Abstract
Development in marsupials is specialized towards an extremely short gestation and highly altricial newborns. As a result, marsupial neonates display morphological adaptations at birth related to functional constraints. However, little is known about the variability of marsupial skull development and its relation to morphological diversity. We studied bony skull development in five marsupial species. The relative timing of the onset of ossification was compared to literature data and the ossification sequence of the marsupial ancestor was reconstructed using squared-change parsimony. The high range of variation in the onset of ossification meant that no patterns could be observed that differentiate species. This finding challenges traditional studies concentrating on the onset of ossification as a marker for phylogeny or as a functional proxy. Our study presents observations on the developmental timing of cranial bone-to-bone contacts and their evolutionary implications. Although certain bone contacts display high levels of variation, connections of early and late development are quite conserved and informative. Bones that surround the oral cavity are generally the first to connect and the bones of the occipital region are among the last. We conclude that bone contact is preferable over onset of ossification for studying cranial bone development.
Marsupials are born at the most altricial (premature) stage of all mammals. A large part of the development that occurs embryonically in other mammals takes place after birth in marsupials, making them a very important group for developmental studies because of the opportunity to observe these later stages in vivo and ex utero1,2,3. With the increased interest in constraints for studies in evolutionary biology4, marsupial development has been extensively studied in light of the marsupial-placental dichotomy5, with a particular focus on the presence6,7 or absence8 of developmental constraints. Very little is known, however, about patterns of development within the marsupial clade and their relation to marsupial morphological diversity.
In addition to the physiological adaptations attributed to the unique life history strategy of marsupials, most adaptations of the marsupial neonate relate to the journey from the vagina into the maternal pouch and the subsequent attachment to the teat immediately after birth, affecting both cranial and postcranial morphology. The postcranium is most notably effected by the neonate’s climb into the pouch, which is unaided by the mother in many species9. In order to make this journey at such a highly altricial state, marsupials have well-developed forelimbs. Conversely, development of the hind limbs is delayed10,11,12,13. These functional requirements of the forelimbs to reach the teat have been shown to constrain variability in forelimb morphotypes14. This constraint has often been used to explain why marsupials do not show the high forelimb disparity as seen in placentals. Placental forelimbs have evolved into specialized structures such as wings (in chiropterans) and flippers (in cetaceans, sirenians, and pinnipeds). Marsupial forelimbs are much more conserved, although specialized adaptations in marsupials occur to some degree (e.g., the marsupial mole Notoryctes)15,16.
Cranial development is mainly affected by functional requirements of early life attached to the teat of the mother. After attachment, the oral region effectively fuses with the teat, which permits a stable position such that further development of the pouch young can occur. This fusion takes place through a swelling of the teat inside the mouth cavity and the bilateral fusion of the lips by a keratinized membrane called the epitrichium11. This membrane also covers the eyes and ears to protect these structures in the neonate until the epitrichium is lost later during development1. In order to suckle and breathe simultaneously, both the nasal cavity and tongue are strongly developed. A cervical swelling supports the head while being attached to the teat11,17. To facilitate these adaptations for suckling, the relative timing of the development of bones and musculature in the oral and facial region is accelerated, whereas the relative timing of the brain is delayed compared to placentals18,19,20. This earlier development of the oral region and the complex suckling behavior led to the consideration that this region is particularly constrained in marsupials. Recently, Goswami et al.21 quantified cranial ontogeny using phenotypic trajectory analysis to compare disparity in placental and marsupial cranial development with a special focus on the oral region. They showed that the development of the bones of this region is indeed constrained in marsupials compared to placentals. However, when considering the entire skull, both groups showed the same amount of cranial disparity.
Although bony skull development in marsupials has been studied in this comparative context among mammals, studies focusing on differences in cranial skeletogenesis within the marsupial clade never included more than two marsupial species22,23. Therefore, in order to further understand the cranial morphological disparity observed within this group, a detailed investigation of the variation of cranial development in a wide range of marsupials was desirable21,24.
The aim of our study was to quantify cranial development of marsupials by establishing 1) the relative timing of the onset of ossification for each bone and 2) the relative timing of each bone-to-bone contact. We considered bone contact, which has previously not been used as a quantitative metric in developmental biology, to be the most reliable proxy to indicate the presence of structurally robust and functionally important bones during skeletal development. Furthermore, since bone contact logically follows some time after the onset of ossification of the respective bones, the former provides information for more progressed developmental stages than the latter. The relative timing and its variation for both bone onset and bone contact could be compared between all skull elements, giving new quantitative insights into marsupial osteogenesis. Finally, by comparing these two different metrics, we were able to determine their general value for studying cranial development.
Methods
Specimens
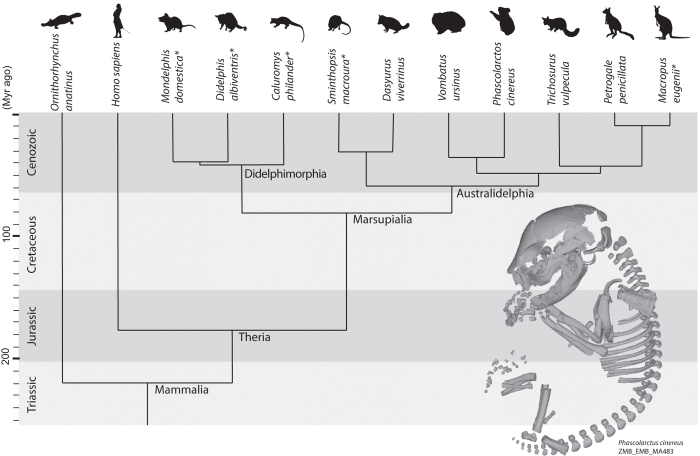
In total, we studied 115 different pouch young in five marsupial species: the common brushtail possum (Trichosurus vulpecula, 35 specimens), the eastern quoll (Dasyurus viverrinus, 23 specimens), the koala (Phascolarctos cinereus, 30 specimens), the common wombat (Vombatus ursinus, 11 specimens), and the brush-tailed rock-wallaby (Petrogale penicillata, 16 specimens) (Fig. 1 and Suppl. Table 1). We have chosen these species out of all 41 marsupial species available in the Hill-Collection25, which is part of the Embryological collection of the Museum für Naturkunde Berlin, Germany26,27. The selected species are represented by a well distributed series of specimens at different relative levels of development, ranging from early pouch young at the beginning of cranial osteogenesis to adult specimens. The studied species represent a phylogenetically diverse subset of marsupials.
