Abstract
More than half of those born after 1960 will develop cancer during their lifetime. Fortunately, owing to improved diagnosis and treatment, cure rates have risen steadily over the last three decades. With an increased survivorship, more will experience adverse effects of cancer therapeutics on the heart. As the oncologist’s focus begins to encompass the issues of cancer survivorship, awareness of the management of cardiac toxicity would be prudent for all physicians looking after patients with cancer.
INTRODUCTION
Cancer and heart disease are the two most common causes of death in Northern Ireland. As cancer survivorship increases, understanding of the links between cancer treatments and cardiovascular disease becomes crucial for all medical practitioners. As cure rates improve, increasing priority can be given to optimising quality of life rather than focusing on prevention of cancer recurrence. Patients may view the adverse effects of cancer treatment in equal concern with the diagnosis of cancer itself, and with the development of new anti-cancer therapies, physicians must be vigilant for the symptoms and signs of emerging cardiovascular problems.
The last decade has seen an exponential growth in the numbers of anti-cancer treatments available, especially systemic anti-cancer treatment (SACT) and radiotherapy. It is these two anti-cancer therapies that are implicated in the emerging field of cardio-oncology, whether the aim of cancer treatment is curative or palliative (Table 1). This article explores the relationship of these treatments to cardiotoxicity, the indicative signs and symptoms and the management options available.
Table 1.
Commonly used cardiotoxic anti-cancer treatments
| Cancer Treatment | Examples |
|---|---|
| Cytotoxic chemotherapy | doxorubicin, epirubicin, cyclophosphamide, 5-fluorouracil, cisplatin, paclitaxel |
| Targeted therapies | trastuzumab, sunitinib, pazopanib |
| Hormonal therapies | tamoxifen, anastrazole, letrozole, goserelin, enzalutamide, abiraterone |
| Radiotherapy | breast, lung, mediastinal lymphoma and oesophageal radiotherapy |
OVERVIEW OF ANTI-CANCER THERAPIES
Radiotherapy delivers energy via ionising radiation to neoplastic cell DNA in such a way that the resultant damage induces cell death preferentially in malignant tissue. Approximately 40% of patients diagnosed with cancer receive radiotherapy1. Radiotherapy may be delivered using external beam radiotherapy (EBRT) (Figure 1), sealed internal sources, for example gold seeds for prostate cancer, brachytherapy and unsealed internal sources, for example, radioactive iodine for thyroid cancer. The key principle of minimising normal tissue irradiation whilst providing the optimum dose to the cancer target dictates that factors such as tumour histology, dimensions and position are used in determining the choice of irradiation method. Since the 1960s, treatment of lung and breast cancer and lymphoma with thoracic radiotherapy has increased cardiovascular morbidity via radiation toxicity2. In general radiotherapy techniques which do not deliver radiation to the heart do not cause cardiotoxicity.
Fig 1.
A linear accelerator for EBRT
The mechanism of action of each of SACT agent depends on the family and the era that they originate from, with newer agents having quite different targets than more established treatments. Cytotoxic chemotherapy is the nomenclature used to describe drugs such as the anthracyclines (eg. doxorubicin) and antimetabolites (eg. 5-fluorouracil) which are generally non-specific and which cause apoptosis and cause cell death by altering key components of cellular metabolism or by directly damaging DNA.
Hormonal therapies, molecular targeting agents and immunotherapies are more specific in their mode of action. Their cardiotoxicity risk can occur via a number of mechanisms:
Direct ‘on-target toxicity’ if the molecular target in the cancer also serves an important role in cardiovascular biology (e.g. trastuzumab.)
Indirect ‘on-target toxicity’ if the molecular target in the cancer is also a modifier of cardiovascular risk leading to the accelerated progression of a second disease (e.g. GnRH agonists for prostate cancer which increase LDL and diabetes risk leading to atherosclerosis and increased risk of myocardial infarction.)
‘Off target toxicity’ where the drug appears to inhibit a range of molecular targets, not only the principle target molecule in the cancer, and inhibition or activation these additional pathways imparts cardiovascular risk (e.g. sunitinib displays both on-target and off-target toxicity.)
EXAMPLES OF CARDIOTOXIC TREATMENTS
External beam radiotherapy
Lung cancer
Curative intent radiotherapy is offered to patients diagnosed with stage I - III non-small cell lung cancer or limited stage small cell lung cancer who have a good Performance Status3. EBRT is often the treatment of choice for patients who decline, or are unsuitable for surgery. Recent progress in radiation technology has the potential to revolutionise lung cancer treatment, with advances such as stereotactic ablative radiotherapy reported to have comparable results with surgical resection4. Radiotherapy also has a role in the control of incurable lung cancer, which affected two thirds of the 1143 total cases diagnosed in Northern Ireland in 20125.
