Abstract
Two new temperate bacteriophages exhibiting a Myoviridae (φB6) and a Siphoviridae (φHER) morphology have been isolated from Streptococcus mitis strains B6 and HER 1055, respectively, and partially characterized. The lytic phage genes were overexpressed in Escherichia coli, and their encoded proteins were purified. The lytAHER and lytAB6 genes are very similar (87% identity) and appeared to belong to the group of the so-called typical LytA amidases (atypical LytA displays a characteristic two-amino-acid deletion signature). although they exhibited several differential biochemical properties with respect to the pneumococcal LytA, e.g., they were inhibited in vitro by sodium deoxycholate and showed a more acidic pH for optimal activity. However, and in sharp contrast with the pneumococcal LytA, a short dialysis of LytAHER or LytAB6 resulted in reversible deconversion to the low-activity state (E-form) of the fully active phage amidases (C-form). Comparison of the amino acid sequences of LytAHER and LytAB6 with that of the pneumococcal amidase suggested that Val317 might be responsible for at least some of the peculiar properties of S. mitis phage enzymes. Site-directed mutagenesis that changed Val317 in the pneumococcal LytA amidase to a Thr residue (characteristic of LytAB6 and LytAHER) produced a fully active pneumococcal enzyme that differs from the parental one only in that the mutant amidase can reversibly recover the low-activity E-form upon dialysis. This is the first report showing that a single amino acid residue is involved in the conversion process of the major S. pneumoniae autolysin. Our results also showed that some lysogenic S. mitis strains possess a lytA-like gene, something that was previously thought to be exclusive to Streptococcus pneumoniae. Moreover, the newly discovered phage lysins constitute a missing link between the typical and atypical pneumococcal amidases known previously.
Bacterial murein hydrolases are enzymes that specifically cleave covalent bonds of the cell wall peptidoglycan. Some murein hydrolases can eventually cause cell lysis and are also designated autolysins. The wide distribution of murein hydrolases in bacteria, particularly of autolysins, has led to the idea that these enzymes participate in a variety of fundamental biological functions, such as synthesis of the cell wall, separation of the daughter cells at the end of the cell division, and genetic transformation (64). Moreover, lytic enzymes are responsible for the irreversible effects caused by β-lactam antibiotics (62, 70).
The major autolysin of Streptococcus pneumoniae is an N-acetylmuramoyl-l-alanine amidase (LytA) that has been well studied from the enzymatic, genetic, and structural viewpoints (17, 38) and is considered a main virulence factor (7). The translation product of the lytA gene is the low-activity form (E-form) of this amidase that is converted to the fully active form (C-form) with choline at low temperature (19, 71). The N-terminal moiety of LytA contains the active center of the enzyme, whereas the C-terminal part, composed of seven repeat units (choline-binding repeats), represents its choline-binding domain responsible for recognition of and attachment to the choline residues of the pneumococcal cell wall teichoic acid. The LytA amidase is also responsible of the characteristic, clinically relevant lysis-prone phenotype exhibited by pneumococcal isolates in the presence of deoxycholate (54, 57).
However, several authors have reported the isolation of pneumococcal strains that do not lyse with deoxycholate (Doc− phenotype) but still harbor a lytA gene (12, 15, 48, 76). These Doc− isolates, which are frequently nontypeable and/or resistant to optochin, are often designated atypical pneumococci, as opposed to the typical strains, which are bile (deoxycholate) soluble (Doc+), synthesize a typeable polysaccharide capsule, and are sensitive to optochin (40). Although the 957-bp lytA alleles (including the termination codon) from typical S. pneumoniae isolates showed only limited genetic variation (0.11 to 3.2%) (75), the atypical pneumococci contained lytA alleles that were very different from those of typical strains (pairwise evolutionary distances of about 20%) (48). However, the lytA alleles from atypical isolates were more than 92% identical to each other. A characteristic signature of atypical lytA alleles (951 bp) is the presence of a 6-bp deletion (ACAGGC) located between nucleotide positions 868 and 873, coding for Thr290-Gly291 in choline-binding repeat 6 (ChBR6) of the wild-type LytA amidase (48). It has been shown that the two-amino-acid deletion was responsible for the inhibitory effect of deoxycholate on the enzymatic activity of the lytic amidases from atypical pneumococci (48).
In addition to the bacterial lytA alleles, temperate phages from S. pneumoniae also harbor lytic genes that are homologous to that of the host. Thus, typical lytA-like genes have been reported in phages isolated from lysogenic, typical pneumococci, that is, the lysin-coding genes from temperate phages HB-3 (hbl) (53), MM1 (mml) (47), and VO1 (lytA) (49). These three genes are very similar to each other (pairwise evolutionary distance ≤ 3%) but diverged both from typical (pairwise evolutionary distance ≈ 14%) and atypical (pairwise evolutionary distance ≈ 25%) lytA alleles. Most interestingly, the pneumococcal phage Dp-1 harbors the pal gene that is a natural chimera, having a 5′ moiety completely different from that of lytA (63). It should be pointed out, however, that the pal gene also has the 6-bp deletion of atypical lytA alleles and that the Pal amidase is also inhibited by deoxycholate. Interestingly, the EJ-1 inducible prophage isolated from an atypical pneumococcal strain harbors a gene (ejl) having the characteristic deletion of atypical lytA alleles (11).
Very recently, the first identification of a temperate phage (SM1) infecting the pneumococcal relative Streptococcus mitis has been reported. SM1 is a siphovirus that also encodes a putative lysin (gp56) that is 72% identical (85% similar) to Pal amidase (65). We report here the isolation and preliminary characterization of two new temperate phages from S. mitis that harbor lytA-like genes in spite of the extended opinion that lytA was exclusive to S. pneumoniae (30). The LytA-like amidases from phages φHER and φB6 have been biochemically characterized and shown to exhibit unexpected properties.
MATERIALS AND METHODS
Bacterial strains, plasmids, phage purification, and growth conditions.
S. mitis strains Hu-o8 (24, 52) and B6 (31) were kindly provided by B. Henrich (Faculty of Biology, University of Kaiserslautern, Germany). Strain Hu-o8 had been deposited in the Félix d'Hérelle Reference Center for Bacterial Viruses under the name S. mitis strain HER 1055 (named HER hereafter). The identity of strains Hu-o8 and HER was confirmed here by determination of a partial nucleotide sequence of 16S rrna, galU, sodA, and six housekeeping genes. Also, these data indicated that strains HER and B6 were indeed different (not shown). The S. pneumoniae strains used were the laboratory strain R6 (lytA+) (3), the ΔlytA mutant M31 (57), and the atypical pneumococcal 101 strain (12, 48). In addition, pneumococcal strain 3870 was used as a source of the ant gene (see below) (4).
Escherichia coli DH5α (25) and DH10B (Life Technologies) were the hosts for recombinant plasmids, and E. coli RB791 (pGL100) was used for the overproduction of the LytAR6 pneumococcal amidase (20). Plasmid pIN-III (lppp-5)-A3 (28) was used as the vector for the overproduction of the phage lytic enzymes. Plasmid pJCP191 carries the pnl gene encoding the pneumococcal pneumolysin (68). E. coli was grown in Luria-Bertani (LB) medium (56), and streptococci were grown in Todd-Hewitt broth supplemented with 0.5% yeast extract (THY) or in C medium (33) supplemented with 0.08% yeast extract and 0.08% bovine serum albumin. The procedure for genetic transformation of E. coli has been described previously (56).
Phage EJ-1 was prepared from atypical pneumococcal strain 101 (12) treated with mitomycin C (75 ng/ml) in the dark, and after lysis of the culture, the phage was purified by two cycles of CsCl density gradient centrifugation, and phage DNA was prepared by treatment of purified phage preparations with sodium dodecyl sulfate (SDS) and proteinase K (11). An identical procedure was used for the purification of the new S. mitis phages (φHER and φB6) and their DNAs.