Figure 1. A scaled phylogeny of the marsupial species studied in the present study, as well as the species from Koyabu et al.29 that were included for the onset of ossification analysis, and two outgroup representatives, one placental (Homo) and one monotreme (Ornithorhynchus).
The species for which all data were taken from Koyabu et al.29 are marked by an asterisk (*). Note however that for the onset of ossification analysis, data for Dasyurus viverrinus and Trichosurus vulpecula were taken from Koyabu et al.29. For T. vulpecula these data were used in addition to data collected directly for this study. In addition, the most relevant clade names are included. The phylogenetic tree is scaled following the divergence times used for the analyses in Mesquite.
μCT-scanning
Because of the rare and historic value of the specimens from the Hill–Collection, we have chosen the non-invasive technique of micro-computed tomography (μCT) to analyze all 115 specimens. To prevent shrinkage, all specimens were wrapped in several layers of synthetic sponge that had been wetted in ethanol. The wrapped specimens were subsequently put into tubes that were closed off with either a cap or tape and secured inside the scanner. All studied specimens were scanned at the Museum für Naturkunde Berlin using a Phoenix nanotom X-ray|s tube at between 50 and 90 kV and 150 to 300 μA, depending on the size of the specimen. For each scan 1000 projections were generated with 750 ms per scan. Effective voxel size ranged between 2 and 3.33 μm. The cone beam reconstruction was performed using the datos|x-reconstruction software (GE Sensing & Inspection Technologies GMBH phoenix|x-ray datos|x 2). Isosurface renderings were produced in VG Studio Max 2.2. Because the settings used for scanning varied depending on the size of each specimens, the gray-scale thresholds used for the isosurface renderings also differed between specimens and were set to include as many bone fragments as possible without including any non-calcified tissue, such as cartilage, skin, or muscle. Isosurfaces were processed as .ply or .stl files using MeshLab 1.3.3328, in which any noise from the scan was removed by deleting isolated pieces by diameter or manually. Images were further edited in Adobe Photoshop CS6 and Adobe Illustrator CS6 (see Fig. 2). For each species, also one adult skull from the Mammal Collection of the Museum für Naturkunde Berlin was μCT-scanned (Suppl. Table 1).
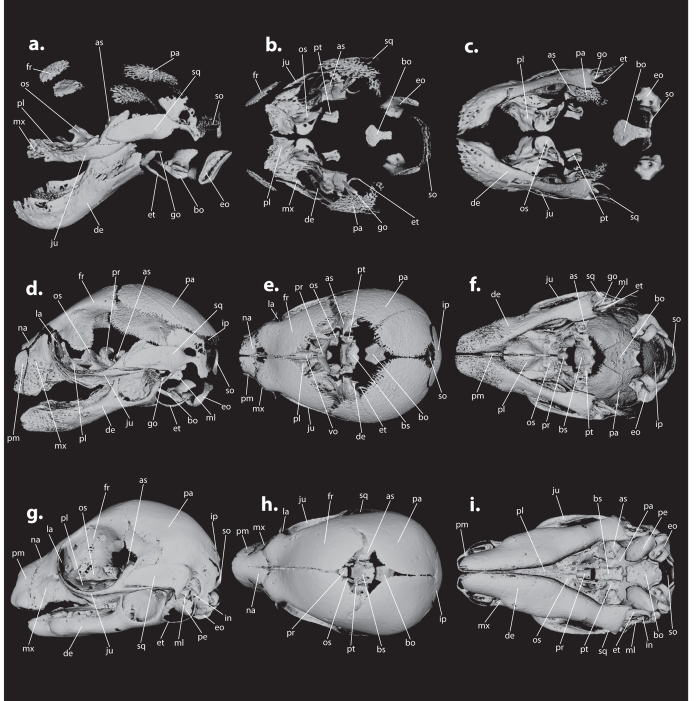
Figure 2. One plate with three different stages of cranial bone development in Petrogale penicillata.
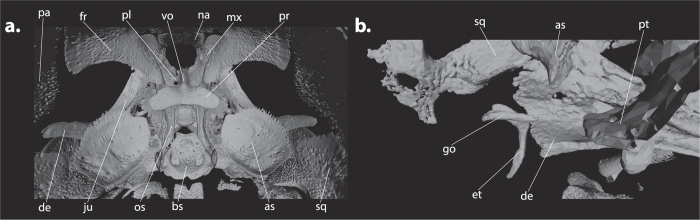
The left lateral side of the skull is shown in a,d, and g, the dorsal view in b,e, and h, and the ventral view in c,f, and i. Images were created using MeshLab 1.3.3 and Adobe Illustrator CS6. a,b, and c represent ZMB_EMB_MA587, the earliest of the three stages; d,e, and f represent ZMB_EMB_MA590, the intermediate stage; and g,h, and i represent ZMB_EMB_MA593A, the most advanced of the three stages illustrated here. Plates of the other four species can be found in the Suppl. Figs 3, 4, 5, and 6. Abbreviations (for all figures): as (alisphenoid), bo (basioccipital), bs (basisphenoid), de (dentary), eo (exoccipital), et (ectotympanic), fr (frontal), go (goniale), in (incus), ip (interparietal), ju (jugal), la (lacrimal), ml (malleus), mx (maxilla), na (nasal), os (orbitosphenoid), pa (parietal), pe (petrosal), pl (palatine), pm (premaxilla), pr (presphenoid), pt (pterygoid), so (supraoccipital), sq (squamosal), vo (vomer).
Analytical framework 1: onset of ossification
We used two approaches to study cranial bone development in marsupials. The first, conventional approach was to study cranial osteogenesis by determining the onset of ossification of 25 bones (Suppl. Tables 4–10). Because no data on the absolute age of the specimens were available, the specimens were ordered based on the bone onset timing and were subsequently ranked with each new rank showing the first appearance of one or more bones (sensu Koyabu et al.29, Suppl. Tables 4–8). A relative scaling was made to create a matrix containing continuous data between 0 and 1 (sensu Werneburg et al.30,31 and Germain and Laurin32; Suppl. Tables 9 and 10). We only included species for which six or more ranks were available in order to decrease the possibility of errors related to sample size29,30. By combining the data on the onset of ossification from our study with data from the literature as assembled by Koyabu et al.29, we compared marsupial cranial osteogenesis in 10 different species; seven species from Koyabu et al.29 and four species from our study, with T. vulpecula being included twice using data from both studies. We excluded the data of D. viverrinus from this analysis because only four different ranks of bone onset could be distinguished. The interparietal, malleus, presphenoid, and incus were not studied by Koyabu et al.29.
