Abstract
RpoS is a conserved alternative sigma factor that regulates the expression of many stress response genes in Escherichia coli. The RpoS regulon is large but has not yet been completely characterized. In this study, we report the identification of over 100 RpoS-dependent fusions in a genetic screen based on the differential expression of an operon-lacZ fusion bank in rpoS mutant and wild-type backgrounds. Forty-eight independent gene fusions were identified, including several in well-characterized RpoS-regulated genes, such as osmY, katE, and otsA. Many of the other fusions mapped to genes of unknown function or to genes that were not previously known to be under RpoS control. Based on the homology to other known bacterial genes, some of the RpoS-regulated genes of unknown functions are likely important in nutrient scavenging.
RpoS, an alternative sigma factor, controls a large regulon that is specifically expressed when the cell is nutrient deprived or is subjected to external stress, such as osmotic shock (32). Genes that are dependent on RpoS have been identified in different contexts from many studies of regulation in various gram-negative bacteria (38). From these studies it is clear that the regulon encodes many proteins that help bacteria adapt to adverse conditions, including those that are involved in nutrient scavenging, DNA repair, protein turnover, and protection from external environmental insult (31).
Characterization of the RpoS regulon has relied on several experimental approaches, including two-dimensional gel electrophoresis (50), identification of genes that are induced by RpoS-related stimuli such as carbon starvation (85), and by identifying gene fusions that depend on RpoS for expression (66). Use of bacterial cDNA microarrays or macroarrays, while potentially useful in identifying additional members of the RpoS regulon, have been employed in only a few studies of Escherichia coli and have not yet been used to directly assess RpoS dependence of genes in isogenic wild-type and rpoS mutant strains. In a previous study (66), our lab introduced an RpoS-null allele into a bank of random operon-lacZ fusions to facilitate the identification of RpoS-dependent genes based solely on RpoS requirement. This resulted in the identification of several new highly RpoS-dependent genes in E. coli (66), and similar approaches have been employed with Salmonella spp. where other RpoS-dependent genes have been identified (37). Lac gene fusions are useful measures of gene expression, because the product, β-galactosidase, is stable and easily assayed (16). This makes lacZ fusions particularly suitable for the study of genes that are expressed in stationary phase, where the stability of the RNA and protein products of the affected gene is not known.
In this study, we report the completion of the identification of reporter fusions identified in a previous genetic screen of lacZ-expressing fusion mutants. In addition, we examined the expression of the RpoS-dependent operon fusions in rich and minimal media and classified the RpoS-dependent genes according to their function.
MATERIALS AND METHODS
Growth conditions.
Expression assays were performed using derivatives of E. coli K-12 (Table 1). Cultures were grown overnight from single colony isolates in M9 minimal medium or Luria-Bertani (LB) medium containing appropriate antibiotics. The rich medium used was LB broth (53). Cell growth was monitored by measuring optical density at 600 nm (OD600) (Novospec II; Pharmacia LKB, Cambridge, United Kingdom). For β-galactosidase expression studies, the cultures were maintained in early exponential phase (OD600 < 0.2) for at least eight generations prior to the start of each experiment. Bacterial cultures were grown at 37°C and shaken at 200 rpm, sampled, and assayed for β-galactosidase activity.
TABLE 1.
E. coli strains and plasmid used in this study
| Strain or plasmid | Genotype or characteristics | Reference |
|---|---|---|
| Strain | ||
| GC4468 | ΔlacU169 rpsL | 68 |
| GC122 | Like GC4468, but φ(rpoS::Tn10) | 67 |
| MC4100 | Δ(argF-lacZ)205 araD139 fibB5301 relA1 RpsL150 thi ptsF25 | 68 |
| HS1002-HS1100 | Like GC4468, but carries RpoS-dependent operon-lacZ fusions | 66 |
| Plasmid | ||
| pMMkatF3 | Carries the rpoS (katF) gene | 54 |
Chemicals and media.
Chemicals were supplied by Fisher Scientific, Ltd. (Toronto, Ontario, Canada), Sigma Chemicals Co. (St. Louis, Mo.), and Gibco BRL (Burlington, Ontario, Canada). Antibiotics, amino acids, sugars, and other nonautoclavable solutions were sterilized by filtration. When required, medium was supplemented with kanamycin, 50 μg/ml; streptomycin, 50 μg/ml; tetracycline, 15 μg/ml; and/or X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside), 50 μg/ml.
Identification of RpoS-dependent promoters.
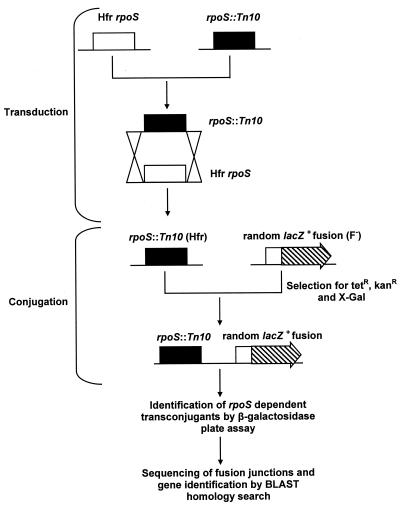
To identify RpoS-dependent lacZ fusions, mutants containing individual random λplacMu insertions were conjugated with an Hfr strain containing an rpoS::Tn10 mutation that is transferred a few minutes after the point of origin during conjugation (66). LacZ+ mutant strains that yielded transconjugants having reduced blue color on X-Gal-containing plates were considered to contain presumptive RpoS-dependent operon fusions. Of several hundred identified mutants, 105 could be efficiently complemented when transformed with an RpoS-expressing plasmid (determined by testing catalase activity in colonies grown on LB plates and flooded with 30% hydrogen peroxide). The fusion mutations were then transduced (53) into GC4468 for further study. A small proportion of the strains in the operon fusion mutant bank carried double fusions (∼2%). During conjugation, loss of a high-expressing lacZ+ allele by recombination occasionally produced transconjugants that were kanamycin resistant (due to retention of a second operon fusion) and possessed reduced lacZ activity. These strains were easily identified by the catalase complementation test and were not studied further. A diagrammatic representation of the protocol used to isolate mutants is shown in Fig. 1.
FIG. 1.
Genetic screening method used to identify RpoS-dependent genes in E. coli.
Enzyme assays.
β-Galactosidase activity was assayed using whole cells with ONPG (o-nitrophenyl-β-d-galactopyranoside) as the substrate (53). Units of activity were calculated as (1,000 × OD420)/(time of incubation [in minutes] × volume [in milliliters] × OD600) and are expressed as Miller units (53).
DNA template preparation and sequencing of λ fusion junctions.
High-titer plate lysates were prepared by UV induction of lysogens (16, 62). DNA was extracted from the phage by phenol:chloroform extraction and ethanol precipitation (49). Bacterial DNA proximal to the λ fusion junction was sequenced by MOBIXlab (McMaster University, Hamilton, Ontario) using a phage Mu c end primer (5′-CCCGAATAATCCAATGTCCTCCCGG-3′) (62). The identities of the sequences obtained were determined by performing alignments using BLASTN (3). The predicted functions of the genes thus identified were either based on SWISSPROT (9) or ECOCYC (40) databases as indicated in the text. To confirm that the 5′ end of the fusion was correct, we designed primers for the 5′ area of the genes identified and for the common lacZ gene. These were used to PCR amplify the 5′ fusion junctions of the integrated mutator phage. All identified genes yielded products consistent with the sequenced 3′ junctions, indicating that no large rearrangements occurred during mutagenesis.
RESULTS
Identification of RpoS-dependent genes.
Previously, several hundred operon lacZ fusion mutants were isolated, of which 105 contained independent RpoS-dependent fusions identified on the basis of reduced β-galactosidase activity in strains carrying an rpoS-null allele (67). RpoS dependence was confirmed by restoration of gene activity following complementation with a functional rpoS gene (66). Duplicate strains that contained RpoS-dependent lacZ fusions in the same gene (albeit in different positions within the gene) were not considered further, and 48 resulting strains with mutations in unique genes were selected for further study. Many of the new lacZ fusions mapped to genes located in polycistronic operons. Although we did not isolate fusions to all RpoS-regulated promoters, the identification of a single RpoS-dependent gene within a predicted or known operon is suggestive that an entire given operon is under RpoS control. When this point is taken into consideration, the number of new RpoS-dependent genes identified in this study is almost 80. As the number of previously known RpoS regulon members is 100 genes (38), the total size of the regulon is approximately 200 members.
