Abstract
Gene products required for in vivo growth and survival of microbial pathogens comprise a unique functional class and may represent new targets for antimicrobial chemotherapy, vaccine construction, or diagnostics. Although some factors governing Staphylococcus aureus pathogenicity have been identified and studied, a comprehensive genomic analysis of virulence functions will be a prerequisite for developing a global understanding of interactions between this pathogen and its human host. In this study, we describe a genetic screening strategy and demonstrate its use in screening a collection of 6,300 S. aureus insertion mutants for virulence attenuation in a murine model of systemic infection. Ninety-five attenuated mutants were identified, reassembled into new pools, and rescreened using the same murine model. This effort identified 24 highly attenuated mutants, each of which was further characterized for virulence attenuation in vivo and for growth phenotypes in vitro. Mutants were recovered in numbers up to 1,200-fold less than wild type in the spleens of systemically infected animals and up to 4,000-fold less than wild type in localized abscess infections. Genetic analysis of the mutants identified insertions in 23 unique genes. The largest gene classes represented by these mutants encoded enzymes involved in small-molecule biosynthesis and cell surface transmembrane proteins involved in small-molecule binding and transport. Additionally, three insertions defined two histidine kinase sensor-response regulator gene pairs important for S. aureus in vivo survival. Our findings extend the understanding of pathogenic mechanisms employed by S. aureus to ensure its successful growth and survival in vivo. Many of the gene products we have identified represent attractive new targets for antibacterial chemotherapy.
Staphylococcus aureus is an important opportunistic pathogen capable of causing a variety of diseases in humans, ranging from localized infections of skin and soft tissue to life-threatening systemic infections (2). Asymptomatic carriage in the human population represents a natural reservoir of S. aureus. Approximately 20% of individuals are persistently colonized, and another 60% of the population is colonized intermittently on the mucosal surface of the anterior nares (32). S. aureus infections can arise under a variety of predisposing conditions that include immune suppression, in-dwelling medical devices, and biomaterial implants. In U.S. hospitals, S. aureus ranks as the leading etiologic agent of hospital-acquired pneumonia and surgical wound infections and the second leading cause of nosocomial infections in intensive care unit patients (2).
Treatment of staphylococcal infections has become increasingly problematic due to the emergence of multidrug-resistant strains, a particular threat being the advent of glycopeptide-resistant isolates (5, 61). These developments highlight the urgent need for new therapeutic agents with novel mechanisms of action. Among the gene products that can be considered as targets for new antimicrobial agents are factors involved specifically in virulence. In the past, studies of S. aureus virulence have focused largely on the contribution of individual factors, and the global regulators of their expression, to pathogenicity in animal or cell culture models of infection (for reviews, see references 7 and 38). Proteins considered to be virulence factors in S. aureus generally fall into two categories: secreted toxins that serve to either degrade host tissues or modulate the host immune system, and cell surface or secreted proteins involved in host cell adhesion, intracellular entry, and immune system evasion (for reviews, see references 3, 16, and 39). Among the regulators of virulence factor gene expression, agrCA and sarA have been most thoroughly studied (6). While virulence factors play an important role in pathogenesis, the contributions of basic catabolic, anabolic, transport, and signaling pathways in S. aureus infection have not been well characterized. These functions must certainly be important in permitting S. aureus to survive and replicate in the variety of distinct in vivo environments encountered by this pathogen (45).
The development of new molecular and genetic methods for the analysis of bacterial pathogenesis has enabled comprehensive screening of bacterial genomes for virulence genes in animal hosts (4). Among these methods are promoter trap strategies, such as in vivo expression technology, a method that selects for genes specifically expressed during infection (40), and differential fluorescence induction, which monitors expression of the green fluorescent protein (gfp) reporter gene as a measure of promoter activity in vivo (55). DNA array hybridization analysis represents a third comprehensive method to evaluate the expression state of individual genes in the host environment (10). These strategies have provided useful information on the in vivo expression of genes which, when combined with subsequent genetic disruption experiments, have allowed the identification of new virulence genes.
A second approach, signature-tagged mutagenesis (STM), relies upon transposons which are marked with unique sequence oligonucleotide tags (24). The unique sequence tags allow mutants to be distinguished by differential hybridization. The strength of STM is that it permits large-scale analysis of insertional mutants for loss of virulence in vivo in an animal-sparing manner. It has been successfully employed to identify virulence genes from a variety of bacterial pathogens (for review, see reference 23), including S. aureus (11, 43). Since it's introduction, STM has been modified by a number of investigators to either significantly improve (31) or eliminate (25) the hybridization process.
In this paper we describe a genetic screening method that employs unique size DNA tags for monitoring the fate of individual bacterial clones within a population. The use of DNA size tags to mark individual cells in a population was first described by Walsh and Cepko, who used the method to study cell fate in the developing rat neocortex (59). We created a set of wild-type, isogenic, DNA size-tagged S. aureus strains that can be detected by multiplex PCR amplification, followed by high-resolution polyacrylamide gel electrophoresis. One advantage of this DNA size marker identification technology (SMIT) is that it circumvents costly and time-consuming probe labeling and hybridization procedures associated with STM. A second advantage of SMIT is that the identifying molecular tag is not linked to the mutagenic agent, thus enabling use of any desired mutagen (J.-P. Zhang, T. Christian, S. Bond, L. Lee, R. Pope, B. Benton, and J. Buysse, Abstr. Gen. Meet. Am. Soc. Microbiol. 2002, abstr. B-314, p. 86, 2002). For organisms in which insertional mutagens are not available, this approach offers the opportunity to screen pooled mutants created by other means. Here we demonstrate the application of SMIT to screen banks of S. aureus Tn551 and Tn917lac insertion mutants for reduced virulence in murine models of infection. Our findings provide insight into the host-bacterium interaction and may provide a useful starting point for identification of new targets for antistaphylococcal therapies.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Strains of S. aureus (Table 1) were cultured in tryptic soy (TS) broth or brain heart infusion (BHI) broth supplemented with erythromycin (Erm) to 2 μg/ml, tetracycline (Tc) to 2 μg/ml, or chloramphenicol (Cm) to 10 μg/ml where appropriate. Solid media were supplemented with 1.5% (wt/vol) agar and contained the same concentrations of antibiotics. For determination of auxotrophies, individual S. aureus mutant strains were inoculated onto chemically defined medium containing supplements essentially as described by Pattee and Neveln (48) with the following exceptions: inorganic phosphate (KPi) was reduced to 1 mM and buffering capacity was supplied by 75 mM HEPES, pH 7.2. Specific auxotrophies were determined by auxanography (12). Derivatives of strains SAM23 (S. aureus 8325-4 wild type cured of prophage) and SAM13 (r−m+ derivative of 8325-4; RN4220; from R. Novick) were employed for all S. aureus studies. Escherichia coli DH5α, employed for all cloning experiments, was cultured in Luria-Bertani medium supplemented with spectinomycin (Spc) to 50 μg/ml or ampicillin to 50 μg/ml where appropriate.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant properties | Reference or source |
|---|---|---|
| S. aureus strains | ||
| SAM23 | 8325-4 | 50 |
| SAM13 | RN4220 (r− m+) | 33 |
| SAM1100 | SAM13/pMP797 | This study |
| E. coli DH5α | F−endA1 hsdR17 recA1 gyrA96 relA1 | Life Technologies, Inc. |
| Plasmids | ||
| pCL84 | S. aureus-E. coli shuttle vector; Tcr Spcr | 36 |
| pC194 | S. aureus plasmid; Cmr | Bacillus Genetic Stock Center |
| pT181 | S. aureus plasmid; Tcr | Bacillus Genetic Stock Center |
| pE194 | S. aureus plasmid; Ermr | Bacillus Genetic Stock Center |
| pYL112Δ19 | S. aureus plasmid carrying L54a int; Cmr | 36 |
| PMP16 | S. aureus-E. coli shuttle vector; Ermr Ampr | 41 |
| pMP202 | pBluescript KS(+)::tet(k); Ampr Tcr | This study |
| pMP797 | pMP16::int | This study |
| pMP820 | S. aureus-E. coli shuttle vector; Cmr Spcr | This study |
| pTV32ts | S. aureus Tn917lac delivery plasmid; Ermr | P. Youngman (unpublished) |
| pI258repA36 Ω1(Tn551) | S. aureus Tn551 delivery plasmid; Ermr | 47 |
| pBluescript KS(+) | E. coli cloning vector; Ampr | Stratagene, Inc. |
Nucleic acid manipulations and plasmids.