Thus, we reported variation in the timing of bone onset among the studied species by presenting the minimal and maximal values for the ossification timing of each bone, as well as by calculating their respective median and 25th and 75th percentiles. Furthermore, we reconstructed the relative ancestral timing of ossification for each bone. We calculated ancestral values using squared-change parsimony analysis33, taking divergence time into consideration in the form of the branch lengths in a molecular time scaled phylogeny. We chose this method of ancestral trait reconstruction over other methods such as event-pairing or Parsimov because this continuous analysis has been convincingly shown to be more reliable32,34. Furthermore, we calculated the 70% confidence intervals (CIs) of the reconstructed timing for each bone to determine the statistical strength of the reconstructions (sensu Werneburg et al.30).
Our molecular time scaled phylogeny was modified from the one used by Koyabu et al.29 by adding the species of the present study using Mesquite 3.0335. Topology and divergence time of the added taxa were determined using The Timetree of Life36. The divergence time of Ma. eugenii and Pe. penicillata were retrieved from http://www.timetree.org/ (2016-11-22) and were scaled to the other divergence times by calculating the deviation between the divergence time found for Potoroidae and Macropodidae from http://www.timetree.org/ and Springer et al.36 (scale = 0.5617).
Analytical framework 2: bone contacts
The second, non-traditional, approach was to study the onset of bone contacts (Suppl. Tables 11–21). This is the first study to quantify initial bone contact (note: cranial suture closure has previously been studied quantitatively, e.g. by Wilson and Sánchez-Villagra, Rager et al., and Wilson37,38,39). Bone contacts were defined as clearly observable contacts between the different cranial bones in the .ply and .stl files. We excluded contacts that were only formed by three or less triangles, because these contacts were likely to be the result of the reconstruction process rather than representing an actual connection between two bones (for further discussion, see Suppl. Fig. 7). We ordered the specimens based on bone contact and ranked them (as for the onset of ossification data, Suppl. Tables 11–15). The contacts were scaled and the median and 25th and 75th percentiles were calculated. Ancestral values and 70% CIs were calculated in Mesquite35, using the same phylogeny as for the onset of ossification analysis. The last common ancestor of all taxa for which bone contact was analyzed was the last common ancestor of all Australidelphia (i.e., Marsupialia excl. the American forms) (Fig. 1).
Results
Onset of ossification
In total, 25 different bones were analyzed for their onset of ossification. In the following, we summarize our results and briefly describe differences to the findings of previously published papers.
The relative timing of all bones in species with more than six ranks are summarized in Fig. 3. The general sequence of ossification found in this study confirms previous studies on marsupials in that the bones of the oral region are the first to ossify (maxilla, premaxilla, and dentary)22,23,29.
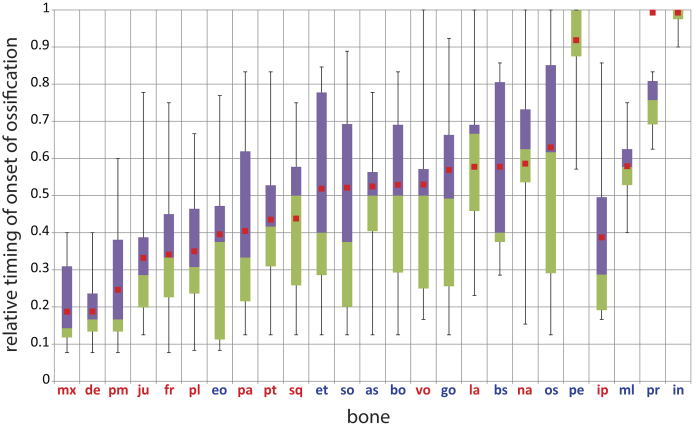
Figure 3. Boxplot comparison showing the range of variation for the relative timing of the onset of ossification.
The 25th percentile is marked by the lower edge of the box, the 75th percentile by the upper end. The median is marked by the boundary between the purple and green parts of the box. The upper and lower whiskers mark the maximal and minimal value, respectively. The reconstructed timing of the onset of ossification for each bone in the marsupial ancestor is shown by the red squares. The bones are ordered by the onset of ossification in the ancestor of all marsupials. Bones colored in red are of dermal origin, bones colored in blue of endochondral origin (sensu Koyabu et al.29 and Hall55). The variation in onset of ossification is very large for all bones and the reconstructed ossification sequence follows previous authors in that the bones surrounding the mouth cavity are among the first bones to ossify22,23,29. For a graphic representation of the onset timing for each individual species see Suppl. Fig. 1. The values for this graph can be found in Suppl. Table 2.
We only included pouch young (=postnatal) specimens in our study because we did not detect ossification centers in any of the fetal specimens of the collection. This supports the observation of Gemell et al.23 that cranial ossification commences distinctly after birth in Isoodon macrourus and Trichosurus vulpecula. However, Clark and Smith22 reported the presence of an already ossified premaxilla, maxilla, dentary, palatine, and pterygoid at birth in Monodelphis domestica and Macropus eugenii. In the case of Ma. eugenii, the presence of these bones at birth does not necessarily contradict the observations of the present study because this species is more precocial than any of the species studied here11. For Mo. domestica, on the other hand, this observation seems more contradictory, since this species only shows an intermediate stage of development at birth among marsupials3,11.
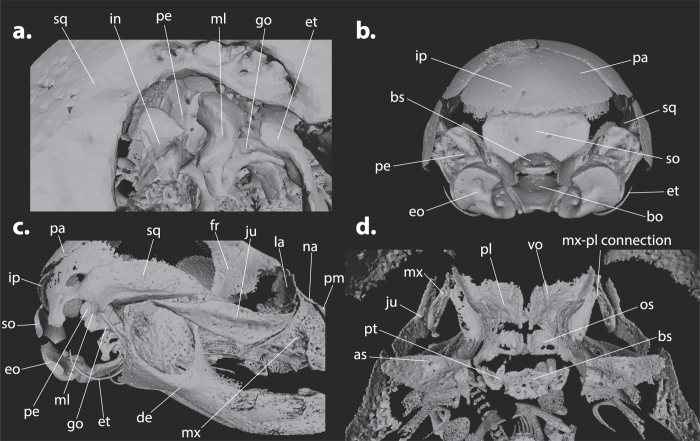
Our μCT-data permitted the identification of bones that were not treated by Koyabu et al.29, namely two middle ear bones (malleus and incus), the interparietal, and the presphenoid (Figs 4a,b and 5). However, these bones have previously been studied, among other cranial bones, in Mo. domestica and Ma. eugenii by Clark and Smith22.