As observed in the general population, patients who are male, older age, African-Caribbean background, have pre-existing cardiac disease or have other co-morbidities experience a greater risk of cardiovascular morbidity following thoracic irradiation6. It is difficult to precisely determine the direct impact of radiotherapy from other risk factors for cardiovascular disease in patients with lung cancer owing to the sizeable impact of smoking. Ischaemic heart disease is more common in patients with tumours of the left lung compared with the right, after radiation treatment6. Recently developed alternative methods of delivery of thoracic radiotherapy have achieved increased survival rates and reduced treatment durations for patients without increasing long-term toxicity7.
Breast cancer
Given the very high local control rates seen in patients with early breast cancer, an increased emphasis on avoidance of toxicity is given. Breast cancer radiotherapy constitutes 30% of a radiotherapy unit’s caseload8. Adjuvant radiotherapy is offered to patients diagnosed with early and locally advanced breast cancer (over a third of patients with breast cancer receive radiotherapy9). However, in previous large overviews of breast cancer using data collected over 40 years, the reduced breast cancer mortality attributable to adjuvant radiotherapy did not always translate into an overall survival gain, probably due to an increased cardiac mortality in patients receiving treatment to the left breast10,11.
Major coronary events after left-sided breast irradiation have a bimodal peak incidence, with many patients affected in the 4 years post-treatment, and others >20 years post-treatment10. It has been shown that unintended cardiac irradiation during left breast treatment occurs in almost one half of patients despite CT-based treatment plans excluding this area12. As seen with lung cancer, cardiotoxicity in breast radiotherapy is dependent upon laterality, with a cardiovascular mortality ratio of 1.33 (left breast versus right) for treatment delivered in the 1970s13. Women aged < 35 years are at greatest risk of radiation toxicity, with a 6.5 x lifetime risk of cardiovascular death compared with the general population14. Recent evidence suggests that for selected older patients, with low risk breast cancer, the cardiovascular risks of radiotherapy may outweigh the potential benefits15. The burden of cardiotoxicity in patients with breast neoplasms has decreased as a consequence of improvements in radiation technology and some investigators have found the effect of tumour laterality has been negligible since 199316,17.
Other thoracic radiotherapy
Mantle radiotherapy describes the extended pattern of radiation fields used to target supra-diaphragmatic lymph node groups, often encompassing the mediastinal and hilar lymphatics. Hodgkin’s disease patients, for whom mantle radiotherapy was primarily used in the past have a higher risk of developing calcific valvular disorders (both stenosis and/or regurgitation)18, myocardial infarction, cardiomyopathy and pericardial disease19. As a result, the relative risk of patients in this group developing heart failure is 4.919. This group of patients are also at risk of symptomatic (usually syncope) and asymptomatic conduction abnormalities20.
A recent review of the limited data on oesophageal cancer found a crude rate of radiation cardiotoxicity in these patients of 10.8%21. Adverse effects in order of increasing incidence were pericardial effusion, ischaemic events and heart failure. A recent review of pacemaker and implantable cardiac defibrillator safety in radiotherapy has acknowledged that certain energies of radiotherapy pose a risk of malfunction and clinical oncologists are awaiting an update in the relevant guidelines22.
Systemic anti-cancer therapy
Anthracyclines
Drugs such as doxorubicin and epirubicin form disruptive cross-links between paired DNA strands. They have an important role in the chemotherapy regime for many cancers including breast, gastrointestinal and blood. Cardiotoxicity due to an anthracycline is seen in 5.4% of breast cancer patients, and has been viewed as dosedependent and related to the cumulative dose given over time23. The increased cardiac event rate is usually observed many years after treatment and is related to a patient’s pre-existing cardiovascular risk factors24. Under the age of 70, anthracycline cardiotoxicity most often manifests as heart failure in breast cancer treatment25. Alternative preparations of the drug can be used to reduce the risk. Liposomal infusions may be less harmful than boluses and cardioprotective agents may be co-administered to further reduce the effect (e.g. angiotensin converting enzyme (ACE) inhibitors, beta blockers, dexrazozane) have been used26,27. Another anthracycline, epirubicin has been associated with mild rises in troponin levels unrelated to ischaemic events in the month following infusion28.