All nucleic acid manipulations were performed according to standard procedures (54) unless stated otherwise. Chromosomal DNA was extracted from S. aureus essentially as described by Pattee and Neveln (48). Oligonucleotides for DNA sequencing and PCR were produced on an ABI 392 synthesizer. PCR amplicons were sequenced with a PRISM dye terminator kit (Applied Biosystems, Inc.) and an ABI 373A automated DNA sequencer. Recombinant constructs were introduced into bacteria either by electroporation with a Gene Pulser (Bio-Rad, Inc.) or by transduction, using the generalized S. aureus transducing phage 80α. Plasmid pMP202 was constructed by ligating a 2.3-kb tet(K)-containing HindIII fragment from pT181 (44) into the HindIII site of pBluescript KS(+) (Stratagene Inc.). Plasmid pCL84, a 6.7-kb S. aureus-E. coli shuttle plasmid containing the phage L54a attP site and pT181 tet(K) gene for selection in S. aureus and the ori and Spc resistance gene sequences derived from pSC101, was the generous gift of Chia Lee (36). Plasmid pMP820 was derived from pCL84 to generate a Cm-selectable S. aureus-E. coli shuttle plasmid. It was constructed by ligating a 4.3-kb AccI/HindIII fragment derived from pCL84 (containing the phage L54a attP site, as well as the ori and Spc resistance gene derived from pSC101) with a 1.6-kb AccI/HindIII cat-containing fragment derived from pC194 (27). To create an S. aureus Erm-selectable plasmid encoding phage L54a integrase, a 1.2-kb BamHI/EcoRI int-containing fragment from pYL112Δ19 (36) was ligated to BamHI/EcoRI-cleaved pMP16 (41), generating pMP797.
Southern hybridization analysis was performed on selected Tn551 and Tn917lac insertion mutants. Genomic DNA preparations were restricted with BglII, HindIII, NdeI, or XbaI, fractionated by agarose gel electrophoresis, and transferred to a nylon membrane (Hybond-N; Amersham). Blots were hybridized under high-stringency conditions with erm-proximal and erm-distal probes homologous to both Tn551 and Tn917lac sequences. Probe DNAs were generated by PCR using pI258repA36 Ω1(Tn551) as template and the following Tn917 (GenBank accession no. M11180) primer pairs: 1.8-kb erm-proximal probes, 5′-GTATCACTTCAGGAGTGATTACATG-3′ and 5′-CTAGTTTATTGACCTTTCCACGG-3′; 850-bp erm-distal probes, 5′-CATATCGAGGTTGCTTCAAC-3′ and 5′-CCCGAGCGCTTAGTGGGAA-3′. Probes were labeled with fluorescein-12-dUTP using a DNA labeling and detection kit (DuPont NEN) and visualized with anti-fluorescein-alkaline phosphatase-conjugated antibody according to the manufacturer's directions.
Isolation and cloning of tags.
Double-stranded DNA tags were generated by restriction of salmon sperm DNA (Sigma) to completion with Sau3AI, separation on a low-melting-temperature agarose gel, and isolation of fragments in the 100- to 600-bp range. The resulting fragments were ligated with BamHI-cleaved and phosphatase-treated pCL84 (36) and isolated by transforming competent E. coli to Spcr. Twenty-five appropriate-size tags were identified by PCR analysis of individual transformants using primers SMIT-F1 (5′-GTCTTACTGTCGGGAATTC) and SMIT-R1 (5′-CCTTTTTCAAATAATCTGCCC). These tags were individually subcloned into the BamHI site of pMP820. The DNA tags are hereafter referred to as SMIT tags.
Construction of SMIT-tagged S. aureus strains.
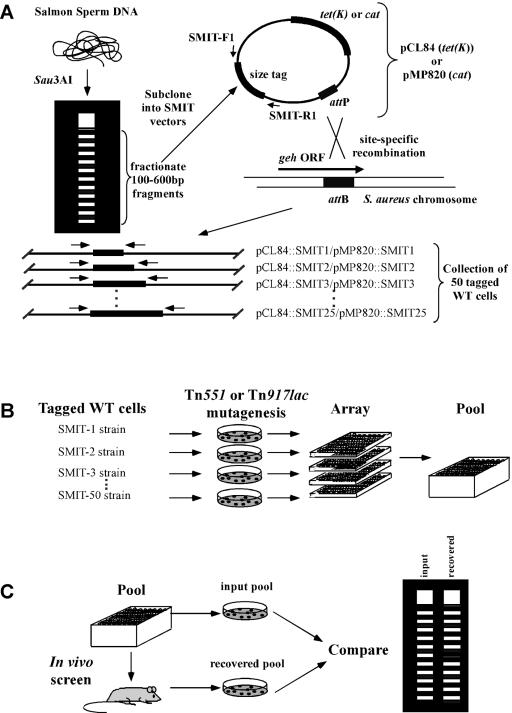
SMIT tags were introduced into the S. aureus chromosome by transforming competent SAM1100 cells to Tc resistance (Tcr) with each of 25 pCL84::SMIT tag clones, or to Cm resistance (Cmr) with each of 25 pMP820::SMIT tag clones. SMIT tags were then mobilized into the virulent S. aureus SAM23 strain by transduction. To confirm integration into the geh locus, culture supernatants derived from individual transformants and transductants were assayed for lipase (geh gene product) activity using commercially available reagents (Sigma). The resulting strain set consisted of 50 tagged SAM23 strains, i.e., 25 individually marked with distinct pCL84::SMIT tags (Tcr) and 25 individually marked with distinct pMP870::SMIT tags (Cmr), at the geh locus.
Construction and screening of S. aureus mutant libraries.
Transposon Tn551 and Tn917lac delivery vectors, pI258repA36Ω1(Tn551) and pTV32ts, respectively, were introduced individually into each of the above 50 tagged SAM23 strains by transduction and verified phenotypically. Transposition was initiated by subculture in TS broth containing Erm at 39°C to early-log-phase growth, followed by selective plating on TS agar containing Erm at 43°C to recover integrants. Approximately 4,200 Tn551 and 2,400 Tn917lac randomly selected mutants were individually archived in 96-well microtiter plates. Pools containing 40 to 50 individual mutants were assembled by combining equivalent CFU of 20 to 25 pCL84::SMIT (Tcr)-tagged mutants and 20 to 25 pMP870::SMIT (Cmr)-tagged mutants.
Mutant pools were screened in the murine model of bacteremia via intraperitoneal challenge (see below). Inocula consisted of 0.2 ml (approximately 104 CFU) of log-phase cells subcultured in BHI broth. Aliquots of inocula were diluted serially and plated selectively to obtain at least 5,000 single colonies on each TS agar containing Tc and TS agar containing Cm (input samples). Viable bacteria were recovered from infected spleens by plating each pool selectively to obtain at least 5,000 single colonies on each TS agar containing Tc and TS agar containing Cm (output samples). Output samples were collected from two to three animals per pool and combined. Genomic DNA was extracted from plated cells and used as template in PCRs with primers SMIT-F1 and SMIT-R1. To reaction mixtures consisting of 1× PCR buffer (50 mM KCl, 1.5 mM MgCl2, 10 mM Tris-HCl; pH 9.0), a 0.2 mM concentration of each of the four deoxynucleoside triphosphates (Pharmacia), and 2.5 U of Taq DNA polymerase (Applied Biosystems, Inc.) were added 50 ng of genomic DNA and a 0.2 μM concentration of each of primers SMIT-F1 and SMIT-R1. Amplifications were performed in a Gene Amp PCR system 9600 thermal cycler (Perkin-Elmer, Inc.) with a program consisting of a denaturation step for 90 s at 96°C followed by 30 cycles of 30 s of denaturation at 95°C, 30 s of annealing at 55°C, and 15 s of extension at 72°C. The reactions were completed by a final extension for 10 min at 72°C. PCR products were fractionated on GeneGel Excel 12.5% polyacrylamide gels (Pharmacia) and subjected to electrophoresis in the GenePhor system (Pharmacia). Amplified DNA tags were visualized by staining with the DNA Silver staining kit (Pharmacia).
Murine infection assays.
All animal experimental protocols and procedures were approved by an institutional animal care and use committee. Two models of staphylococcal systemic infection were employed. Inocula were prepared by growing bacteria to mid-log phase in BHI broth. For systemic infections disseminated to the spleen, bacterial inocula were administered by the intraperitoneal route. Bacteria were washed and diluted in BHI broth and mixed 1:1 with a 4% (wt/vol) sterile solution of Brewer's yeast in BHI broth, and inocula of approximately 104 CFU in 0.2 ml were injected intraperitoneally into female BALB/c mice (Charles River Laboratories, Inc.), aged 6 to 8 weeks. At 48 h postinfection, animals were sacrificed and spleens were harvested and homogenized aseptically in phosphate-buffered saline (PBS; per liter, NaCl, 7.65 g; Na2HPO4, 1.27 g; NaH2PO4, 0.1 g; KH2PO4, 0.21 g; pH 7.2). For systemic infections disseminated to the kidney, bacterial inocula were administered by the intravenous route. Bacteria were washed and diluted in PBS, and inocula of approximately 107 CFU in 0.1 ml were injected into the lateral tail vein of female BALB/c mice, aged 6 to 8 weeks. At 7 days postinfection, animals were sacrificed and kidneys were harvested and homogenized aseptically in PBS. Viable bacteria were recovered from infected tissue by plating onto TS agar containing antibiotics where appropriate. Inoculating doses were verified in all cases by determining CFU on tryptic soy agar.
The method of Ford et al. (14) was employed for establishing abscess infections. Inocula were prepared by growing bacteria to post-exponential phase (35°C, 18 h) in BHI broth, after which they were washed once in PBS, diluted in PBS, and mixed 1:1 with a 20-mg/ml sterile solution of Cytodex 1 beads (Sigma) in PBS. Inocula of approximately 105 CFU in 0.1 ml were injected intramuscularly into the thigh muscle of male Swiss-Webster mice, aged 6 to 8 weeks. At 3 to 5 days postinfection, animals were sacrificed and the thigh muscle was dissected and homogenized aseptically in PBS. Viable bacterial cells were recovered from infected tissue by plating onto TS agar containing antibiotics. Inoculating doses were verified by determining CFU on TS agar.