Quantitative expression of lacZ fusions.
To quantify the relative expression levels and RpoS dependence of the 48 lacZ fusion strains, β-galactosidase activity was assayed in rpoS+ and rpoS mutant strains grown in rich and minimal media (Table 2). All fusion strains were confirmed to be RpoS dependent in rich and minimal media in stationary phase, with the exception of the strain carrying a fusion in the argH-oxyR intergenic region, which was RpoS dependent only in rich media. RpoS dependence of the fusions in rich media ranged from 2- to 40-fold, with 80% of the strains showing greater than 5-fold dependence (Table 2). Similarly, in minimal media, 80% of the strains showed greater than fivefold dependence in stationary phase (Table 2).
TABLE 2.
Growth-phase-dependent expression of RpoS-dependent operon-lacZ fusions in strains grown in rich and minimal mediaa
| Strain | Gene | β-Galactosidase activity (Miller units) in:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LB
|
M9 glucose
|
||||||||||||
| Exponential phase
|
Stationary phase
|
Exponential phase
|
Stationary phase
|
||||||||||
| WT | rpoS mutant | RpoS dependent | WT | rpoS mutant | RpoS dependent | WT | rpoS mutant | RpoS dependent | WT | rpoS mutant | RpoS dependent | ||
| HS1002 | yjbJ | 6.2 | 3.2 | 2 | 74.4 | 3.4 | 22 | 20.2 | 3.6 | 6 | 53.7 | 0.3 | 180 |
| HS1006 | yjbE | 4.2 | 2.1 | 2 | 45.2 | 2.1 | 22 | 23.9 | 1.0 | 24 | 63.3 | 1.1 | 58 |
| HS1008 | aldB | 5.0 | 1.8 | 3 | 91.4 | 4.3 | 22 | 4.3 | 0.3 | 14 | 8.2 | 0.4 | 21 |
| HS1010 | gabP | 5.1 | 2.8 | 2 | 111 | 4.7 | 24 | 6.9 | 0.5 | 14 | 26.8 | 1.5 | 18 |
| HS1011 | ygaU | 15.4 | 18.9 | 1 | 144 | 33.5 | 4 | 58.9 | 6.9 | 9 | 198 | 16.0 | 12 |
| HS1012 | ygdI | 13.8 | 3.9 | 4 | 54.5 | 7.6 | 7 | 8.4 | 4.7 | 2 | 21.3 | 4.0 | 5 |
| HS1014 | katE | 12.7 | 1.9 | 7 | 80.3 | 2.0 | 40 | 23.4 | 0.3 | 78 | 79.4 | 1.2 | 66 |
| HS1019 | yqhE | 7.4 | 10.5 | 2 | 91.4 | 12.2 | 8 | 20.2 | 3.4 | 6 | 48.3 | 4.6 | 11 |
| HS1020 | mltB | 12.3 | 38.1 | 0.3 | 90.1 | 52.5 | 2 | 3.5 | 5.3 | 1 | 26.1 | 16.3 | 2 |
| HS1022 | narY | 3.4 | 2.5 | 1 | 28.3 | 3.0 | 10 | 0.9 | 1.2 | 1 | 5.4 | 2.4 | 2 |
| HS1024 | ygaF | 3.1 | 10.2 | 0.3 | 49.7 | 18.8 | 3 | 2.3 | 1.0 | 2 | 16.9 | 2.5 | 7 |
| HS1026 | ybaY | 29.8 | 10.1 | 3 | 243 | 16.4 | 15 | 16.1 | 1.5 | 11 | 132 | 11.0 | 12 |
| HS1028 | yjgR | 14.4 | 7.4 | 2 | 63.0 | 11.5 | 6 | 3.5 | 1.5 | 2 | 27.6 | 8.1 | 3 |
| HS1033 | ydaM | 8.6 | 0.9 | 10 | 35.2 | 2.2 | 16 | 20.8 | 2.1 | 10 | 38.2 | 1.5 | 25 |
| HS1035 | ydcS | 6.7 | 2.4 | 3 | 21.7 | 3.9 | 6 | 2.0 | 1.1 | 2 | 18.9 | 12.7 | 2 |
| HS1036 | ydcK | 0.7 | 1.8 | 0.4 | 46.2 | 3.0 | 15 | 5.2 | 1.9 | 3 | 26.7 | 3.1 | 9 |
| HS1037 | gabD-gabT | 0.7 | 0.6 | 1 | 11.0 | 1.3 | 8 | 1.9 | 0.3 | 6 | 3.7 | 0.6 | 6 |
| HS1042 | yhjY | 11.7 | 3.6 | 3 | 66.8 | 4.5 | 15 | 3.0 | 1.2 | 3 | 29.1 | 3.2 | 9 |
| HS1045 | yhiN | 16.1 | 11.1 | 1 | 60.1 | 13.0 | 5 | 12.7 | 1.6 | 8 | 16.3 | 2.5 | 7 |
| HS1049 | yeaG | 11.7 | 2.2 | 5 | 42.2 | 3.3 | 13 | 12.3 | 0.5 | 25 | 13.7 | 1.4 | 10 |
| HS1050 | phnP | 17.4 | 5.4 | 3 | 51.1 | 6.5 | 8 | 2.7 | 1.0 | 3 | 2.2 | 1.4 | 2 |
| HS1054 | yebF | 23.0 | 2.7 | 9 | 225 | 5.5 | 41 | 44.6 | 3.6 | 13 | 87.7 | 3.6 | 25 |
| HS1056 | yodC | 7.1 | 1.9 | 4 | 166 | 4.0 | 41 | 9.78 | 0.9 | 11 | 23.6 | 1.4 | 17 |
| HS1057 | gabD | 4.6 | 3.7 | 1 | 245 | 15.7 | 16 | 12.4 | 1.3 | 10 | 27.1 | 2.1 | 13 |
| HS1059 | talA | 3.2 | 3.3 | 1 | 106 | 5.3 | 20 | 13.3 | 0.9 | 15 | 42.6 | 1.6 | 26 |
| HS1061 | aroM | 2.1 | 2.1 | 1 | 74.8 | 5.0 | 15 | 1.81 | 0.3 | 6 | 31.9 | 0.4 | 78 |
| HS1063 | ugpE | 2.7 | 3.5 | 1 | 47.0 | 8.1 | 6 | 7.3 | 0.6 | 12 | 10.8 | 1.9 | 6 |
| HS1064 | nlpA-yicM | 5.9 | 7.8 | 1 | 30.7 | 11.1 | 3 | 8.8 | 6.1 | 1 | 23.1 | 6.0 | 4 |
| HS1066 | argH-oxyR | 12.3 | 14.8 | 1 | 30.0 | 18.9 | 2 | 88.3 | 81.4 | 1 | 102 | 102 | 1 |
| HS1067 | ybiO | 2.4 | 1.0 | 2 | 11.9 | 1.0 | 13 | 7.0 | 0.4 | 18 | 15.8 | 0.6 | 26 |
| HS1071 | yhjG | 12.8 | 6.0 | 2 | 159 | 9.4 | 17 | 27.7 | 1.8 | 15 | 95.7 | 6.3 | 15 |
| HS1072 | argH | 9.2 | 12.4 | 1 | 33.9 | 18.3 | 2 | 42.8 | 24.5 | 2 | 90.8 | 17.9 | 5 |
| HS1073 | yliI | 2.5 | 1.0 | 3 | 20.3 | 1.2 | 17 | 13.4 | 0.4 | 34 | 22.0 | 0.6 | 37 |
| HS1075 | ugpC | 2.7 | 1.3 | 2 | 26.0 | 1.3 | 20 | 3.2 | 0.7 | 5 | 9.9 | 1.1 | 9 |
| HS1077 | yhiV | 2.1 | 1.9 | 1 | 101 | 3.3 | 31 | 13.2 | 1.2 | 11 | 86 | 22.5 | 4 |
| HS1078 | uspB | 4.5 | 5.6 | 1 | 30.0 | 7.1 | 4 | 21.1 | 2.0 | 11 | 66.6 | 8.2 | 8 |
| HS1079 | ldcC | 4.9 | 3.4 | 1 | 29.8 | 4.5 | 7 | 18.1 | 1.3 | 14 | 46.6 | 4.3 | 11 |
| HS1080 | appB | 2.7 | 0.9 | 3 | 112 | 4.6 | 25 | 8.4 | 1.6 | 5 | 9.3 | 1.3 | 7 |
| HS1081 | yhiU | 6.0 | 2.1 | 3 | 112 | 2.9 | 39 | 34.0 | 2.5 | 14 | 117 | 14.0 | 8 |
| HS1082 | aidB | 8.8 | 3.0 | 3 | 138 | 3.8 | 36 | 22.2 | 1.0 | 20 | 111 | 3.4 | 33 |
| HS1084 | yehX | 3.6 | 1.5 | 2 | 58.3 | 2.1 | 28 | 23.1 | 1.0 | 23 | 55.5 | 2.6 | 21 |
| HS1090 | yphA | 6.0 | 2.7 | 2 | 64.5 | 2.7 | 24 | 25.2 | 1.0 | 25 | 67.5 | 3.7 | 18 |
| HS1091 | osmY | 15.9 | 6.0 | 3 | 264 | 7.5 | 35 | 110 | 2.3 | 48 | 210 | 3.3 | 64 |
| HS1092 | yfcG | 3.3 | 1.2 | 3 | 62.3 | 1.7 | 37 | 16.3 | 0.6 | 27 | 67.9 | 1.3 | 52 |
| HS1094 | otsA | 8.5 | 3.1 | 3 | 165 | 5.4 | 31 | 80.1 | 2.3 | 35 | 159.8 | 2.8 | 57 |
| HS1095 | ecnB | 15.0 | 7.3 | 2 | 144 | 6.3 | 23 | 48.9 | 1.9 | 26 | 134.0 | 1.8 | 74 |
| HS1099 | yhjD | 29.2 | 37.9 | 1 | 172 | 33.6 | 5 | 47.3 | 20.9 | 2 | 87.4 | 9.5 | 34 |
| HS1100 | yjiN | 8.6 | 6.2 | 1 | 66.8 | 15.6 | 4 | 30.2 | 4.0 | 8 | 52.2 | 9.5 | 6 |
