAACGATGGTAAGCATTGG 3′ | Amplify amyA |
| AMY-ECO | 5′ CCGGAATTCCGTTTGCAATTAGCTTCCC 3′ | Amplify amyA |
| β Fwd. | 5′ TTCAGTTGCCAAGGTTGG 3′ | Amplify atpD |
| β Rev. | 5′ AAAGGTGGTAAAGTCGGC 3′ | Amplify atpD |
| Plasmids | ||
| pGEM-T | Promega | |
| pUC:CAT | R. A. Burne (University of Florida) | |
| pGEM-7Zf+ | Promega | |
| pDL278 | 16, 17 | |
| pDL556/CAT | This study | Full-length atpP-CAT fusion |
| pDL680/CAT | This study | Short-length atpP-CAT fusion |
| pDLCAT | This study | Promoterless CAT fusion |
| pUC-amy | This study | amyA clone |
| pUC-amy556 | This study | Full-length atpP-CAT fusion in amyA |
| pUC-amy680 | This study | Short-length atpP-CAT fusion in amyA |
| pUC-amyCAT | This study | Promoterless CAT fusion in amyA |
| pBGK | 31 | S. mutans integration vector |
Restriction sites are denoted by the underlined sequence. Double-underlined sequence denotes the ATG start site of the atp operon.
FIG. 1.
Slot blots of RNA prepared from S. mutans UA159 grown at steady state at pH 5 or 7. Total cellular RNA samples were serially diluted as shown and were probed with the β-subunit-specific probe.
ATPase operon transcriptional rate is greater at pH 5 than at pH 7.
Three reporter gene fusions were constructed using PCR (25), both in multicopy (plasmid-borne) and single-copy (chromosomal) fusions, to determine the transcriptional activity of the operon. These included (i) an intact promoter fragment of the S. mutans GS-5 ATPase operon fused to a cat gene (contained on pDL556/CAT and in S. mutans UR123); (ii) a fragment containing the −10 and −35 sequences and promoter sequence that extended only into the predicted inverted DNA repeat proximal to the structural gene (carried on pDL680/CAT and in S. mutans UR124); and (iii) a promoterless cat gene fusion (contained on pDLCAT and in S. mutans UR122). The appropriate clones of promoter fragments were verified by nucleotide sequencing (15, 26) and were subsequently subcloned into pDL278 (16, 17), resulting in the constructs listed above (detailed in Fig. 2).
FIG. 2.
Location of predicted motifs in the intergenic space upstream of the S. mutans GS-5 ATPase operon. Motif analysis (MEME) of the intergenic spaces was conducted as described previously (3) using the San Diego Center for Supercomputing facility (http://meme.sdsc.edu/meme/website/). The three motifs ([1], [2] and [3]) are shown with their respective positional probabilities. Plus signs indicate the presence of the predicted motif in the sense strand; negative signs indicate an antisense direction to the motif. The thick arrow indicates the positioning of the short-length promoter, and the dashed arrow indicates positioning of the full-length promoter portion of the two cat fusions (described in the text). The thin arrows indicate the position of predicted inverted DNA repeats. Double-underlined sequence indicates the −35 region, and single-underlined sequence denotes the −10 region. The transcriptional start site of the promoter is shown as a bold G (29). Nucleotide sequence information for the ATPase promoters was derived from GenBank deposits with the following accession numbers: S. mutans GS-5 (U31170); S. sanguis 10904 (AF001955); S. pyogenes M1 (AE004092) (9); S. mutans UA159 (NC004350) (1).
Single-copy fusions were created by integrating the promoter-chloramphenicol acetyltransferase (CAT) fusions (described above) into the amyA locus (1) of the chromosome and by using plasmid pBGK (31) as a transformation control. Genotypes of recombinant strains UR122, UR123, and UR124 (detailed above) were confirmed by PCR.
Initially, CAT assays were performed on protein extracts of the plasmid-borne cat fusion-bearing strains grown in batch culture to obtain data regarding transcription of the atp operon during growth in the absence of pH control. CAT activity (27) and protein concentrations (7) were determined as described previously. As expected, the streptococcal strains containing the promoterless control plasmid, pDLCAT, showed no CAT activity (Table 2). Expression of the fusions in S. mutans UA159 showed that the shorter construct (pDL680/CAT) resulted in less CAT activity than the full-length fusion (pDL556/CAT) (Table 2). The decreased levels of CAT activity seen with the shorter fusion suggested that the entire promoter region (including both inverted repeat regions) played a role in the expression of the atp operon in both S. mutans and S. sanguis.
TABLE 2.