CI determinations.
Competitive index (CI) measures were determined essentially as described by Chiang et al., (8) with the following modifications. For competitive infection assays, mutant strains and the wild-type strain (SAM23) were grown separately to post-exponential phase (35°C, 18 h) in BHI broth and washed and diluted in BHI broth. Each mutant was mixed with the wild type at a concentration of 5 × 103 CFU/ml each (104 CFU/ml total). To determine the exact input ratio, serial dilutions of this mixture were plated onto TS agar (to determine total CFU) and TS agar containing Erm (to determine mutant CFU). For in vitro CI determinations, 4-ml aliquots of this mixture were incubated for 18 h at 35°C in a shaking incubator. Output ratios were determined by plating aliquots of serially diluted cultures as described above to determine both total CFU and mutant CFU. For in vivo CI determinations, 0.2-ml aliquots of the mutant-wild-type mixture were combined 1:1 with Brewer's yeast and used as inocula for intraperitoneal challenge in the murine systemic infection model as described above. Output ratios were determined by plating aliquots of infected tissue homogenates as described above to determine both total CFU and mutant CFU. The CI is defined as the output ratio of mutant to wild type divided by the input ratio of mutant to wild type.
Identification of transposon insertion sites.
To identify Tn551 and Tn917lac insertion sites, chromosomal DNA was prepared from strains of interest and subjected to Southern hybridization analysis. Inverse PCR was used to isolate the DNA sequences flanking Tn551 and Tn917lac insertions, essentially as described previously (52). Chromosomal DNA from strains of interest was digested with any of several restriction enzymes that cut at least once within the transposon sequence. The cleaved DNA was self-ligated and used as template in PCRs with the following primer pairs. For amplification of erm-proximal junction sequences, primer 917-12 (5′-GAGAGATGTCACCGTCAAG) was used in combination with one of the following primers, depending upon the restriction enzyme used for template preparation (HindIII, 5′-GGCTAAAAGACATTCCAGGTAAG; NdeI, 5′-TCTTATGTGAGTCGACAGC; XbaI/BglII, 5′-CATATCGAGGTTGCTTCAAC). For amplification of erm-distal junction sequences, primer 917-17 (5′-GGGAGCATATCACTTTTCTTG) was used in combination with one of the following primers in the manner described above (HindIII, 5′-CATTCCGTCTGAAGCAGTG; NdeI, 5′-TCTTGTGAATCACGTGTCC; XbaI/BglII, 5′-TCATTCCAAACACCTGATCAG). Purified amplification products were sequenced directly.
Bioinformatic analysis of transposon insertion sites.
Sequences obtained from erm-proximal and erm-distal junctions of each mutant clone were used to query the S. aureus COL genome sequence database (The Institute for Genomic Research) and locate sites of Tn551 and Tn917lac insertion. Both GCG (version 8.0.1; Genetics Computer Group, Inc.) and Vector NTI (version 6.0; InforMax, Inc.) suites of software were used to assemble and analyze sequence data. Similarity searches were performed with BLAST programs (1) using potential open reading frames (ORFs) to query the S. aureus N315 genome (34; http://www.ncbi.nlm.nih.gov/cgi-bin/Entrez/framik?db=Genome&gi=179) as well as protein (http://www.ncbi.nlm.nih.gov/BLAST/and) and nucleotide (http://www.ncbi.nlm.nih.gov/Microb_blast/unfinishedgenome.html) databases. Amino acid similarity was determined by the GAP function of GCG, using the entire S. aureus and comparator ORFs.
RESULTS
Size tag library and strain construction.
A library of DNA tags was constructed by inserting different-sized DNA fragments (100 to 600 bp), derived by Sau3AI restriction of salmon sperm DNA, into pCL84, an S. aureus integration-E. coli shuttle vector that confers Tc resistance in S. aureus (36) (Fig. 1A). Oligonucleotide primers flanking the cloning site were used in a PCR screen of transformed E. coli colonies to identify 25 tag clones that could be amplified uniformly in multiplex PCRs and resolved adequately by polyacrylamide gel electrophoresis. Each of these 25 plasmid clones was individually introduced into the S. aureus 8325-4 (SAM23) chromosome by transformation of SAM1100 to Tcr and subsequently crossed into SAM23 by transduction. Since the plasmid vector pCL84 directs integration specifically and accurately to the geh locus in SAM1100 (see Materials and Methods), the resulting library contained 25 isogenic strains, each of which was genetically tagged in a manner that permitted precise identification of the tag by PCR amplification and polyacrylamide gel electrophoresis. We refer to this method of genetic tagging as DNA SMIT.
FIG. 1.
Isolation of DNA tags, strain construction, mutagenesis, and screening strategy. (A) Isolation of DNA SMIT tags and integration into the S. aureus chromosome. Tags were generated by digestion of salmon sperm DNA with Sau3AI, size fractionated by agarose gel electrophoresis, and isolated by cloning into the BamHI site of pCL84 (see Materials and Methods). Twenty-five unique tag clones were selected, and the tags were individually subcloned into the BamHI site of pMP820. Schematic maps of S. aureus integrative plasmids pCL84 and pMP820 are shown. The 50 tag clones, 25 constructed in pCL84 and 25 constructed in pMP820, were introduced individually into the S. aureus 8325-4 chromosome at the geh locus (see Materials and Methods), creating 50 isogenic SMIT-tagged S. aureus strains. (B) Generation of S. aureus insertion mutant banks and pools. Transposon delivery plasmids pI258repA36 Ω1(Tn551) and pTV32ts, introduced individually into each of the 50 tagged S. aureus strains, were used to create banks of 50 Tn551 and 50 Tn917lac insertion mutants, respectively. Pools of up to 50 S. aureus mutants were generated by combining 25 pCL84::SMIT-tagged mutants with 25 pMP820::SMIT-tagged mutants. (C) In vivo screening strategy. Inocula were prepared from subcultured mutant pools and used to establish systemic infections in mice. At 48 h postchallenge, animals were sacrificed and spleens were dissected and homogenized. Viable bacterial cells were isolated by plating aliquots of inoculum (input) and spleen (recovered) samples from each pool of mutant cells. Extracted chromosomal DNA was used as template for PCR amplification of SMIT tags, which were subsequently resolved by polyacrylamide gel electrophoresis and visualized by staining with silver.
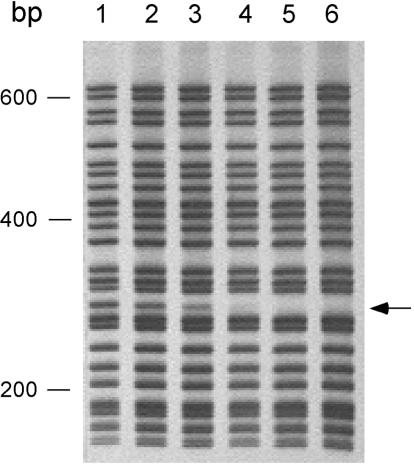
Control experiments were performed to determine whether introduction of SMIT tags into S. aureus cells impaired growth and whether the tags were genetically stable. In order to determine whether inactivation of S. aureus geh by pCL84 integration affected growth or virulence, isogenic geh+/geh::pCL84 strain pairs were compared in kinetic growth experiments and in murine infection models. Aside from the lack of expression of Geh (lipase) from the geh mutant strain, we were unable to detect any phenotypic consequence of geh inactivation (data not shown). We next evaluated the collection of 25 S. aureus geh::pCL84-SMIT-tagged strains for genetic stability. Strains were cultured individually to post-exponential phase, pooled, and used as inoculum for nutrient broth culture and murine infection models (see Materials and Methods). Equivalent quantities of all 25 input strains were recovered from both overnight broth cultures as well as infected murine tissues (data not shown), leading us to conclude that SMIT tags were genetically stable upon prolonged growth both in vitro and in vivo. Finally, limiting dilution PCR experiments were performed to establish quantitative standards by which negatively selected strains might be compared (Fig. 2). We determined that 4-fold reduction of a given strain in a 25-member pool was readily observed and that the signal diminished beyond detection when a given strain was diluted greater than 16-fold.
FIG. 2.
Limiting dilution SMIT PCR amplification and detection. Genomic DNA was isolated from 25 pooled pCL84::SMIT-tagged S. aureus strains grown individually and combined in equal portions (lane 1) or combined so that 24 strains were represented equally and one strain (arrow) was represented at 50% (lane 2), 25% (lane 3), 12.5% (lane 4), 6.25% (lane 5), or 3.12% (lane 6) relative to each of the other 24 strains. Products of tag amplification reactions were resolved by polyacrylamide gel electrophoresis and visualized by staining with silver. Positions of migration of double-stranded 100-bp-ladder DNA size markers are indicated (in base pairs).
In order to assemble pools consisting of greater than 25 strains, we created a second library of the same 25 SMIT tags mentioned above. Each tag was individually subcloned into a newly constructed S. aureus integration-E. coli shuttle vector, pMP820, that confers Cmr in S. aureus. Like pCL84, pMP820 directs integration specifically to the geh locus. Each of the 25 pMP820-SMIT clones was individually introduced into the SAM23 chromosome by transformation and transduction as described above. When S. aureus cells harboring both geh::pCL84-SMIT (Tcr) tags and geh::pMP820-SMIT (Cmr) tags were pooled and cocultured, each tag set could be resolved by plating selectively on medium containing either Tc or Cm, respectively (data not shown).