Strains were grown overnight in LB broth, subcultured, and maintained in the appropriate media for eight generations prior to sampling. Cultures were sampled at exponential phase (OD600 was 0.3 for rich media and 0.2 for minimal media) and stationary phase (OD600 was 1.5 for rich media and 1.3 for minimal media). Cultures of strain HS1072 grown in M9 glucose medium were supplemented with 20 μg of arginine/ml. Values are the averages of three independent experiments, and the coefficient of variation was less than 10%.
As expected for RpoS-dependent genes, in rich media, gene fusions in rpoS+ strains were expressed to much higher levels in stationary phase than in exponential phase (Table 2). β-Galactosidase activity was 2- to 66-fold higher in stationary phase. Expression levels for the fusions in an rpoS mutant background were generally low in exponential and stationary phases, confirming that the stationary phase dependence exhibited in the rpoS+ strains is due to the presence of RpoS rather than other regulatory factors.
Residual expression levels for several identified genes remained high in an rpoS mutant background. This was the case for rpoS mutants carrying fusions in ygaU, mltB, and yhjD (Table 2) and in the argH-oxyR intergenic region (Table 2) suggesting that these genes are not solely RpoS-dependent. Other regulators, in particular other sigma factors, may recognize the promoters of these genes and activate their transcription. The nucleotide sequence of promoters recognized by RpoS are similar to those recognized by RpoD, the housekeeping sigma factor for RNA polymerase, and in fact some RpoS-dependent promoters can also be activated by RpoD in vitro (11). In support of the hypothesis that these genes are regulated by other factors, gene fusions in rpoS mutant strains that had a high level of expression in stationary phase also tended to have a similarly high level of expression in exponential phase (Table 2). Many RpoS-dependent genes are regulated by other transcription factors (Table 3); for example, the promoters of genes ygaU and yhjD are known to be activated by Lrp (77).
TABLE 3.
RpoS-dependent genes and their known or predicted functionsd
| Genea | Blattner no. | Functionb | Other regulator(s) | Previously reported as RpoS dependentc | RpoS dependence in:
|
Reference(s) | |
|---|---|---|---|---|---|---|---|
| Rich Medium | Minimal Medium | ||||||
| ybaY | b0453 | Unknown | No | 15 | 12 | ||
| gabP | b2663 | GABA transporter protein | GabC(−), Nac(+) | Yes | 24 | 18 | 55, 66, 93 |
| ugpE | b3451 | G-3-P transporter membrane bound component | PhoBR(+) | No | 6 | 6 | 15 |
| ugpC | b3450 | ATP binding protein of G-3-P transporter | PhoBR(+) | No | 20 | 9 | 15 |
| ydcS | b1440 | Putative binding protein of ABC transporter | Nac(+) | Yes | 6 | 2 | 93 |
| yehX | b2129 | Hypothetical ATP-binding protein of ABC transporter | No | 28 | 21 | ||
| narY | b1467 | Beta subunit of nitrate reductase-II | Yes | 10 | 2 | 13 | |
| appB | b0979 | Subunit of cytochrome bd-II oxidase | AppR(+) | Yes | 25 | 7 | 23 |
| IdcC | b0186 | Lysine decarboxylase | Yes | 7 | 11 | 47, 79 | |
| aidB | b4187 | Induced by alkylating agents; protects DNA from alkylation damage | Yes | 36 | 33 | 45, 46 | |
| katE | b1732 | Hydroperoxidase II | Lrp(+) | Yes | 40 | 66 | 74 |
| uspB | b3494 | Universal stress protein B; required for ethanol resistance | Yes | 4 | 8 | 26 | |
| osmY | b4376 | Periplasmic protein induced by high osmolarity; function unknown | Lrp(−), Crp(−), IHF(−) | Yes | 35 | 64 | 20, 90, 91 |
| yhiV | b3514 | Multidrug efflux pump | EvgA(+), Lrp(+) | No | 31 | 4 | 56, 57, 74, 78 |
| yhiU | b3513 | Multidrug efflux pump | EvgA(+) | Yes | 39 | 8 | 56, 57, 78 |
| mltB | b2701 | Membrane-bound lytic transglycosylase B | No | 2 | 2 | 25 | |
| otsA | b1896 | Trehalose phosphate synthase | Lrp(+) | Yes | 31 | 57 | 72, 74 |
| ecnB | Bacteriolytic lipoprotein entericidin B | EnvZ/OmpR(−) | Yes | 23 | 74 | 12 | |
| argH | b3960 | Argino-succinate lyase | ArgR(−) | No | 2 | 5 | 77 |
| aroM | b0390 | Unknown; may play a role in aromatic amino acid biosynthesis | TyrR(+) | No | 15 | 78 | 24 |
| yhjY | b3548 | Putative lipase | No | 15 | 9 | ||
| yqhE | b3012 | 2,5-diketo-d-gluconic acid reductase A; catalyzes the conversion of 2,5-KGDR to 2-KLG | No | 8 | 11 | 92 | |
| aldB | b3588 | Aldehyde dehydrogenase B; converts lactaldehyde and NAD+ to lactate and NADH | Yes | 22 | 21 | 74, 87 | |
| gabD | B2661 | Succinate-semialdehyde dehydrogenase | GabC(−), Nac(+) | No | 16 | 13 | 55, 93 |
| phnP | B4092 | Membrane-bound subunit of carbon-phosphorus lyase complex | PhnF and PhnO(+) | No | 8 | 2 | 51 |
| talA | B2464 | Transaldolase A; converts Sed-7-P and Gly-3-P to Fru-6-P and Ery-4-P | CreBC(+) | No | 20 | 26 | 7, 70 |
| yfcG | B2302 | Putative glutathione S-transferase | SdiA(+) | No | 37 | 52 | 84 |
| yliI | B0837 | Putative dehydrogenase | Yes | 17 | 37 | 66 | |
| yjbJ | B4045 | Unknown | No | 22 | 179 | ||
| yjbE | B4026 | Unknown | No | 22 | 58 | ||
| ygaU | B2665 | Unknown | Lrp(+) | No | 4 | 12 | 74 |
| ygdI | B2809 | Unknown | No | 7 | 5 | ||
| ygaF | B2660 | Unknown | No | 3 | 7 | ||
| yjgR | B4263 | Unknown | No | 6 | 3 | ||
| ydaM | B1341 | Unknown | No | 16 | 23 | ||
| ydcK | B1428 | Unknown | No | 15 | 9 | ||
| yhiN | B3492 | Unknown | No | 5 | 7 | ||
| yebF | B1847 | Unknown | No | 41 | 25 | ||
| yjiN | B4336 | Unknown | No | 4 | 6 | ||
| yeaG | B1783 | Unknown | Lrp(+) | Yes | 13 | 10 | 63, 74 |
| yodC | B1957 | Unknown | No | 41 | 17 | ||
| ybiO | B0808 | Unknown | No | 13 | 26 | ||
| yhjG | B3524 | Unknown | No | 17 | 15 | ||
| yphA | B2543 | Unknown | No | 24 | 18 | ||
| yhjD | B3522 | Unknown | Lrp(+) | Yes | 5 | 34 | 63 |
Genes were identified using the BLAST algorithm (3).