Trastuzumab
Trastuzumab is a monoclonal antibody directed towards the HER2 epidermal growth factor receptor and is licensed for use in metastatic breast cancer patients with over-expression of this receptor29. The associated type of cardiac dysfunction differs from that seen with classic cytotoxic drugs in that it is observed early (i.e. within weeks of commencing treatment), in a range of severities, independent of dose and it has a mainly reversible pattern without any ultrastructure changes30. In a study of 179 breast cancer patients, 44% of patients developed a cardiac event (defined as New York Heart Association (NYHA) III or IV heart failure or a reduction in ejection fraction) during the year of treatment, and almost a tenth had a second31. A total of 7% patients in this study had their trastuzumab discontinued, though discontinuing treatment did not affect the rates of a second cardiac event occurring. Prior or concomitant radiation therapy doesn’t influence trastuzumab cardiotoxicity32, but anthracycline therapy does33.
Other SACTs
Intercalating agents such as cyclophosphamide employ a similar mechanism of action to anthracyclines (disruption of DNA ‘unzipping’ in replicating cancer cells) and are associated with early heart failure (one week post-treatment) and haemorrhagic myocarditis34.
Antimetabolite drugs such as 5-fluorouracil (5-FU), have a number of mechanism of actions, but exert their main effect by triggering apoptosis in malignant cells by a process called a thymineless death35. These drugs may cause acute chest pain during or shortly after administration which occurs through the mechanism of coronary artery vasospasm36. Recognition of this is important for patients on capecitabine and infusional 5-FU as on-ongoing administration of those agents must be stopped until the symptoms have settled and cardiac investigations are completed.
Table 2.
Commonly used cardiotoxic SACT and their adverse cardiovascular effects
| Drug / Family of Drugs | Cardiotoxicities |
|---|---|
| Anthracyclines | Heart failure (late and irreversible) |
| Trastuzumab | Heart failure (early and reversible) |
| Cyclophosphamide | Heart failure (early), haemorrhagic myocarditis |
| Antimetabolites | Coronary vasopasm, acute coronary syndromes |
| Platinum | Acute coronary syndromes |
| Taxanes | Heart block, bradycardia, tachyarrhythmia |
| Bevacizumab | Hypertension, thromboembolism |
| Sunitinib, sorafenib, pazopanib | Hypertension, heart failure, myocardial infarction |
Platinum-based intercalating agents may also cause this symptom. Unlike the pattern seen in antimetabolites, the risk continues for many years, and cisplatin can be detected in the serum at 20 years37.
Taxane drugs such as paclitaxel and docetaxel disturb the microtubules needed to separate original and fresh copies of DNA in the dividing cell. These are commonly used in ovarian and breast cancer treatment and have been linked to heart block, bradycardia and tachyarrhythmias with a cumulative incidence of 0.5%38.
Hormonal treatments have not been consistently shown to increase the risk of cardiac events despite interfering with lipid metabolism23.
Bevacizumab, an agent targeting vascular endothelial growth factor receptors used in colorectal cancer, has been linked to cardiotoxicity via hypertension and thromboembolism39. Sunitinib targets this receptor in renal cell carcinoma and was strongly associated with cardiotoxicity in one large study40. Over a tenth of patients experienced cardiovascular events including myocardial infarction and heart failure, over a quarter had an asymptomatic decrease in ejection fraction of 10% or more and almost one half of patients were diagnosed with hypertension following drug administration.
DIAGNOSIS OF HEART FAILURE
Whilst oncological treatments may cause a range of adverse cardiovascular conditions, the commonest is heart failure. Heart failure is a complex syndrome in which the ability of the heart to maintain the circulation of blood is impaired41. Approximately half of patients die within 4 years42 however mortality rates are improving43.
Biomarkers of cardiac injury (e.g. cardiac troponin I concentration) have been shown to identify patients at risk for anthracycline cardiotoxicity and predict a poor response to cardiac therapy44,45. The finding of a significantly raised B-type natriuretic peptide level (BNP or its precursor fragment NT-proBNP) provides evidence of heart failure with high sensitivity46. Whilst not useful in an unselected cohort of patients receiving SACT, NT-proBNP trends can identify at-risk patients treated with anthracyclines47.
Echocardiography is an excellent tool for identifying the patient with heart failure. A reduction in left ventricular ejection fraction (LVEF) is a late finding and may occur only after a significant amount of myocardial damage has occurred. Advanced echocardiographic techniques such as global longitudinal strain measurement may detect left ventricular impairment earlier than conventional LVEF48. Deteriorations of 13.7% or more from baseline warrant continued increased clinical vigilance with fair sensitivity (88%) and specificity (71%)49.