CAT activity in batch cultures of S. mutans UA159 and S. sanguis 10904 cultures containing S. mutans atp promoter-fusion plasmidsa
| Strain | CAT activity (μmol min−1 mg−1) |
|---|---|
| S. mutans UA159(pDL556/CAT) | 12.1 ± 1.3 a |
| S. mutans UA159(pDL680/CAT) | 4.7 ± 0.7 a |
| S. mutans UA159(pDLCAT) | 0.0 ± 0.0 |
| S. sanguis 10904(pDL556/CAT) | 11.6 ± 1.2 b |
| S. sanguis 10904(pDL680/CAT) | 4.0 ± 0.2 b |
| S. sanguis 10904(pDLCAT) | 0.0 ± 0.0 |
Data represent assays performed on cells grown in duplicate with at least six replicates for each extract. Letters indicate statistically different values, between pairs, using two-tailed Student's t test with P ≤ 0.05 and 95% confidence interval.
The identical strains were also grown in the chemostat under pH-controlled conditions. The strains were grown first at pH 7.0, under glucose-limiting conditions, and then the pH was lowered to pH 5.0 or 6.0 for S. mutans and S. sanguis, respectively. CAT activity data from S. mutans showed that the longer fusion constructs again expressed greater CAT activity compared to that of the shorter fusion, though the differences were somewhat less pronounced (Table 3). These observations were in agreement with the data obtained from the batch-grown cells.
TABLE 3.
CAT activity in steady-state cultures of S. mutans UA159 and S. sanguis 10904 containing S. mutans atp promoter-fusion plasmidsa
| Strain | CAT activity (μmol min−1 mg−1) |
|---|---|
| S. mutans UA159(pDL556/CAT), pH 7 | 7.9 ± 0.7 a,b |
| S. mutans UA159(pDL556/CAT), pH 5 | 10.2 ± 0.9 a,c |
| S. mutans UA159(pDL680/CAT), pH 7 | 6.1 ± 1.1 d,b |
| S. mutans UA159(pDL680/CAT), pH 5 | 7.7 ± 0.3 d,c |
| S. mutans UA159(pDLCAT), pH 7 | 0.0 ± 0.0 |
| S. mutans UA159(pDLCAT), pH 5 | 0.0 ± 0.0 |
| S. sanguis 10904(pDL556/CAT), pH 7 | 3.7 ± 0.2 e,f |
| S. sanguis 10904(pDL556/CAT), pH 6 | 6.2 ± 0.3 e,g |
| S. sanguis 10904(pDL680/CAT), pH 7 | 1.7 ± 0.1 h,f |
| S. sanguis 10904(pDL680/CAT), pH 6 | 3.3 ± 0.5 h,g |
| S. sanguis 10904(pDLCAT), pH 7 | 0.0 ± 0.0 |
| S. sanguis 10904(pDLCAT), pH 6 | 0.0 ± 0.0 |
Data represent assays performed on cells grown in duplicate with at least six replicates for each extract. Letters indicate statistically different values, between pairs, using two-tailed Student's t test with P ≤ 0.05 and 95% confidence interval.
Following the experiments with multicopy, plasmid-borne reporter fusions, we examined the activities of the strains bearing single-copy constructs. Results were similar to those seen with the episomal strains (Table 4).
TABLE 4.
Single-copy S. mutans atp promoter-fusion CAT activity in steady-state cultures of S. mutansa
| Strain | CAT activity (μmol min−1 mg−1) |
|---|---|
| S. mutans UR122, pH 7 | 0.0 ± 0.0 |
| S. mutans UR122, pH 5 | 0.0 ± 0.0 |
| S. mutans UR123, pH 7 | 0.7 ± 0.0 a,b |
| S. mutans UR123, pH 5 | 1.0 ± 0.0 a,c |
| S. mutans UR124, pH 7 | 0.4 ± 0.0 d,b |
| S. mutans UR124, pH 5 | 0.7 ± 0.1 d,e |
Data represents assays performed in triplicate for each extract. Letters indicate statistically different values, between pairs, using two-tailed Student's t test with P ≤ 0.05 and 95% confidence interval.
Overall, the data indicated that the full-length fusion was likely to be necessary for optimal expression of the atp promoter. Nevertheless, the effects of extracellular pH on ATPase transcription were still manifested in the short fusion. This observation supports the notion that pH-mediated regulation may be exerted near the structural genes, possibly by an alternative sigma factor acting at a core region containing the −35 and −10 sequences, as postulated for S. pneumoniae (20). The transcriptional activity of the fusions of the S. mutans ATPase promoter in the S. sanguis background indicates the likelihood that regulation of the ATPase is similar in both organisms.