Functions were assigned to each gene based on either SWISSPROT or ECOCYC databases.
In E. coli.
G-3-P, glyceraldehyde-3-phosphate; Sed-7-P, sedoheptulose-7-phosphate; Fru-6-P, fructose-6-phosphate; Ery-4-P, erythrose-4-phosphate.
Classification of RpoS-dependent genes.
All proteins encoded by the E. coli genome have been grouped into 22 classes based on their known or predicted functions (14). The 48 RpoS-regulated genes identified in this study encode proteins that fall into 12 of these functional classes (Table 4), many of them (17 genes) into the class of hypothetical, unclassified, and unknown proteins.
TABLE 4.
Classification of proteins encoded by the RpoS-dependent genes identified based on Blattner functional categories (14)
| Functional category(ies) | No. of proteins in each category | Protein(s) |
|---|---|---|
| Cell structure | 1 | YbaY |
| Transport and binding proteins | 3 | GabP, UgpE, UgpC |
| Putative transport proteins | 2 | YdcS, YehX |
| Energy metabolism | 3 | NarY, AppB, LdcC |
| DNA replication, recombination, modification, and repair | 1 | AidB |
| Cell processes (including adaptation and protection) | 8 | KatE, UspB, OsmY, YhiV, YhiU, MltB, OtsA, EcnB |
| Amino acid biosynthesis and metabolism | 2 | ArgH, AroM |
| Fatty acid and phospholipid metabolism | 1 | YhjY |
| Carbon compound catabolism | 2 | YqhE, AldB |
| Central intermediary metabolism | 4 | GabD, PhnP, TalA, YfcG |
| Putative enzymes | 1 | Ylil |
| Hypothetical, unclassified, unknown | 17 | YjbJ, YjbE, YgaU, Ygdl, YgaF, YjgR, YdaM, YdcK, YhiN, YebF, YjiN, YeaG, YodC, YbiO, YhjG, YphA, YhjD |
Cell processes (including adaptation and protection).
Of the 48 genes identified in this study, eight are in this class (cell processes), underscoring the importance of RpoS-dependent genes in adaptation. From the expression data (Table 2), it is clear that genes required for stress protection, such as otsA, osmY, and katE, are expressed highly in both minimal and rich media, especially in stationary phase, and are among the most highly RpoS dependent.
Two of the eight genes, yhiV and mltB, are newly identified as RpoS dependent in this study (Table 4). The yhiV gene along with yhiU (previously reported to be RpoS dependent [4]) codes for a multidrug efflux pump, overexpression of which increases resistance to a variety of antimicrobial compounds (56). In addition to RpoS, these are positively regulated by the response regulator EvgA of the EvgSA two-component regulatory system (57). The mltB gene codes for a membrane-bound lytic transglycosylase required for mucopeptide recycling during cell wall growth in E. coli (25).
Six genes of this class are known to be RpoS controlled. The katE gene encodes catalase (hydroperoxidase II), which converts highly reactive hydrogen peroxide into oxygen and water (81). The uspB gene codes for a 14-kDa protein that may play a role in sensing or changing membrane composition during stationary phase (26). Mutations in uspB render the cell sensitive to ethanol in stationary phase, and overexpression causes cell death in stationary phase (26). Mutations in the osmY gene, which encodes a periplasmic protein induced during high osmolarity, increase the susceptibility of E. coli to osmotic stress (91). The otsA gene forms a two-member trehalose biosynthetic operon with otsB (72). Biosynthesis of trehalose is activated in response to high osmolarity and upon entry into stationary phase (72). Trehalose can serve as both an osmoprotectant and as a carbon source (72).
The ecnB gene codes for a bacteriolytic lipoprotein known as entericidin B (12). Along with ecnA, ecnB forms an antidote/toxin gene pair termed an addiction module that induces programmed cell death of bacterial populations in stationary phase (12). Expression of the ecnB gene is induced by high osmolarity in stationary phase, positively regulated by RpoS (66) and negatively regulated by the EnvZ/OmpR two-component regulator (12).
Cell structure.
In stationary phase, changes occur in cellular morphology as well as in membrane and cell wall composition of E. coli (36). A distinctive feature of stationary-phase cells is an oval cell morphology, a consequence of RpoS-mediated induction of bolA (65). RpoS also regulates other cell-structure-related genes, such as ftsQAZ, required for cell septum formation (69); csgAB, required for the formation of curli fimbriae (59); and cfa, required for cyclopropane fatty acid synthesis, which accumulates in the cell membrane during stationary phase and has been implicated in acid resistance (19).
Expression of ybaY, encoding a cell-structure-related protein required for glycolipid/polysaccharide metabolism (40), was also found to be RpoS dependent in this study.
Transport and binding proteins.
Operon fusions to two new operons belonging to the transport and binding class were identified, ugpBAECQ and ydcSTUV. ugpC and ugpE are members of the ugpBAECQ operon, which codes for a binding protein-dependent ATP-binding cassette (ABC) transporter (15). The ugp operon is also a member of the Pho regulon that is regulated by PhoBR in response to external phosphate limitation (83). UgpB to UgpQ are required for phosphate scavenging and for utilization of glycerol-3-phosphate as a sole carbon source (15), suggestive of a role for RpoS in nutrient scavenging.
The ydcS gene encodes a putative binding protein-dependent ABC transporter (40) that is similar in amino acid sequence to polyamine transporters PotD and PotF. Although the function of this gene has not been confirmed, microarray analysis has revealed that ydcS, and the entire ydcSTUV operon, is positively regulated by the nitrogen assimilation control protein Nac (93), which induces genes required for nitrogen scavenging under nitrogen-limited conditions. The gabP gene, previously reported to be RpoS controlled (66), codes for a γ-aminobutyric acid (GABA) permease (55).
DNA replication, recombination, modification, and repair.
In stationary phase, the E. coli chromosome is stabilized by nucleoid-associated proteins, including Dps (86) and H-NS (5). Dps is one of the most abundant proteins during stationary phase, and the dps gene is positively controlled by RpoS (2). Dps cocrystallizes with DNA, forming a ferritin-like structure which effectively deprives the cell of iron required for the Fenton reaction, which can lead to oxidative damage (86). H-NS, another nucleoid-associated protein that is controlled by RpoS (10), compacts DNA (22) and can modulate gene expression (5). Under starvation conditions, endogenous DNA-methylating agents are produced in E. coli by nitrosation reactions that lead to methylation of DNA (76). Methylated DNA mispairs with thymine during DNA replication, leading to GC-to-AC transition mutations (76). There are various mechanisms to counteract DNA damage during stationary phase. One of these is by the induction of the adaptive response by the Ada protein that acts as a methyl transferase; in its methylated form, Ada acts as a transcriptional regulator of the Ada operon genes ada, aidB, alkA, and alkB (44). The aidB gene is known to be RpoS dependent (80). Expression of the aidB gene is also induced by alkylating agents, anaerobiosis, and acetate at acidic pH (45). Though the exact function of AidB is not known, it may neutralize nitrosoguanidines or their intermediates (46). Microarray analysis of mitomycin C-treated E. coli cells has revealed that numerous stationary-phase genes, including that encoding RpoS, are induced by mitomycin C treatment, confirming the role of RpoS in alleviating DNA damage (42).
Energy metabolism.