Construction and screening of insertion mutant libraries.
Two S. aureus mutant banks, one consisting of approximately 4,200 Tn551 insertion mutants and a second consisting of approximately 2,400 Tn917lac mutants, were constructed for in vivo screening (Fig. 1B). Tn551 mutants were isolated following introduction of plasmid pI258repA36Ω1(Tn551) individually into each of the 50 SAM23 SMIT-tagged strains and plating at the nonpermissive temperature on medium containing Erm. Approximately 84 randomly selected Tn551 mutants were collected from each of the 50 tagged strains (4,200 mutants total), archived into microtiter dishes, and subsequently combined into 84 pools consisting of up to 50 mutants each. Tn917lac mutants were similarly isolated following introduction of plasmid pTV32ts into each of the 50 SAM23 SMIT-tagged strains. Forty-eight randomly selected Tn917lac mutants were collected from each of the 50 tagged strains (2,400 mutants total), archived, and combined into pools. To verify that our mutagenesis procedures resulted in random, single transposon insertions, chromosomal DNA from five arbitrarily selected Tn551 mutants derived from each of eight strains (40 mutants total) was analyzed by Southern hybridization using Tn551 as a probe (data not shown). Our results indicated that each mutant possessed a single Tn551 insertion. Insertion hot spots were not observed and, moreover, mutants isolated from a single SMIT-tagged strain had a sibling rate of less than 15%.
We selected a murine model of systemic infection for mutant screening because it measures the ability of S. aureus to survive exposure to a range of host environments. In this model, bacteria are introduced by intraperitoneal inoculation, whereupon they disseminate by the lymphatic system to the bloodstream and are able to colonize and replicate in organ tissues (19). The progression of bacteremia in this model, including bacteriologic and pathological changes, seeding of multiple organs, and cell-mediated and inflammatory cytokine host responses, parallel many events that occur in human disease (53, 56, 63). When we examined kidney tissue recovered from animals infected with wild-type S. aureus, we observed renal histopathology analogous to human pyelonephritis.
The number of mutant clones that can be screened in a pool is restricted by the number of clones that can simultaneously establish infection and persist within the host. Previous reports describing screens of S. aureus mutants by STM suggested that up to 96 individual clones could be reproducibly recovered from murine models of abscess, wound, and systemic infection (11, 43). We performed control experiments using pools of unmutagenized SMIT-tagged strains to (i) evaluate the time course of infection, (ii) optimize variables such as bacterial inoculation and duration of infection, and (iii) determine the ideal target organ for bacterial recovery. Results from these experiments demonstrated that inoculating doses at or near the 50% lethal dose (104 CFU) were required to establish infection by all input strains (corresponding to approximately 200 CFU per clone in a pool of 50) in both spleen and kidney when infected tissue was recovered 48 h postchallenge. We also determined that bacterial counts of at least 105 CFU per g of tissue were required to ensure recovery of all members in a pool. In order to reduce the occurrence of false positives in our mutant screening, each pool was screened in at least two animals and output samples derived from spleen tissue were combined prior to analysis.
Our initial screen of 6,300 insertion mutants in mice identified 339 in vivo attenuated mutants (5.4% of the mutants screened). Representative screening results obtained from one pool are shown in Fig. 3. Attenuated mutants were visually identified by comparing the signal intensities of tags amplified from the input pools to those amplified from the combined recovered pools. Clones present in the input pools that were significantly reduced (greater than fourfold reduction) in the recovered pools were selected for further analysis. Of the 339 mutants that fulfilled this criterion, 95 yielded no detectable signal in the recovered pool (greater than 16-fold reduction). These 95 mutants were considered to be more severely attenuated than the remaining 282 mutants and were the subject of further investigation. To confirm virulence attenuation in a competitive environment, each of the 95 mutants was individually subcultured and reassembled into new pools for screening in mice. In this second round of in vivo screening, each mutant was assembled into at least two pools; each pool consisted of eight mutant and four tagged wild-type strains. Pools were screened in the murine systemic infection model, and mutants that were attenuated in all pools were selected for further studies. This exercise led to the identification of 24 mutants that were consistently attenuated in competitive infections.
FIG. 3.

Identification of virulence-attenuated mutants. Products of tag amplification reactions were resolved by polyacrylamide gel electrophoresis and visualized by staining with silver. Shown are input tags (lane I) and recovered tags (lane R) derived from a pool (same pool for both panels) plated on TSA medium containing Tc (A) or containing Cm (B). Attenuated mutants in panel B are indicated by arrows. Positions of migration of double-stranded 100-bp-ladder DNA size markers are indicated (in base pairs).
Identification and analysis of genes required for in vivo survival.
Southern hybridization analysis determined that each of the 24 mutants had arisen from a single transposon insertion into a unique site in the chromosome. Sites of transposon insertion in each mutant were identified by inverse PCR and DNA sequence determination (see Materials and Methods). Sequences were used to query the S. aureus COL genome sequence determined by The Institute for Genomic Research and identify complete ORFs. Insertions in 23 unique ORFs were identified. These were used to query the N315 genome as well as public protein and nucleotide databases for homologous gene products and revealed that our mutant collection contained insertions in genes encoding a variety of distinct cellular functions. The mutants can be grouped into four functional gene classes, including small-molecule biosynthetic enzymes, cell surface binding and transport proteins, signal transduction systems, and anaerobic energy generation, as well as several conserved hypothetical proteins of unknown function (Table 2). Nine of the 24 strains had transposon insertions in genes required for amino acid or purine biosynthesis, 5 of which (IVT626, IVT627, IVT15, IVT638, and IVT173) affected genes required for biosynthesis of aspartate or aspartate family amino acids and 2 of which (IVT36 and IVT285) affected genes in tyrosine and tryptophan biosynthesis, respectively. Another large group of mutants, 6 of 24, harbored insertions in previously described or putative transmembrane transporters (IVT35, IVT133, IVT176, IVT207, IVT291, and IVT631). Two independent insertions (IVT59 and IVT84) were isolated in different regions of the coding sequence of a putative sensor histidine kinase, arlS (17). In all, 10 previously identified S. aureus genes and 13 novel genes encoding proteins of putative or unknown function were identified as essential for full virulence. Mutations in genes encoding secreted virulence factors, such as the hemolysins, were not isolated. It is believed that strains harboring such mutations may be complemented in trans during mixed culture infections and, hence, are expected to be underrepresented in our screen for attenuated mutants (9).
TABLE 2.
Attenuated S. aureus strains identified by SMIT
| Classification | Strain | Gene (organism)a | Lengthb | Insertion sitec | Strain N315 ORF | Putative or known function |
|---|---|---|---|---|---|---|
| Biosynthesis of small molecules | IVT15 | lysC | 401 | 196 | SA1225 | Aspartate kinase |
| IVT36 | tyrA (B. halodurans) | 366 | 263 | SA1197 | Prephenate dehydrogenase | |
| IVT173 | dapB | 230 | 132 | SA1228 | Dihydrodipicolinate synthase | |
| IVT252 | hipO | 383 | 367 | SA1230 | Hippurate hydrolyase | |
| IVT285 | trpF | 211 | 53 | SA1203 | Phosphoribosylanthranilate isomerase | |
| IVT626 | aspB (B. circulans) | 428 | 154 | SA1749 | Aspartate aminotransferase | |
| IVT627 | pycA (B. halodurans) | 1,073 | 589 | SA0963 | Pyruvate carboxylase | |
| IVT636 | pyrC (B. halodurans) | 425 | 131 | SA1044 | Dihydroorotase | |
| IVT638 | asd | 329 | 91 | SA1226 | Aspartate semialdehyde dehydrogenase | |
| Cell surface binding and transport | IVT35 | vga | 642 | 512 | SA1852 | Streptogramin resistance ABC-type transporter |
| IVT133 | yflS (B. subtilis) | 472 | 159 | SA2486 | Predicted 2-oxoglutarate/malate translocator | |
| IVT176 | yuiF (B. subtilis) | 438 | 46 | SA0804 | Predicted permease | |
| IVT207 | braB | 448 | 2 | SA1239 | Branched-chain amino acid transporter | |
| IVT291 | pstS (B. anthracis) | 327 | 15 | SA1221 | Phosphate ABC transporter binding protein | |
| IVT631 | gabP | 484 | 400 | SA1169 | Gamma-aminobutyrate permease | |
| Signal transduction | IVT24 | saeS | 353 | 278 | SA0660 | Regulator of exotoxin gene expression |
| IVT59 | arlS | 451 | 401 | SA1246 | Regulator of extracellular proteases | |
| IVT84 | arlS | 451 | 118 | SA1246 | Regulator of extracellular proteases | |
| Energy | IVT40 | narJ (S. carnosus) | 200 | 151 | SA2183 | Anaerobic nitrate reductase, delta subunit |
| Unknown | IVT27 | SE0526 (S. epidermidis) | 164 | 161 | SA0699 | |
| IVT29 | SE1059 (S. epidermidis) | 255 | 251 | SA1209 | ||
| IVT42 | yitL (B. subtilis) | 300 | 279 | SA1223 | ||
| IVT171 | LMO2587 (L. monocytogenes) | 282 | 92 | SA2478 | ||
| IVT211 | yneP (B. subtilis) | 155 | 113 | SA1185 | Predicted thioesterase |
Previously described S. aureus gene designation or name of the gene and source organism encoding the ORF with highest similarity.