Figure 4.
(a) Right lateral view of the ear region of Trichosurus vulpecula ZMB_EMB_MA400 showing the incus, malleus, goniale, and dorsal part of the ectotympanic. In most species the goniale detaches from its surrounding bones at the final stages of cranial bone development (Suppl. Tables 11–15). The connection of the goniale to the ectotympanic is the result of their close proximity across most of their length (see also Fig. 5B). Similarly, this is likely also the case for the connection of the malleus and incus. (b) Posterior view of the skull of T. vulpecula ZMB_EMB_MA400 showing a large interparietal. In T. vulpecula, the interparietal has a very early onset and large size early in development, being much larger than the supraoccipital. It appears to generally have one center of ossification, although intraspecific variation occurs. It remains very prominent in the adult skull, even connecting to the squamosal bone, and forms the posterior part of the cranial dome. In Dasyurus viverrinus, the interparietal can be clearly distinguished in the earliest developmental rank (ZMB_EMB_MA759) but cannot be found in more developed specimens, indicating a very early fusion of the interparietal with the supraoccipital. In Phascolarctos cinereus, the interparietal generally has three ossification centers; otherwise its development is similar to T. vulpecula, although it does not make contact with the squamosal. In contrast to the other species, the interparietal ossifies relatively very late in Vombatus ursinus. It is comparatively very small throughout cranial development, shows two centers of ossification, and most likely fuses completely with the parietals. The interparietal appears to have one ossification center in Petrogale penicillata (0.38), but otherwise seems to follow the same general developmental pattern as in Ph. cinereus and T. vulpecula. (c) Lateral view of the skull of Pe. penicillata ZMB_EMB_MA564 showing the jugal, maxilla and squamosal bones that form the jugal arch. (d) Dorsal view of the sphenoid region of Ph. cinereus ZMB_EMB_MA495B showing the clear distinction of the individual bones, as well as the presence of early ossification centers of both vomers. Furthermore, the early connection between the maxilla and palatine can be observed.
Figure 5.
(a) Posterodorsal view of the sphenoid region of Petrogale penicillata ZMB_EMB_MA585 showing the shape and location of the presphenoid. This bone is often overlooked in osteological studies; it originates from two different ossification centers at the anterior part of the braincase. Its ventral part connects successively to the orbitosphenoid, vomer, and basisphenoid, whereas the dorsal part forms two winglike lateral extensions that first connect to the frontal and at a later stage to the alisphenoid. Regarding the presphenoid, Clark and Smith22 reported that it connects to the orbitosphenoid almost immediately after the individual ossification of both bones. Although the orbitosphenoid is generally the first bone the presphenoid connects to in our data, it occurs late in development (median: 0.79), and the orbitosphenoid first connects to the maxilla (0.14), alisphenoid (0.69) pterygoid (0.69), and frontal (0.73) before connecting to the presphenoid. (b) Medial view of the middle ear region of Pe. penicillata ZMB_EMB_MA587 showing the close proximity of the goniale and ectotympanic.
According to Clark and Smith22, the incus is the last bone to ossify, with the exception of the stapes, the latter of which has not been treated in our study because it could not be distinguished in the μCT-scans. This concurs with our results, in which the incus is the last bone to ossify (median: 1.00; potentially excluding the stapes, which was not analyzed herein). Furthermore, Clark and Smith22 reported that the malleus ossifies 11 and 21 days after birth in Mo. domestica and Ma. eugenii, respectively, which is relatively late. This could also be confirmed for the relative timing of the malleus in our study (median: 0.58; Fig. 3).
The interparietal is present as a postparietal bone (Fig. 4b) and ossifies eight days after birth in Ma. eugenii and and three days after birth in Mo. domestica22, which is relatively earlier than in Vombatus ursinus, but later than in T. vulpecula, Dasyurus viverrinus, Phascolarctos cinereus, and Petrogale penicillata (Fig. 3).
The onset of ossification data show a large amount of variation. The variation in relative onset of ossification is more than 75% in six of the 25 bones, namely in the ossification of the supraoccipital (min-max: 0.13–0.89; Fig. 3), vomer (0.17–1.00), goniale (0.13–0.92), lacrimal (0.23–1.00), nasal (0.15–1.00), and orbitosphenoid (0.13–1.00). The incus, which was only studied in four of the 10 species, is the only bone that shows less than 25% variation (0.90–1.00) (Fig. 3).
The 70% confidence intervals for the reconstructed ancestral sequence of ossification are very broad. Only the squamosal (70% lower CI: 0.30 – reconstructed value: 0.4–70% upper CI: 0.57), vomer (0.35–0.5–0.71), goniale (0.40–0.6–0.70), and petrosal (0.83–0.92–0.95) are relatively narrow (Suppl. Table 2). Overall, this indicates a very high uncertainty for the reconstructed onset of ossification values. As such, any comparison with the reconstructions of the ancestral ossification sequence of Mammalia by Koyabu et al.29 has to be handled with care. However, compared to the mammalian ancestor reconstructed by Koyabu et al.29, the premaxilla, squamosal, pterygoid, and nasal ossified distinctly earlier in the marsupial ancestor as reconstructed herein. The exoccipital ossified distinctly later in the marsupial ancestor.
Bone contacts
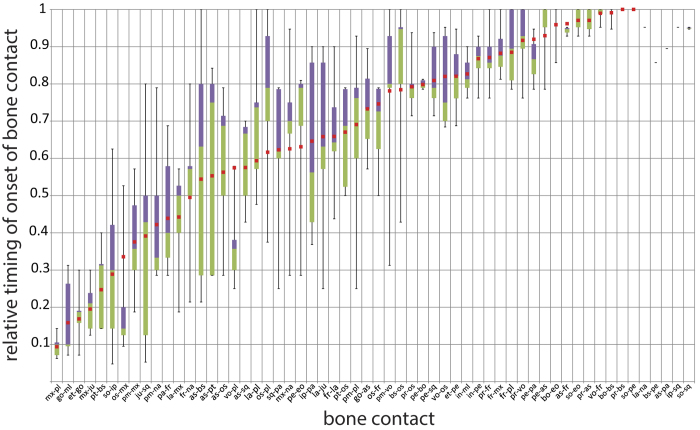
In total, 64 different bone contacts were defined, out of which 50 contacts were observed in all species and 14 contacts could only be observed in certain species (see Suppl. Tables 17–19). A boxplot of the relative timing of the bone contacts and the reconstructed bone contact sequence for the ancestor of the marsupials is shown in Fig. 6. In comparison to the reconstructed ancestral values for the onset of ossification, the reconstructed bone contact values show very narrow confidence intervals (Suppl. Table 3).