PCR amplification, cloning, Southern blotting, nucleotide sequencing, plasmid construction, and site-directed mutagenesis.
Routine DNA manipulations were performed essentially as described (56). DNA fragments were purified with the Geneclean II kit (Bio 101). The nucleotide sequence was determined by the dideoxy chain termination method (59) with an automated ABI Prism 3700 DNA sequencer (Applied Biosystems). An internal fragment of the phage lysin genes was amplified with oligonucleotide primers Fag1 (219 5′-ggaattCGTTGGGGGCGGTTGGAATGC-3′) and Fag2 (489/c 5′-cgggatcCTGCTYACGGCTAATGCCCC-3′) (46). The numbers in parentheses indicate the position of the first nucleotide of the primer in the sequence reported previously (21) (accession no. M13812) (starting at the first nucleotide of the lytA gene), and c means that the sequence corresponds to the complementary strand. Lowercase letters indicate nucleotides introduced to construct appropriate restriction sites (shown in italics).
Direct sequencing with whole phage DNA as a template was performed with primers deduced from the sequence previously determined. Multilocus sequence typing was carried out as described elsewhere (14). We also used primers 63f/1387r to amplify and sequence the 16S rRNA gene (41), G-GalUD/G-GalUR for determination of a partial nucleotide sequence of the informative galU allele of the S. mitis strains (42), and antUP/antDOWN to amplify the ant gene (4). All primers for PCR amplification and nucleotide sequencing were synthesized in-house on a Beckman model Oligo 1000M synthesizer. The lytA probe (pGL100) was labeled with the DIG luminescent detection kit (Boehringer Mannheim). Southern blots and hybridizations were carried out according to the manufacturer's instructions.
To clone lytAB6 and lytAHER under the control of the strong lppp-5, lacPO promoter-operator, we first amplified the corresponding genes with primers H3-FHU5′ (5′-ccaagcttATGGATATTGATACAAGTAGAC-3′) and BA-FHU3′ (5′-cgcggatccYTATTTWGTWGTAATC-3′). Afterwards, the amplified DNA fragments were digested with HindIII and BamHI and ligated to pIN-III (lppp-5)-A3 previously treated with the same enzymes, and the ligation mixture was used to transform E. coli DH5α. Recombinant clones harboring pLytAB6 or pLytAHER were selected among the ampicillin-resistant transformants, and the inserts of those plasmids were sequenced.
A Val317-Thr mutation was introduced into the lytAR6 allele by site-directed mutagenesis. To do that, the lytAR6 gene (from pGL100) was PCR amplified with oligonucleotides lytA100/X (5′-GTTGTTTTAATTCTAGATAAGGAG-3′) and lytAr/B (c) (5′-gggatccATTATTATTTTGTTGTAATCAAGCC-3′) (the changed nucleotides are italic), digested with XbaI and BamHI, and ligated to pIN-III (lppp-5)-A3 previously treated with the same enzymes, and the ligation mixture was used to transform E. coli DH10B. A recombinant clone harboring pIN-lytAR6(T) overexpressing the mutated amidase, LytAR6(T), was selected among the ampicillin-resistant transformants. The accuracy of the construction was checked by completely sequencing the insert of the recombinant plasmid.
Overproduction, purification of amidases, conversion of the low-activity catalytic form of LytA, and measurement of the enzymatic activity.
E. coli DH5α cells harboring either pLytAB6 or pLytAHER were grown with vigorous shaking at 30°C in LB supplemented with ampicillin (100 μg/ml) to an A600 of ≈0.6. Isopropyl-β-d-thiogalactopyranoside (IPTG) (50 μM) was added, and the incubation was continued for 4 h. For overproduction of LytAR6(T) we followed the same procedure except that incubation was carried out at 37°C and, after the addition of IPTG, the culture was shaken overnight. After centrifugation (10,000 × g, 10 min), the cells were suspended in 20 mM sodium phosphate (NP) buffer (pH 6.9) and broken in a French pressure cell press, and the insoluble material obtained after centrifugation at 100,000 × g for 1 h at 4°C was discarded. The supernatant was applied to a DEAE-cellulose column equilibrated in NP buffer, the contaminating proteins were washed out with the same buffer containing 1.5 M NaCl, and the amidases were eluted from the column with NP buffer containing 1.5 M NaCl and 2% choline chloride (58). The pure protein preparations were stored frozen at −20°C with or without a previous dialysis step against NP buffer (see below).
Pneumococcal cell walls were radioactively labeled with [methyl-3H]choline as described (44). Assays for cell wall lytic (amidase) activity were carried out according to standard conditions described elsewhere with labeled cell walls as the substrate (27), except that the pH and the reaction buffer were adjusted to the optimum for each enzyme (see below). Amidase “conversion” was tested by incubating the enzyme at 0°C for 5 min with either choline-containing pneumococcal cell walls or 2% choline chloride prior to shifting the mixture to 37°C. Nonconverted amidases were assayed by addition to a suspension of radioactively labeled cell walls previously warmed to 37°C. One unit of amidase activity was defined as the amount of enzyme that catalyzed the hydrolysis (solubilization) of 1 μg of cell wall material in 10 min. Radioactively labeled pneumococcal cell walls were incubated with the pure enzymes, and the degradation products were analyzed by gel filtration as previously described (44).
Bioinformatic analysis.
DNA and protein sequences were analyzed with the Genetics Computer Group software package (version 10.0) (11). Pairwise evolutionary distances (estimated number of substitutions per 100 bases) were determined with the Distances program with the correction adequate to each case. Multiple sequence alignments were created with Pileup or Clustal W (69). Sequence comparisons used the EMBL/UniProt databases and the FASTA (50) or BLAST (1) program. Bioinformatic predictions for transmembrane domains (32) or signal sequence processing (SIGNALP) (5) were carried out at the Center for Biological Sequence Analysis server (http://www.cbs.dtu.dk/services). Three-dimensional modeling of the C-terminal choline-binding repeats of phage amidases was carried out with the GENO3D program (9) run at the Pôle Bio-Informatique Lyonnais server (http://geno3d-pbil.ibcp.fr) with the crystal structure of the C-terminal domain of LytA (17) as a model.
Miscellaneous techniques.
CsCl-purified phage preparations were negatively stained with 1% uranyl acetate in carbon-reinforced, Formvar-coated copper grids (300 mesh). Micrographs were taken on a LEO 910 transmission electron microscope working at 80 kV. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out with the buffer system described by Laemmli (34) with 10 or 15% polyacrylamide gels, and protein bands were visualized by staining with Coomassie brilliant blue R250. N-terminal sequence analyses were carried out according to a published procedure (66). Sedimentation equilibrium experiments with amidases were performed in an Optima XL-A analytical ultracentrifuge (Beckman Instruments) as previously described (73). Anti-EJ-1 serum was prepared by repeated injections of purified phage preparations, as previously reported (35). The preparation of an antiserum against LytAR6 and the technique used in double immunodiffusion studies have been reported previously (39). Western blot analysis was performed according to a published procedure (58). Pulsed-field gel electrophoresis was carried out as described elsewhere (2). The SmaI-digested S. pneumoniae R6 genome (22) was used as molecular size markers.
Nucleotide sequence accession numbers.
The nucleotide sequences determined in this study have been deposited in the EMBL, GenBank, and DBJ databases. The 16S rrna, aroE, ddh, ddl, galU, gdh, recP, and spi alleles have been assigned accession numbers AJ617796 to AJ617814, respectively, and the nucleotide sequences of the lytic genes of phages φHER and φB6 have been assigned accession numbers AJ617815 and AJ617816, respectively.
RESULTS
Characterization of new S. mitis phages and their lysogenic strains.