Gene ORF length (condons).
Codon site of transposon insertion.
Phenotypic characterization of attenuated mutants.
Prior to investigating the nature and extent of virulence defects exhibited by each of the 24 prioritized mutants, it was necessary to confirm that the observed virulence attenuation of each mutant was linked to the transposon insertion. This was accomplished by back-crossing each insertion into SAM23 by phage-mediated transduction, followed by Southern blotting and PCR analyses to confirm sites of insertion (data not shown). Each of these newly constructed mutants was used for all further experimentation. We investigated each mutant for apparent auxotrophies and performed mixed-culture competitive in vitro growth and competitive in vivo infection assays. To assess apparent auxotrophies, individual mutants were inoculated onto solid chemically defined minimal medium. Consistent with our bioinformatic analyses, all clones with mutations in genes encoding known biosynthetic enzymes or homologs failed to grow on minimal medium. In all but two instances, the specific auxotrophies exhibited by each of these mutants were consistent with the defined genetic lesions (Table 3). The exceptions were mutants IVT207 and IVT252, strains that carry mutations in a branched-chain amino acid transporter gene, braB, and in the gene encoding hippurate hydrolase, hipO, respectively, for which we observed apparent lysine auxotrophies.
TABLE 3.
Phenotypic and competitive virulence analysis of SMIT mutants
| Strain | Disrupted gene or homolog | Apparent auxotropy | CIa
|
|
|---|---|---|---|---|
| In vitro | In vivo | |||
| IVT15 | lysC | Lysine | 0.84 | 0.32 |
| IVT24 | saeS | 0.36 | 0.15 | |
| IVT27 | ORF | 0.31 | 0.11 | |
| IVT29 | ORF | 0.82 | 0.32 | |
| IVT35 | ydiF | 0.94 | 0.40 | |
| IVT36 | tyrA | Tyrosine | 0.79 | 0.28 |
| IVT40 | narJ | 0.70 | 0.67 | |
| IVT42 | yitL | 0.29 | 0.25 | |
| IVT59 | arlS | 0.25 | 0.10 | |
| IVT133 | yflS | 1.06 | 0.14 | |
| IVT171 | LMO2587 | 1.36 | 0.63 | |
| IVT173 | dapB | Lysine | 0.84 | 0.24 |
| IVT176 | yuiF | 0.92 | 0.16 | |
| IVT207 | braB | Lysine | 0.84 | 0.29 |
| IVT211 | yneP | 0.44 | 0.28 | |
| IVT252 | hipO | Lysine | 1.10 | 0.20 |
| IVT285 | trpF | Tryptophan | 0.89 | 0.59 |
| IVT291 | phoX | 0.92 | 0.67 | |
| IVT626 | aspB | Aspartate | 0.73 | 0.001 |
| IVT627 | pycA | Aspartate | 0.91 | 0.02 |
| IVT631 | gabP | 0.82 | 0.03 | |
| IVT636 | pyrC | Uracil | 0.34 | 0.19 |
| IVT638 | asd | Lysine | 0.80 | 0.26 |
CI determinations were relative to isogenic wild-type SAM23 (see Materials and Methods).
Mixed-culture competitive growth and infection experiments are sensitive measures of growth and virulence attenuation, respectively, in which effects associated with changes in the medium or the host environment are internally controlled (8). With each mutant strain, we performed in vitro CI determinations in rich broth medium in order to identify mutations that conferred general growth disadvantages unrelated to in vivo fitness (Table 3). We also performed in vivo CI determinations, using the murine systemic infection model, to confirm and quantify virulence attenuation of each mutant strain. The results of these studies (Table 3) indicated that our collection of mutants exhibited a wide range of phenotypes in the mouse CI assay. One insertion, IVT42, conferred a general growth disadvantage apparently unrelated to virulence. Several mutants exhibited modest virulence attenuation (in vivo CI of >0.2), including mutations affecting lysine biosynthesis (IVT15, IVT173, and IVT638), aromatic amino acid biosynthesis (IVT36 and IVT285), anaerobic energy generation (IVT40), and cell surface binding-transport functions (IVT35, IVT207, and IVT291). Among the most severely virulence-attenuated mutants (in vivo CI, <0.2) were those affecting aspartic acid biosynthesis (IVT626 and IVT627), pyrimidine biosynthesis (IVT636), signal transduction (IVT24 and IVT59), and integral membrane transport proteins, including a putative 2-oxoglutarate/malate symporter (IVT133), a putative gamma-aminobutyrate permease (IVT631), and a predicted permease of unknown biochemical function (IVT176).
To determine whether virulence attenuation was influenced by competition with the coinfecting wild-type strain in mixed-culture infections, we ascertained virulence of selected mutants in noncompetitive infections established by individual mutant strains. For these studies we assessed virulence in both systemic and abscess murine infections, models that measure the ability of a bacterium to survive two distinct host environments. The systemic infection model measures the ability of the bacterium to adapt to the host environment, survive innate host defense systems, disseminate, and colonize and persist within the spleen. The abscess model of focal infection, in contrast to the systemic infection model, does not require dissemination. During abscess formation bacterial growth is curtailed by influx of polymorphonuclear leukocytes as well as oxygen and nutrient limitation. Eight mutants exhibiting in vivo CI values of less than 0.2 were subjected to these analyses. The results (Table 4) indicated that the virulence attenuation detected in competitive infections is recapitulated in pure culture infections. In systemic infections, six mutants (IVT24, IVT59, IVT133, IVT626, IVT627, and IVT636) were recovered from spleen tissue in relative yields comparable to the mutant/wild-type ratio observed in the mouse CI assay, while two mutants (IVT631 and IVT176) showed approximately 20- and 200-fold greater reductions, respectively, in recovery compared to the mouse CI assay (Table 4). In abscess infections five mutants (IVT24, IVT59, IVT176, IVT626, and IVT631) were attenuated to levels comparable to those observed in systemic infections, one mutant (IVT627) was attenuated almost 100-fold greater than observed in systemic infections, and two mutants (IVT133 and IVT636) were mildly attenuated.
TABLE 4.
Virulence attenuation of selected SMIT mutants
| Strain | Disrupted gene or homolog | Spleena | Abscessb |
|---|---|---|---|
| IVT24 | saeS | −1.26 | −1.40 |
| IVT59 | arlS | −1.63 | −1.95 |
| IVT133 | yflS | −1.49 | −0.29 |
| IVT176 | yuiF | −3.09 | −2.59 |
| IVT626 | aspB | −2.47 | −3.28 |
| IVT627 | pycA | −1.65 | −3.60 |
| IVT631 | gabP | −2.82 | −3.14 |
| IVT636 | pyrC | −0.47 | −0.20 |
Mean log10 CFU per gram of tissue difference relative to isogenic WT control in disseminated systemic infection of the spleen.
Mean log10 CFU per gram of tissue difference relative to isogenic WT control in abscess infection.
DISCUSSION
The purpose of this study was to identify, on a genomic scale, genes that are required for full virulence of S. aureus. To screen for virulence genes in vivo, we developed a genetic screening methodology that employs unique size DNA tags to monitor the fate of individual clones within a bacterial population and a procedure for creating random, unlinked insertion mutants. Mutant screening was performed using a murine systemic infection model, because the course of progression of this disease in the mouse has been well documented and closely parallels disease progression in humans. In order to help eliminate false positives, we screened mutant banks for attenuated virulence in a two-round screening procedure. To confirm the relevance of virulence attenuation observed in mutant pools, we performed quantitative assessments of virulence in competitive and noncompetitive systemic infections. We anticipated that some factors important for S. aureus virulence during systemic infection might also be required for full virulence in other models of infection, and so we tested this hypothesis directly by examining virulence of selected systemic infection attenuated mutants in a mouse model of abscess formation. Through this effort we have identified a broad range of potential S. aureus virulence functions, including enzymes involved in aspartate and pyrimidine biosynthesis, three putative transmembrane transport proteins, and two response regulator-histidine kinase two-component system signal transduction gene pairs.
The largest class of mutants affected biosynthesis of small molecules, particularly amino acid and pyrimidine biosynthetic functions (Table 2). Disruption of aspartate biosynthesis by insertional inactivation of either the gene encoding aspartate aminotransferase, aspB (IVT626), or the gene encoding pyruvate carboxylase, pycA (IVT627), resulted in the most pronounced virulence attenuation observed for mutations in this class. Virulence of the aspB mutant was reduced 1,000-fold in competitive systemic infections, nearly 300-fold in noncompetitive systemic infections, and nearly 2,000-fold in noncompetitive abscess infections, while virulence of the pycA mutant was reduced 50-fold in competitive systemic infections, 45-fold in noncompetitive systemic infections, and nearly 4,000-fold in noncompetitive abscess infections (Tables 3 and 4). The products of pycA and aspB execute successive steps in the biosynthesis of aspartate from pyruvate. Pyruvate carboxylase (PycA) catalyzes the ATP-dependent carboxylation of pyruvate to form oxaloacetate, a substrate for aspartate aminotransferase (AspB). AspB catalyzes transfer of the α-amino group from l-glutamate to oxaloacetate, yielding l-aspartate and α-ketoglutarate. Bioinformatic analysis suggests two possible biosynthetic routes to aspartate encoded by the S. aureus N315 genome: AspB-catalyzed transamination of oxaloacetate, and asparaginase (ansA gene product, SA1310)-catalyzed deamination of asparagine. Our observation that the aspB mutant is growth attenuated in vivo suggests that ansA does not contribute significantly to in vivo fitness, likely because there is not sufficient free asparagine available in the host environment. We determined that the aspB mutant required 125 μg of asparagine/ml to fully remediate growth in aspartate-free chemically defined medium, approximately 10-fold higher than the concentration of free asparagine in human serum, 11 μg/ml, reported recently (51). That both aspB and pycA mutants were attenuated to a greater extent in noncompetitive abscess infections compared to systemic infections (Table 4) suggests that aspartate might be significantly more limiting in the abscess environment.