Figure 6. Boxplot comparison showing the range of variation for the relative timing of the bone contacts.
The reconstructed timing of bone-to-bone contact of the last common ancestor of all studied species (Australidelphia) is marked by the red squares. The bone contacts are ordered based on the bone contact sequence in the last common ancestor. The bone contacts clearly show less variation in timing than bone onset (see Fig. 3). The contacts of the occipital and middle ear regions, as well as the contacts of the petrosal and the maxilla-palatine contact show especially little variation. The values for this graph can be found in Suppl. Table 3.
Most bones that connect at an early stage are bones surrounding the mouth cavity [maxilla-palatine (median: 0.10; Fig. 6), maxilla-jugal (0.21), orbitosphenoid-maxilla (0.14), premaxilla-maxilla (0.36), and premaxilla-nasal (0.33) and the goniale and the bones to which it connects: goniale-malleus (0.10) and ectotympanic-goniale (0.19)].
The bones that are among the last to make contact are the bones that make up the back of the skull, particularly the bones of the occipital region [supraoccipital-petrosal (median: 1.00), basioccipital-basisphenoid (1.00), supraoccipital-exoccipital (1.00), basioccipital-exoccipital (1.00), petrosal-alisphenoid (1.00), and petrosal-parietal (0.87)]. The very late connection between the vomer and the frontal is created by the formation of the nasal septum (1.00).
The bone contact data also show a high degree of variation (Fig. 6). However, whereas for the onset of ossification variation is high in all bones, this is not the case for bone contact, where the bones that connect very early or late show a much lower range of variation than the intermediate contacts.
Contacts such as the jugal-squamosal (min-max: 0.05–0.80; Fig. 6), interparietal-parietal (0.37–0.90), supraoccipital-interparietal (0.05–0.63), alisphenoid-basisphenoid (0.21–1.00), and maxilla-nasal (0.29–0.95) show a particularly high range of variation. The high variation in the connection of the interparietal with the parietal and the supraoccipital is obviously effected by the high interspecific variation in the size and shape of the interparietal40.
Most bones that connect in the sphenoid region show a high range of variation as well (Fig. 6). The alisphenoid-basisphenoid (min-max: 0.21–1.00) connection shows most variation, but the alisphenoid-pterygoid (0.29–0.84), alisphenoid-orbitosphenoid (0.29–0.79), and basisphenoid-orbitosphenoid (0.43–1.00) connections are also highly variable. The pterygoid-basisphenoid (0.14–0.40) and pterygoid-orbitosphenoid (0.50–0.79) show a much lower range of variation.
The earliest and one of the most conserved connections is the maxilla-palatine connection (median: 0.10; min-max: 0.06–0.14). The petrosal connects to many different bones and these contacts are quite conserved [petrosal-basioccipital (min-max: 0.79–0.81; Fig. 6), petrosal-squamosal (0.71–0.94), ectotympanic-petrosal (0.69–0.95), incus-petrosal (0.76–0.93), petrosal-parietal (0.79–0.95), petrosal-alisphenoid (0.79–1.00), and supraoccipital-petrosal (1.00–1.00)] and are established very late in development.
The connections of the occipital region also occur very late and show a small range of variation [basioccipital-exoccipital (min-max: 0.86–1.00), supraoccipital-exoccipital (0.93–1.00), and basioccipital-basisphenoid (0.95–1.00)] (Fig. 4b and 6). In the ear region, the connections of goniale and malleus (min-max: 0.07–0.31), goniale and ectotympanic (0.07–0.30), and malleus and incus (0.76–0.90) are very conserved (Fig. 4a).
The presphenoid (Fig. 5a) ossifies relatively late (onset of ossification median: 0.76) and connects successively to the orbitosphenoid (bone contact median: 0.79), frontal (0.86), vomer (0.93), alisphenoid (1.00), and basisphenoid (1.00) (Figs 3 and 6). These connections also occur generally late in development and are quite conserved [presphenoid-orbitosphenoid (min-max: 0.71–0.94), presphenoid-frontal (0.76–1.00), presphenoid-vomer (0.76–1.00), and presphenoid-alisphenoid (0.93–1.00)], especially the presphenoid-basisphenoid connection (1.00–1.00), which only develops in the last rank for all species.
Discussion
Our study revealed a large variation in the onset of ossification of all cranial bones (Fig. 3). In contrast, bone to bone contacts are constrained in many cases (Fig. 6). The calculated confidence intervals for the reconstructed ancestral values are much broader for the onset of ossification than for bone contact, showing that onset of ossification reconstructions are comparatively much less reliable (Suppl. Tables 2 and 3). This challenges many traditional studies that use the onset of ossification as their marker for phylogeny or as a functional proxy. Because onset of ossification per definition precedes bone contact, one could argue that there is an inherent correlation between onset and first contact timing and that it seems contradictory that, within the process of bone development, a highly variable phase is followed by a much more conserved phase. In part, the variability of contact timing is diminished simply because we could observe a greater number of bone contacts: 64 bone contacts compared to 25 bones observed. This larger amount of contacts logically leads to a larger number of different ranks from which the relative scaling is established (Suppl. Tables 9 and 10 for the onset of ossification and Suppl. Tables 16–21 for bone contact), thereby diminishing the randomness. Mathematically speaking, bone number is inverse to contact variability. As such, the absolute randomness of both datasets are not comparable, as is supported by the narrower confidence intervals calculated for bone contact compared to onset of ossification.
Nonetheless, we had expected to find conservation in the onset of ossification in marsupials as they are strongly affected by constraints in early postnatal development related to early delivery and the tight fixation to the mother’s teat. However, the high variation observed likely indicates that bones offer only very little functional support to the head during early postnatal development in marsupials. Actually, cranial osteogenesis has yet to start at birth in all of the studied species and instead the cartilaginous chondocranium is strongly developed within a very short time span in the neonate to withstand ex utero conditions in general and the forces associated with suckling activities in particular41.
The early ossification of the premaxilla, maxilla, dentary, palatine, and pterygoid in marsupials has been suggested by Clark and Smith22 to represent an adaptation to suckling and the attachment to the teat. However, these bones are the first to ossify in the crania of monotreme and placental mammals (and sauropsids) as well29,42. Monotremes do not suckle but instead lick the milk from a field enclosed by skin folds in which the milk is collected43. Therefore, instead of being a specialized adaptation to suckling, early ossification of the bones surrounding the mouth cavity seems to be the ancestral condition in mammals, as was reconstructed by Koyabu et al.29.