A range of similarities between different alleles and those included in the data banks indicated that B6 and HER belong to the S. mitis species (see Materials and Methods), in agreement with previous speciation determinations carried out with standard biochemical techniques (31, 52). Recently, Balsalobre and coworkers reported a gene (ant) that was present in S. mitis and Streptococcus oralis strains but absent in typical pneumococcal isolates (4). Southern blot analysis revealed that ant was present in strain B6 but not in HER (not shown). On the other hand, typical pneumococci (but no other species of the mitis group of streptococci) harbor the pnl gene encoding the pneumolysin (hemolysin), a major virulence factor (7). Southern blot experiments with pJCP191 failed to reveal any evidence of the presence of a pnl-like gene in either HER or B6 (not shown).
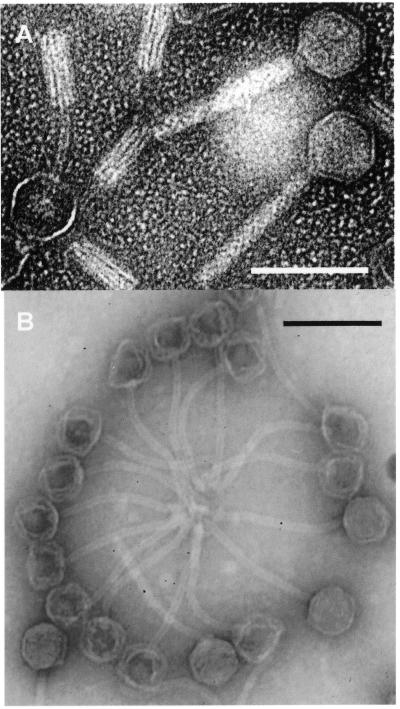
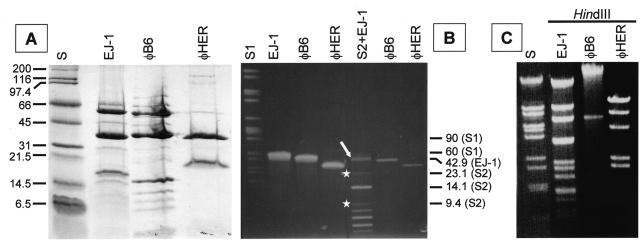
Strain HER and B6 grown in THY broth did lyse when induced with mitomycin C and phage particles could be observed in the electron microscope (Fig. 1). However, whereas Myoviridae virions were seen in mitomycin C-induced purified preparations from B6 (φB6) (Fig. 1A), siphoviruses (φHER) could be purified from strain HER (Fig. 1B). SDS-PAGE of purified virions (Fig. 2A) showed that the structural proteins of φB6 were similar to those from the EJ-1 phage and completely different from those from φHER. The gels were slightly overloaded to allow the visualization of minor bands. Western blot analysis with an EJ-1 antiserum failed to reveal any similarity with the structural proteins of φHER, and only the 36-kDa protein band of φB6 showed a slight reaction with the antiserum (not shown). Proteinase K-treated DNA prepared from φB6 and φHER virions and subjected to pulsed-field gel electrophoresis (Fig. 2B) showed that φHER DNA has the smallest genome (ca. 32 kb) whereas φB6 DNA has an intermediate size (ca. 40 kb), between that of EJ-1 (42.9 kb) and φHER. Moreover, HindIII digestion of phage DNAs fully confirmed that EJ-1, φB6, and φHER were different phages (Fig. 2C). In addition, a predicted size of 33 kb was obtained for φHER DNA, which agrees with the size calculated by pulsed-field gel electrophoresis. Note that the largest band seen in the HindIII digest from φHER DNA corresponds to a double fragment.
FIG. 1.
Electron micrographs of phage particles purified from mitomycin C-induced cultures of S. mitis strains B6 (A) and HER (B). The bar represents 100 nm.
FIG. 2.
Characterization of phages φB6 and φHER and their DNAs. (A) SDS-PAGE (15% gels) showing the structural virion proteins. The proteins of EJ-1 are shown for comparison. The molecular mass (in kilodaltons) of the standards (lane S) is indicated at the left. (B) Pulsed-field gel electrophoresis of proteinase K-treated phage DNAs. Two different amounts of DNA were loaded in the same gel. In the well labeled S2+EJ-1, EJ-1 DNA (open arrow) was mixed with a size standard mixture (S2) consisting of λ DNA digested with either HindIII (white stars) or BstEII. The size (in kilobases) of several DNA bands is indicated at the right. S1, SmaI-digested S. pneumoniae R6 DNA. (C) Agarose (0.7%) gel electrophoresis of phage DNAs digested with HindIII. S, BstEII-digested λ DNA.
S. mitis phages harbor a lytAR6-like gene.
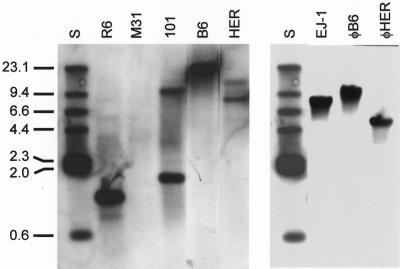
Hakenbeck and coworkers recently observed that the lytA gene reacted at a low but significant level in two out of five S. mitis strains tested by hybridization with S. pneumoniae oligonucleotide microarrays (24). One of the strains giving positive results was HER. To confirm and extend this result, Southern blot hybridization of chromosomal DNAs from S. mitis strains B6 and HER was carried out with the lytAR6 allele as the probe. The results shown in Fig. 3 (left panel) revealed that both strains harbor a lytA-like gene. HindIII-digested DNAs prepared from three pneumococcal strains (two typical and one atypical) were used as controls. A 1.2-kb fragment (strain R6) and two fragments (1.7 and 9.4 kb) (strain 101) were found to hybridize with the probe. This hybridization bands correspond to the lytAR6, lytA101, and the ejl genes, respectively (11, 12). As expected, no hybridization band was found with M31 DNA, a strain completely deleted of the lytA gene (57). Additional experiments with HindIII-digested phage DNA confirmed that φB6 and φHER harbor a gene that hybridized with lytA (Fig. 3, right panel). Whether the largest positive band observed when strain HER DNA was hybridized with the probe corresponds to a different gene or represents a partial digestion product is not known.
FIG. 3.
Southern blot hybridization analyses of DNAs from S. mitis strains B6 and HER and their phages. Chromosomal DNAs (left panel) prepared from the indicated strains were digested with HindIII and hybridized with a digoxigenin-labeled lytA gene. DNAs prepared from either M31 (ΔlytA) or the lysogenic strain 101 were used as controls. In the right panel, phage DNAs were digested with HindIII, blotted, and hybridized as above. The sizes (in kilobases) of the restriction fragments of HindIII-digested, biotinylated λ DNA (S) are indicated.
Cloning and sequencing of φB6 and φHER lytA-like genes
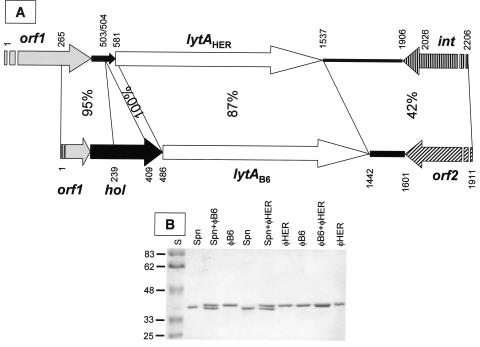
PCR amplification reactions were carried out with a variety of oligonucleotide primers designed on the basis of bacterial and phage lytA-like alleles included in the EMBL database (20 October 2003, last date accessed). Seventeen typical and 17 atypical bacterial alleles and four phage gene sequences of ≥900 bp were taken into consideration in this analysis (not shown). A pair of primers (Fag1 and Fag2) were finally used to amplify an internal fragment (about 270-bp long) of both genes. The determination of the nucleotide sequence of the amplification products allowed us to design new primers for obtaining the complete nucleotide sequence of lytAB6, lytAHER, and their flanking regions with the corresponding mature phage DNA as template (Fig. 4A).
FIG. 4.