Slot blot analysis of ATPase mRNA in plasmid-borne, fusion-bearing strains.
To ensure that ATPase message levels correlated with CAT activity in the fusion-bearing strains, experiments were conducted to estimate the effect of pH on the level of F-ATPase-specific mRNA. Total RNA, prepared from cells harvested from chemostat cultures of S. mutans and S. sanguis strains containing the fusion plasmids, was treated prior to blotting as previously described (26). Treated samples were serially diluted into aliquots as indicated in Fig. 1, and they were applied to duplicate membranes. The first blot of each pair was probed with a 16S rRNA-encoding DNA probe, as previously described (28), to establish a baseline for RNA loading and to allow for a comparative analysis of the β-subunit (atpD)-probed RNA. The second blot was probed with a 404-bp atpD probe amplified from the S. mutans atp operon using the β Fwd and β Rev primers (29). Bound, radiolabeled probe was estimated with a PhosphorImager (Molecular Dynamics). Data from the S. mutans blots indicated that F-ATPase message expression was higher in cells grown at pH 5.0 (Table 5), which was in agreement with the CAT activity. The negative control strain, containing pDLCAT, showed levels of ATPase message similar to those seen in the full-length (pDL556/CAT) fusion strains, indicating that the plasmid-borne fusions did not interfere with chromosomal transcription of the ATPase operon.
TABLE 5.
RNA transcript levels of atpD in steady-state cultures of S. mutans UA159 and S. sanguis 10904 containing S. mutans atp promoter fusion plasmids
| Strain | Phosphorimager units (10−7)a |
|---|---|
| S. mutans UA159(pDL556/CAT), pH 7 | 1.2 ± 0.2 a |
| S. mutans UA159(pDL556/CAT), pH 5 | 3.2 ± 0.9 b |
| S. mutans UA159(pDL680/CAT), pH 7 | 0.9 ± 0.1 c,d |
| S. mutans UA159(pDL680/CAT), pH 5 | 1.7 ± 0.2 c,e |
| S. mutans UA159(pDLCAT), pH 7 | 1.3 ± 0.4 f |
| S. mutans UA159(pDLCAT), pH 5 | 3.6 ± 0.5 f |
| S. sanguis 10904(pDL556/CAT), pH 7 | 0.1 ± 0.1 a |
| S. sanguis 10904(pDL556/CAT), pH 6 | 0.3 ± 0.1 b |
| S. sanguis 10904(pDL680/CAT), pH 7 | 1.7 ± 0.0 d |
| S. sanguis 10904(pDL680/CAT), pH 6 | 2.2 ± 0.0 e |
| S. sanguis 10904(pDLCAT), pH 7 | 0.1 ± 0.0 |
| S. sanguis 10904(pDLCAT), pH 6 | 0.3 ± 0.1 |
Units are presented for the RNA probed with atpD as normalized data obtained using a radiolabeled 16S rRNA probe. Letters indicate statistically different values, between pairs, using two-tailed Student's t test with P ≤ 0.05 and 95% confidence interval.
Results from S. sanguis strains showed that mRNA, specific for atpD at least, was expressed at similar levels in S. mutans (Table 5) such that ATPase-encoding RNA levels were higher in cells grown at a pH value of 6.0 than in the cells grown at pH 7.0.
Control experiments, estimating the copy number of the plasmid-borne fusions, showed that differences between strains were not statistically significant (data not shown).
ATPase promoter in S. mutans contains repeated patterns.
Our observations indicated the possibility of two types of domains in the atp promoter that were conserved between S. mutans and S. sanguis. Subsequent motif searches (2, 3) were conducted, with 6, 10, 15, 25, or 50 bp as the maximum window size and 3 or 6 bp as the minimum window size. The analyses resulted in the prediction of three motifs with significant probabilities in streptococcal atp operon promoters. Motif 1, 5′-TAGGACTAGACTAAGCAAGCGCA-3′, and motif 2, 5′-AAAGTTGTGAAGGGGAATTCA-3′, were the more distributed patterns, each occurring more than once in the promoter sequences. The third motif, 5′-AAAATTTTGAAGGAGAATCC-3′, was located immediately upstream of the coding region of the operon, inside the transcriptional start site at −29 (Fig. 2).