The in vivo attenuation noted for aspB and pycA mutants corresponds to deficiencies in the ability to synthesize aspartate. These mutants require exogenous aspartate to grow on chemically defined medium (Table 3). Supplementation of minimal medium with aspartate to 50 μg/ml completely remediated the growth defects of aspB and pycA mutants. The concentration of free aspartate in mammalian tissues (less than 6 μg/ml in normal human serum [51]) is apparently not sufficient to support S. aureus growth in the absence of a functioning aspartate biosynthesis pathway and seems a likely explanation for the avirulence observed for aspB and pycA mutants. Moreover, the bacterium must also be unable to procure aspartate-containing peptides in vivo in a quantity sufficient to fulfill the aspartate requirement of these mutants.
Disruption of pyrimidine biosynthesis also attenuated S. aureus virulence. A single Tn917 insertion mutation in the gene encoding dihydroorotase, pyrC (IVT636), reduced virulence fivefold in the competitive systemic infection model and threefold in the noncompetitive systemic infection model, while virulence attenuation in the abscess infection model was negligible (Tables 3 and 4). Dihydroorotase catalyzes the third of six enzymatic steps in the biosynthesis of UMP from glutamine and aspartate precursors. Supplementation of minimal medium with uracil to 1 μg/ml was sufficient to completely restore growth of the pyrC mutant, indicating the presence of an efficient transporter and intact pyrimidine salvage pathway. Indeed, pyrC is the third gene of a seven-gene cluster encoding enzymes for pyrimidine nucleotide biosynthesis as well as the putative uracil permease gene, pyrP. These data might suggest that an intact de novo pyrimidine biosynthesis pathway contributes to full bacterial virulence and that the presence of an intact pathway for salvaging pyrimidines allows the pyrC mutant to survive the host environment with only modest diminution of virulence. Considering that the human plasma concentration of uracil (less than 0.05 μg/ml [21]) is insufficient to support the salvage pathway when organisms are cultured in minimal medium, however, requires an alternative interpretation. We examined growth of isogenic wild-type and pyrC mutant cells in serum and discovered that the pyrC mutant was unable to grow in either human or mouse serum. To determine whether the pyrC mutant genotype and phenotype were stable during growth in vivo, pyrC mutant cells were recovered from infected mouse spleen and abscess tissues and evaluated; all isolates retained the uracil− phenotype. We therefore conclude that the bacterium must be able to procure uracil and/or pyrimidine nucleotides from host cellular sources, possibly through cytolysis of host cell tissues mediated by the vast armamentarium of exoenzymes and exotoxins secreted by this bacterium.
The second largest class of mutants affected cell surface binding and transport proteins from a variety of known (IVT35 [vga], IVT207 [braB], IVT291 [phoX], and IVT631 [gabP]) and predicted (IVT133 [yflS]) functions, as well as one predicted permease of unknown function (IVT176 [yuiF]) (Table 2). These transporters may allow the cell to scavenge for distinct nutrients or cofactors or, alternatively, may function in solute transport responses to osmotic or other environmental stresses. We tested the latter hypothesis by examining each of the mutants in this class for growth in the presence of high (2 M) NaCl and found that IVT207 grew poorly in both solid and liquid high-salt media (ca. 20% growth rate reduction in liquid high-salt medium). This was not surprising, given that inactivation of a parologous S. aureus gene, brnQ, encoding a 442-amino-acid branched-chain amino acid carrier protein with 74% amino acid identity to BraB (IVT207), was reported by Vijaranakul et al. to result in osmosensitivity (57). However, we also observed that the braB::Tn551 insertion in IVT207 conferred auxotrophy for lysine (Table 2). Transduction experiments determined that both lysine auxotrophy and high salt sensitivity were tightly linked with the braB::Tn551 insertion. Since the operon encoding diaminopimelate (DAP) and lysine biosynthetic enzymes (DAP operon) maps approximately 4 kb 3′ of braB, the possibility of polar effects from the braB::Tn551 insertion seems low. It remains possible that IVT207 harbors an independent mutation in the DAP operon that cotransduces with braB at high frequency.
Disruption of two transmembrane transporters resulted in dramatic attenuation of virulence. Loss of function of gabP in IVT631, encoding a predicted gamma-aminobutyrate transporter, resulted in 33-fold attenuation in competitive systemic infections, 660-fold attenuation in noncompetitive systemic infections, and nearly 1,400-fold attenuation in noncompetitive abscess infections. Likewise, the yuiF mutation in IVT176, encoding a predicted 11-pass transmembrane protein with similarity to small-molecule permeases, attenuated virulence approximately 6-fold in competitive systemic infections, over 1,200-fold in noncompetitive systemic infections, and nearly 400-fold in noncompetitive abscess infections. We were unable to determine in vitro phenotypes (e.g., auxotrophy, altered chemical sensitivity, altered growth rate) for either IVT631 or IVT176. Disruption of a third transporter, yflS (IVT133), attenuated virulence differentially in pure culture systemic and abscess infections (Table 4). These findings suggest either a higher demand for the putative dicarboxylate substrate (oxoglutarate and/or malate) and/or repression of the tricarboxylic acid cycle during systemic infection.
Of particular interest was the discrepancy in virulence attenuation that we observed for yuiF and gabP mutants between mixed and pure culture infections. These isolates appeared to be partially complemented by wild-type cells in mixed culture infections, as evidenced by the 200- and 20-fold increases in recovery of yuiF and gabP mutants, respectively, from spleens of animals infected with mixed compared to pure cultures (Tables 3 and 4). These were the only two mutants for which trans-complementation was noted, and they suggest that the functions of GabP and YuiF are not limited to nutrient acquisition from the host. It is possible that GabP and YuiF functions are required in early steps of establishing infection, such as colonization, or that these gene products may function in bacterial cell-cell communication in the host. These may be new members of an expanding class of permease-like proteins that function as signal-transducing environmental sensors (15, 30).
The third largest class of genes identified in our screen encodes proteins of unknown biochemical function, all of which possess putative orthologs in other bacterial species (Table 2). All but one of the mutants in this class, IVT27, were modestly attenuated for virulence. Because the insertion in IVT27 occurred near the C terminus (codon 161) of the predicted 164-codon ORF and was therefore potentially polar on the neighboring gene (pepT; SA0698), together with the observed in vitro growth defect (Table 3; in vitro CI of 0.31), we did not pursue further characterization of this strain.
The ability of S. aureus to adapt to and cause disease at distinct anatomical sites suggests that virulence mechanisms are modulated in response to environmental cues. Two-component sensor kinase-response regulators are one of the major mechanisms of signal transduction employed by bacteria, allowing for coordinate control of gene expression in response to diverse environmental stimuli (26). We isolated insertion mutations in two distinct two-component system sensor kinase genes, arlS (IVT59 and IVT92) and saeS (IVT24). Each of these mutations attenuated virulence approximately 10-fold in competitive systemic infections (Table 3) and between 10- and 100-fold in noncompetitive systemic and abscess infections (Table 4). These two genes were identified in previous mutant screens for fluoroquinolone hypersensitivity (arlS) and exotoxin production (saeS), but their role in virulence in vivo has not yet been determined (17, 22). A large body of literature has described the role of the AgrCA two-component system in S. aureus virulence, virulence gene expression, and quorum sensing (for review, see reference 46). More recently, genetic interplay between agrCA and other two-component systems, including arlRS and saeRS, has been demonstrated (18, 22). While the ligand responsible for activating AgrC, the AgrD-derived octapeptide, has been well defined (28, 29), the environmental signals to which other S. aureus sensor kinases respond have not been identified. Our results suggest important individual roles for both ArlRS and SaeRS two-component systems in S. aureus virulence in vivo.