Schematic representation of the lysin genes from phages φHER and φB6 and their flanking regions, and analysis of the purified S. mitis phage lysins. (A) Arrows show the genes and their direction of transcription. The nucleotide identity between different regions of both sequences is indicated. Broken arrows correspond to incomplete open reading frames. The putative deleted holin gene from φHER is shown as a narrow, solid arrow. The nucleotide positions are also indicated. (B) The purified LytA amidases from S. pneumoniae R6, φB6, and φHER were analyzed in SDS-10% polyacrylamide gels alone or in combination. The molecular mass (in kilodaltons) of the standards (S) is indicated at the left.
The lytAHER and lytAB6 genes were very similar (87% nucleotide identity), have an identical size (957 bp, including the termination codon), and the putatively encoded proteins (318 amino acids) have predicted molecular masses of 36,782 and 36,919 Da, respectively. Immediately upstream of lytAB6, a gene (hol) putatively encoding a 111-amino-acid protein was found. This gene was 85% identical to the holin 2 gene (orf51) from the pneumococcal phage MM1 (47). In φHER, however, the putative holin gene showed an internal 169-bp deletion (Fig. 4A). Holins have been grouped into two classes, according to the number of potential transmembrane domains. Class I members have the potential to form three transmembrane domains whereas class II members can form only two transmembrane domains (74). In contrast, HolB6 is predicted to form only one transmembrane domain (from Phe7 to Val24). This region might also correspond to a cleavable signal sequence with a potential signal processing site located between Ala26 and Val27, as predicted by bioinformatic analysis (not shown). Similar features have recently been described for the RI protein, the bacteriophage T4 antiholin (51). Consequently, the hol gene product is most probably an antiholin. An incomplete open reading frame (orf1) potentially encoding a protein of unknown function was located upstream of the putative holin genes. A putative integrase gene (int) was found downstream of lytAHER whereas an open reading frame (orf2) encoding a putative transposase DDE domain (Pfam01609) was located at an equivalent position in the φB6 genome.
Evolutionary considerations.
As already mentioned, the lytA genes of the S. mitis phages described here do not have the 6-bp deletion characteristic of atypical alleles. Consequently, they can be considered typical lytA alleles. However, a number of additional data could be obtained on the basis of multiple alignments and evolutionary tree reconstruction techniques. The current databases contain 40 different lytA-like allele sequences of at least 900 bp in length (2 December 2003, last date accessed), 34 from bacteria (17 typical alleles) and six from phages (including four pneumococcal phages and φB6 and φHER reported here). Analysis of a full alignment of the alleles revealed three additional features of the lytA alleles. The phage alleles diverged from the bacterial alleles mainly at their 5′ ends (not shown). Nearly 50% of the nucleotides from positions 1 to 66 of the phage genes differed from those of the bacterial alleles. This characteristic had been reported previously for the hbl gene from phage HB-3 (53). Interestingly, the lytAHER and lytAB6 alleles showed identical sequences in this region.
Whatmore and Dowson (75) first pointed out that several typical bacterial lytA alleles appeared to have a limited mosaic distribution, suggesting a localized recombination event with similar phage genes, namely hbl and/or ejl. Our results confirmed and extended this proposal because we found that, between positions 441 and 465, the nucleotide sequence of lytAHER is identical to that of six typical bacterial alleles (accession nos. M13812, AE007483, AF45844, AJ243401, AJ243403, and AJ243405) (unpublished observations). In addition to the 6-bp deletion already mentioned, all the atypical lytA alleles showed characteristic nucleotide substitutions (111 positions) that differed from those found, conserved, in typical alleles. In these positions, most of the nucleotides of the amidase genes from phages HB-3, VO1, and MM1 (up to 85 positions) correspond to those of typical bacterial alleles, whereas in lytAHER and lytAB6 only about 53% of the 111 positions coincide with those characteristic of typical alleles (not shown).
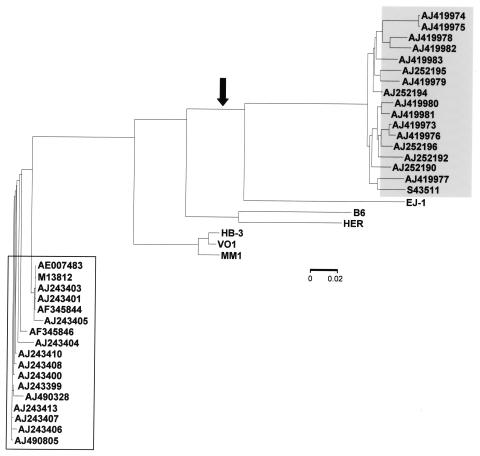
Pairwise evolutionary distances were calculated for all (bacterial and phage) lytA alleles. Since phage genes evolve faster than their bacterial counterparts, it was not surprising to find that the phage alleles were the most divergent compared with either typical (pairwise evolutionary distance ≥ 14%) or atypical (pairwise evolutionary distance ≥ 21%) bacterial alleles (not shown). In particular, lytAB6 and lytAHER diverged more than 15% even when comparisons where done among phage genes. On the contrary, the lysin genes from phages HB-3, VO1, and MM1 were quite similar to each other (pairwise evolutionary distance ≤ 3%). Phage lysin genes appeared to form three independent clades (HB-3/VO1/MM1, B6/HER, and EJ-1) when a neighbor-joining tree was calculated (Fig. 5). Interestingly, typical and atypical bacterial lytA alleles form two independent and consistent clades. From the phylogenetic tree, it could also be predicted that the event that produced the 6-bp deletion characteristic of atypical alleles took place before the separation of lineages leading to ejl and bacterial atypical alleles and that the phage lysin gene largely diverged subsequently.
FIG. 5.
Phylogenetic tree of the bacterial and phage lytA alleles. The neighbor-joining phylogenetic tree (phylogram) shows the evolutionary relationships between lytA alleles. Open and gray boxes indicate the positions of typical and atypical bacterial alleles, respectively. They are identified by their accession numbers. The arrow indicates the event causing the 6-bp deletion characteristic of atypical lytA alleles, including ejl. The scale represents the number of nucleotide substitutions per site.
Biochemical characterization of the LytAB6 and LytAHER amidases.
To biochemically characterize the lytA gene products from both phages, proteins were overexpressed in E. coli transformants harboring either pLytAB6 or pLytAHER and purified to electrophoretic homogeneity (Fig. 4B). N-terminal amino acid sequencing of both proteins (MDIDTSRLRT) confirmed that deduced from the nucleotide sequences. As reported for the pneumococcal amidase, the translation products of lytAB6 and lytAHER are low-activity forms (E-forms) of these proteins that are converted to their fully active forms (C-forms) by incubation with choline at low temperature (not shown). Analyses of the degradation products generated by both proteins were consistent with an N-acetylmuramoyl-l-alanine amidase activity (not shown).
Although both proteins could be differentiated from the LytAR6 amidase (Mr 36,544) by SDS-PAGE analysis (Fig. 4B), a double immunodiffusion test revealed an identical precipitin band between LytAR6 and the phage lysins (not shown). Nevertheless, the optimum pH for activity of both phage lysins was more acidic (ca. 5.5) than that of LytAR6 (ca. 6.5) (Table 1). When tested at their optimal pHs, the phage amidases showed only a slightly reduced specific activity compared to that of LytAR6 (Table 1). It should be noted that, although the cell walls of S. mitis have a choline-containing C-polysaccharide identical to that of S. pneumoniae, they also contain a unique teichoic acid-like polymer (6), which might account for this slight difference in activity. Interestingly, 1 h after induction with mitomycin C, the addition of 1% deoxycholate immediately caused the lysis of the cells, although uninduced cultures of S. mitis B6 and HER strains do not lyse with 1% deoxycholate (data not shown), indicating that LytAB6 and LytAHER were active against the peptidoglycan of their own host and not only against heterologous S. pneumoniae cell walls.
TABLE 1.