One traditional approach to deal with the timing of ossification is to speculate a correlation of developmental timing and adult prominence of an element. The related “rule of thumb” states that the earlier a structure occurs in development, the larger or the more differentiated the structure appears in the adult stage and the later it occurs, the smaller or the less differentiated the structure appears44,45,46,47,48,49,50. This is because earlier appearing structures have more time to develop than later appearing structures. This “rule of thumb” has been tested by Werneburg et al.51, revealing that only in very few cases a clear correlation exists between ontogenetic timing and adult appearance of a structure. There are many possible reasons for this, including different speeds of development between different elements. Also, single elements can have faster and slower phases of development. Moreover, a comparative quantification of adult bone prominence and complexity is very difficult, which makes a correlation to the relative timing of the first appearance of the structures very complicated. The whole issue becomes even more challenging when considering the observed high variation in the onset of ossification among the very similar species of our study. The petrosal bone, for example, ossifies very late and also makes contact with other bones late in development (Figs 3 and 6). The relative timing in the onset of ossification is comparatively conserved and the petrosal increases in size rapidly to connect to a number of other bones. This contradicts the “early equals important rule” that bones that ossify earlier are larger and more differentiated in the adult stage. Simultaneously, the very late onset of ossification and subsequent rapid growth after onset explains the conserved connections of the petrosal with other bones.
Given the results of our study, a well supported discussion can only be drawn on ontogenetic structures of little variation among species. This will consequently result in the discussion of highly conserved developmental processes such as the observed earlier ossification of the malleus in comparison to the incus. This pattern probably recapitulates the development of the articular (malleus) bone in synapsid ancestors, which, as a part of the mandible, would have ossified earlier than the quadrate (=incus)52 (Fig. 3).
We observed high variation for most bone contacts. However, certain contacts were conserved (Fig. 6). These were generally established either quite early or late in development. The bones of the oral region, as well as the goniale and its surrounding bones, connect early and in doing so, they correspond to the (generally) early ossification of these bones.
In many cases, the observed variation in bone contacts can primarily be explained through characteristics of individual bones during ontogeny that do not necessarily constitute any detectable evolutionary value, such as shape and proximity to other bones. In other cases, the absence or presence of variation in the timing of bone contacts seems to indicate the functional requirements of these connections during ontogeny. Therefore, the only way to infer the meaning of the observed timing of bone contacts is to interpret every contact based on the individual development of each bone and its relationships with the other bones surrounding it.
The ectotympanic, for example, is located near the posterior end of the dentary in mammals and is homologous to the angular in non-mammalian amniotes53. In all studied species, it shows an early and conserved connection to the goniale, which is homologous to the prearticular, with which it forms a ring-like structure from which the tympanic membrane or eardrum is spanned (Figs 4c and 5b)52. The incus-malleus connection, which facilitates the advanced hearing in mammals and is therefore also functionally important, is also quite conserved (Fig. 6).
The maxilla-palatine contact, the contacts of the occipital region, the petrosal contacts, and the middle ear bone contacts are particularly constrained and show little variation (Fig. 6). The earliest and one of the most conserved connections is the maxilla-palatine connection; this might be an important adaptation for suckling as it supports the palatine region (Fig. 4d). Similarly, the late timing of a conserved bone-to-bone connection could also indicate the functional role of a structure. Namely, the late and constrained connections of the occipital region could indicate that this area does not have an important function for cervical support during suckling early in development. Therefore the cervical support provided by the swelling of non-ossified tissue is likely sufficient11.
Most bones that connect in the sphenoid region show a high range of variation (Fig. 6). This overall high variability is most likely related to the proximity of these connections to each other (Fig. 4d, but see also Suppl. Fig. 7B). Because of their close proximity, a small variation in the position of the bones can result in relatively large differences in the onset of bone contact.
It is important to note that the jugal-squamosal contact is highly variable. Its connection implies the formation of the jugal arch, which supports part of the jaw musculature (mm. masseter et zygomandibularis) and the formation of the eye socket54. Therefore, the jugal arch is an important structure for both feeding and perception (Fig. 4c). The high variation observed in this connection, however, seems to indicate that the jugal arch is not of strong functional importance during marsupial development in the pouch. The early and constrained maxilla-jugal connection, however, which connects the anterior part of the jugal arch, contrasts with this very variable jugal-squamosal contact (Fig. 4c and 6).
The high variation in the maxilla-nasal connection could be explained in that this connection is generally very small, as the nasal mainly connects to the premaxilla to form a connection with the lower part of the skull. Therefore, the maxilla-nasal connection most likely does not constitute an important supporting function and is dependent on the size and formation of the snout (see Suppl. Fig. 7D).
The late connections of certain bones to the frontal (frontal-maxilla, frontal-palatine, alisphenoid-frontal, and formation of the nasal septum: vomer-frontal) (Fig. 6) represent the connection of the upper and lower parts of the cranium, which are further formed during early development by the premaxilla and nasal and in the middle of development by the squamosal and parietal, anteriorly and posteriorly, respectively.
The bones in the oral region have both an early onset of ossification and are among the first bones to connect (Figs 3 and 6). Overall, this might indicate that in the altricial marsupial neonate suckling forces require more structural support from the cranial bones of the oral region than the cervical region requires for the connection between the body and the head. The support for this connection is already largely provided by the cervical swelling in the early neonate11,17.
Whereas the early onset of ossification is very variable and not unique to marsupials (see above), the bone contact formations are more conservative. The latter result in similar interpretations as provided for ossification in the past11,22,23,29 but having greater statistic support. However, in order to further substantiate such a statement, the timing of bone contacts in the other two major mammalian clades has to be investigated in the future.
Conclusion
The results of this study indicate that studying bone contact is a very suitable marker for studying cranial osteogenesis, since it offers more data than the onset of ossification and is less variable partly because of this. By observing and interpreting the timing of every bone contact individually, particularly for contacts that show little variation, its timing and degree of variation can give valuable insights into phylogeny and functional requirements early in development (e.g., the early connection of the middle ear bones and the maxilla-palatine connection). Furthermore, bone contacts give insight into much later stages of development than can be observed through studying onset of ossification alone. Therefore, bone contact should be considered an important proxy in studies of cranial osteogenesis in vertebrates.
