Abstract
The nucleotide sequence of the Clostridium cellulovorans xynB gene, which encodes the XynB xylanase, consists of 1,821 bp and encodes a protein of 607 amino acids with a molecular weight of 65,976. XynB contains a typical N-terminal signal peptide of 29 amino acid residues, followed by a 147-amino-acid sequence that is homologous to the family 4-9 (subfamily 9 in family 4) carbohydrate-binding domain. Downstream of this domain is a family 10 catalytic domain of glycosyl hydrolase. The C terminus separated from the catalytic domain by a short linker sequence contains a dockerin domain responsible for cellulosome assembly. The XynB sequence from mass spectrometry and N-terminal amino acid sequence analyses agreed with that deduced from the nucleotide sequence. XynB was highly active toward xylan, but not active toward carboxymethyl cellulose. The enzyme was optimally active at 40°C and pH 5.0. Northern hybridizations revealed that xynB is transcribed as a monocistronic 1.9-kb mRNA. RNA ligase-mediated rapid amplification of 5′ cDNA ends by PCR (RLM-5′RACE PCR) analysis of C. cellulovorans RNA identified a single transcriptional start site of xynB located 47 bp upstream from the first nucleotide of the translation initiation codon. Alignment of the xynB promoter region provided evidence for highly conserved sequences that exhibited strong similarity to the σA consensus promoter sequences of gram-positive bacteria. Expression of xynB mRNA increased from early to middle exponential phase and decreased during the early stationary phase when the cells were grown on cellobiose. No alternative promoter was observed by RLM-5′RACE PCR and reverse transcriptase PCR analyses during expression. The analysis of the products from xylan hydrolysis by thin-layer chromatography indicated its endoxylanase activity. The results suggest that XynB is a consistent and major cellulosomal enzyme during growth on cellulose or xylan.
Hemicellulose is the second most abundant renewable polysaccharide in nature after cellulose (3). β-1,4-Xylan is a major component of hemicellulose and has a backbone of β-1,4-linked d-xylopyranoside residues replaced by acetyl, arabinosyl, and uronyl side chains (50). The complete breakdown of xylan requires the action of several hydrolytic enzymes (3). The most important hydrolytic enzyme is endoxylanase (1,4-β-d-xylan xylanohydrolase) (EC 3.2.1.8). Xylanases have been widely detected in bacteria and fungi, and their properties have been characterized (3, 52). On the basis of the amino acid sequences of catalytic domains, xylanases have been classified into two groups: family 10 and family 11 of glycosyl hydrolases (25).
Clostridium cellulovorans ATCC 35296 (44) produces a highly active polysaccharolytic complex termed the cellulosome, in which several cellulases are tightly bound to a scaffolding protein called CbpA (1). Doi et al. (11) and Tamaru et al. (11, 49) have characterized the genes necessary for degradation of the plant cell wall of this bacterium. However, until now, only one xylanase gene, xynA, encoding a family 11 glycosyl hydrolase, had been cloned and expressed in Escherichia coli in agreement with the presence of xylanase activity in the cellulosome (27, 28).
In this paper, we describe the nucleotide sequence, expression, and characterization of the xynB gene, a family 10 glycosyl hydrolase, encoding one of the consistent components of the cellulosome and corresponding to component P6 of a subpopulation of the C. cellulovorans cellulosome (35). In addition, we performed a detailed transcriptional analysis of the xynB promoter and its regulation by growth phase in order to gain some understanding of the pattern of expression of this gene in C. cellulovorans. We also characterized the XynB enzyme purified from a recombinant E. coli strain.
MATERIALS AND METHODS
Bacterial strains and fosmid.
C. cellulovorans ATCC 35296, used for isolation of the cellulosome fraction, was described previously (42). The E. coli strain used was EPI300 (Epicentre). A fosmid (Epicentre) containing 50-kb DNA fragments was used for determination of the DNA sequence of xynB and the production of the recombinant enzyme.
Nucleic acid isolation.
Chromosomal DNA of C. cellulovorans was isolated by using the genomic DNA purification kit (Promega) according to the manufacturer's instructions. Total RNA was extracted from C. cellulovorans broth cultures using the QIAGEN RNeasy kit with the additional step of treatment with RNAlater solution (Ambion) and RNase-free DNase (Promega) according to the manufacturers' instructions.
Fosmid library construction and screening.
The C. cellulovorans fosmid library was constructed as follows. Briefly, genomic DNA was randomly sheared by passing it through a syringe needle. The ends of the sheared DNA were then repaired, and the DNA fragments were separated on 0.8% agarose in 1× TAE (1× TAE is 40 mM Tris, 20 mM acetic acid, and 1 mM EDTA) overnight at 30 V. Then 50-kb fragment pools were gel purified and cloned into the vector EpiFOS (Epicentre) according to the manufacturer's instructions. Ligated DNA was packaged with the Epicentre MaxPlax lambda packaging extract and used to transfect E. coli EPI300 cells (Epicentre). Transfected cells were selected on Luria-Bertani (LB) agar containing 12.5 μg of chloramphenicol per ml. The procedure yielded 2,000 recombinant clones. The LB agar plates, on which the recombinant E. coli bacteria were grown, were overlaid with soft agar containing 0.3% xylan (birchwood; Sigma) and 0.7% agar in 25 mM sodium acetate buffer (pH 6.0). After incubation at 37°C for 16 h, the plates were stained with 0.3% Congo red and destained with 1 M NaCl. The clones that formed halos were selected as xylanase-positive colonies. The xylanase-positive clones were picked and restreaked to confirm the formation of clearing zones around the colonies on LB agar overlaid with soft agar containing xylan.
Construction of the mutant library and DNA sequencing.
The EZ::TN <oriV/KAN-2> insertion system (Epicentre), based on in vitro Tn5 transposition (18), was used to generate insertion knockout mutants of xynB. The xylanase-positive clone was incubated with transposase and an EZ::TN <oriV/KAN-2> transposon with a kanamycin resistance marker under the conditions suggested by the manufacturer (Epicentre). After the reaction, the reaction mixture was used to transform TransforMax EPI300 electrocompetent E. coli, and the knockout (xylanase-negative) transformants were selected on LB plates with 0.3% xylan, 50 μg of kanamycin per ml, and 12.5 μg of chloramphenicol per ml. The transposon insertion sites of mutant clones were mapped by nucleotide sequencing with the transposon-specific flanking primers (Epicentre).
RLM-RACE PCR.