TEXT BOX 1. Application of natriuretic peptides to CTRCD.
| - | raised natriuretic peptide level is evidence of heart failure (highly sensitive) |
| - | elevated NT-proBNP after an anthracycline identifies patients at risk of CTRCD |
| - | CTRCD is not limited to high-dose regimes |
| - | persistent elevations after 72 hrs predict progressive decline in left ventricular failure |
| - | raised levels are associated with a greater risk of developing trastuzumab CTRCD |
CANCER THERAPEUTICS-RELATED CARDIAC DYSFUNCTION
Cancer Therapeutics-Related Cardiac Dysfunction (CTRCD) is defined by the European Society of Cardiology (ESC) as a decrease in LVEF of 10% or more, to a value less than the normal range50. CTRCD can further be divided into those who develop symptoms of overt heart failure and those who have an asymptomatic decline in systolic function. The ESC also sub-classifies systolic dysfunction by the degree of reversibility following clinical intervention:
Reversible (returns to within 5% of baseline)
Partly reversible (>10% improvement, >5% below baseline)
Irreversible (<10% improvement, >5% below baseline).
It is felt that the most important factor is demonstration of reversibility.
All patients should be treated with an ACE inhibitor/angiotensin II receptor blocker (ARB) and a beta blocker licensed for heart failure. Some of these drugs have been assessed specifically in CTRCD patients, and may be preferable to others in the same drug class, as not all agents seem to be beneficial51.
In patients with early evidence of left ventricular impairment during cancer treatment, introduction of ACE inhibitors and possibly beta blockers may be helpful to recover cardiac function, and offer cardioprotection against further cancer treatment if required - particularly those with a history of cardiac disease. Different strategies are currently employed in various specialist centres with Cardio-Oncology services, including surveillance with echocardiography and cardiac biomarkers, early detection and cardioprotection during chemotherapy or cardiotoxic targeted molecular therapies. Involvement of the Heart Failure Specialist Team is essential.
TRASTUZUMAB
It is important to appreciate that the majority of the 44% patients developing cardiotoxicity after trastuzumab had mild or no symptoms31. The implication of previous or concurrent anthracycline is also worth recognising - this triples the risk31. Whilst no study has been designed to test this link specifically, a recent very large population-based study indicates that sequential therapy (anthracycline followed by trastuzumab) is more dangerous (4 x hazard ratio) than concomitant treatment (2 x hazard ratio)52. It is postulated that the cumulative oxidative damage from an anthracycline is negatively modulated in the vulnerable by trastuzumab via its direct mechanism of action on the HER2 pathway and some data suggests that a delay in initiating trastuzumab allows the myocytes to recover before a “second hit”44.
TEXT BOX 2. Recommended actions for patients undergoing cardiotoxic anticancer therapy.
| BEFORE COMPLETING TREATMENT | AFTER COMPLETING TREATMENT |
|---|---|
| Be aware of the cardiotoxic potential of patients’ anti-cancer treatment plan Investigate new or changing cardiovascular symptoms | |
| Ensure patients are aware of the potential for heart problems during/after cancer treatment Monitor weight, lipid profile, glycaemic control during hormonal therapies Update the Oncologist early with relevant investigation results and medication changes |
Regular and relevant cardiac function testing no later than 6 months following high-risk treatments and if normal/asymptomatic Approach metastatic and non-metastatic patients with identical monitoring Liaise with a cardiologist if there is a suspicion of cardiotoxicity |
New elevations in cardiac troponin I were commonly seen after the first cycle, resolved within 3 months with patients developing cardiovascular symptoms by 8 months. In the same study, baseline troponin elevation patterns (following cytotoxic chemotherapy and prior to trastuzumab) were consistent with the hypothesis that the conventional time to commence trastuzumab may be during a period of myocyte vulnerability52. Therefore abnormal troponin values should prompt clinicians to follow up symptoms actively whilst a Cardio-oncology opinion is awaited. There is less data about using BNP for diagnosis and prediction of CTRCD with trastuzumab, compared with anthracyclines. Exploratory analyses have established that NT-BNP can be raised in absence of impaired LVEF on echocardiogram 53, suggesting LVEF is not sensitive enough to detect subclinical damage from SACT. The Australian CATS (Cardiotoxicity of Adjuvant Trastuzumab) study should address this need when it is reported 54.