Additional Information
How to cite this article: Spiekman, S. N. F. and Werneburg, I. Patterns in the bony skull development of marsupials: high variation in onset of ossification and conserved regions of bone contact. Sci. Rep. 7, 43197; doi: 10.1038/srep43197 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
We are grateful to Kristin Mahlow for advice and help with the μCT facilities at the Museum für Naturkunde Berlin. We thank Peter Giere and Christiane Funk for access to the Hill Collection and the Zoological collection at this museum. In addition, we acknowledge Nadia B. Fröbisch, Johannes Müller, and all the colleagues of their research groups for discussions, as well as Vera Weisbecker and Marcelo R. Sánchez-Villagra for valuable insights. In particular, we would like to thank Daisuke Koyabu for further improving the manuscript. Finally, Michael K. Richardson and Rui Diogo are thanked for their support and Borja Esteve-Altava and one anonymous reviewer are thanked for their constructive recommendations. S.S. was funded by an Institute of Biology Leiden Travel Grant for Master of Science research project abroad, Curatorenfonds Leiden University, Quintusfonds by student society Algemene Leidse Studentenvereniging Quintus, and the Erasmus Programme; I.W. was funded by the Swiss National Science Foundation Advanced Postdoc Mobility Grant (P300P3_158526 and P300PA_164720). Publication costs were covered partially by the Museum für Naturkunde Berlin, Open-Access-Publikationsfonds der Leibniz-Gemeinschaft, and mainly by the ‘Deutsche Forschungsgemeinschaft & Open Access Publishing Fund of University of Tübingen’.
Footnotes
The authors declare no competing financial interests.
Author Contributions The project was conceived by S.S. and I.W. S.S. collected the data and created the figures. S.S. and I.W. wrote the manuscript. Both authors have read and approved the final manuscript.
References
- Tyndale-Biscoe C. H. Life of Marsupials. CSIRO Publishing, Collingwood (2005). [Google Scholar]
- Tyndale-Biscoe C. H. & Renfree M. Reproductive physiology of marsupials. Cambridge University press, Cambridge (1987). [Google Scholar]
- Werneburg I. & Spiekman S. N. F. Mammalian Embryology. Zoology Frank Zachos, Asher, Robert (eds). Mammalia. Series: The Handbook of Zoology/Handbuch der Zoologie (online), from http://www.degruyter.com/view/Zoology/bp_027590-2_7. Retrieved 5 Dec. 2016.
- Gould S. J. The panda’s thumb: More reflections in natural history. WW Norton & company (1980). [Google Scholar]
- Sánchez-Villagra M. R. Why are there Fewer Marsupials than Placentals? On the Relevance of Geography and Physiology to Evolutionary Patterns of Mammalian Diversity and Disparity. Journal of Mammalian Evolution 20, 279–290 (2013). [Google Scholar]
- Lillegraven J. A. Biological Considerations of the Marsupial-Placental Dichotomy. Evolution 29, 707–722 (1975). [DOI] [PubMed] [Google Scholar]
- Lillegraven J. A., Thompson S. D., McNab B. K. & Patton J. L. The origin of eutherian mammals. Biological Journal of the Linnean Society 32, 281–336 (1987). [Google Scholar]
- Kirsch J. A. W. The Six-Percent Solution: Second Thoughts on the Adaptedness of the Marsupialia. American Scientist 65, 276–288 (1977). [PubMed] [Google Scholar]
- Gemmell R. T., Veitch C. & Nelson J. Birth in marsupials. Comparative Biochemistry and Physiology Part B 131, 621–630 (2002). [DOI] [PubMed] [Google Scholar]
- Bininda-Emonds O. R. P. et al. Forelimb-hind limb developmental timing across tetrapods. BMC Evolutionary Biology 7 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R. L. & Hall L. S. Structural Adaptations of the Newborn Marsupial. In The Developing Marsupial: Models for Biomedical Research, by Tyndale-Biscoe C. H. & Janssens P. A., Springer-Verlag, Berlin (1988). [Google Scholar]
- Sears K. E. Differences in the timing of prechrondrogenic limb in Mammals: the Marsupial-Placental dichotomy resolved. Evolution 63, 2193–2200 (2009). [DOI] [PubMed] [Google Scholar]
- Weisbecker V., Goswami A., Wroe S. & Sánchez-Villagra M. R. Ossification Heterochrony in the Therian Postcranial Skeleton and the Marsupial-Placental Dichotomy. Evolution 62, 2027–2041 (2008). [DOI] [PubMed] [Google Scholar]
- Sears K. E. Constraints on the Morphological Evolution of Marsupial Shoulder Girdles. Evolution 58, 2353–2370 (2004). [DOI] [PubMed] [Google Scholar]
- Goswami A., Weisbecker V. & Sánchez-Villagra M. R. Developmental Modularity and the Marsupial-Placental Dichotomy. Journal of Experimental Zoology 312B, 186–195 (2009). [DOI] [PubMed] [Google Scholar]
- Kelly E. M. & Sears K. E. Limb specialization in living marsupial and eutherian mammals: constraints on mammalian limb evolution. Journal of Mammalogy 92, 1038–1049 (2011). [Google Scholar]
- Hill J. P. & Osman-Hill W. C. The growth-stages of the pouch-young of the Native Cat (Dasyurus viverrinus) together with observations on the anatomy of the new-born young. The Transactions of the Zoological Society of London 28, 349–352 (1955). [Google Scholar]
- Smith K. K. The evolution of mammalian development. Bulletin of the Museum of Comparative Zoology 156, 119–135 (2001). [Google Scholar]
- Smith K. K. Craniofacial development in marsupial mammals: developmental origins of evolutionary change. Developmental Dynamics 235, 1181–1193 (2006). [DOI] [PubMed] [Google Scholar]
- Nunn C. L. & Smith K. K. Statistical analyses of developmental sequences: the craniofacial region in marsupial and placental mammals. The American Naturalist 152, 82–101 (1998). [DOI] [PubMed] [Google Scholar]
- Goswami A. et al. Do Developmental Constraints and High Integration Limit the Evolution of the Marsupial Oral Apparatus? Integrative and Comparative Biology icw039 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C. T. & Smith K. K. Cranial Osteogenesis in Monodelphis domestica (Didelphidae) and Macropus eugenii (Macropodidae). Journal of Morphology 215, 119–149 (1993). [DOI] [PubMed] [Google Scholar]
- Gemmell R. T., Johnston G. & Bryden M. M. Osteogenesis in two marsupial species, the bandicoot Isoodon macrourus and the possum Trichosurus vulpecula. Journal of Anatomy 159, 155–164 (1988). [PMC free article] [PubMed] [Google Scholar]
- Goswami A., Milne N. & Wroe S. Biting through constraints: cranial morphology, disparity and convergence across living and fossil carnivorous mammals. Proceedings of the Royal Society B 278, 1831–1839 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. M. S. James Peter Hill. 1873-1954. Biographical Memoirs of Fellows of the Royal Society 1, 100–117 (1955). [Google Scholar]
- Giere P. & Zeller U. Die Embryologische Sammlung/The embryological collection. Berlin Annual Report Jahresbericht 2006 15 (2006). [Google Scholar]
- Richardson M. K. & Narraway J. A treasure house of comparative embryology. The International Journal of Developmental Biology 43, 591–602 (1999). [PubMed] [Google Scholar]
- Cignoni P., Corsini M. & Ranzuglia G. MeshLab: an open-source 3D mesh processing system. ERCIM News 73, 45–46 (2008). [Google Scholar]
- Koyabu D. et al. Mammalian skull heterochrony reveals modular evolution and a link between cranial development and brain size. Nature Communications 5, 1–9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werneburg I., Koyabu D., Laurin M. & Sánchez-Villagra M. R. Evolution of organogenesis and the origin of altriciality in mammals. Evolution & Development 18, 229–244 (2016). [DOI] [PubMed] [Google Scholar]
- Werneburg I., Laurin M., Koyabu D., Ascarrunz E. & Sánchez-Villagra M. R. Reconstruction of life history traits in early amniotes. 86. Annual meeting of Paläontologische Gesellschaft. Schiffweiler, September 14th–17th 2015. Veröffentlichungen des Zentrums für Biodokumentation (ZfB) (4/2015), 57 (2015).