Comparison of the biochemical properties of the pneumococcal and S. mitis phage amidases
| Enzyme | Sp act, U 10−5/mg of protein (%)a | Optimum pH for activity | Activity after dialysis (%)b | Activity (%) after treatmentc with:
|
Choline 50% inhibitory concn (mM)d
|
||
|---|---|---|---|---|---|---|---|
| 1% deoxycholate | 1% Triton X-100 | Deconverted | Converted | ||||
| LytAR6 | 13.0 (100) | 6.5 | 100 | 100 | 120 | 5 | 20 |
| LytAB6 | 4.4 (33.8) | 5.5 | 10 | 2 | 32 | 2 | 20 |
| LytAHER | 4.6 (35.4) | 5.5 | 10 | 3 | 33 | 0.4 | 10 |
| LytAR6(T)e | 12.7 (100) | 6.5 | 10 | 30 | 90 | 3 | 20 |
Activity values are the averages of at least three independent determinations with pneumococcal cell walls as the substrate. Each enzyme was assayed at its optimal pH (6.5 in 20 mM sodium phosphate buffer for LytAR6 and 5.5 in 0.1 M sodium acetate for LytAB6 and LytAHER). The percentage of LytAK6 activity is shown in parentheses.
Enzymes were dialyzed in the cold against the appropriate buffer for at least 3 h and assayed without any conversion step. Percentages were calculated with respect to the activity of the corresponding nondialyzed enzyme.
Percentages were calculated with respect to that of untreated LytAR6.
For LytAR6, unconverted and converted mean crude E-form and purified C-form amidase, respectively, whereas for phage proteins those terms refer to dialyzed and dialyzed and then converted enzymes, respectively. Deconverted data for LytAR6 are from a previous publication (8).
LytAR6(T) is LytAR6 in which Val317 has been replaced by Thr.
As already mentioned, deoxycholate was capable of inhibiting in vitro the enzymatic activity of atypical amidases (either bacterial or phage EJ-1 enzymes), and this effect was attributed to the two-amino-acid deletion characteristic of this type of enzyme (48). However, LytAB6 and LytAHER, which do not have any deletion, were also inhibited in vitro by 1% deoxycholate but not when 1% Triton X-100 was used instead of deoxycholate (Table 1).
Additional biochemical differences between the phage and R6 amidases were revealed when the relationship between the enzymes and their ligand (the choline) were investigated. It should be noted that the primary translation product of the lytA gene shows reduced amidase activity and is commonly referred to as the E-form amidase (19, 71). The transformation of the E-form enzyme to the fully active amidase (the so-called C-form) is named conversion (see Materials and Methods) and takes place in vivo once the enzyme interacts with choline residues of the cell wall teichoic acids. Conversion can also be carried out in vitro upon incubation of the E-amidase in the cold with choline-containing cell walls (71) or 2% choline chloride (8). In the latter case, and before incubation at 37°C to evaluate catalytic activity, the assay mixture has to be diluted because choline concentrations higher than 0.03% also inhibit the enzyme activity by preventing the attachment of the enzyme to the cell wall (23).
It has also been observed that LytA is converted to the active C-form during purification in DEAE-cellulose (60) or equivalent chromatographic supports (8, 20) because elution of the amidase is carried out with 2% choline chloride. It is important to underline that the conversion process of the pneumococcal amidases (either typical or atypical) cannot be reversed by dialysis except in the case of the Ejl amidase (11). Dialyzed Ejl appears to be mostly monomeric (55), whereas analytical ultracentrifugation (73) and crystallization studies (16) revealed that the enzymatically active, C-form LytAR6 is a dimer formed by hydrophobic interactions among residues located at the most C-terminal choline-binding repeats of LytA (ChBR6 and ChBR7). Full activity was found when purified LytAB6 and LytAHER were assayed under the standard conditions discussed above without any previous dialysis, but, remarkably, both enzymes lost between 70 and 90% of this activity after a short (3-h) dialysis against NP buffer. Nevertheless, the loss of activity was completely reversible, as found when the dialyzed enzyme preparations were preincubated with choline-containing cell walls (or 2% choline chloride) before a shift to 37°C (Table 1). Sedimentation equilibrium ultracentrifugation (not shown) demonstrated that the deconverted phage enzymes were in a monomeric state, whereas mostly dimers were formed upon incubation with 2% choline.
In a different set of experiments, dialyzed LytAB6 and LytAHER were first reconverted by incubation with pneumococcal cell walls and then received increasing amounts of choline chloride and were immediately shifted to 37°C. As shown in Table 1 the choline 50% inhibitory concentration varied from about 10 mM for LytAHER to 20 mM for the other amidases, well within the values previously reported for the active C-form of LytAR6 (8, 12, 23). However, about 20% activity remained in the case of phage amidases, even at 2% choline (data not shown). Besides, when the dialyzed enzymes were incubated at 0°C with cell walls together with choline prior to being shifted to 37°C, LytAHER was more strongly inhibited that the other amidases (Table 1), suggesting less efficient anchoring of this enzyme to the heterologous pneumococcal cell walls. A similar property has been described for Ejl (11). Whether this behavior represents an intrinsic property of these phage lysins or reflects the existence of chemical and/or steric differences between the cell wall peptidoglycan of S. pneumoniae and that of S. mitis remains to be investigated.
Fundamental role of Val317 in LytAR6 conversion.
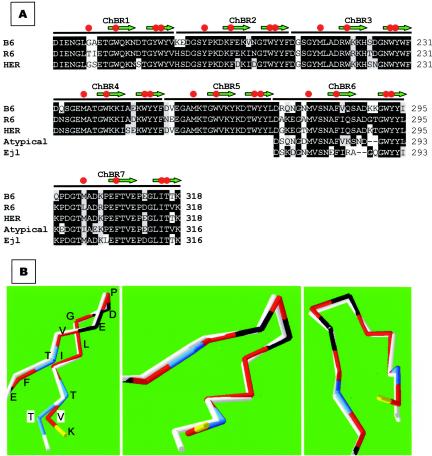
Interestingly, many of the amino acid differences between LytAR6 and the phage amidases described here are located in the last two choline-binding repeats (ChBR6 and ChBR7), which are those involved in dimerization (see above) (Fig. 6A). Of particular interest was the finding that one of the key residues implicated in the hydrophobic interactions of ChBR7 in both monomers is Val317 (16). When the three-dimensional structure of ChBR7 of the S. mitis phage amidases was modeled, taking the crystal structure of the choline-binding domain of LytAR6 (C-LytA) (16, 17) as the template, a different spatial orientation of the C terminus of the molecule could be predicted (Fig. 6B).
FIG. 6.
Amino acid sequence conservation at the choline-binding domain of typical and atypical amidases. (A) The sequences of the choline-binding domains of LytAB6 (B6) and LytAHER (HER) were compared to that of LytAR6 (R6). The last two choline-binding repeats from Ejl and a consensus sequence from an atypical enzyme (48) were also aligned. Amino acid residues that coincide with those of LytAR6 are shown in black boxes, and conserved substitutions are indicated in a gray background. Red circles and green arrows indicate choline binding residues and the portions of the sequence that form the first and second strands of the hairpins, respectively. (B) Predicted three-dimensional folding of the C-terminal part of LytAB6 (white line). For simplicity, only the α-carbon chains are shown. The Thr317 residue characteristic of LytAB6, LytAHER, and Ejl is highlighted in blue and boxed (T). The corresponding Val residue (V) in LytAR6 is also boxed. The folding of LytAR6 has been experimentally determined (17), and the residues are labeled as follows: acid, black; basic, yellow; polar, blue; nonpolar, red. Three different rotations of the model are shown.
It is conceivable that the Val317 to Thr change might alter the three-dimensional folding at the region that participates in the dimer conformation of the active form of the enzyme, in some way altering the stability of the active dimer. Nevertheless, the existence of other neighboring amino acid differences among the enzymes precluded a direct acceptance of that suggestion. As a direct test of our proposal, site-directed mutagenesis was performed to change Val317 to Thr in LytAR6. As predicted, LytAR6(T) was rapidly converted to the low-activity E-form of the pneumococcal amidase upon a brief period of dialysis in a way similar to that reported here for the S. mitis phage amidases (Table 1). A preliminary analytical ultracentrifugation analysis confirmed that mostly monomers or dimers were present in deconverted and reconverted preparations of LytAR6(T). Quite unexpectedly, LytAR6(T) was partly inhibited by deoxycholate, although about 30% of initial enzyme activity remained after treatment with 1% deoxycholate (Table 1). No other biochemical differences among the wild-type and mutant LytA amidases could be found.