A model for the regulation of the operon could include two or three types of sites for a regulatory protein(s): one for full transcriptional activity of the operon, and a second domain, closer to the structural genes, which may confer pH sensitivity for transcriptional upregulation. Such a model of regulation could then be described as a single protein interacting at all of the predicted sites but with differing affinities. The incremental increases in ATPase activity, observed with incremental decreases in extracellular pH (19), could be explained by the successive loss or gain of protein binding at the motif sites. Alternatively, multiple proteins could be involved with atp operon regulation, reflecting entirely different binding sites and roles for proteins binding at motifs 1, 2, and 3. The motifs predicted in this study must be considered preliminary models that will become refined as our understanding of streptococcal gene regulation improves. A similar approach recently identified motifs of approximately 40 bp in size for OxyR binding sites in Escherichia coli (32), including one such site that was dsbG proximal, that is, inside the −35 region.
We have demonstrated that the S. mutans ATPase operon is regulated by a mechanism that includes DNA elements located upstream of the putative −35 site. The regulatory scheme appears to include sensitivity to environmental pH, which also seems to be conserved in other streptococci, though S. sanguis is known to be less aciduric than S. mutans (30). The mechanism through which the potential regulatory sequences exert their effects remains to be determined.
Acknowledgments
We thank R. E. Marquis, R. A. Burne, and Y.-Y. M. Chen for helpful discussion throughout. We also thank Raymond C. Ogawa for assistance with the RNA experiments and E. Fozo for help with the statistical analysis.
The work was supported by grants from the NIH, National Institute for Dental and Craniofacial Research (DE-06127, DE-11549), and the Cariology Training Program (T32-DE07165) (W.L.K.).
REFERENCES
- 1.Ajdic, D., W. M. McShan, R. E. McLaughlin, G. Savic, J. Chang, M. B. Carson, C. Primeaux, R. Tian, S. Kenton, H. Jia, S. Lin, Y. Qian, S. Li, H. Zhu, F. Najar, H. Lai, J. White, B. A. Roe, and J. J. Ferretti. 2002. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl. Acad. Sci. USA 99:14434-14439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey, T. L., and C. Elkan. 1994. Fitting a mixture model by expectation maximization to discover motifs in biopolymers, p. 28-36. In R. Altman, D. Brutlag, P. Karp, R. Lathrop, and D. Searls (ed.), Second International Conference on Intelligent Systems for Molecular Biology, vol. 1. AAAI Press, Menlo Park, Calif. [PubMed]
- 3.Bailey, T. L., and M. Gribskov. 1998. Methods and statistics for combining motif match scores. J. Comput. Biol. 5:211-221. [DOI] [PubMed] [Google Scholar]
- 4.Belli, W. A., and R. E. Marquis. 1991. Adaptation of Streptococcus mutans and Enterococcus hirae to acid stress in continuous culture. Appl. Environ. Microbiol. 57:1134-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belli, W. A., and R. E. Marquis. 1994. Catabolite modification of acid tolerance of Streptococcus mutans GS-5. Oral Microbiol. Immun. 9:29-34. [DOI] [PubMed] [Google Scholar]
- 6.Bender, G. R., S. V. Sutton, and R. E. Marquis. 1986. Acid tolerance, proton permeabilities, and membrane ATPases of oral streptococci. Infect. Immun. 53:331-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 8.Dashper, S. G., and E. C. Reynolds. 1992. pH regulation by Streptococcus mutans. J. Dent. Res. 71:1159-1165. [DOI] [PubMed] [Google Scholar]
- 9.Ferretti, J. J., W. M. McShan, D. Ajdic, D. J. Savic, G. Savic, K. Lyon, C. Primeaux, S. Sezate, A. N. Suvorov, S. Kenton, H. S. Lai, S. P. Lin, Y. Qian, H. G. Jia, F. Z. Najar, Q. Ren, H. Zhu, L. Song, J. White, X. Yuan, S. W. Clifton, B. A. Roe, and R. McLaughlin. 2001. Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc. Natl. Acad. Sci. USA 98:4658-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fozo, E. M., and R. G. Quivey Jr. 2004. Shifts in the membrane fatty acid profile of Streptococcus mutans enhance survival in acidic conditions. Appl. Environ. Microbiol. 70:929-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibbons, R. J., K. S. Berman, P. Knoettner, and B. Kapsimalis. 1966. Dental caries and alveolar bone loss in gnotobiotic rats infected with capsule forming streptococci of human origin. Arch. Oral Biol. 11:549-560. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton, I. R., and N. D. Buckley. 1991. Adaptation by Streptococcus mutans to acid tolerance. Oral Microbiol. Immun. 6:65-71. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi, H. 1985. A proton-translocating ATPase regulates pH of the bacterial cytoplasm. J. Biol. Chem. 260:72-76. [PubMed] [Google Scholar]
- 14.Kobayashi, H., T. Suzuki, and T. Unemoto. 1986. Streptococcal cytoplasmic pH is regulated by changes in amount and activity of a proton-translocating ATPase. J. Biol. Chem. 261:627-630. [PubMed] [Google Scholar]
- 15.Kuhnert, W. L., and R. G. Quivey Jr. 2003. Genetic and biochemical characterization of the F-ATPase operon from Streptococcus sanguis 10904. J. Bacteriol. 185:1525-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LeBlanc, D. J., and L. N. Lee. 1991. Replication function of pVA380-1, p. 235-239. In G. M. Dunny, P. P. Cleary, and L. L. McKay (ed.), Genetics and molecular biology of streptococci and enterococci. American Society for Microbiology, Washington, D.C.