Several of the genes we identified had a minor contribution toward virulence of S. aureus but have been identified as important virulence determinants in other pathogens. Three mutants (IVT15 [lysC], IVT173 [dapB], and IVT638 [asd]) possessed insertions in genes involved in DAP biosynthesis that resulted in modest virulence attenuation (Table 3). This is in marked contrast to the significant attenuation of virulence observed for DAP auxotrophic mutants of Salmonella enterica serovar Typhimurium, Shigella flexneri, and Legionella pneumophila (13), as well as the observation that the aspartate kinase gene ask in Mycobacterium smegmatis, a paralog of S. aureus lysC, is essential for viability (49). In these organisms DAP is not only a key intermediate to lysine biosynthesis but also a constituent of the cell wall peptidoglycan. In contrast, S. aureus and many other gram-positive bacteria incorporate l-lysine in place of DAP into peptidoglycan. The contribution of a functional DAP biosynthetic pathway toward S. aureus virulence should not be understated, however, given that these genes have previously been identified in screens for virulence in mice (asd) as well as in distinct in vivo surrogate assays (asd, lysC, and dapB) (11, 35, 43, 58, 62). We also determined that loss of the dissimilatory nitrate reductase (IVT40 [narJ]) had little impact on virulence (Table 3). This is in contrast to the recent report that nitrate respiration appears to play an important role in the virulence of Mycobacterium bovis BCG (60). Using a standard assay to detect production of nitrite from nitrate, we confirmed that the narJ::Tn917lac insertion in IVT40 eliminated nitrate reductase function. These results suggest that if S. aureus encounters an anaerobic environment in vivo, energy generation must proceed by a route(s) that does not require respiration using nitrate as the terminal electron acceptor.
Our analysis is not saturating despite screening 6,300 mutants, greater than twice the number of S. aureus genes. This is evidenced by the fact that most of the mutants isolated in our screen were unique. In addition, previous S. aureus STM screens conducted by Mei et al. (43) and Coulter et al. (11) analyzed 1,248 and 1,520 mutants, respectively, and the overlap between the three studies is limited. Only one annotated gene, asd, was reported in common to all three studies. Of the most highly attenuated mutants we identified (Table 4), only two, saeS and arlS, described previously identified S. aureus genes (17).
Recent advances in molecular microbiology have enabled comprehensive genetic screening for gene products required for full virulence. In recent years STM has been applied successfully to identify virulence-related genes in a variety of pathogenic bacteria (for review, see reference 42). Despite being a powerful approach, STM as it was originally described requires time-consuming probe labeling and hybridization procedures and commits the investigator to a predetermined transposon or insertion sequence element as a mutagen (24). Several investigators have described modifications that overcome some of these obstacles, including application of nonradioactive filter hybridization (20), hybridization to high-density oligonucleotide arrays (31), uniplex PCR (37), and polymorphic tags (25), to eliminate the requirement for hybridization. In this report we describe a novel method for genetically tagging microbial cells in a nonmutagenic fashion and a procedure for monitoring the fate of individual strains in a population by multiplex PCR followed by direct visualization of the amplification products. Separation of the genetic tagging and mutagenesis processes permits mutagenesis to be performed with any number of agents and is thus applicable to systems that lack tools for random insertional mutagenesis (D. Biek, personal communication).
The findings reported here expand our understanding of the microbial-host interaction during S. aureus infection. Critical metabolic and transmembrane transport functions that are described provide clues to the nutritional requirements of the bacterium and may indicate new opportunities for design of antimetabolite chemotherapies. Moreover, determination of substrates for the putative transmembrane transporters of unknown function may afford identification of heretofore-unrecognized nutritional requirements. And finally, the complex role of environmental signal transduction in bacterial virulence is highlighted. Determination of both the environmental stimuli as well as effector functions of both of the sensor kinase-response regulator gene pairs we have described will surely enlighten our understanding of the pathogenic process.
Acknowledgments
We thank Keith A. Bostian and Patrick Martin for helpful discussions. We thank Chia Y. Lee (University of Kansas) for providing plasmids pCL84 and pYL112D19, Phil Youngman for providing plasmid pTV32ts, and John Iandolo (University of Oklahoma) for providing numerous S. aureus strains.
REFERENCES
- 1.Altschul, S., W. Gish, W. Miller, E. Myers, and D. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. 1999. National nosocomial infections surveillance (NNIS) system report, data summary from January 1990-May 1999, issued June 1999. Am. J. Infect. Control 27:520-532. [DOI] [PubMed] [Google Scholar]
- 3.Bohach, G. A., D. J. Fast, R. D. Nelson, and P. M. Schlievert. 1990. Staphylococcal and streptococcal pyrogenic toxins involved in toxic shock syndrome and related illnesses. Crit. Rev. Microbiol. 17:251-272. [DOI] [PubMed] [Google Scholar]
- 4.Buysse, J. M. 2001. The role of genomics in antibacterial target discovery. Curr. Med. Chem. 8:1713-1726. [DOI] [PubMed] [Google Scholar]
- 5.Chang, S., D. M. Sievert, J. C. Hageman, M. L. Boulton, F. C. Tenover, F. P. Downes, S. Shah, J. T. Rudrik, G. R. Pupp, W. J. Brown, D. Cardo, and S. K. Fridkin. 2003. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N. Engl. J. Med. 348:1342-1347. [DOI] [PubMed] [Google Scholar]
- 6.Cheung, A., J. Koomey, C. Butler, S. Projan, and V. Fischetti. 1992. Regulation of exoprotein expression in Staphylococcus aureus by a locus (sar) distinct from agr. Proc. Natl. Acad. Sci. USA 89:6462-6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung, A., S. Projan, and H. Gresham. 2002. The genomic aspect of virulence, sepsis, and resistance to killing mechanisms in Staphylococcus aureus. Curr. Infect. Dis. Rep. 4:400-410. [DOI] [PubMed] [Google Scholar]
- 8.Chiang, S., and J. Mekalanos. 1998. Use of signature-tagged transposon mutagenesis to identify Vibrio cholerae genes critical for colonization. Mol. Microbiol. 27:797-805. [DOI] [PubMed] [Google Scholar]
- 9.Chiang, S., J. Mekalanos, and D. Holden. 1999. In vivo genetic analysis of bacterial virulence. Annu. Rev. Microbiol. 53:129-154. [DOI] [PubMed] [Google Scholar]
- 10.Chuang, S.-E., D. Daniels, and F. Blattner. 1993. Global regulation of gene expression in Escherichia coli. J. Bacteriol. 175:2026-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coulter, S. N., W. R. Schwan, E. Y. Ng, M. H. Langhorne, H. D. Ritchie, S. Westbrock-Wadman, W. O. Hufnagle, K. R. Folger, A. S. Bayer, and C. K. Stover. 1998. Staphylococcus aureus genetic loci impacting growth and survival in multiple infection environments. Mol. Microbiol. 30:393-404. [DOI] [PubMed] [Google Scholar]
- 12.Davis, R., D. Botstein, and J. Roth. 1980. Advanced bacterial genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 13.Fields, P. I., R. V. Swanson, C. G. Haidaris, and F. Heffron. 1986. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc. Natl. Acad. Sci. USA 83:5189-5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ford, C., J. Hamel, D. Stapert, and R. Yancey. 1989. Establishment of an experimental model of a Staphylcoccus aureus abscess in mice by use of dextran and gelatin microcarriers. J. Med. Microbiol. 28:259-266. [DOI] [PubMed] [Google Scholar]
- 15.Forsberg, H., and P. O. Ljungdahl. 2001. Sensors of extracellular nutrients in Saccharomyces cerevisiae. Curr. Genet. 40:91-109. [DOI] [PubMed] [Google Scholar]
- 16.Foster, T. J., and D. McDevitt. 1994. Surface-associated proteins of Staphylococcus aureus: their possible roles in virulence. FEMS Microbiol. Lett. 118:199-205. [DOI] [PubMed] [Google Scholar]
- 17.Fournier, B., and D. Hooper. 2000. A new two-component regulatory system involved in adhesion, autolysis, and extracellular proteolytic activity of Staphylococcus aureus. J. Bacteriol. 182:3955-3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fournier, B., A. Klier, and G. Rapoport. 2001. The two-component system ArlS-ArlR is a regulator of virulence gene expression in Staphylococcus aureus. Mol. Microbiol. 41:247-261. [DOI] [PubMed] [Google Scholar]
- 19.Frimodt-Moller, N. 1993. The mouse peritonitis model: present and future use. J. Antimicrob. Chemother. 31:55-60. [DOI] [PubMed] [Google Scholar]
- 20.Fuller, T., M. Kennedy, and D. Lowery. 2000. Identification of Pasteurella multocida virulence genes in a septicemic mouse model using signature-tagged mutagenesis. Microb. Pathog. 29:25-38. [DOI] [PubMed] [Google Scholar]
- 21.Garg, M. B., J. C. Sevester, J. A. Sakoff, and S. P. Ackland. 2002. Simple liquid chromatographic method for the determination of uracil and dihydrouracil plasma levels: a potential pretreatment predictor of 5-fluorouracil toxicity. J. Chromatogr. B 774:223-230. [DOI] [PubMed] [Google Scholar]
- 22.Giraudo, A., H. Rampone, A. Calzolari, and R. Nagel. 1996. Phenotypic characterization and virulence of a sae− agr− mutant of Staphylococcus aureus. Can. J. Microbiol. 42:120-123. [DOI] [PubMed] [Google Scholar]
- 23.Handfield, M., and R. Levesque. 1999. Strategies for isolation of in vivo expressed genes from bacteria. FEMS Microbiol. Rev. 23:69-91. [DOI] [PubMed] [Google Scholar]
- 24.Hensel, M., J. Shea, C. Gleeson, M. Jones, E. Dalton, and D. Holden. 1995. Simultaneous identification of bacterial virulence genes by negative selection. Science 269:400-403. [DOI] [PubMed] [Google Scholar]
- 25.Hidalgo-Grass, C., M. Ravins, M. Dan-Goor, J. Jaffe, A. E. Moses, and E. Hanski. 2002. A locus of group A Streptococcus involved in invasive disease and DNA transfer. Mol. Microbiol. 46:87-99. [DOI] [PubMed] [Google Scholar]
- 26.Hoch, J., and T. Silhavy. 1995. Two-component signal transduction. American Society for Microbiology, Washington, D.C.