DISCUSSION
Genetic transformation is envisaged as the main mechanism of horizontal gene transfer among the mitis group streptococci, leading to rapid spread of β-lactam (13) and fluoroquinolone (18) resistance, whereas the contribution of phages, either lytic or temperate, to the dissemination of clinically relevant genes has not been so well established. Although some clues on the recombination between the S. pneumoniae lytA gene and those from some pneumococcal phages have been reported (53, 75), the S. mitis phages described here expand all previous data on the role of phages in dissemination of the pneumococcal lytA genes among alpha-hemolytic streptococci in the same habitat. In sharp contrast to previous results reporting the presence of atypical lytA genes in some S. mitis isolates (76), strains HER and B6 harbor temperate prophages encoding full-size pneumococcal LytA amidases.
Nevertheless, the phage lysins described here showed several biochemical characteristics that differ from those of typical and atypical amidases previously described. Their optimal pH for activity (5.5) was significantly lower than that of a typical pneumococcal amidase (Table 1). This might represent an adaptation to a more acidic environment, since it is recognized that S. mitis is more acid tolerant than S. pneumoniae (67). Besides, LytAB6 and LytAHER were strongly inhibited in vitro by deoxycholate (Table 1), a property previously thought to be exclusive to amidases lacking Thr290 and Gly291 (48). Consequently, it is clear that point mutations, not only deletions, can result in an in vitro deoxycholate-sensitive amidase. In fact, a single mutation, Val317 to Thr, also caused a deoxycholate-sensitive enzyme (Table 1).
It has been reported previously that some pneumococcal transformants synthesizing an in vitro deoxycholate-sensitive LytA were susceptible to deoxycholate-induced lysis in vivo (48). This also appears to be the case for the S. mitis strains studied here, as illustrated by the observation that cultures of S. mitis B6 and HER strains lysed by the addition of 1% deoxycholate 1 h after induction with mitomycin C. This result also showed that, under noninducing conditions, the prophage lytA genes were repressed. In addition to mitomycin C, prophage induction can be promoted by a variety of physical and chemical agents, including antibiotics such as fluoroquinolones (78). Since the high inflammatory potential of cell wall fragments, particularly the stem peptides of the peptidoglycan that are released by LytA, is widely recognized (43, 72), phage-induced lysis of S. mitis strains B6 and HER, either spontaneous or provoked by antibiotic treatment, might confer increased virulence on these strains.
A prominent peculiarity of the pneumococcal LytA amidases, the need for conversion to get full enzymatic activity, was shared by the S. mitis phage enzymes described here. However, and in sharp contrast to the irreversibility of the conversion process exhibited by the pneumococcal LytA amidases, LytAB6 and LytAHER behaved as reversible enzymes. Site-directed mutagenesis of Val317 of LytAR6 to Thr demonstrated in a direct experimental way that Val317 is a key amino acid in the dimerization/conversion process (Table 1). Whether the Val137 to Thr substitution in LytAR6 might be relevant for the biology of S. pneumoniae is currently under study.
The pneumococcal genome has the potential to encode up to 15 choline-binding proteins and some additional choline-binding proteins have been shown to be encoded by pneumococcal phages (37). However, the three-dimensional structure of only two choline-binding proteins is currently known: the choline-binding domain of LytAR6 (C-LytA) (16, 17) and the Cpl-1 lysozyme encoded by phage Cp-1 (26). Cpl-1, which is synthesized in vivo in an enzymatically active form, only crystallized in the absence of choline, that is, after a dialysis step following its purification on DEAE-cellulose (26). In contrast, C-LytA only gave crystals when choline was present, and this domain and the complete LytAR6 enzyme were completely denatured after extensive dialysis to remove choline.
The availability of the new S. mitis phage amidases together with the mutant LytAR6(T) pneumococcal amidase that can be easily deconverted may allow new crystallization trials to determine the three-dimensional structure of this important S. pneumoniae virulence factor. In addition, the successful use of phage lytic enzymes, as an alternative to antibiotics, to combat drug-resistant bacteria has recently been documented in the case of S. pneumoniae (29, 37), Streptococcus pyogenes (45), Bacillus anthracis (61), and enterococci (77). The availability of the purified lytic enzymes coded by S. mitis phages reported here opens the possibility of exploring this new therapeutic approach in the case of S. mitis, a major cause of endocarditis.
Acknowledgments
This work was supported by grants from the Dirección General de Investigación Científica y Técnica (BCM2003-00074) and from Redes Temáticas de Investigación Cooperativa (G03/103 and C03/14) (Ministerio de Sanidad y Consumo). P. Romero is the recipient of an FPI fellowship from the Ministerio de Ciencia y Tecnología.
We are grateful to P. García, M. Moscoso, and D. Llull for helpful comments and critical reading of the manuscript and to A. G. de la Campa for advice on the amplification of the ant gene. We thank E. Cano for skillful technical assistance.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arrecubieta, C., R. López, and E. García. 1994. Molecular characterization of cap3A, a gene from the operon required for the synthesis of the capsule of Streptococcus pneumoniae type 3: sequencing of mutations responsible for the unencapsulated phenotype and localization of the capsular cluster on the pneumococcal chromosome. J. Bacteriol. 176:6375-6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avery, O. T., C. M. MacLeod, and M. McCarty. 1944. Studies on the chemical nature of the substance inducing transformation of pneumococcal types. Induction of transformation by a deoxyribonucleic acid fraction isolated from pneumococcus type III. J. Exp. Med. 79:137-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balsalobre, L., M. J. Ferrándiz, J. Liñares, F. Tubau, and A. G. de la Campa. 2003. Viridans group streptococci are donors in horizontal transfer of topoisomerase IV genes to Streptococcus pneumoniae. Antimicrob. Agents Chemother. 47:2072-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bendtsen, J. D., H. Nielsen, G. von Heijne, and U. B. Skov-Sørensen. 2000. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340:783-795. [DOI] [PubMed] [Google Scholar]
- 6.Bergstrom, N., P. E. Jansson, M. Kilian, and U. B. Skov-Sørensen. 2000. Structures of two cell wall-associated polysaccharides of a Streptococcus mitis biovar 1 strain. A unique teichoic acid-like polysaccharide and the group O antigen which is a C-polysaccharide in common with pneumococci. Eur. J. Biochem. 267:7147-7157. [DOI] [PubMed] [Google Scholar]
- 7.Berry, A. M., and J. C. Paton. 2000. Additive attenuation of virulence of Streptococcus pneumoniae by mutation of the genes encoding pneumolysin and other putative pneumococcal virulence proteins. Infect. Immun. 68:133-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Briese, T., and R. Hakenbeck. 1985. Interaction of the pneumococcal amidase with lipoteichoic acid and choline. Eur. J. Biochem. 146:417-427. [DOI] [PubMed] [Google Scholar]
- 9.Combet, C., M. Jambon, G. Deleage, and C. Geourjon. 2002. Geno3D: automatic comparative molecular modelling of protein. Bioinformatics 18:213-214. [DOI] [PubMed] [Google Scholar]