RNA ligase-mediated rapid amplification of cDNA ends by PCR (RLM-RACE PCR) was performed with total RNA extracted from a C. cellulovorans culture grown on cellobiose and was used to determine single or multiple transcription start points. Rapid amplification of 5′ cDNA ends (5′RACE) was performed using the FirstChoice RLM-RACE kit (Ambion) according to the manufacturer's instructions with the following exceptions. The nested PCR conditions for 5′ outer PCR were as recommended, with 10 pmol of gene-specific outer primer xynB-5′-Outer (5′-GAACAGACGTATTGAAAGCA), 1.25 U of SuperTaq polymerase (Ambion), 10 pmol of 5′RACE outer primer (5′-GCTGATGGCGATGAATGAACACTG) (Ambion), 1× SuperTaq PCR buffer (Ambion), 100 μM deoxynucleoside triphosphates, 1 ng of first-strand cDNA reaction mixture per μl, and H2O to a volume of 50 μl. PCR was performed as follows: (i) 4 min at 94°C; (ii) 35 cycles, with 1 cycle consisting of 30 s at 94°C, 30 s at 60°C, and 40 s at 72°C; and (iii) 7 min at 72°C. 5′ inner PCR was performed, as recommended, with 10 pmol of gene-specific inner primer xynB-5′-Inner (5′-CTCTATTAATAAGTAGCACCTTCG) and 10 pmol of 5′RACE inner primer (5′-CGCGGATCCGAACACTGCGTTTGCTGGCTTTGATG) (Ambion) using the same conditions as for the 5′ outer PCR. PCR products were run on 2% agarose gels and sequenced.
RT-PCR analysis.
Reverse transcriptase (RT) reactions were performed in a final volume of 20 μl, which contained 5 mM MgCl2, RT buffer (10 mM Tris-HCl [pH 9.0], 50 mM KCl, 0.1% Triton X-100), 1 mM each of the four deoxynucleoside triphosphates, 1 U of recombinant RNasin RNase inhibitor (Promega), 15 U of avian myeloblastosis virus RT (Promega), 0.25 μM oligonucleotide primer, and 10 μg of substrate RNA. Reaction mixtures were incubated at 42°C for 60 min, and reactions were terminated by heating the mixtures at 95°C for 5 min, followed by incubation on ice for 5 min. The cDNA products were then amplified in 50-μl PCR mixtures using 1 μl of the RT reaction mixture as the template.
Northern blot analysis.
RNA samples (5 μg) were denatured in RNA sample buffer at 65°C for 10 min. RNA sample buffer consists of 250 μl of formamide, 83 μl of 37% (wt/vol) formaldehyde, 83 μl of 6× loading dye (Promega), 50 μl of 10× MOPS (morpholinepropanesulfonic acid) buffer (10× MOPS buffer is 20 mM MOPS, 5 mM sodium acetate, and 1 mM EDTA [pH 7.0]), and 34 μl of distilled water. RNA samples were separated through 1% agarose gels in MOPS buffer with 2% (vol/vol) formaldehyde. DNA probes were synthesized by PCR using specific oligonucleotides derived from the C. cellulovorans sequence as a template (Table 1). The probes were nonradioactively labeled by random priming using the digoxigenin (DIG) High Prime kit (Roche). To add the correct amount of probe for hybridization, a serial dilution of each probe (0.05 to 10 pg) was spotted on a nylon membrane, and the labeling sensitivity (amount of labeled DNA per spot) was determined. RNA was transferred overnight to a positively charged nylon membrane (Roche) by capillary transfer using 20× SSC (20× SSC is 0.3 M NaCl plus 0.03 M sodium citrate [pH 7]). Hybridization was performed for 16 h at 50°C in DIG Eazy Hyb buffer solution (Roche). The membrane was washed and specific transcripts on the blots were detected by using the DIG luminescence detection kit (Roche) according to the protocol recommended for the kit.
TABLE 1.
Oligonucleotide primers used in this study
| Primer | Sequence (5′→3′) | Gene | Nucleotide positiona | Useb |
|---|---|---|---|---|
| xynB-F | GTATTATATTTCTATAAAAGACC | xynB | 269-291 | NB, RT |
| xynB-R | TGCTTTCAATACGTCTGTTC | xynB | 1027-1051 | NB, RT |
| xynB-F1 | GTTCTTAAGTTCACAAATACTCGAG | xynB | 46-70 | RT |
| xynB-F2 | GATTTTCATATTATTATACTTAATTTTA | xynB | 198-228 | RT |
| 5′-Inner | CGAAGGTGCTACTTATTAATAGAG | xynB | 410-433 | RACE |
| 5′-Outer | TGCTTTCAATACGTCTGTTC | xynB | 563-582 | RACE |
| xynA-F | TGTTAGCCTCTTCTGC | xynA | 6389-6404 | NB |
| xynA-R | GATTCCAAGTGCCATAGC | xynA | 6722-6739 | NB |
| cbpA-exgS-F | ATGCAAAAAAAGAAATCGCTG | cbpA-exgS | 883-903 | NB |
| cbpA-exgS-R | GGTTGATGTTGGGCTTGCTGTT | cbpA-exgS | 1215-1236 | NB |
| arfA-F | ATGGAGGATTTTGGGTTGGG | arfA | 553-572 | NB |
| arfA-R | TCGGTGACTCTCCATC | arfA | 809-906 | NB |
The nucleotide position refers to the relevant position within the xynB sequence (GenBank accession no. AY604045), xynA sequence (28) (GenBank accession no. AF435978), cbpA-exgS sequence (43) (GenBank accession no. M73817), or arfA sequence (26) (GenBank accession no. AY128945).
NB, Northern blot analysis; RT, RT-PCR; RACE, RLM-5′ RACE.
Preparation of the recombinant enzyme.
The recombinant enzyme was isolated from culture supernatants of a xylanase-positive clone or a knockout clone. The culture supernatants were obtained by centrifugation of cultures grown overnight (1,000 ml) in LB medium containing chloramphenicol (12.5 μg/ml). The supernatants were concentrated by 80% ammonium sulfate saturation and dialyzed. The dialyzed enzyme was concentrated with Ultrafree Biomax (10-kDa cutoff; Millipore). The concentration of fractionated protein was measured by the method of Bradford (5) with a protein assay kit from Bio-Rad, using bovine serum albumin as the standard.