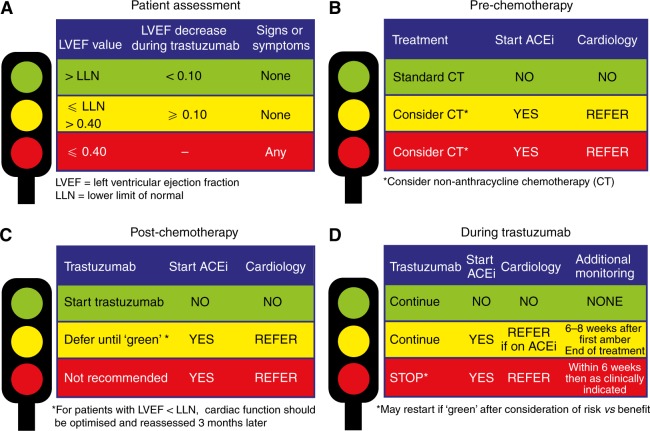
Protocols for managing trastuzumab cardiotoxicity have been developed55,56. In the event of a severe drop in LVEF or the development of clinical heart failure, cessation of trastuzumab is clearly indicated. For cases with mild subclinical dysfunction, continuation of therapy with careful LVEF monitoring and protective medical therapy may be appropriate. Figure 2 is the traffic light triage approach suggested by the National Cancer Research Institute55. The traffic light system guides clinicians when to increase monitoring, when to refer to Cardiology and when to continue/discontinue trastuzumab and ACE inhibitors.
Fig 2.
NCRI Traffic Light System
The clinical outcome of trastuzumab cardiotoxicity is more favourable than anthracyclines and in many cases, rechallenge may be safely carried out44. In the PRADA study, patients randomised to cardioprotection with candesartan or metoprolol suffered less cardiotoxicity from trastuzumab with candesartan (no benefit from metoprolol observed)50. Future studies should seek to clarify the need for cardioprotection as trastuzumab becomes increasingly prescribed not only for breast but other tumour types.
CONCLUSIONS
Cardiovascular disease and cancer are very common treatable conditions. Survival rates for cancer have increased significantly over the last three decades. Increased survivorship increases the chances of developing treatment related complications such as cardiovascular disease. Both oncologists and non-oncologists need to be aware of the clinical syndromes and the potential treatments available for cardiovascular disease related to cancer treatment. The development of new specialist Cardio-Oncology services can help oncologists, haematologists and primary care access specialist care for cancer patients before, during and after treatment with cardiotoxic therapy.
REFERENCES
- 1.Cancer Research UK . About radiotherapy. [Internet] London: Cancer Research UK; 2014. [Updated 2014; cited 2015]. Available online: http://www.cancerresearchuk.org/about-cancer/cancers-in-general/treatment/radiotherapy/about/what-radiotherapy-is Last accessed September 2016. [Google Scholar]
- 2.Stewart JR, Fajardo LF. Radiation-induced heart disease: an update. Prog Cardiovasc Dis. 1984;27(3):173–94. doi: 10.1016/0033-0620(84)90003-3. [DOI] [PubMed] [Google Scholar]
- 3.NICE Clinical Guideline 121. [Internet] London: National Institute of Clinical Excellence; 2005. [Updated 2011; cited 2015]. Available online from: https://www.nice.org.uk/guidance/cg121. Last accessed March 2016. [Google Scholar]
- 4.Chang J, Senan S, Paul M, Mehran R, Louie A, Balter P, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-smallcell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16(6):630–7. doi: 10.1016/S1470-2045(15)70168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cancer Research UK . Lung cancer incidence statistics. [Internet] London: National Institute of Clinical Excellence; 2005. [Updated 2011; cited 2015]. Available online from: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/lungcancer/incidence#heading-Zero. Last accessed March 2016. [Google Scholar]
- 6.Hardy D, Liu C, Cormier J, Xia R, Du X. Cardiac toxicity in association with chemotherapy and radiation therapy in a large cohort of older patients with non-small-cell lung cancer. Ann Oncol. 2010;21(9):1825–33. doi: 10.1093/annonc/mdq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saunders M, Dische S, Barrett A, Harvey A, Gibson D, Palmar M. Continuous hyperfractionated accelerated radiotherapy (CHART) versus conventional radiotherapy in non-small-cell lung cancer: a randomised multicentre trial. CHART Steeting Committee. Lancet. 1997;350(9072):161–5. doi: 10.1016/s0140-6736(97)06305-8. [DOI] [PubMed] [Google Scholar]