- 10.Devereux, J., P. Haeberli, and O. Smithies. 1984. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 12:387-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Díaz, E., R. López, and J. L. García. 1992. EJ-1, a temperate bacteriophage of Streptococcus pneumoniae with a Myoviridae morphotype. J. Bacteriol. 174:5516-5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Díaz, E., R. López, and J. L. García. 1992. Role of the major pneumococcal autolysin in the atypical response of a clinical isolate of Streptococcus pneumoniae. J. Bacteriol. 174:5508-5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dowson, C. G., V. Barcus, S. King, P. Pickerill, A. Whatmore, and M. Yeo. 1997. Horizontal gene transfer and the evolution of resistance and virulence determinants in Streptococcus. J. Appl. Microbiol. 83:42S-51S. [DOI] [PubMed] [Google Scholar]
- 14.Enright, M. C., and B. G. Spratt. 1998. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology 144:3049-3060. [DOI] [PubMed] [Google Scholar]
- 15.Fenoll, A., J. V. Martínez-Suárez, R. Muñoz, J. Casal, and J. L. García. 1990. Identification of atypical strains of Streptococcus pneumoniae by a specific DNA probe. Eur. J. Clin. Microbiol. Infect. Dis. 9:396-401. [DOI] [PubMed] [Google Scholar]
- 16.Fernández-Tornero, C., E. García, R. López, G. Giménez-Gallego, and A. Romero. 2002. Two new crystal forms of the choline-binding domain of the major pneumococcal autolysin: insights into the dynamics of the active homodimer. J. Mol. Biol. 321:163-173. [DOI] [PubMed] [Google Scholar]
- 17.Fernández-Tornero, C., R. López, E. García, G. Giménez-Gallego, and A. Romero. 2001. A novel solenoid fold in the cell wall anchoring domain of the pneumococcal virulence factor LytA. Nat. Struct. Biol. 8:1020-1024. [DOI] [PubMed] [Google Scholar]
- 18.Ferrándiz, M. J., A. Fenoll, J. Liñares, and A. G. de la Campa. 2000. Horizontal transfer of parC and gyrA in fluoroquinolone-resistant clinical isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 44:840-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.García, E., J. L. García, C. Ronda, P. García, and R. López. 1985. Cloning and expression of the pneumococcal autolysin gene in Escherichia coli. Mol. Gen. Genet. 201:225-230. [DOI] [PubMed] [Google Scholar]
- 20.García, J. L., E. García, and R. López. 1987. Overproduction and rapid purification of the amidase of Streptococcus pneumoniae. Arch. Microbiol. 149:52-56. [DOI] [PubMed] [Google Scholar]
- 21.García, P., J. L. García, E. García, and R. López. 1986. Nucleotide sequence and expression of the pneumococcal autolysin gene from its own promoter in Escherichia coli. Gene 43:265-272. [DOI] [PubMed] [Google Scholar]
- 22.Gasc, A. M., L. Kauc, P. Barraille, M. Sicard, and S. Goodgal. 1991. Gene localization, size, and physical map of the chromosome of Streptococcus pneumoniae. J. Bacteriol. 173:7361-7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giudicelli, S., and A. Tomasz. 1984. Attachment of pneumococcal autolysin to wall teichoic acids, an essential step in enzymatic wall degradation. J. Bacteriol. 158:1188-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hakenbeck, R., N. Balmelle, B. Weber, C. Gardès, W. Keck, and A. de Saizieu. 2001. Mosaic genes and mosaic chromosomes: intra- and interspecies genomic variation of Streptococcus pneumoniae. Infect. Immun. 69:2477-2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanahan, D. 1983. Studies on transformation of E. coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 26.Hermoso, J. A., B. Monterroso, A. Albert, B. Galán, O. Ahrazem, P. García, M. Martínez-Ripoll, J. L. García, and M. Menéndez. 2003. Structural basis for selective recognition of pneumococcal cell wall by modular endolysin from phage Cp-1. Structure 11:1239-1249. [DOI] [PubMed] [Google Scholar]
- 27.Höltje, J. V., and A. Tomasz. 1976. Purification of the pneumococcal N-acetylmuramyl-L-alanine amidase to biochemical homogeneity. J. Biol. Chem. 251:4199-4207. [PubMed] [Google Scholar]
- 28.Inouye, S., and M. Inouye. 1985. Up-promoter mutations in the lpp gene of Escherichia coli. Nucleic Acids Res. 13:3101-3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jado, I., R. López, E. García, A. Fenoll, J. Casal, and P. García. 2003. Phage lytic enzymes as therapy of antibiotic-resistant Streptococcus pneumoniae infection in a murine sepsis model. J. Antimicrob. Chemother. 52:967-973. [DOI] [PubMed] [Google Scholar]
- 30.Kawamura, Y., R. A. Whiley, S.-E. Shu, T. Ezaki, and J. M. Hardie. 1999. Genetic approaches to the identification of the mitis group within the genus Streptococcus. Microbiology 145:2605-2613. [DOI] [PubMed] [Google Scholar]
- 31.König, A., R. R. Reinert, and R. Hakenbeck. 1998. Streptococcus mitis with unusually high level resistance to β-lactam antibiotics. Microb. Drug Resist. 4:45-49. [DOI] [PubMed] [Google Scholar]
- 32.Krogh, A., B. Larsson, G. von Heijne, and E. L. L. Sonnhammer. 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305:567-580. [DOI] [PubMed] [Google Scholar]
- 33.Lacks, S., and R. D. Hotchkiss. 1960. A study of the genetic material determining an enzyme activity in Pneumococcus. Biochim. Biophys. Acta 39:508-517. [DOI] [PubMed] [Google Scholar]
- 34.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 35.Lam, J. S., and L. M. Mutharia. 1994. Antigen-antibody reactions, p. 104-132. In P. Gerhardt, R. G. E. Murray, W. A. Wood, and N. R. Krieg (ed.), Methods for general and molecular bacteriology. American Society for Microbiology, Washington, D.C.
- 36.Loeffler, J. M., D. Nelson, and V. A. Fischetti. 2001. Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science 294:2170-2172. [DOI] [PubMed] [Google Scholar]
- 37.López, R., and E. García. 2004. Recent trends on the molecular biology of pneumococcal capsules, lytic enzymes, and bacteriophage. FEMS Microbiol. Rev. doi:10.1016/j.femsre.2004.1005.1002. [DOI] [PubMed]
- 38.López, R., E. García, P. García, and J. L. García. 1997. The pneumococcal cell wall degrading enzymes: a modular design to create new lysins? Microb. Drug Resist. 3:199-211. [DOI] [PubMed] [Google Scholar]
- 39.Lopez, R., E. Garcia, P. Garcia, C. Ronda, and A. Tomasz. 1982. Choline-containing bacteriophage receptors in Streptococcus pneumoniae. J. Bacteriol. 151:1581-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lund, E., and J. Henrichsen. 1978. Laboratory diagnosis, serology and epidemiology of Streptococcus pneumoniae. Methods Microbiol. 12:241-262. [Google Scholar]
- 41.Marchesi, J. R., T. Sato, A. J. Weightman, T. A. Martin, J. C. Fry, S. J. Hiom, and W. G. Wade. 1998. Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl. Environ. Microbiol. 64:795-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mollerach, M., and E. García. 2000. The galU gene of Streptococcus pneumoniae that codes for a UDP-glucose pyrophosphorylase is highly polymorphic and suitable for molecular typing and phylogenetic studies. Gene 260:77-86. [DOI] [PubMed] [Google Scholar]
- 43.Moreillon, P., and P. A. Majcherczyk. 2003. Proinflammatory activity of cell-wall constituents from gram-positive bacteria. Scand. J. Infect. Dis. 35:632-641. [DOI] [PubMed] [Google Scholar]
- 44.Mosser, J. L., and A. Tomasz. 1970. Choline-containing teichoic acid as a structural component of pneumococcal cell wall and its role in sensitivity to lysis by an autolytic enzyme. J. Biol. Chem. 245:287-298. [PubMed] [Google Scholar]
- 45.Nelson, D., L. Loomis, and V. A. Fischetti. 2001. Prevention and elimination of upper respiratory colonization of mice by group A streptococci by using a bacteriophage lytic enzyme. Proc. Natl. Acad. Sci. USA 98:4107-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Obregón, V. 2002. Autolisinas y bacteriófagos como factores de atipicidad en Streptococcus pneumoniae. Ph.D. thesis. Universidad Complutense de Madrid, Madrid, Spain.