SDS-PAGE, zymogram, and mass spectrometry analyses.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed with a 10% polyacrylamide gel by the method of Laemmli et al. (29). Proteins were fixed in the gels by soaking in a solution containing 40% (vol/vol) methanol and 10% (vol/vol) acetic acid for approximately 1 h and subsequently visualized by Coomassie blue staining (Genomic Solutions). The zymograms for xylanase and carboxymethyl cellulase were performed by using a 0.1% (wt/vol) concentration of each substrate incorporated into the polyacrylamide. After SDS-PAGE, the gels were renatured in renaturation buffer (100 mM succinic acid, 10 mM CaCl2, 1 mM dithiothreitol [pH 6.3]) for 2 h at 25°C with gentle shaking. The renatured gel was then incubated in fresh renaturation buffer for 1 h at 37°C with gentle shaking. The clearing zones corresponding to enzyme activities were visualized with 0.3% (wt/vol) Congo red (stained for 10 min and destained with 1 M NaCl solution) (4). Mass spectrometric analysis was performed to identify the XynB protein on cellulosomes, and recombinant proteins were separated by SDS-PAGE as described previously (19).
Analysis of reaction products.
Xylan degradation products were determined by thin-layer chromatography (TLC) on precoated TLC sheets (silica gel; Whatman) with acetone-ethyl acetate-acetic acid (2:1:1, vol/vol/vol) (34). The plates were visualized by spraying with a 1:1 (vol/vol) mixture of 0.2% methanolic orcinol and 20% sulfuric acid.
Nucleotide sequence accession number.
The xynB gene sequence was submitted to GenBank and has been assigned accession number AY604045.
RESULTS AND DISCUSSION
Analysis of DNA sequence and transcriptional start site of C. cellulovorans xynB.
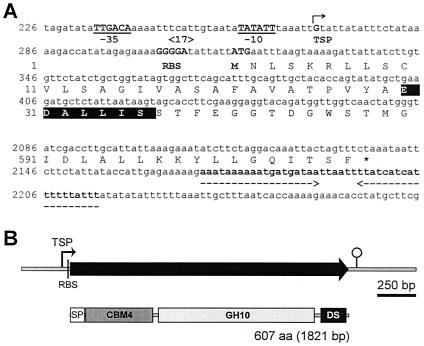
Figure 1 shows the C. cellulovorans xynB coding sequence and flanking regions. There is an open reading frame composed of 1,821 nucleotides encoding a protein of 607 amino acids with a predicted molecular weight of 65,976. The assigned ATG initiation codon at nucleotide position 316 is preceded by a conserved putative ribosome binding site (GGGGA). The reading frame is terminated by the stop codon TAA at position 2136.
FIG. 1.
Nucleotide and deduced amino acid sequences (A) and modular structure (B) of xynB. (A) Untranslated regions (lowercase letters) and putative promoter regions (consensus −10 and −35 regions, gap 17 bp) (underlined) are indicated. The transcription start point (+1) (TSP) is indicated by the bent arrow. The putative ribosome binding site (RBS) and the first codon (M) are indicated. The stop codon (asterisk) and a palindrome (broken arrows facing each other below the sequence) are indicated. The deduced amino acid sequence corresponding to the experimentally determined N-terminal sequence (35) is shown by white letters on a black background. Additional sequences upstream and downstream of xynB can be accessed at GenBank accession no. AY604045. (B) The putative signal peptide (SP) identified using SignalP prediction software (37) is boxed. A family 4-9 carbohydrate-binding domain (CBM4) is shaded and followed by a lightly shadowed family 10 catalytic domain of glycosyl hydrolases (GH10). The DS (or dockerin) domain is shown. aa, amino acids.
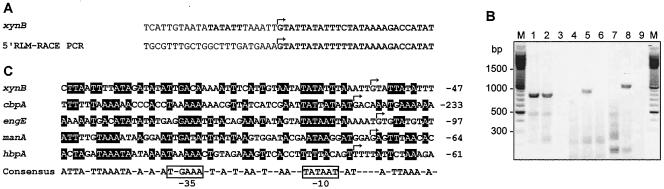
In order to localize the promoter, the transcription start site of xynB was determined by RLM-5′RACE PCR analysis. An 165-bp PCR fragment (5′-Inner/5′RACE inner primer) was generated for DNA sequencing of the region upstream of the transcription start site of xynB. One clear ending sequence was identified 47 bp upstream from the A of the proposed ATG start codon, and it was obtained by DNA sequencing of the RLM-5′RACE PCR product (Fig. 2A). In addition, this transcription start point was confirmed by RT-PCR analysis using multiple primer combinations (xynB-F1/xynB-R, xynB-F2/xynB-R, and xynB-F/xynB-R) (Fig. 2B and Table 1). The xynB mRNA start point suggested a putative promoter sequence, TTGACA and TATATT, with a 17-bp spacing between them (Fig. 2C). These sequences show high homologies to the consensus promoter sequences for σ70 factor found in E. coli, i.e., TTGACA and TATAAT with a 17-bp spacing (39). The consensus sequence of xynB and previously determined C. cellulovorans promoters (21) at −10 (5′-T-GAAA-3′) and at −35 (5′-TATAAT-3) are homologous to elements recognized by Bacillus subtilis (σA) and E. coli (σ70) RNA polymerases (7, 56) (Fig. 2C), and the consensus sequence contained only one mismatch to the E. coli σ70 −10 consensus sequence (23). The spacing between the −35 and −10 regions was 17 bp, which is the optimal spacing observed for B. subtilis and E. coli promoter sequences (7, 22). The close similarity of the promoter to the consensus σA sequence suggests that the xynB promoter, if not subjected to any regulatory constraints, would act as a strong promoter in vivo (10, 23). In addition, the xynB promoter was very similar to previously determined C. cellulovorans promoters (1, 21).
FIG. 2.
Determination and alignment of putative xynB promoter located −10 and −35 bases upstream of the start point of transcription. (A) The transcription start point was determined by sequencing the RLM-5′RACE PCR product and indicated by the bent arrows. (B) Agarose gel electrophoresis of RT-PCR products. The oligonucleotide primers used in RT-PCR analysis of promoter region were xynB-F1 and xynB-R (lanes 1 to 3), xynB-F2 and xynB-R (lanes 4 to 6), and xynB-F and xynB-R (lanes 7 to 9) (more details in Table 1). RT-PCR analysis was performed using total RNA (lanes 1, 4, and 7) that was isolated from C. cellulovorans in the medium containing cellobiose. In controls, the reactions were performed with DNA template (lanes 2, 5, and 8) or in the absence of RT (lanes 3, 6, and 9). The positions of molecular size markers (M) (in base pairs) are shown to the left of the gel. (C) Transcription start points are indicated by the bent arrows. The nucleotide numbering begins from the first codon shown on the right. The consensus sequence derived from this alignment is given at the bottom. It consists of nucleotides that are present in any given position in more than 50% of the sequences. Promoter sequence nucleotides that match those of the consensus sequence are shown as white letters on a black background. The coordinates are those in the published nucleotide sequences for cbpA (43) (GenBank accession no. M73817), engE (48) (GenBank accession no. AF105331), and manA and hbpA (47, 49) (GenBank accession no. AF132735).