- 8.Taylor C, Kirby A. Cardiac side effects from breast cancer radiotherapy. Clin Oncol (R Coll Radiol) 2015;27(11):621–9. doi: 10.1016/j.clon.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Darby SC, McGale P, Taylor CW, Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 2005;6(8):557–65. doi: 10.1016/S1470-2045(05)70251-5. [DOI] [PubMed] [Google Scholar]
- 10.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 11.Darby S, McGale P, Peto R, Granath F, Hall P, Ekbom A. Mortality from cardiovascular disease more than 10 years after radiotherapy for breast cancer: nationwide cohort study of 90 000 Swedish women. BMJ. 2003;326(7383):256–7. doi: 10.1136/bmj.326.7383.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goody RB, O’Hare J, McKenna K, Dearey L, Robinson J, Bell P, et al. Unintended cardiac irradiation during left-sided breast cancer radiotherapy. Br J Radiol. 2013;86(1022) doi: 10.1259/bjr.20120434. 20120434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bao J, Yu K-D, Jiang Y-Z, Shao Z-M, Di G-H. The effect of laterality and primary tumour site on cancer-specific mortality in breast cancer: a SEER population-based study. PLoS ONE. 2014;9(4):e94815. doi: 10.1371/journal.pone.0094815. Available online from: http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0094815. Last accessed: September 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hooning M, Botma A, Aleman B, Baaijens M, Bartelink H, Klijn J, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst. 2007;99(5):365–75. doi: 10.1093/jnci/djk064. [DOI] [PubMed] [Google Scholar]
- 15.Marks L, Yu X, Prosnitz R, Zhou S, Hardenbergh P, Blazing M, et al. The incidence and functional consequences of RT-associated cardiac perfusion defects. Int J Radiat Oncol Biol Phys. 2005;63(1):214–23. doi: 10.1016/j.ijrobp.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 16.Henson K, McGale P, Taylor C, Darby S. Radiation-related mortality from heart disease and lung cancer more than 20 years after radiotherapy for breast cancer. Br J Cancer. 2013;108(1):179–82. doi: 10.1038/bjc.2012.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doyle J, Neuget A, Jacobson J, Wang J, McBride R, Grann A, et al. Radiation therapy, cardiac risk factors and cardiac toxicity in early-stage breast cancer patients. Int J Radiat Oncol Biol Phys. 2007;68(1):82–93. doi: 10.1016/j.ijrobp.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 18.Cutter DJ, Schaapveld M, Darby SC, Hauptmann M, van Nimwegen FA, Krol AD, et al. Risk of valvular heart disease after treatment for Hodgkin lymphoma. J Natl Cancer Inst. 2015;107(4) doi: 10.1093/jnci/djv008. djv008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ng AK. Review of the cardiac long-term effects of therapy for Hodgkin’s lymphoma. Br J Haem. 2011;154(1):23–31. doi: 10.1111/j.1365-2141.2011.08713.x. [DOI] [PubMed] [Google Scholar]
- 20.Heidenreich PA, Hancock SL, Lee BK, Mariscal CS, Schnittger I. Asymptomatic cardiac disease following mediastinal irradiation. J Am Coll Cardiol. 2003;42(4):743–9. doi: 10.1016/s0735-1097(03)00759-9. [DOI] [PubMed] [Google Scholar]
- 21.Beukema J, van Luijk P, Widder J, Langendijk J, Muijs C. Is cardiac toxicity a relevant issue in radiation treatment of oesophageal cancer? Radiother Oncol. 2015;114(1):85–90. doi: 10.1016/j.radonc.2014.11.037. [DOI] [PubMed] [Google Scholar]
- 22.Zaremba T, Jakobsen A, Søgaard M, Thøgersen A, Riahi S. Radiotherapy in patients with pacemakers and implantable cardioverter defibrillators: a literature review. Europace. 2016;18:479–91. doi: 10.1093/europace/euv135. [DOI] [PubMed] [Google Scholar]
- 23.Valachis A, Nilsson C. Cardiac risk in the treatment of breast cancer: assessment and management. Breast Cancer (Dove Press) 2015;7:21–35. doi: 10.2147/BCTT.S47227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinder MC, Duan Z, Goodwin JS, Hortobagyi GN, Giordano SH. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J Clin Oncol. 2007;25(25):3808–15. doi: 10.1200/JCO.2006.10.4976. [DOI] [PubMed] [Google Scholar]