- 47.Obregón, V., J. L. García, E. García, R. López, and P. García. 2003. Genome organization and molecular analysis of the temperate bacteriophage MM1 of Streptococcus pneumoniae. J. Bacteriol. 185:2362-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Obregón, V., P. García, E. García, A. Fenoll, R. López, and J. L. García. 2002. Molecular peculiarities of the lytA gene isolated from clinical pneumococcal strains that are bile-insoluble. J. Clin. Microbiol. 40:2545-2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Obregón, V., P. García, R. López, and J. L. García. 2003. VO1, a temperate bacteriophage of the type 19A multiresistant epidemic 8249 strain of Streptococcus pneumoniae: analysis of variability of lytic and putative C5 methyltransferase genes. Microb. Drug Resist. 9:7-15. [DOI] [PubMed] [Google Scholar]
- 50.Pearson, W. R. 1990. Rapid and sensitive sequence comparison with FASTP and FASTA. Methods Enzymol. 183:63-98. [DOI] [PubMed] [Google Scholar]
- 51.Ramanculov, E., and R. Young. 2001. An ancient player unmasked: T4 rI encodes a t-specific antiholin. Mol. Microbiol. 41:575-583. [DOI] [PubMed] [Google Scholar]
- 52.Reichmann, P., E. Varon, J. Liñares, F. Alcaide, F. C. Tenover, L. McDougal, S. Swidsinski, and R. Hakenbeck. 1997. A global gene pool for high-level cephalosporin resistance in commensal Streptococcus spp. and Streptococcus pneumoniae. J. Infect. Dis. 176:1001-1012. [DOI] [PubMed] [Google Scholar]
- 53.Romero, A., R. López, and P. García. 1990. Sequence of the Streptococcus pneumoniae bacteriophage HB-3 amidase reveals high homology with the major host autolysin. J. Bacteriol. 172:5064-5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ronda, C., J. L. García, E. García, J. M. Sánchez-Puelles, and R. López. 1987. Biological role of the pneumococcal amidase. Cloning of the lytA gene in Streptococcus pneumoniae. Eur. J. Biochem. 164:621-624. [DOI] [PubMed] [Google Scholar]
- 55.Sáiz, J. L., C. López-Zumel, B. Monterroso, J. Varea, J. L. R. Arrondo, I. Iloro, J. L. García, J. Laynez, and M. Menéndez. 2002. Characterization of Ejl, the cell-wall amidase coded by the pneumococcal bacteriophage Ej-1. Protein Sci. 11:1788-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 57.Sánchez-Puelles, J. M., C. Ronda, J. L. García, P. García, R. López, and E. García. 1986. Searching for autolysin functions. Characterization of a pneumococcal mutant deleted in the lytA gene. Eur. J. Biochem. 158:289-293. [DOI] [PubMed] [Google Scholar]
- 58.Sánchez-Puelles, J. M., J. M. Sanz, J. L. García, and E. García. 1992. Immobilization and single-step purification of fusion proteins using DEAE-cellulose. Eur. J. Biochem. 203:153-159. [DOI] [PubMed] [Google Scholar]
- 59.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sanz, J. M., R. López, and J. L. García. 1988. Structural requirements of choline derivatives for ‘conversion’ of pneumococcal amidase. A new single-step procedure for purification of this autolysin. FEBS Lett. 232:308-312. [DOI] [PubMed] [Google Scholar]
- 61.Schuch, R., D. Nelson, and V. A. Fischetti. 2002. A bacteriolytic agent that detects and kills Bacillus anthracis. Nature 418:884-889. [DOI] [PubMed] [Google Scholar]
- 62.Severin, A., and A. Tomasz. 2000. The peptidoglycan of Streptococcus pneumoniae, p. 179-195. In A. Tomasz (ed.), Streptococcus pneumoniae—molecular biology and mechanisms of disease. Mary Ann Liebert, Inc., Larchmont, N.Y.
- 63.Sheehan, M. M., J. L. García, R. López, and P. García. 1997. The lytic enzyme of the pneumococcal phage Dp-1: a chimeric lysin of intergeneric origin. Mol. Microbiol. 25:717-725. [DOI] [PubMed] [Google Scholar]
- 64.Shockman, G. D., and J.-V. Höltje. 1994. Microbial peptidoglycan (murein) hydrolases, p. 131-166. In J.-M. Ghuysen and R. Hakenbeck (ed.), Bacterial cell wall. Elsevier, Amsterdam, The Netherlands.
- 65.Siboo, I. R., B. A. Bensing, and P. M. Sullam. 2003. Genomic organization and molecular characterization of SM1, a temperate bacteriophage of Streptococcus mitis. J. Bacteriol. 185:6968-6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Speicher, D. W. 1994. Methods and strategies for the sequence analysis of proteins on PVDF membranes. Methods 6:262-273. [Google Scholar]
- 67.Svensäter, G., M. Borgström, G. H. W. Bowden, and S. Edwardsson. 2003. The acid-tolerant microbiota associated with plaque from initial caries and healthy tooth surfaces. Caries Res. 37:395-403. [DOI] [PubMed] [Google Scholar]
- 68.Taira, S., E. Jalonen, J. C. Paton, M. Sarvas, and K. Runeberg-Nyman. 1989. Production of pneumolysin, a pneumococcal toxin, in Bacillus subtilis. Gene 77:211-218. [DOI] [PubMed] [Google Scholar]
- 69.Thompson, J. D., D. G. Higgins, and T. J. Ginson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tomasz, A., and W. Fischer. 2000. The cell wall of Streptococcus pneumoniae, p. 191-200. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. ASM Press, Washington, D.C.
- 71.Tomasz, A., and M. Westphal. 1971. Abnormal autolytic enzyme in a pneumococcus with altered teichoic acid composition. Proc. Natl. Acad. Sci. USA 68:2627-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tuomanen, E. I. 2000. Pathogenesis of pneumococcal inflammation: otitis media. Vaccine 19(Suppl. 1):S38-S40. [DOI] [PubMed] [Google Scholar]
- 73.Usobiaga, P., F. J. Medrano, M. Gasset, J. L. Garcia, J. L. Saiz, G. Rivas, J. Laynez, and M. Menendez. 1996. Structural organization of the major autolysin from Streptococcus pneumoniae. J. Biol. Chem. 271:6832-6838. [DOI] [PubMed] [Google Scholar]
- 74.Wang, I.-N., D. L. Smith, and R. Young. 2000. Holins: the protein clocks of bacteriophage infection. Annu. Rev. Microbiol. 54:799-825. [DOI] [PubMed] [Google Scholar]
- 75.Whatmore, A. M., and C. G. Dowson. 1999. The autolysin-encoding gene (lytA) of Streptococcus pneumoniae displays restricted allelic variation despite localized recombination events with genes of pneumococcal bacteriophage encoding cell wall lytic enzymes. Infect. Immun. 67:4551-4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Whatmore, A. M., A. Efstratiou, A. P. Pickerill, K. Broughton, G. Woodard, D. Sturgeon, R. George, and C. G. Dowson. 2000. Genetic relationships between clinical isolates of Streptococcus pneumoniae, Streptococcus oralis, and Streptococcus mitis: characterization of “atypical” pneumococci and organisms allied to S. mitis harboring S. pneumoniae virulence factor-encoding genes. Infect. Immun. 68:1374-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yoong, P., R. Schuch, D. Nelson, and V. A. Fuschetti. 2004. Identification of a broadly active lytic enzyme with lethal activity against antibiotic-resistant Enterococcus faecalis and Enterococcus faecium. J. Bacteriol. 186:4808-4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang, X., A. D. McDaniel, L. E. Wolf, G. T. Keusch, M. K. Waldor, and D. W. K. Acheson. 2000. Quinolone production induces Shiga toxin-encoding bacteriophages, toxin production, and death of mice. J. Infect. Dis. 181:664-670. [DOI] [PubMed] [Google Scholar]