Northern hybridizations of the RNA with a xynB probe showed a single transcript of 1.9 kb (data not shown). The size of this mRNA agreed with the size of the xynB gene (1,821 bp) and indicated that xynB was a monocistronic gene. On the basis of the size of xynB mRNA and the location of the transcription start site, a putative transcription terminator that consists of a 44-bp palindromic sequence, corresponding to an mRNA hairpin loop with a ΔG of −67.6 kcal/mol (6, 45), followed by a long poly(U) tail (AUAUAUAUUUUUU) was found downstream of the TGA termination codon at nucleotide position 2171 (Fig. 1A). This structure is similar to the rho factor-independent terminator of E. coli (39).
Transcriptional analysis of xynB.
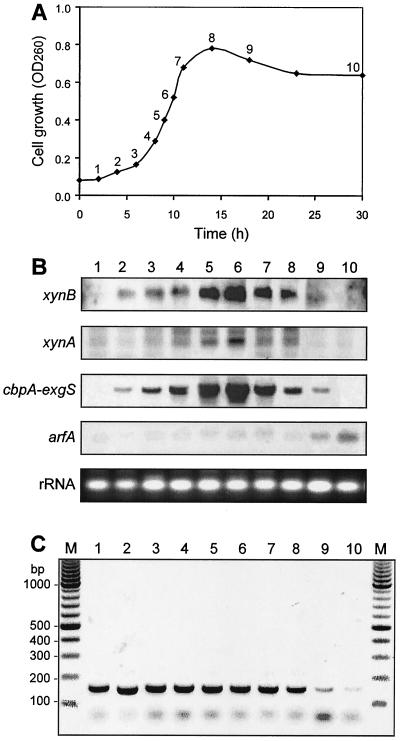
To find alternative promoters and determine whether the xynB gene along with C. cellulovorans genes are regulated coordinately, changes in the level of mRNA expressed were monitored during cultivation of C. cellulovorans on cellobiose as the sole carbon source. RNA was prepared from the culture at different stages of growth. During the culture for 30 h, the pH was not changed and 0.5% (wt/vol) cellobiose was not consumed totally by C. cellulovorans (data not shown). The RNA was subjected to Northern blot analyses using primers that were specific to the xynB, xynA, cbpA-exgS, and arfA genes. These genes represent the major subunits (cbpA-exgS) (17, 32, 43), xylanase gene (xynA) (28), and a noncellulosomal α-l-arabinofuranosidase gene, arfA (26). A semiquantitative measure of the level of xynB mRNA, using DIG-labeled probes and RNA isolated at different times during cell growth, was obtained by Northern blot analysis (Fig. 3A and B). The intensities of the bands were close approximations of their relative abundance. The levels of xynB mRNA increased simultaneously from early to middle exponential phase and dramatically decreased during the early stationary phase when the cells were grown on cellobiose (Fig. 3A, points 1 to 9, and B, lanes 1 to 9). Like xynB gene expression, cells contained high levels of cbpA, exgS, and xynA mRNAs during most of the exponential growth phase with the level peaking at the middle of the exponential phase (Fig. 3A and B). In contrast, the arfA transcripts increased during the middle of the stationary phase (Fig. 3A and B, lane 10) and peaked when the cells reached the stationary growth phase (Fig. 3A and B, lanes 9 and 10).
FIG. 3.
Relative levels (B) and size (C) of transcripts of xynB at different growth phases (A) on cellobiose culture. (A) C. cellulovorans growth curve. OD260, optical density at 260 nm. (B) Northern blot analyses were conducted with the same concentration of RNA (5 μg) isolated from C. cellulovorans cultured grown on 0.5% cellobiose as the sole carbon source. Ethidium bromide staining of rRNA is shown as a loading control. (C) RLM-5′RACE PCR analysis for putative promoter location. Primers specific for xynB were used to amplify fragments by PCR. In negative controls, the reactions were performed in the absence of RT or RNA templates (data not shown). The numbers of the lanes in panels B and C are the numbers of the growth curve points in panel A. See Materials and Methods for details. The positions of molecular size markers (M) (in base pairs) are shown to the left of the gel.
RLM-5′RACE PCR analysis was performed using primers 5′-Inner and 5′-Outer (Fig. 1A), which were specific for identifying the putative promoter. One clear band (165 bp) of the nested mRNA product from the RLM-5′RACE PCR was found during the early to late exponential phase (Fig. 3C) and disappeared during the stationary phase (Fig. 3A and C). The fact that nested PCR did not produce several bands indicates no alternative transcriptional start site. A negative control using the RNA that was not reverse transcribed was included to ensure that there was no contaminating DNA present in the RNA preparation (data not shown).
The mRNA of xynB and the mRNAs of cbpA-exgS and xynA are coordinately expressed, whereas the time course of expression of these genes (xynB, cbpA-exgS, and xynA) was greatly different from that of the noncellulosomal α-l-arabinofuranosidase gene (arfA). The transcriptional data also showed that whereas xynB and cbpA-exgS from cells grown on cellobiose were efficiently expressed, relatively low levels of xynA and arfA transcripts were present. In addition, the xynB gene and major cellulase genes, such as cbpA-exgS and engE, were constitutively expressed (19-21). On the basis of a general model for induction of xylanase expression in Trichoderma reesei, a sensor enzyme may be constitutively expressed; this enzyme hydrolyzes xylan into oligosaccharides that can enter the bacterium and activates the expression of all the xylanase genes (33, 57). It is possible that one or more of the constitutively expressed C. cellulovorans xylanases functions as the sensor enzyme. Cellulose and xylan are closely associated in nature, and it appears that C. cellulovorans has mechanisms to ensure efficient utilization of both types of polymers. Previous studies also suggest that a common regulatory mechanism may exist at the transcriptional level for cellulase and xylanase induction by cellobiose in this organism (19, 20). Cellulose metabolites, such as cellobiose, or a derivative of cellobiose may act as an inducer and may bind to a receptor protein in a signal transduction pathway, and this pathway would then lead to cellulase induction.