- 27.Horinouchi, S., and B. Weisblum. 1982. Nucleotide sequence and functional map of pC194, a plasmid that specifies inducible chloramphenicol resistance. J. Bacteriol. 150:815-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ji, G., R. Beavis, and R. P. Novick. 1997. Bacterial interference caused by autoinducing peptide variants. Science 276:2027-2030. [DOI] [PubMed] [Google Scholar]
- 29.Ji, G., R. C. Beavis, and R. P. Novick. 1995. Cell density control of staphylococcal virulence mediated by an octapeptide pheromone. Proc. Natl. Acad. Sci. USA 92:12055-12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kadner, R. J., C. A. Webber, and M. D. Island. 1993. The family of organo-phosphate transport proteins includes a transmembrane regulatory protein. J. Bioenerg. Biomembr. 25:637-645. [DOI] [PubMed] [Google Scholar]
- 31.Karlyshev, A., P. Oyston, K. Williams, G. Clark, R. Titball, W. Winzeler, and B. Wren. 2001. Application of high-density array-based signature-tagged mutagenesis to discover novel Yersinia virulence-associated genes. Infect. Immun. 69:7810-7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kluytmans, J., A. van Belkum, and H. Verbrugh. 1997. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 10:505-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kreiswirth, B., S. Lofdahl, M. Betley, M. O'Reilly, P. Schlievert, M. Bergdoll, and R. Novick. 1983. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305:709-712. [DOI] [PubMed] [Google Scholar]
- 34.Kuroda, M., T. Ohta, I. Uchiyama, T. Baba, H. Yuzawa, I. Kobayashi, L. Cui, A. Oguchi, K. Aoki, Y. Nagai, J. Lian, T. Ito, M. Kanamori, H. Matsumaru, A. Maruyama, H. Murakami, A. Hosoyama, Y. Mizutani-Ui, N. K. Takahashi, T. Sawano, R. Inoue, C. Kaito, K. Sekimizu, H. Hirakawa, S. Kuhara, S. Goto, J. Yabuzaki, M. Kanehisa, A. Yamashita, K. Oshima, K. Furuya, C. Yoshino, T. Shiba, M. Hattori, N. Ogasawara, H. Hayashi, and K. Hiramatsu. 2001. Whole genome sequencing of methicillin-resistant Staphylococcus aureus. Lancet 357:1225-1240. [DOI] [PubMed] [Google Scholar]
- 35.Lammers, A., E. Kruijt, C. van de Kuijt, P. J. M. Nuijten, and H. E. Smith. 2000. Identification of Staphylococcus aureus genes expressed during growth in milk: a useful model for selection of genes important in bovine mastitis? Microbiology 146:981-987. [DOI] [PubMed] [Google Scholar]
- 36.Lee, C. Y., S. L. Buranen, and Z.-H. Ye. 1991. Construction of single-copy integration vectors for Staphylococcus aureus. Gene 103:101-105. [DOI] [PubMed] [Google Scholar]
- 37.Lehoux, D., F. Sanschagrin, and R. Levesque. 1999. Defined oligonucleotide tag pools and PCR screening in signature-tagged mutagenesis of essential genes from bacteria. BioTechniques 26:473-480. [DOI] [PubMed] [Google Scholar]
- 38.Lowy, F. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532. [DOI] [PubMed] [Google Scholar]
- 39.Lowy, F. 2000. Is Staphylococcus aureus an intracellular pathogen? Trends Microbiol. 8:341-343. [DOI] [PubMed] [Google Scholar]
- 40.Mahan, M., J. Slauch, and J. Mekalanos. 1993. Selection of bacterial virulence genes that are specifically induced in host tissues. Science 259:686-688. [DOI] [PubMed] [Google Scholar]
- 41.Martin, P., T. Li, D. Sun, D. Biek, and M. Schmid. 1999. Role in cell permeability of an essential two-component system in Staphylococcs aureus. J. Bacteriol. 181:3666-3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mecsas, J. 2002. Use of signature-tagged mutagenesis in pathogenesis studies. Curr. Opin. Microbiol. 5:33-37. [DOI] [PubMed] [Google Scholar]
- 43.Mei, J., F. Nourbakhsh, C. Ford, and D. Holden. 1997. Identification of Staphylococcus aureus virulence genes in a murine model of bacteraemia using signature-tagged mutagenesis. Mol. Microbiol. 26:399-407. [DOI] [PubMed] [Google Scholar]
- 44.Mojumdar, M., and S. Khan. 1988. Characterization of the tetracycline resistance gene of plasmid pT181 of Staphylococcus aureus. J. Bacteriol. 170:5522-5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Musher, D. M., N. Lamm, R. O. Darouiche, E. J. Young, R. J. Hamill, and G. C. Landon. 1994. The current spectrum of Staphylococcus aureus infection in a tertiary care hospital. Medicine (Baltimore) 73:186-208. [DOI] [PubMed] [Google Scholar]
- 46.Novick, R. P. 2003. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol. Microbiol. 48:1429-1449. [DOI] [PubMed] [Google Scholar]
- 47.Novick, R., I. Edelman, M. Schwesinger, A. Gross, E. Swanson, and P. Pattee. 1979. Genetic translocation in Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 76:400-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pattee, P., and D. Neveln. 1975. Transformation analysis of three linkage groups in Staphylococcus aureus. J. Bacteriol. 124:201-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pavelka, M. S., Jr., and W. R. Jacobs, Jr. 1996. Biosynthesis of diaminopimelate, the precursor of lysine and a component of peptidoglycan, is an essential function of Mycobacterium smegmatis. J. Bacteriol. 178:6496-6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peng, H., R. Novick, B. Kreiswirth, J. Kornblum, and P. Schlievert. 1988. Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J. Bacteriol. 170:4365-4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pitkanen, H. T., S. S. Oja, K. Kemppainen, J. M. Seppa, and A. A. Mero. 2003. Serum amino acid concentrations in aging men and women. Amino Acids 24:413-421. [DOI] [PubMed] [Google Scholar]
- 52.Rich, J., and D. Willis. 1990. A single oligonucleotide can be used to rapidly isolate DNA sequences flanking a transposon Tn5 insertion by the polymerase chain reaction. Nucleic Acids Res. 18:6673-6676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rozalska, B., and T. Wadstrom. 1993. Interferon-gamma, interleukin-1 and tumour necrosis factor-alpha synthesis during experimental murine staphylococcal infection. FEMS Immunol. Med. Microbiol. 7:145-152. [DOI] [PubMed] [Google Scholar]
- 54.Sambrook, J., E. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 55.Valdivia, R., and S. Falkow. 1997. Fluorescence-based isolation of bacterial genes expressed within host cells. Science 277:2007-2011. [DOI] [PubMed] [Google Scholar]
- 56.Verdrengh, M., and A. Tarkowski. 1997. Role of neutrophils in experimental septicemia and septic arthritis induced by Staphylococcus aureus. Infect. Immun. 65:2517-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vijaranakul, U., A. Xiong, K. Lockwood, and R. Jayaswal. 1998. Cloning and nucleotide sequencing of a Staphylococcus aureus gene encoding a branched-chain amino-acid transporter. Appl. Environ. Microbiol. 64:763-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vriesema, A., H. Beekhuizen, M. Hamdi, A. Soufan, et al. 2000. Altered gene expression in Staphylococcus aureus upon interaction with human endothelial cells. Infect. Immun. 68:1765-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walsh, C., and C. L. Cepko. 1992. Widespread dispersion of neuronal clones across functional regions of the cerebral cortex. Science 255:434-440. [DOI] [PubMed] [Google Scholar]
- 60.Weber, I., C. Fritz, S. Ruttkowski, A. Kreft, and F. C. Bange. 2000. Anaerobic nitrate reductase (narGHJI) activity of Mycobacterium bovis BCG in vitro and its contribution to virulence in immunodeficient mice. Mol. Microbiol. 35:1017-1025. [DOI] [PubMed] [Google Scholar]
- 61.Whitener, C. J., S. Y. Park, F. A. Browne, L. J. Parent, K. Julian, B. Bozdogan, P. C. Appelbaum, J. Chaitram, L. M. Weigel, J. Jernigan, L. K. McDougal, F. C. Tenover, and S. K. Fridkin. 2004. Vancomycin-resistant Staphylococcus aureus in the absence of vancomycin exposure. Clin. Infect. Dis. 38:1049-1055. [DOI] [PubMed] [Google Scholar]
- 62.Wiltshire, M., and S. Foster. 2001. Identification and analysis of Staphylococcus aureus components expressed by a model system of growth in serum. Infect. Immun. 69:5198-5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yao, L., J. Berman, S. Factor, and F. Lowy. 1997. Correlation of histopathologic and bacteriologic changes with cytokine expression in an experimental murine model of bacteremic Staphylococcus aureus infection. Infect. Immun. 65:3889-3895. [DOI] [PMC free article] [PubMed] [Google Scholar]