E. coli, a facultative anaerobe, has two terminal oxidases, cytochrome bo, encoded by the cyo operon, and cytochrome bd, encoded by the cyd operon (6). The cyo operon operates under conditions of high oxygen concentration and has low affinity to oxygen, while cyd has high affinity to oxygen and operates under microaerobic conditions (28). A third cytochrome oxidase encoded by appB has been identified (73) and was found (this study and reference 23) to be RpoS dependent. The appB gene is in an operon with appA (encoding pH 2.5 acid phosphatase) and encodes a cytochrome bd-type oxidase (73). In addition to RpoS, appB is regulated by AppY (6), which itself is subject to regulation by RpoS (6). What might be the physiological significance of RpoS upregulation of cytochrome oxidases during stationary phase? In stationary phase, the density of cells in a culture increases, and, as a consequence, the amount of oxygen available to the cells is reduced. Under these microaerobic conditions, upregulation of cytochrome bd II and AppB, which have a high affinity for oxygen, may be required for efficient electron transport.
In the absence of oxygen as an electron acceptor, E. coli preferentially uses nitrate as an electron acceptor (28). E. coli has three functional nitrate reductases, one periplasmic nitrate reductase (NapA) and two membrane-bound nitrate reductases, nitrate reductase A (NRA) and nitrate reductase Z (NRZ) (71). Nitrate reductase Z is encoded by members of the narZYWV operon (71). The RpoS-dependent narY gene codes for the beta subunit of nitrate reductase Z, which functions as an electron acceptor during anaerobic growth on nitrate in E. coli (18).
The ldcC gene, encoding lysine decarboxylase C (47), which is classified as an energy metabolism function in E. coli (14), was previously shown to be RpoS dependent (89). LdcC catalyzes the decarboxylation of lysine to cadaverine (47) and may be necessary for utilization of lysine as a nutrient source in stationary phase.
Amino acid biosynthesis and metabolism.
Two amino acid biosynthetic genes, argH and aroM, were found to be RpoS regulated. The argH gene, the terminal member of the argCBH operon, codes for arginino-succinate lyase and is required for arginine biosynthesis in E. coli (21). Arginine biosynthesis is likely important during stationary phase, because this amino acid is a precursor for synthesis of polyamines (29), which protect DNA from oxidative damage by scavenging free radicals (30). Other arginine metabolic functions are controlled by RpoS, including the catabolic astCADBE operon, which is required for growth in arginine as a sole nitrogen source (43). This operon is also controlled by RpoN (43). Some of the well-known amino acid catabolic operons that have protective functions in E. coli are arginine decarboxylase (encoded by adiA) and glutamate decarboxylase (encoded by gadABC) (17). These operons are part of the acid resistance (AR) system in E. coli and are regulated by RpoS (17).
The aroM gene, which is highly RpoS dependent, particularly in minimal medium (Table 2), forms an operon with aroL that codes for shikimate kinase II (24). This enzyme is required for the conversion of shikimate to shikimate-3-phosphate, which is ultimately converted to chorismate by the aroA and aroC gene products (61). Chorismate is the branching point for the synthesis of three aromatic amino acids: tyrosine, phenylalanine, and tryptophan (61). Though aroM is cotranscribed with aroL, the function of AroM is not unknown (24).
Carbon compound catabolism.
Two RpoS-dependent genes identified in this study, yqhE and aldB, are in the carbon compound catabolism class. The aldB gene, previously identified as RpoS dependent (87), codes for a broad-spectrum aldehyde dehydrogenase that converts aldehydes to their corresponding acids. AldB may thus detoxify aldehydes produced during stationary phase (87). The yqhE gene, found to be RpoS dependent in this study, encodes 2,5-diketo-d-gluconic acid reductase A (92). This enzyme is part of the ketogluconate utilization pathway that converts 2,5-diketo-d-gluconate into 2-keto-l-gluconate, which can then be converted to d-gluconate and further catabolized through the Entner-Doudoroff and pentose-phosphate pathways (92).
Other catabolic genes regulated by RpoS include treA (34) and glgS (33). In addition to its role as an osmoprotectant, trehalose, synthesized by the products of otsBA (39), is converted to glucose by trehalose, which is encoded by treA (72), and thus can be used as a carbon source. The glgS gene is required for glycogen synthesis and is under the positive control of RpoS (33). Carbon-starved E. coli cells accumulate glycogen, which acts as a carbon reserve during starvation (36). Regulation by RpoS has been proposed to provide an alternative mechanism of carbon catabolite control in E. coli (64), especially in stationary-phase metabolism.
Central intermediary metabolism.
We found four genes, gabD, phnP, talA, and yfcG, which fall into the central intermediary metabolism functional group, none of which have been previously reported to be RpoS dependent. The gab operon (gabDTP) is required for conversion of gamma-amino butyric acid (GABA) into glutamate and succinate (55). Succinate and glutamate thus produced can be utilized as a carbon source via the trichloroacetic acid (TCA) cycle. Glutamate protects cells against acid stress, and this pathway may be the only mechanism by which internal glutamate pools are generated (35). The phnP gene, along with 13 other genes, codes for phosphonate transport and catabolism (88). Although E. coli cannot utilize phosphonates as carbon sources, they can be used as a sole phosphorus source (88).
The talA gene codes for transaldolase A of the pentose phosphate pathway and forms an operon with tktB (70). Intermediates of the pentose phosphate pathway serve as precursors for the biosynthesis of aromatic amino acids and also for heptoses found in lipopolysaccharides (70). The reason why talA is regulated by RpoS is not clear; however, talA may replenish the glycolytic pathway during stationary phase by providing the necessary intermediates from the pentose phosphate pathway. The yfcG gene codes for a putative glutathione S-transferase (GST) (40) and is highly RpoS dependent, especially in minimal medium (Table 2). In eukaryotes, GSTs function as detoxifying enzymes (82). The role of GST in prokaryotes is not well characterized, but it has been implicated in the degradation of xenobiotics (82).
Hypothetical, unclassified, unknown.
Of the 48 RpoS-dependent genes identified in this study, 17 have unknown or hypothetical function. It is not surprising to find a majority of genes in this class, because 30% of E. coli genes do not yet have an assigned function (14). The yjbJ gene has a very high RpoS dependence in stationary phase, especially when grown in minimal media (Table 2), and is one of the most abundant proteins produced in stationary phase (48). Moreover, this gene has homology similarities to csbD of Bacillus subtilis, which is also regulated by a stress sigma factor (1). Some of the RpoS-dependent genes of this class have recently been shown to be upregulated under different growth conditions. The yeaG gene is induced in artificial seawater medium (63), while ybaF is induced under in vivo conditions in mice (41). Though the physiological functions of these genes were not revealed by these studies, the growth conditions mentioned above are stressful conditions, emphasizing that some of these RpoS-regulated genes may have a role in adaptation or in counteracting stress.
DISCUSSION
In this study, we employed a mutational screen to identify genes that require RpoS for expression. Because this screen did not rely on a phenotypic property of the identified gene, the class of genes identified is likely less biased than might be obtained by using other screens dependent on phenotypic differences (such as carbon starvation or osmotic shock). This was found to be important, as many of the genes identified have paralogous functions whose activity may be masked by the presence of another gene product (e.g., NarG or NarZ). There are several published RpoS-dependent paralogs, including NarZ (nitrate reductase) (18) and Ldc (lysine decarboxylase) (89), that comprise only a small percentage of the cell's total activity. There are many other paralogous functions that are controlled by RpoS, and collectively this suggests that expressing a subset of key duplicated metabolic functions is an important physiological imperative in stationary-phase cultures. These functions include polyamine transport (through PotGHIJ), balancing the activities of glycolytic and oxidative pentose pathways (through TalA/TktB), acid resistance (through lysine decarboxylase), drug efflux (YhiUV), and the use of alternative electron acceptors (NarZ).
In some cases, RpoS-regulated functions may form redundant or alternate metabolite cycles. For example, both arginine biosynthetic (argH, this study) and catabolic genes (astCADBE [8, 27]) are RpoS dependent (albeit weakly). Similarly, the RpoS-dependent gabDTP operon (52) with glutamate decarboxylases (gadAB [17]) could comprise a cycle that produces fumarate from succinate, similar to the succinate dehydrogenase step of the TCA cycle but with less energy conserved as chemical-reducing power. In both cases, concomitant decarboxylation likely confers acid resistance. Trehalose synthesis and catabolism, encoded by RpoS-dependent treA and otsAB, respectively, may allow the cell to accumulate a compatible solute for catabolic purposes when the cell is starved of preferred carbon sources.