- Germain D. & Laurin M. Evolution of ossification sequences in salamanders and urodele origins assessed through event-pairing and new methods. Evolution & Development 11, 170–190 (2009). [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Phylogenies and the comparative method. The American Naturalist 125, 1–15 (1985). [DOI] [PubMed] [Google Scholar]
- Laurin M. & Germain D. Developmental characters in phylogenetic inference, and their absolute timing information. Systematic Biology 60, 630–644 (2011). [DOI] [PubMed] [Google Scholar]
- Maddison W. P. & Maddison D. R. Mesquite: a modular system for evolutionary analysis. http://mesquiteproject.org Version 2.75 (2011).
- Springer M. S., Krajewski C. W. & Meredith R. W. Marsupials (Metatheria). Pp. 466–470 in The Timetree of Life, Hedges S. B. & Kumar S. Eds (Oxford University Press, 2009). (2009). [Google Scholar]
- Rager L., Hautier L., Forasiepi A. M., Goswami A. & Sánchez-Villagra M. R. Timing of Cranial Suture Closure in Placental Mammals: Phylogenetic Patterns, Intraspecific Variation, and Comparison with Marsupials. Journal of Morphology 275, 125–140 (2014). [DOI] [PubMed] [Google Scholar]
- Wilson L. A. B. Cranial Suture Closure Patterns in Sciuridae: Heterochrony and Modularity. Journal of Mammalian Evolution 21, 257–268 (2014). [Google Scholar]
- Wilson L. A. B. & Sánchez-Villagra M. R. Heterchrony and patterns of cranial suture closure in hystricognath rodents. Journal of Anatomy 214, 339–354 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyabu D., Maier W. & Sánchez-Villagra M. R. Paleontological and developmental evidence resolve the homology and dual embryonic origin of a mammalian skull bone, the interparietal. PNAS 109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier W. The ontogenetic development of the orbitotemporal region in the skull of Monodelphis domestica (Didelphidae, Marsupialia), and the problem of the mammalian alisphenoid. Mammalia depicta 13, 71–90 (1987). [Google Scholar]
- Maier W. On the evolutionary biology of early mammals - with methodological remarks on the interaction between ontogenetic adaptation and phylogenetic transformation. Zoologischer Anzeiger 238, 55–74 (1999). [Google Scholar]
- Manger P. R., Hall L. S. & Pettigrew J. D. The development of the external features of the platypus (Ornithorhynchus anatinus). Philosophical Transactions of the Royal Society B: Biological Sciences 353, 1115–1125 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley J. Problems of relative growth. Methuen & Co., London (1932).
- Maxwell E. E. Comparative ossification and development of the skull in palaeognathous birds (Aves: Palaeognathae). Zoological Journal of the Linnean Society 156, 184–200 (2009). [Google Scholar]
- Maxwell E. E. & Larsson H. C. E. Comparative ossification sequence and skeletal development of the postcranium of palaeognathous birds (Aves: Palaeognathae). Zoological Journal of the Linnean Society 157, 169–196 (2009). [Google Scholar]
- Mehnert E. Kainogenesis als Ausdruck differenter phylogenetischer Energien. Gustav Fischer, Jena (1897). [Google Scholar]
- Mehnert E. Biomechaniker schlossen aus dem Principe der Organogenese. Gustav Fischer, Jena (1898). [Google Scholar]
- Sánchez-Villagra M. R., Goswami A., Weisbecker V., Mock O. & Kuratani S. Conserved relative timing of cranial ossification patterns in early mammalian evolution. Evolution & Development 10, 519–530 (2008). [DOI] [PubMed] [Google Scholar]
- Werneburg I. & Sánchez-Villagra M. R. Skeletal heterochrony is associated with the anatomical specializations of snakes among squamate reptiles. Evolution 69, 254–263 (2015). [DOI] [PubMed] [Google Scholar]
- Werneburg I., Polachowski K. M. & Hutchinson M. N. Bony skull development in the Argus monitor (Squamata, Varanidae, Varanus panoptes) with comments on developmental timing and adult anatomy. Zoology 118, 255–280 (2015). [DOI] [PubMed] [Google Scholar]
- Maier W. Phylogeny and Ontogeny of Mammalian Middle Ear Structures. Netherlands Journal of Zoology 40, 55–74 (1990). [Google Scholar]
- Maier W. & Ruf I. Evolution of the mammalian middle ear: a historical review. Journal of Anatomy 228, 270–283 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diogo R. & Abdala V. Muscles of Vertebrates: Comparative Anatomy, Evolution, Homologies and Development. CRC Press 1st Edition (2010). [Google Scholar]
- Hall B. K. Bones and Cartilage: Developmental and Evolutionary Skeletal Biology. Academic Press Elsevier 2nd Edition (2015). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.