Amino acid sequences and domains of XynB.
A previous study of C. cellulovorans showed that the bacterium produces high levels of xylanase activity when grown on xylan and concluded that at least four xylanase genes were located on the C. cellulovorans chromosome (27, 35). The xylanase subunit, XynA, was found recently, and its xylanase activity is ascribed to a family 11 catalytic domain (28). In this work, we have located xynB from the fosmid clone which contained a 50-kb insert DNA without xynA. High sequence identity to XynB is observed with XynC of C. thermocellum (44%) (24), XynB of Polyplastron multivesiculatum (38%) (9), and XynA of Thermotoga neapolitana (35%) (59).
Figure 1B shows schematically the molecular architecture of XynB. The deduced N-terminal sequence of 29 amino acids contains a sequence similar to the signal peptide sequences found in prokaryotic secretory proteins, which have a short region rich in positively charged amino acids (lysine-5 and arginine-6), followed by a sequence of predominantly hydrophobic residues (leucine-7, -8, and -12; valine-11, -17, and -23; alanine-14, -18, -20, -22, and -24; and isoleucine-16), a residue breaking the secondary structure (proline-26), and a cleavage site ending with alanine-29 (37).
Comparison of the amino acid sequence of XynB with those registered in SWISS PROT and GenBank databases clearly revealed that the mature XynB protein consisted of three distinct functional domains. The N-terminal domain of the mature form of XynB, 147 amino acid residues downstream of the signal peptide, is homologous with the family 4-9 (subfamily 9 in family 4) carbohydrate-binding domain of other xylanases (Fig. 4A), i.e., 37% sequence identity with XynC of Clostridium thermocellum (24), 33% identity with XynD of Ruminococcus flavefaciens (14), and 32% identity with XynA of Thermotoga maritima (55).
FIG.4.
Alignment of domains of Xyn proteins from different species. (A) Alignment of family 4-9 carbohydrate-binding domains of C. cellulovorans (C.v) XynB (C.v-XynB) (GenBank accession no. AY604045), Clostridium thermocellum (C.t) XynC (BAA21516) (24), Thermoanaerobacterium saccharolyticum (T.s) XynA (P36917) (30), Thermotoga maritima (T.m) XynA (Q60037) (55), Clostridium stercorarium (C.s) XynB (P40942) (16), Ruminococcus albus (R.a) XynA (BAB39493) (36), C. thermocellum (C.t) XynY (P51584) (15), and Ruminococcus flavefaciens (R.f) XynD (Q53317) (14). (B) Alignment of family 10 catalytic domains of C. cellulovorans (C.v) XynB (C.v-XynB) (GenBank accession no. AY604045), R. flavefaciens (R.f) XynA (P29126) (58), C. thermocellum (C.t) XynY (P51584) (15), Butyrivibrio fibrisolvens (B.f) XynB (P26223) (31), Caldicellulosiruptor sp. strain Rt8B.4 (C.r) XynA (P40944) (13), Bacillus halodurans (B.h) XynA (P07528) (46), Clostridium stercorarium (C.s) XynB (P40942) (16), Thermoanaerobacterium saccharolyticum (T.s) XynA (P36917) (30), C. thermocellum (C.t) XynX (P38535), Geobacillus stearothermophilus (G.s) XynA (P45703) (2), Thermotoga maritima (T.m) XynA (Q60037) (55), and Thermotoga neapolitana (T.n) XynA (Q60042) (59). The two catalytic Glu residues are marked by vertical arrows. (C) Alignment of dockerin domains of XynB, EngB, EngE, EngH, EngK, EngL, EngM, EngY, ExgS, ManA, PelA, and XynA of C. cellulovorans. Gaps introduced to maximize alignment are indicated by dashes. Amino acids that are conserved in more than 50% of the sequences are indicated by white letters on a black background. Residues that are identical in all enzymes are indicated by an asterisk. Numbers at the start of the respective lines refer to amino acid residues; all sequences are numbered from Met-1 of the peptide.
The family 10 catalytic domain of XynB, extending from positions 193 to 532, exhibited extensive sequence homology with the catalytic domains of the other xylanases in family 10 glycosyl hydrolase domains (Fig. 4B), i.e., 52% sequence identity with XynA of R. flavefaciens (58), 45% identity with XynY of C. thermocellum (15), and 38% identity with XynB of Butyrivibrio fibrisolvens (31). The catalytic domains of these enzymes are highly conserved in eight regions and presumably serve to form the overall structure of these enzymes (Fig. 4B), suggesting that these xylanases have diverged from a common evolutionary ancestor. In addition, two glutamic acid residues (Fig. 4B), which have been confirmed as the catalytic amino acids by site-directed mutagenesis and crystallography in Cellulomonas fimi, C. thermocellum, and Streptomyces lividans (8, 51, 53, 54), were conserved in two identical regions as the nucleophile and the proton donor, and it is assumed that the highly conserved histidine-427 of XynB presumably serves to stabilize the transition state with an oxocarbonium ion character (41).
The third domain in XynB is a dockerin domain located in the C terminus of the peptide. Dockerins that consist of a pair of well-conserved 22-residue repeats are highly conserved in cellulases and xylanases from C. cellulovorans (Fig. 4C) and play a role in cellulosome assembly by docking the various catalytic subunits to the scaffolding protein CbpA (11, 12, 38). Murashima et al. (35) fractionated the cellulosome of C. cellulovorans into subpopulations by ion-exchange chromatography. One subpopulation with high cell wall degrading activity contained an enzyme subunit called P6 with strong xylanase activity, and the N-terminal amino acid sequence of P6 was identical to that of XynB reported in this study. We also found XynB on cellulosomal enzymes isolated from different culture conditions, for example, not only in cultures grown on xylan as the sole carbon source but also in cultures grown on cellulose and cellobiose (data not shown). These findings along with the presence of a dockerin domain suggest that XynB is a consistent and major catalytic subunit of the cellulosome of C. cellulovorans.
Identification and characterization of XynB in recombinant protein.