- 17.LeBlanc, D. J., L. N. Lee, A. Abu, and A. Jaibat. 1992. Molecular, genetic, and functional analysis of the basic replicon of pVA380-1, a plasmid of oral streptococcal origin. Plasmid 28:130-145. [DOI] [PubMed] [Google Scholar]
- 18.Loesche, W. J. 1986. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 50:353-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma, Y., T. M. Curran, and R. E. Marquis. 1997. Rapid procedure for acid adaptation of oral lactic-acid bacteria and further characterization of the response. Can. J. Microbiol. 43:143-148. [DOI] [PubMed] [Google Scholar]
- 20.Martin-Galiano, A. J., M. J. Ferrandiz, and A. G. de la Campa. 2001. The promoter of the operon encoding the F0F1 ATPase of Streptococcus pneumoniae is inducible by pH. Mol. Microbiol. 41:1327-1338. [DOI] [PubMed] [Google Scholar]
- 21.McDermid, A. S., A. S. McKee, D. C. Ellwood, and P. D. Marsh. 1986. The effect of lowering the pH on the composition and metabolism of a community of nine oral bacteria grown in a chemostat. J. Gen. Microbiol. 132:1205-1214. [DOI] [PubMed] [Google Scholar]
- 22.Murchison, H. H., J. F. Barrett, G. A. Cardineau, and R. Curtiss III. 1986. Transformation of Streptococcus mutans with chromosomal and shuttle plasmid (pYA629) DNAs. Infect. Immun. 54:273-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pierais, V. L., and G. J. Barcak. 1999. Development of E. coli host strains tolerating unstable DNA sequences on ColE1 vectors. Focus 21:18-19. [Google Scholar]
- 24.Quivey, R. G., Jr., W. L. Kuhnert, and K. Hahn. 2000. Adaptation of oral streptococci to low pH. Adv. Microb. Physiol. 42:239-274. [DOI] [PubMed] [Google Scholar]
- 25.Quivey, R. G., Jr., R. C. Faustoferri, W. A. Belli, and J. S. Flores. 1991. Polymerase chain reaction amplification, cloning, sequence determination and homologies of streptococcal ATPase-encoding DNAs. Gene 97:63-68. [DOI] [PubMed] [Google Scholar]
- 26.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning. A laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 27.Shaw, W. V. 1979. Chloramphenicol acetyltransferase activity from chloramphenicol-resistant bacteria. Methods Enzymol. 43:737-755. [DOI] [PubMed] [Google Scholar]
- 28.Slots, J., A. Ashimoto, M. J. Flynn, G. Li, and C. Chen. 1995. Detection of putative periodontal pathogens in subgingival specimens by 16S ribosomal DNA amplification with the polymerase chain reaction. Clin. Infect. Dis. 20:S304-S307. [DOI] [PubMed] [Google Scholar]
- 29.Smith, A. J., R. G. Quivey, Jr., and R. C. Faustoferri. 1996. Cloning and nucleotide sequence analysis of the Streptococcus mutans membrane-bound, proton-translocating ATPase operon. Gene 183:87-96. [DOI] [PubMed] [Google Scholar]
- 30.Sturr, M. G., and R. E. Marquis. 1992. Comparative acid tolerances and inhibitor sensitivities of isolated F-ATPases of oral lactic acid bacteria. Appl. Environ. Microbiol. 58:2287-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wen, Z. T., and R. A. Burne. 2001. Construction of a new integration vector for use in Streptococcus mutans. Plasmid 45:31-36. [DOI] [PubMed] [Google Scholar]
- 32.Zheng, M., X. Wang, B. Doan, K. A. Lewis, T. D. Schneider, and G. Storz. 2001. Computation-directed identification of OxyR DNA binding sites in Escherichia coli. J. Bacteriol. 183:4571-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]