- 25.Bird BR, Swain SM. Cardiac toxicity in breast cancer survivors: review of potential cardiac problems. Clin Cancer Res. 2008;14(1):14–24. doi: 10.1158/1078-0432.CCR-07-1033. [DOI] [PubMed] [Google Scholar]
- 26.Van Dalen E, Caron HN, Dickinson HO, Kremer LC. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database Syst Rev. 2011;15(6) doi: 10.1002/14651858.CD003917.pub4. CD003917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bosch X, Rovira M, Sitges M, Domenech A, Ortiz-Perez JT, de-Caralt TM, et al. Enalapril and carvedilol for preventing chemotherapyinduced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies) J Am Coll Cardiol. 2013;61(23):2355–62. doi: 10.1016/j.jacc.2013.02.072. [DOI] [PubMed] [Google Scholar]
- 28.Feola M, Garrone O, Occelli M, Fancini A, Biggi A, Visconti G, et al. Cardiotoxicity after anthracycline chemotherapy in breast carcinoma: Effects on left ventricular ejection fraction, troponin I and brain natriuretic peptide. Int J Cardio. 2011;148(2):194–8. doi: 10.1016/j.ijcard.2009.09.564. [DOI] [PubMed] [Google Scholar]
- 29.Piccart-Gebhart MJ, Procter M, Leylan-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–72. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 30.Ewer MS, Lippman SM. Type II chemotherapy-related cardiac dysfunction: time to recognise a new entity. J Clin Onc. 2005;23(15):2900–2. doi: 10.1200/JCO.2005.05.827. [DOI] [PubMed] [Google Scholar]
- 31.Farolfi A, Melegari E, Aquilina M, Scarpi E, Ibrahim T, Maltoni R, et al. Trastuzumab-induced cardiotoxicity in early breast cancer patients: a retrospective study of possible risk and protective factors. Heart. 2013;99(9):634–9. doi: 10.1136/heartjnl-2012-303151. [DOI] [PubMed] [Google Scholar]
- 32.Marinko T, Dolenc J, Bilban-Jakopin C. Cardiotoxicity of concomitant radiotherapy and trastuzumab for early breast cancer. Radiol Oncol. 2014;48(2):105–12. doi: 10.2478/raon-2013-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that over-expresses HER2. N Engl J Med. 2001;344(11):783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 34.Bovelli D, Plataniotis G, Roila F. Cardiotoxicity of chemotherapeutic agents and radiotherapy-related heart disease: ESMO Clinical Practice Guidelines. Ann Oncol. 2010;21(Suppl 5):v277–82. doi: 10.1093/annonc/mdq200. [DOI] [PubMed] [Google Scholar]
- 35.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3(5):330–8. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 36.Ryberg M. Recent advances in cardiotoxicity of anticancer therapies. Am Soc Clin Oncol Educ Book. 2012:555–9. doi: 10.14694/EdBook_AM.2012.32.40. [DOI] [PubMed] [Google Scholar]
- 37.Gietema JA, Meinardi MT, Messerschmidt J, Gelevert T, Alt F, Uges DR, et al. Circulating plasma platinum more than 10 years after cisplatin treatment for testicular cancer. Lancet. 2000;355(9209):1075–6. doi: 10.1016/s0140-6736(00)02044-4. [DOI] [PubMed] [Google Scholar]
- 38.Schlitt A, Jordan K, Vordermark D, Schwamborn J, Langer T, Thomssen C. Cardiotoxicity and oncological treatments. Dtsch Arztebl Int. 2014;111(10):161–8. doi: 10.3238/arztebl.2014.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berardi R, Caramanti M, Savini A, Chiorrini S, Perantoni C, Onofri A, et al. State of the art for cardiotoxicity due to chemotherapy and to targeted therapies: a literature review. Crit Rev Oncol Hematol. 2013;88(1):75–86. doi: 10.1016/j.critrevonc.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 40.Chu TF, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowski D, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007;370(9604):2011–9. doi: 10.1016/S0140-6736(07)61865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.NICE Clinical Guideline; 108. [Internet] London: National Institute of Clinical Excellence; 2010. [Updated 2015; cited 2015]. Available online: https://www.nice.org.uk/guidance/cg108. Last accessed September 2016. [Google Scholar]
- 42.Dickstein K1, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, et al. ESC European Society of Cardiology. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur Heart J. 2008;19(19):2388–442. doi: 10.1093/eurheartj/ehn309. [DOI] [PubMed] [Google Scholar]