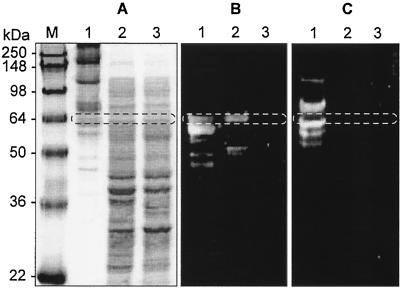
The gene product of xynB was recovered from the supernatant of E. coli EPI300 grown on LB by ammonium sulfate precipitation. The cellulosomal proteins were purified from C. cellulovorans grown on xylan-containing medium by a cellulose affinity column, HiLoad 26/60 Superdex 200 prep grade column and RESOURCE Q (35, 42). We analyzed the recombinant proteins by zymogram analysis and compared them with the cellulosomal protein. The recombinant proteins gave one major band in the zymogram with xylan, and the molecular weight of the enzyme was estimated to be 65,000 (Fig. 5B, lane 2), which appeared to be the same size as the mature XynB deduced from the nucleotide sequence (65,976 Da) (Fig. 1). A band with an apparent molecular weight of 65,000 was detected in the cellulosomal proteins purified from C. cellulovorans (Fig. 5A, lane 1). The size of the protein was in good agreement with that of the full-length XynB produced by recombinant E. coli and the size calculated from the deduced amino acid sequence.
FIG. 5.
Expression of XynB in C. cellulovorans and E. coli. Gels were stained with Coomassie blue (A) or stained for xylanase (B) or carboxymethyl cellulase (C) activity. Lanes: M, protein mass standards; 1, cellulosomal proteins of C. cellulovorans; 2, supernatant of recombinant E. coli; 3, supernatant of knockout XynB recombinant E. coli. The putative XynB (65,976 Da) is circled.
The mass spectrometry technique was also used for the identification of proteins separated by SDS-PAGE. The mass spectra of XynB showed the amino acid sequences of tryptic peptides LPLLFDGNYYPKPAFDSVVK, LVPTSDYYTPGYALGDVNNDGK, IYAWDVVNECYLDGGNLR, YFDIGCAATPSEVSLQVAK, ENFSDTGATVSK, and EASAWNLVYGDDSYIDNAFTYAR, which perfectly matched the entire sequence of XynB. The N-terminal amino acid sequence of this protein (65.3 kb) was previously identified as Glu-Asp-Ala-Leu-Leu-Ile-Ser (35), which was found in the deduced amino acid sequence of XynB at amino acid positions 31 to 36 (Fig. 1A). However, one additional smaller band was found at a molecular size of 52,000 (Fig. 5B, lane 2), and it is likely that the xylanase obtained here arose from the parental protein by partial proteolysis. The XynB protein showed specific activity toward xylan and no activity toward carboxymethyl cellulose (Fig. 5C). The profiles based on SDS-PAGE, mass spectrometry, and zymogram analyses suggest that XynB is one of the major components of the cellulosome.
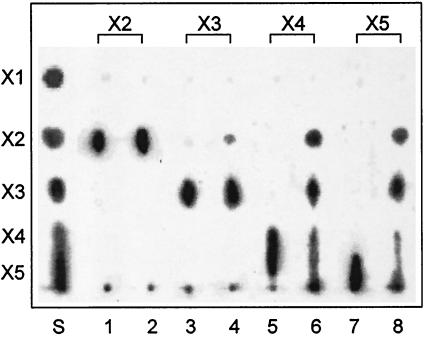
The optimum temperature for activity was found to be 40°C at pH 6.0 (data not shown). The optimum pH for activity was found to be pH 5 when the enzyme activity was assayed at 37°C in sodium acetic buffer solutions at various pHs (data not shown). The action of the enzyme on xylooligosaccharides was qualitatively analyzed. As shown in Fig. 6, XynB hydrolyzed xylotetraose and xylopentaose to yield mainly xylobiose and xylotriose, along with very small amounts of xylose. When xylan was treated with the enzyme, xylobiose and xylotriose were produced as end products accompanied by small amounts of xylose (data not shown). In contrast, this enzyme was less active toward xylotriose and not active at all toward xylobiose (Fig. 6).
FIG. 6.
TLC of hydrolysis products from xylooligosaccharides. Each xylooligosaccharide (xylobiose to xylopentaose) (2 mg) was incubated with either knockout recombinant XynB (1 μg) (lanes 1, 3, 5, and 7) or recombinant XynB (1 μg) (lanes 2, 4, 6, and 8) for 16 h, and the hydrolysates were analyzed by TLC. S, authentic oligosaccharides; X1, xylose; X2, xylobiose; X3, xylotriose; X4, xylotetraose; X5, xylopentaose.
The general characteristics of XynB, including temperature and pH for maximum activity, are very similar to those of xylanases (3). XynB hydrolyzed xylan substrates, releasing xylobiose and xylotriose as the major products. In addition, TLC analysis with xylooligosaccharides showed that all the xylooligosaccharides (xylotriose, xylotetraose, and xylopentaose) were hydrolyzed except for xylobiose, suggesting that XynB hydrolyzes xylan in a manner typical of many endo-ββ-1,4-xylanases (3, 40). XynB as a xylanase in the cellulosome should contribute to the degradation of the xylan present in plant cell walls, allowing the cellulosome access to cellulose chains that are buried in xylan and are not readily accessible unless xylan is hydrolyzed and removed.
Acknowledgments
This research was supported in part by a grant from the Research Institute of Innovative Technology for the Earth (RITE) and by U.S. Department of Energy grant DDF03-92ER20069.