The relatively large size of the RpoS regulon has made its complete characterization difficult. There are several approaches by which members of a regulon can be characterized. Two-dimensional gel electrophoresis can be used to identify differentially expressed proteins based on mass and charge (58). Limitations to this approach include difficulty in identifying proteins that are expressed at very low levels, masking of one protein spot by another, leading to an underestimation of the total number of proteins separated, and loss of detectable protein spots from treatment of the gels under denaturing conditions (58).
Microarray and cDNA macroarray analyses can also potentially be used to identify members of a large regulon like RpoS. To date, however, there have been only a few reports where these tools have been used to identify regulon members by comparing RNA expression profiles of wild-type and isogenic regulator-minus mutants (60, 74). Such methodology has identified several genes that are dependent on Lrp in stationary phase (74). Interestingly, the majority of these were previously identified as RpoS dependent. Here, we extend the number of Lrp-regulated genes identified that are also regulated by RpoS to include talA, ydcS, and yjbJ. Array methodology can be employed to examine gene expression under different growth conditions, and this may also indirectly provide information about a regulon. Macroarrays have been used (75) to identify many genes that are upregulated in minimal medium as opposed to rich media. As expected, many of these, especially those that are involved in adaptation and protection, are RpoS dependent (75). Array analysis, though exhaustive, yields expression results that are more conservative than either Northern analyses or reporter fusions (unpublished data). The results of this study should be a useful independent measure to validate future array studies.
Unlike microarray analysis, the genetic screening technique used in this study provides a direct estimate of gene expression levels, because the expression of the lacZ reporter gene is primarily a function of the promoter activity of the gene into which lacZ is inserted (16). Moreover, as a reporter, lacZ is a sensitive indicator of gene expression. Genes with low expression levels can be detected easily by β-galactosidase assays, and direct sequencing of the fusion junctions makes this technique useful (16). In addition, insertion of lacZ into a gene generally inactivates the gene, which can then be exploited to study gene function. Many E. coli strains used in expression studies, including GC4468 and its derivatives, carry a very large deletion (approximately 80 kb) in the region of the lac operon, beginning at 6.2 min. Thus, genes in this region, including any that may be regulated by RpoS, cannot be studied in these strains. These drawbacks notwithstanding, we have employed this technique successfully to characterize genes of the RpoS regulon and have identified 48 genes under RpoS control.
In summary, we have identified a large number of genes that are dependent on RpoS for expression, particularly in stationary phase in cultures grown in rich media. The functions of the identified genes are consistent with previous suggestions that the RpoS regulon is required for nutrient scavenging, pH homeostasis, and protection from oxidative stress. Further insight into the mechanisms by which bacterial cells cope with suboptimal conditions may be gained from learning the functions of the genes identified here whose physiological role is not yet known. RpoS and the genes that it controls are conserved among many gram-negative bacteria; therefore, such studies are likely to reveal fundamental information regarding the adaptive physiology of other bacteria in addition to E. coli.
Acknowledgments
We thank T. Dong, J. Gilles, A. Hughes, Y. Li, and S. Yun for technical assistance and for comments on the manuscript. We also gratefully acknowledge the long-standing cooperation of R. A. Morton in this study.
This study was supported by an operating grant to H.E.S. from the Natural Sciences and Engineering Council (NSERC) of Canada.
REFERENCES
- 1.Akbar, S., S. Y. Lee, S. A. Boylan, and C. W. Price. 1999. Two genes from Bacillus subtilis under the sole control of the general stress transcription factor σB. Microbiology 145(Part 5):1069-1078. [DOI] [PubMed] [Google Scholar]
- 2.Almiron, M., A. J. Link, D. Furlong, and R. Kolter. 1992. A novel DNA-binding protein with regulatory and protective roles in starved Escherichia coli. Genes Dev. 6:2646-2654. [DOI] [PubMed] [Google Scholar]
- 3.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 4.Altuvia, S., D. Weinstein-Fischer, A. Zhang, L. Postow, and G. Storz. 1997. A small, stable RNA induced by oxidative stress: role as a pleiotropic regulator and antimutator. Cell 90:43-53. [DOI] [PubMed] [Google Scholar]
- 5.Atlung, T., and H. Ingmer. 1997. H-NS: a modulator of environmentally regulated gene expression. Mol. Microbiol. 24:7-17. [DOI] [PubMed] [Google Scholar]
- 6.Atlung, T., K. Knudsen, L. Heerfordt, and L. Brondsted. 1997. Effects of σS and the transcriptional activator AppY on induction of the Escherichia coli hya and cbdAB-appA operons in response to carbon and phosphate starvation. J. Bacteriol. 179:2141-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avison, M. B., R. E. Horton, T. R. Walsh, and P. M. Bennett. 2001. Escherichia coli CreBC is a global regulator of gene expression that responds to growth in minimal media. J. Biol. Chem. 276:26955-26961. [DOI] [PubMed] [Google Scholar]
- 8.Baca-DeLancey, R. R., M. M. South, X. Ding, and P. N. Rather. 1999. Escherichia coli genes regulated by cell-to-cell signaling. Proc. Natl. Acad. Sci. USA 96:4610-4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bairoch, A., and R. Apweiler. 2000. The SWISS-PROT protein sequence database and its supplement TrEMBL in 2000. Nucleic Acids Res. 28:45-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barth, M., C. Marschall, A. Muffler, D. Fischer, and R. Hengge-Aronis. 1995. Role for the histone-like protein H-NS in growth phase-dependent and osmotic regulation of sigma S and many sigma S-dependent genes in Escherichia coli. J. Bacteriol. 177:3455-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Becker, G., and R. Hengge-Aronis. 2001. What makes an Escherichia coli promoter sigma(S) dependent? Role of the −13/−14 nucleotide promoter positions and region 2.5 of sigma(S). Mol. Microbiol. 39:1153-1165. [DOI] [PubMed] [Google Scholar]
- 12.Bishop, R. E., B. K. Leskiw, R. S. Hodges, C. M. Kay, and J. H. Weiner. 1998. The entericidin locus of Escherichia coli and its implications for programmed bacterial cell death. J. Mol. Biol. 280:583-596. [DOI] [PubMed] [Google Scholar]
- 13.Blasco, F., C. Iobbi, J. Ratouchniak, V. Bonnefoy, and M. Chippaux. 1990. Nitrate reductases of Escherichia coli: sequence of the second nitrate reductase and comparison with that encoded by the narGHJI operon. Mol. Gen. Genet. 222:104-111. [DOI] [PubMed] [Google Scholar]
- 14.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 15.Boos, W. 1998. Binding protein-dependent ABC transport system for glycerol 3-phosphate of Escherichia coli. Methods Enzymol. 292:40-51. [DOI] [PubMed] [Google Scholar]
- 16.Bremer, E., T. J. Silhavy, J. M. Weisemann, and G. M. Weinstock. 1984. Lambda placMu: a transposable derivative of bacteriophage lambda for creating lacZ protein fusions in a single step. J. Bacteriol. 158:1084-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castanie-Cornet, M. P., T. A. Penfound, D. Smith, J. F. Elliott, and J. W. Foster. 1999. Control of acid resistance in Escherichia coli. J. Bacteriol. 181:3525-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang, L., L. I. Wei, J. P. Audia, R. A. Morton, and H. E. Schellhorn. 1999. Expression of the Escherichia coli NRZ nitrate reductase is highly growth phase dependent and is controlled by RpoS, the alternative vegetative sigma factor. Mol. Microbiol. 34:756-766. [DOI] [PubMed] [Google Scholar]
- 19.Chang, Y. Y., and J. E. Cronan. 1999. Membrane cyclopropane fatty acid content is a major factor in acid resistance of Escherichia coli. Mol. Microbiol. 33:249-259. [DOI] [PubMed] [Google Scholar]
- 20.Colland, F., M. Barth, R. Hengge-Aronis, and A. Kolb. 2000. Sigma factor selectivity of Escherichia coli RNA polymerase: role for CRP, IHF and Lrp transcription factors. EMBO J. 19:3028-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cunin, R., N. Glansdorff, A. Pierard, and V. Stalon. 1986. Biosynthesis and metabolism of arginine in bacteria. Microbiol. Rev. 50:314-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dame, R. T., C. Wyman, and N. Goosen. 2000. H-NS mediated compaction of DNA visualised by atomic force microscopy. Nucleic Acids Res. 28:3504-3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dassa, J., H. Fsihi, C. Marck, M. Dion, M. Kieffer-Bontemps, and P. L. Boquet. 1991. A new oxygen-regulated operon in Escherichia coli comprises the genes for a putative third cytochrome oxidase and for pH 2.5 acid phosphatase (appA). Mol. Gen. Genet. 229:341-352. [DOI] [PubMed] [Google Scholar]
- 24.DeFeyter, R. C., B. E. Davidson, and J. Pittard. 1986. Nucleotide sequence of the transcription unit containing the aroL and aroM genes from Escherichia coli K-12. J. Bacteriol. 165:233-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ehlert, K., J. V. Holtje, and M. F. Templin. 1995. Cloning and expression of a murein hydrolase lipoprotein from Escherichia coli. Mol. Microbiol. 16:761-768. [DOI] [PubMed] [Google Scholar]
- 26.Farewell, A., K. Kvint, and T. Nystrom. 1998. uspB, a new σS-regulated gene in Escherichia coli which is required for stationary-phase resistance to ethanol. J. Bacteriol. 180:6140-6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fraley, C. D., J. H. Kim, M. P. McCann, and A. Matin. 1998. The Escherichia coli starvation gene cstC is involved in amino acid catabolism. J. Bacteriol. 180:4287-4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gennis, R. B. and V. Stewart. 1996. Respiration, p. 217-261. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, D.C.