- 43.Mehta PA, Dubrey SW, McIntyre HF, Walker DM, Hardman SM, Sutton GF, et al. Improving survival in the 6 months after diagnosis of heart failure in the past decade: population-based data from the UK. Heart. 2009;95(22):1851–6. doi: 10.1136/hrt.2008.156034. [DOI] [PubMed] [Google Scholar]
- 44.Cardinale D, Colombo A, Torrisi R, Sandri MT, Civelli M, Salvatici M, et al. Trastuzumab-induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J Clin Oncol. 2010;28(25):3910–6. doi: 10.1200/JCO.2009.27.3615. [DOI] [PubMed] [Google Scholar]
- 45.Cardinale D, Sandri MT, Colombo A, Colombo N, Boeri M, Lamantia G, et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high dose chemotherapy. Circulation. 2004;109(22):2749–54. doi: 10.1161/01.CIR.0000130926.51766.CC. [DOI] [PubMed] [Google Scholar]
- 46.Weber M, Hamm C. Role of B-type natriuretic peptide (BNP) and NTproBNP in clinical routine. Heart. 2006;92(6):843–9. doi: 10.1136/hrt.2005.071233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daugaard G, Lassen U, Bie P, Pederson EB, Jensen KT, Abildgaard U, et al. Natriuretic peptides in the monitoring of anthracycline induced reduction in left ventricular ejection fraction. Eur J Heart Fail. 2005;7(1):87–93. doi: 10.1016/j.ejheart.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 48.Mornoş C, Manolis AJ, Cozma D, Kouremenos N, Zacharopoulou I, Ionac A. The value of left ventricular global longitudinal strain assessed by three-dimensional strain imaging in the early detection of anthracyclinemediated cardiotoxicity. Hellenic J Cardiol. 2014;55(3):235–44. [PubMed] [Google Scholar]
- 49.Plana J, Galderisi M, Barac A, Ewer M, Ky B, Scherrer-Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2014;15(10):1063–93. doi: 10.1093/ehjci/jeu192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gulati G, Heck SL, Hansen Ree AH, Hoffmann P, Schulz-Menger J, Fagerlands MW, et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 x 2 factorial, randomised, placebocontrolled, double-blind clinical trial of candesartan or metoprolol. Euro Heart J. doi: 10.1093/eurheartj/ehw022. Epub 2016 Apr 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thavendiranathan P, Abdel-Qadir H, Fischer HD, Camacho X, Amir E, Austin P, et al. Breast cancer therapy–related cardiac dysfunction in adult women treated in routine clinical practice: a population-based cohort study. J Clin Oncol. 2016;34(19):2239–46. doi: 10.1200/JCO.2015.65.1505. [DOI] [PubMed] [Google Scholar]
- 52.Ewer MS, Ewer SM. Troponin I provides insight into cardiotoxicity and the anthracycline-trastuzumab interaction. J Clin Oncol. 2010;28(25):3901–4. doi: 10.1200/JCO.2010.30.6274. [DOI] [PubMed] [Google Scholar]
- 53.Goel S, Simes RJ, Beith JM. Exploratory analysis of cardiac biomarkers in women with normal cardiac function receiving trastuzumab for breast cancer. Asia Pac J Clin Oncol. 2011;7(3):276–80. doi: 10.1111/j.1743-7563.2011.01422.x. [DOI] [PubMed] [Google Scholar]
- 54.Beth J. Royal Prince Alfred Hospital, Sydney. Prediction of cardiotoxicity using serum n-terminal pro-b-type natriuretic peptide in breast cancer patients receiving adjuvant trastuzumab. ClinicalTrials.gov [Internet] [cited 2016 June 2016]. Available from: https://clinicaltrials.gov/ct2/show/NCT00858039.
- 55.Jones AL, Barlow M, Barrett-Lee PJ, Canney PA, Gilmour IM, Robb SD, et al. Management of cardiac health in trastuzumab treated patients with breast cancer: updated United Kingdom National Research Institute recommendations for monitoring. Br J Cancer. 2009;100(5):684–92. doi: 10.1038/sj.bjc.6604909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kerkhove D, Fontaine C, Droogmans S, De Greve J, Tanaka K, Van De Veire N, et al. How to monitor cardiac toxicity of chemotherapy: time is muscle. Heart. 2014;100(15):1208–17. doi: 10.1136/heartjnl-2013-303815. [DOI] [PubMed] [Google Scholar]