REFERENCES
- 1.Attwood, G. T., H. P. Blaschek, and B. A. White. 1994. Transcriptional analysis of the Clostridium cellulovorans endoglucanase gene, engB. FEMS Microbiol. Lett. 124:277-284. [DOI] [PubMed] [Google Scholar]
- 2.Baba, T., R. Shinke, and T. Nanmori. 1994. Identification and characterization of clustered genes for thermostable xylan-degrading enzymes, β-xylosidase and xylanase, of Bacillus stearothermophilus 21. Appl. Environ. Microbiol. 60:2252-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beg, Q. K., M. Kapoor, L. Mahajan, and G. S. Hoondal. 2001. Microbial xylanases and their industrial applications: a review. Appl. Microbiol. Biotechnol. 56:326-338. [DOI] [PubMed] [Google Scholar]
- 4.Bequin, P. 1983. Detection of cellulase activity in polyacrylamide gels using Congo red-stained agar replicas. Anal. Biochem. 131:333-336. [DOI] [PubMed] [Google Scholar]
- 5.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 6.Breslauer, K. J., R. Frank, H. Blocker, and L. A. Marky. 1986. Predicting DNA duplex stability from the base sequence. Proc. Natl. Acad. Sci. USA 83:3746-3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang, B. Y., Y. T. Shyu, and R. H. Doi. 1992. The interaction between Bacillus subtilis sigma-A (σA) factor and RNA polymerase with promoters. Biochimie 74:601-612. [DOI] [PubMed] [Google Scholar]
- 8.Derewenda, U., L. Swenson, R. Green, Y. Wei, R. Morosoli, F. Shareck, D. Kluepfel, and Z. S. Derewenda. 1994. Crystal structure, at 2.6-A resolution, of the Streptomyces lividans xylanase A, a member of the F family of β-1,4-d-glycanases. J. Biol. Chem. 269:20811-20814. [PubMed] [Google Scholar]
- 9.Devillard, E., C. Bera-Maillet, H. J. Flint, K. P. Scott, C. J. Newbold, R. J. Wallace, J. P. Jouany, and E. Forano. 2003. Characterization of XYN10B, a modular xylanase from the ruminal protozoan Polyplastron multivesiculatum, with a family 22 carbohydrate-binding module that binds to cellulose. Biochem. J. 373:495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doi, R. H. 1991. Regulation of gene expression, p. 15-39. In U. N. Streips and R. E. Yasbin (ed.), Modern microbial genetics. Wiley-Liss, New York, N.Y.
- 11.Doi, R. H., A. Kosugi, K. Murashima, Y. Tamaru, and S. O. Han. 2003. Cellulosomes from mesophilic bacteria. J. Bacteriol. 185:5907-5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doi, R. H., and Y. Tamaru. 2001. The Clostridium cellulovorans cellulosome: an enzyme complex with plant cell wall degrading activity. Chem. Rec. 1:24-32. [DOI] [PubMed] [Google Scholar]
- 13.Dwivedi, P. P., M. D. Gibbs, D. J. Saul, and P. L. Bergquist. 1996. Cloning, sequencing and overexpression in Escherichia coli of a xylanase gene, xynA from the thermophilic bacterium Rt8B.4 genus Caldicellulosiruptor. Appl. Microbiol. Biotechnol. 45:86-93. [DOI] [PubMed] [Google Scholar]
- 14.Flint, H. J., J. Martin, C. A. McPherson, A. S. Daniel, and J. X. Zhang. 1993. A bifunctional enzyme, with separate xylanase and β(1,3-1,4)-glucanase domains, encoded by the xynD gene of Ruminococcus flavefaciens. J. Bacteriol. 175:2943-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fontes, C. M. G. A., G. P. Hazlewood, E. Morag, J. Hall, B. H. Hirst, and H. J. Gilbert. 1995. Evidence of a general role for non-catalytic thermostabilizing domains in xylanases from thermophilic bacteria. Biochem. J. 307:151-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukumura, M., K. Sakka, K. Shimada, and K. Ohmiya. 1995. Nucleotide sequence of the Clostridium stercorarium xynB gene encoding an extremely thermostable xylanase, and characterization of the translated product. Biosci. Biotechnol. Biochem. 59:40-46. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein, M. A., M. Takagi, S. Hashida, O. Shoseyov, R. H. Doi, and I. H. Segel. 1993. Characterization of the cellulose-binding domain of the Clostridium cellulovorans cellulose-binding protein A. J. Bacteriol. 175:5762-5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goryshin, I. Y., and W. S. Reznikoff. 1998. Tn5 in vitro transposition. J. Biol. Chem. 273:7367-7374. [DOI] [PubMed] [Google Scholar]
- 19.Han, S. O., H. Y. Cho, H. Yukawa, M. Inui, and R. H. Doi. 2004. Regulation of expression of cellulosomes and noncellulosomal (hemi)cellulolytic enzymes in Clostridium cellulovorans during growth on different carbon sources. J. Bacteriol. 186:4218-4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han, S. O., H. Yukawa, M. Inui, and R. H. Doi. 2003. Regulation of expression of cellulosomal cellulase and hemicellulase genes in Clostridium cellulovorans. J. Bacteriol. 185:6067-6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han, S. O., H. Yukawa, M. Inui, and R. H. Doi. 2003. Transcription of Clostridium cellulovorans cellulosomal cellulase and hemicellulase genes. J. Bacteriol. 185:2520-2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harley, C. B., and R. P. Reynolds. 1987. Analysis of E. coli promoter sequences. Nucleic Acids Res. 15:2343-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hawley, D. K., and W. R. McClure. 1983. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 11:2237-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayashi, H., K.-I. Takagi, M. Fukumura, T. Kimura, S. Karita, K. Sakka, and K. Ohmiya. 1997. Sequence of xynC and properties of XynC, a major component of the Clostridium thermocellum cellulosome. J. Bacteriol. 179:4246-4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henrissat, B., and A. Bairoch. 1996. Updating the sequence-based classification of glycosyl hydrolases. Biochem. J. 316:695-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kosugi, A., K. Murashima, and R. H. Doi. 2002. Characterization of two noncellulosomal subunits, ArfA and BgaA, from Clostridium cellulovorans that cooperate with the cellulosome in plant cell wall degradation. J. Bacteriol. 184:6859-6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kosugi, A., K. Murashima, and R. H. Doi. 2001. Characterization of xylanolytic enzymes in Clostridium cellulovorans: expression of xylanase activity dependent on growth substrates. J. Bacteriol. 183:7037-7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kosugi, A., K. Murashima, and R. H. Doi. 2002. Xylanase and acetyl xylan esterase activities of XynA, a key subunit of the Clostridium cellulovorans cellulosome for xylan degradation. Appl. Environ. Microbiol. 68:6399-6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 30.Lee, Y. E., S. E. Lowe, and J. G. Zeikus. 1993. Gene cloning, sequencing, and biochemical characterization of endoxylanase from Thermoanaerobacterium saccharolyticum B6A-RI. Appl. Environ. Microbiol. 59:3134-3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin, L. L., and J. A. Thomson. 1991. Cloning, sequencing and expression of a gene encoding a 73 kDa xylanase enzyme from the rumen anaerobe Butyrivibrio fibrisolvens H17c. Mol. Gen. Genet. 228:55-61. [DOI] [PubMed] [Google Scholar]