- 29.Gerard, F., A. M. Dri, and P. L. Moreau. 1999. Role of Escherichia coli RpoS, LexA and H-NS global regulators in metabolism and survival under aerobic, phosphate-starvation conditions. Microbiology 145(Part 7):1547-1562. [DOI] [PubMed] [Google Scholar]
- 30.Ha, H. C., N. S. Sirisoma, P. Kuppusamy, J. L. Zweier, P. M. Woster, and R. A. Casero, Jr. 1998. The natural polyamine spermine functions directly as a free radical scavenger. Proc. Natl. Acad. Sci. USA 95:11140-11145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hengge-Aronis, R. 1996. Regulation of gene expression during entry into stationary phase, p. 1497-1512. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, D.C.
- 32.Hengge-Aronis, R. 2002. Signal transduction and regulatory mechanisms involved in control of the sigma(S) (RpoS) subunit of RNA polymerase. Microbiol. Mol. Biol. Rev. 66:373-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hengge-Aronis, R., and D. Fischer. 1992. Identification and molecular analysis of glgS, a novel growth-phase-regulated and rpoS-dependent gene involved in glycogen synthesis in Escherichia coli. Mol. Microbiol. 6:1877-1886. [DOI] [PubMed] [Google Scholar]
- 34.Hengge-Aronis, R., W. Klein, R. Lange, M. Rimmele, and W. Boos. 1991. Trehalose synthesis genes are controlled by the putative sigma factor encoded by rpoS and are involved in stationary-phase thermotolerance in Escherichia coli. J. Bacteriol. 173:7918-7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hersh, B. M., F. T. Farooq, D. N. Barstad, D. L. Blankenhorn, and J. L. Slonczewski. 1996. A glutamate-dependent acid resistance gene in Escherichia coli. J. Bacteriol. 178:3978-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huisman, G. W., D. A. Siegele, M. A. Zambrano, and R. Kolter. 1996. Morphological and physiological changes during stationary phase, p. 1672-1682. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, D.C.
- 37.Ibanez-Ruiz, M., V. Robbe-Saule, D. Hermant, S. Labrude, and F. Norel. 2000. Identification of RpoS σS-regulated genes in Salmonella enterica serovar Typhimurium. J. Bacteriol. 182:5749-5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishihama, A. 2000. Functional modulation of Escherichia coli RNA polymerase. Annu. Rev. Microbiol. 54:499-518. [DOI] [PubMed] [Google Scholar]
- 39.Kaasen, I., P. Falkenberg, O. B. Styrvold, and A. R. Strom. 1992. Molecular cloning and physical mapping of the otsBA genes, which encode the osmoregulatory trehalose pathway of Escherichia coli: evidence that transcription is activated by katF (appR). J. Bacteriol. 174:889-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karp, P. D., M. Riley, M. Saier, I. T. Paulsen, J. Collado-Vides, S. M. Paley, A. Pellegrini-Toole, C. Bonavides, and S. Gama-Castro. 2002. The EcoCyc database. Nucleic Acids Res. 30:56-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khan, M. A., and R. E. Isaacson. 2002. Identification of Escherichia coli genes that are specifically expressed in a murine model of septicemic infection. Infect. Immun. 70:3404-3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khil, P. P., and R. D. Camerini-Otero. 2002. Over 1000 genes are involved in the DNA damage response of Escherichia coli. Mol. Microbiol. 44:89-105. [DOI] [PubMed] [Google Scholar]
- 43.Kiupakis, A. K., and L. Reitzer. 2002. ArgR-independent induction and ArgR-dependent superinduction of the astCADBE operon in Escherichia coli. J. Bacteriol. 184:2940-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Landini, P., and S. J. Busby. 1999. Expression of the Escherichia coli ada regulon in stationary phase: evidence for rpoS-dependent negative regulation of alkA transcription. J. Bacteriol. 181:6836-6839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Landini, P., L. I. Hajec, L. H. Nguyen, R. R. Burgess, and M. R. Volkert. 1996. The leucine-responsive regulatory protein (Lrp) acts as a specific repressor for sigma s-dependent transcription of the Escherichia coli aidB gene. Mol. Microbiol. 20:947-955. [DOI] [PubMed] [Google Scholar]
- 46.Landini, P., L. I. Hajec, and M. R. Volkert. 1994. Structure and transcriptional regulation of the Escherichia coli adaptive response gene aidB. J. Bacteriol. 176:6583-6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lemonnier, M., and D. Lane. 1998. Expression of the second lysine decarboxylase gene of Escherichia coli. Microbiology 144(Part 3):751-760. [DOI] [PubMed] [Google Scholar]
- 48.Link, A. J., D. Phillips, and G. M. Church. 1997. Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J. Bacteriol. 179:6228-6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 50.McCann, M. P., J. P. Kidwell, and A. Matin. 1991. The putative sigma factor KatF has a central role in development of starvation-mediated general resistance in Escherichia coli. J. Bacteriol. 173:4188-4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Metcalf, W. W., and B. L. Wanner. 1993. Mutational analysis of an Escherichia coli fourteen-gene operon for phosphonate degradation, using TnphoA′ elements. J. Bacteriol. 175:3430-3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Metzner, M., J. Germer, and R. Hengge. 2004. Multiple stress signal integration in the regulation of the complex sigma S-dependent csiD-ygaF-gabDTP operon in Escherichia coli. Mol. Microbiol. 51:799-811. [DOI] [PubMed] [Google Scholar]
- 53.Miller, J. H. 1992. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 54.Mulvey, M. R., P. A. Sorby, B. L. Triggs-Raine, and P. C. Loewen. 1988. Cloning and physical characterization of katE and katF required for catalase HPII expression in Escherichia coli. Gene 73:337-345. [DOI] [PubMed] [Google Scholar]
- 55.Niegemann, E., A. Schulz, and K. Bartsch. 1993. Molecular organization of the Escherichia coli gab cluster: nucleotide sequence of the structural genes gabD and gabP and expression of the GABA permease gene. Arch. Microbiol. 160:454-460. [DOI] [PubMed] [Google Scholar]
- 56.Nishino, K., and A. Yamaguchi. 2002. EvgA of the two-component signal transduction system modulates production of the yhiUV multidrug transporter in Escherichia coli. J. Bacteriol. 184:2319-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nishino, K., and A. Yamaguchi. 2001. Analysis of a complete library of putative drug transporter genes in Escherichia coli. J. Bacteriol. 183:5803-5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oliver, S. G. 2002. Functional genomics: lessons from yeast. Philos. Trans. R. Soc. Lond. B Biol. Sci. 357:17-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Olsen, A., A. Arnqvist, M. Hammar, S. Sukupolvi, and S. Normark. 1993. The RpoS sigma factor relieves H-NS-mediated transcriptional repression of csgA, the subunit gene of fibronectin-binding curli in Escherichia coli. Mol. Microbiol. 7:523-536. [DOI] [PubMed] [Google Scholar]
- 60.Oshima, T., H. Aiba, Y. Masuda, S. Kanaya, M. Sugiura, B. L. Wanner, H. Mori, and T. Mizuno. 2002. Transcriptome analysis of all two-component regulatory system mutants of Escherichia coli K-12. Mol. Microbiol. 46:281-291. [DOI] [PubMed] [Google Scholar]
- 61.Pittard, A. J. 1996. Biosynthesis of aromatic amino acids, p. 458-484. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, D.C.
- 62.Roy, R. N., S. Mukhopadhyay, L. I. Wei, and H. E. Schellhorn. 1995. Isolation and sequencing of gene fusions carried by lambda placMu specialized transducing phage. Nucleic Acids Res. 23:3076-3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rozen, Y., T. K. Dyk, R. A. LaRossa, and S. Belkin. 2001. Seawater activation of Escherichia coli gene promoter elements: dominance of rpoS control. Microb. Ecol. 42:635-643. [DOI] [PubMed] [Google Scholar]
- 64.Saier, M. H., Jr. 1998. Multiple mechanisms controlling carbon metabolism in bacteria. Biotechnol. Bioeng. 58:170-174. [DOI] [PubMed] [Google Scholar]
- 65.Santos, J. M., P. Freire, M. Vicente, and C. M. Arraiano. 1999. The stationary-phase morphogene bolA from Escherichia coli is induced by stress during early stages of growth. Mol. Microbiol. 32:789-798. [DOI] [PubMed] [Google Scholar]
- 66.Schellhorn, H. E., J. P. Audia, L. I. Wei, and L. Chang. 1998. Identification of conserved, RpoS-dependent stationary-phase genes of Escherichia coli. J. Bacteriol. 180:6283-6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schellhorn, H. E., and H. M. Hassan. 1988. Transcriptional regulation of katE in Escherichia coli K-12. J. Bacteriol. 170:4286-4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schellhorn, H. E., and V. L. Stones. 1992. Regulation of katF and katE in Escherichia coli K-12 by weak acids. J. Bacteriol. 174:4769-4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sitnikov, D. M., J. B. Schineller, and T. O. Baldwin. 1996. Control of cell division in Escherichia coli: regulation of transcription of ftsQA involves both rpoS and SdiA-mediated autoinduction. Proc. Natl. Acad. Sci. USA 93:336-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sprenger, G. A. 1995. Genetics of pentose-phosphate pathway enzymes of Escherichia coli K-12. Arch. Microbiol. 164:324-330. [DOI] [PubMed] [Google Scholar]
- 71.Stewart, V. 1993. Nitrate regulation of anaerobic respiratory gene expression in Escherichia coli. Mol. Microbiol. 9:425-434. [DOI] [PubMed] [Google Scholar]
- 72.Strom, A. R., and I. Kaasen. 1993. Trehalose metabolism in Escherichia coli: stress protection and stress regulation of gene expression. Mol. Microbiol. 8:205-210. [DOI] [PubMed] [Google Scholar]
- 73.Sturr, M. G., T. A. Krulwich, and D. B. Hicks. 1996. Purification of a cytochrome bd terminal oxidase encoded by the Escherichia coli app locus from a Δcyo Δcyd strain complemented by genes from Bacillus firmus OF4. J. Bacteriol. 178:1742-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tani, T. H., A. Khodursky, R. M. Blumenthal, P. O. Brown, and R. G. Matthews. 2002. Adaptation to famine: a family of stationary-phase genes revealed by microarray analysis. Proc. Natl. Acad. Sci. USA 99:13471-13476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tao, H., C. Bausch, C. Richmond, F. R. Blattner, and T. Conway. 1999. Functional genomics: expression analysis of Escherichia coli growing on minimal and rich media. J. Bacteriol. 181:6425-6440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Taverna, P., and B. Sedgwick. 1996. Generation of an endogenous DNA-methylating agent by nitrosation in Escherichia coli. J. Bacteriol. 178:5105-5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tian, G., D. Lim, J. Carey, and W. K. Maas. 1992. Binding of the arginine repressor of Escherichia coli K12 to its operator sites. J. Mol. Biol. 226:387-397. [DOI] [PubMed] [Google Scholar]
- 78.Tsukagoshi, N., and R. Aono. 2000. Entry into and release of solvents by Escherichia coli in an organic-aqueous two-liquid-phase system and substrate specificity of the AcrAB-TolC solvent-extruding pump. J. Bacteriol. 182:4803-4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Van Dyk, T. K., B. L. Ayers, R. W. Morgan, and R. A. LaRossa. 1998. Constricted flux through the branched-chain amino acid biosynthetic enzyme acetolactate synthase triggers elevated expression of genes regulated by rpoS and internal acidification. J. Bacteriol. 180:785-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Volkert, M. R., L. I. Hajec, Z. Matijasevic, F. C. Fang, and R. Prince. 1994. Induction of the Escherichia coli aidB gene under oxygen-limiting conditions requires a functional rpoS (katF) gene. J. Bacteriol. 176:7638-7645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.von Ossowski, I., M. R. Mulvey, P. A. Leco, A. Borys, and P. C. Loewen. 1991. Nucleotide sequence of Escherichia coli katE, which encodes catalase HPII. J. Bacteriol. 173:514-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vuilleumier, S., and M. Pagni. 2002. The elusive roles of bacterial glutathione S-transferases: new lessons from genomes. Appl. Microbiol. Biotechnol. 58:138-146. [DOI] [PubMed] [Google Scholar]
- 83.Wanner, B. L., and B. D. Chang. 1987. The phoBR operon in Escherichia coli K-12. J. Bacteriol. 169:5569-5574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wei, Y., J. M. Lee, D. R. Smulski, and R. A. LaRossa. 2001. Global impact of sdiA amplification revealed by comprehensive gene expression profiling of Escherichia coli. J. Bacteriol. 183:2265-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weichart, D., R. Lange, N. Henneberg, and R. Hengge-Aronis. 1993. Identification and characterization of stationary phase-inducible genes in Escherichia coli. Mol. Microbiol. 10:407-420. [PubMed] [Google Scholar]
- 86.Wolf, S. G., D. Frenkiel, T. Arad, S. E. Finkel, R. Kolter, and A. Minsky. 1999. DNA protection by stress-induced biocrystallization. Nature 400:83-85. [DOI] [PubMed] [Google Scholar]
- 87.Xu, J., and R. C. Johnson. 1995. aldB, an RpoS-dependent gene in Escherichia coli encoding an aldehyde dehydrogenase that is repressed by Fis and activated by Crp. J. Bacteriol. 177:3166-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yakovleva, G. M., S. K. Kim, and B. L. Wanner. 1998. Phosphate-independent expression of the carbon-phosphorus lyase activity of Escherichia coli. Appl. Microbiol. Biotechnol. 49:573-578. [DOI] [PubMed] [Google Scholar]
- 89.Yamamoto, Y., Y. Miwa, K. Miyoshi, J. Furuyama, and H. Ohmori. 1997. The Escherichia coli ldcC gene encodes another lysine decarboxylase, probably a constitutive enzyme. Genes Genet. Syst. 72:167-172. [DOI] [PubMed] [Google Scholar]
- 90.Yim, H. H., R. L. Brems, and M. Villarejo. 1994. Molecular characterization of the promoter of osmY, an rpoS-dependent gene. J. Bacteriol. 176:100-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yim, H. H., and M. Villarejo. 1992. osmY, a new hyperosmotically inducible gene, encodes a periplasmic protein in Escherichia coli. J. Bacteriol. 174:3637-3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yum, D. Y., B. Y. Lee, and J. G. Pan. 1999. Identification of the yqhE and yafB genes encoding two 2,5-diketo-d-gluconate reductases in Escherichia coli. Appl. Environ. Microbiol. 65:3341-3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zimmer, D. P., E. Soupene, H. L. Lee, V. F. Wendisch, A. B. Khodursky, B. J. Peter, R. A. Bender, and S. Kustu. 2000. Nitrogen regulatory protein C-controlled genes of Escherichia coli: scavenging as a defense against nitrogen limitation. Proc. Natl. Acad. Sci. USA 97:14674-14679. [DOI] [PMC free article] [PubMed] [Google Scholar]