- 32.Liu, C. C., and R. H. Doi. 1998. Properties of exgS, a gene for a major subunit of the Clostridium cellulovorans cellulosome. Gene 211:39-47. [DOI] [PubMed] [Google Scholar]
- 33.Mach, R. L., and S. Zeilinger. 2003. Regulation of gene expression in industrial fungi: Trichoderma. Appl. Microbiol. Biotechnol. 60:515-522. [DOI] [PubMed] [Google Scholar]
- 34.Morag, E., E. A. Bayer, and R. Lamed. 1990. Relationship of cellulosomal and noncellulosomal xylanases of Clostridium thermocellum to cellulose-degrading enzymes. J. Bacteriol. 172:6098-6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murashima, K., A. Kosugi, and R. H. Doi. 2002. Determination of subunit composition of Clostridium cellulovorans cellulosomes that degrade plant cell walls. Appl. Environ. Microbiol. 68:1610-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura, M., T. Nagamine, A. Takenaka, R. I. Aminov, K. Ogata, K. Tajima, H. Matsui, Y. Benno, and H. Itabashi. 2002. Molecular cloning, nucleotide sequence and characteristics of a xylanase gene (xynA) from Ruminococcus albus 7. Anim. Sci. J. 73:347-352. [Google Scholar]
- 37.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. A neural network method for identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Int. J. Neural Syst. 8:581-599. [DOI] [PubMed] [Google Scholar]
- 38.Park, J. S., Y. Matano, and R. H. Doi. 2001. Cohesin-dockerin interactions of cellulosomal subunits of Clostridium cellulovorans. J. Bacteriol. 183:5431-5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenberg, M., and D. Court. 1979. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu. Rev. Genet. 13:319-353. [DOI] [PubMed] [Google Scholar]
- 40.Saha, B. C. 2003. Hemicellulose bioconversion. J. Ind. Microbiol. Biotechnol. 30:279-291. [DOI] [PubMed] [Google Scholar]
- 41.Sakka, K., Y. Kojima, T. Kondo, S. Karita, K. Ohmiya, and K. Shimada. 1993. Nucleotide sequence of the Clostridium stercorarium xynA gene encoding xylanase A: identification of catalytic and cellulose binding domains. Biosci. Biotechnol. Biochem. 57:273-277. [DOI] [PubMed] [Google Scholar]
- 42.Shoseyov, O., and R. H. Doi. 1990. Essential 170-kDa subunit for degradation of crystalline cellulose by Clostridium cellulovorans cellulase. Proc. Natl. Acad. Sci. USA 87:2192-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shoseyov, O., M. Takagi, M. A. Goldstein, and R. H. Doi. 1992. Primary sequence analysis of Clostridium cellulovorans cellulose binding protein A. Proc. Natl. Acad. Sci. USA 89:3483-3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sleat, R., R. A. Mah, and R. Robinson. 1984. Isolation and characterization of an anaerobic, cellulolytic bacterium, Clostridium cellulovorans sp. nov. Appl. Environ. Microbiol. 48:88-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sugimoto, N., S. Nakano, M. Katoh, A. Matsumura, H. Nakamuta, T. Ohmichi, M. Yoneyama, and M. Sasaki. 1995. Thermodynamic parameters to predict stability of RNA/DNA hybrid duplexes. Biochemistry 34:11211-11216. [DOI] [PubMed] [Google Scholar]
- 46.Takami, H., K. Nakasone, Y. Takaki, G. Maeno, R. Sasaki, N. Masui, F. Fuji, C. Hirama, Y. Nakamura, N. Ogasawara, S. Kuhara, and K. Horikoshi. 2000. Complete genome sequence of the alkaliphilic bacterium Bacillus halodurans and genomic sequence comparison with Bacillus subtilis. Nucleic Acids Res. 28:4317-4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tamaru, Y., and R. H. Doi. 2000. The engL gene cluster of Clostridium cellulovorans contains a gene for cellulosomal manA. J. Bacteriol. 182:244-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tamaru, Y., and R. H. Doi. 1999. Three surface layer homology domains at the N terminus of the Clostridium cellulovorans major cellulosomal subunit EngE. J. Bacteriol. 181:3270-3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tamaru, Y., S. Karita, A. Ibrahim, H. Chan, and R. H. Doi. 2000. A large gene cluster for the Clostridium cellulovorans cellulosome. J. Bacteriol. 182:5906-5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomson, J. A. 1993. Molecular biology of xylan degradation. FEMS Microbiol. Lett. 104:65-82. [DOI] [PubMed] [Google Scholar]
- 51.Tull, D., S. G. Withers, N. R. Gilkes, D. G. Kilburn, R. A. Warren, and R. Aebersold. 1991. Glutamic acid 274 is the nucleophile in the active site of a “retaining” exoglucanase from Cellulomonas fimi. J. Biol. Chem. 266:15621-15625. [PubMed] [Google Scholar]
- 52.Uffen, R. L. 1997. Xylan degradation: a glimpse at microbial diversity. J. Ind. Microbiol. Biotechnol. 19:1-6. [Google Scholar]
- 53.Wang, Q., D. Tull, A. Meinke, N. R. Gilkes, R. A. Warren, R. Aebersold, and S. G. Withers. 1993. Glu280 is the nucleophile in the active site of Clostridium thermocellum CelC, a family A endo-β-1,4-glucanase. J. Biol. Chem. 268:14096-14102. [PubMed] [Google Scholar]
- 54.White, A., S. G. Withers, N. R. Gilkes, and D. R. Rose. 1994. Crystal structure of the catalytic domain of the β-1,4-glycanase cex from Cellulomonas fimi. Biochemistry 33:12546-12552. [DOI] [PubMed] [Google Scholar]
- 55.Winterhalter, C., P. Heinrich, A. Candussio, G. Wich, and W. Liebl. 1995. Identification of a novel cellulose-binding domain within the multidomain 120 kDa xylanase XynA of the hyperthermophilic bacterium Thermotoga maritima. Mol. Microbiol. 15:431-444. [DOI] [PubMed] [Google Scholar]
- 56.Young, M., N. P. Minton, and W. L. Staudenbauer. 1989. Recent advances in the genetics of the clostridia. FEMS Microbiol. Rev. 5:301-325. [DOI] [PubMed] [Google Scholar]
- 57.Zeilinger, S., R. L. Mach, M. Schindler, P. Herzog, and C. P. Kubicek. 1996. Different inducibility of expression of the two xylanase genes xyn1 and xyn2 in Trichoderma reesei. J. Biol. Chem. 271:25624-25629. [DOI] [PubMed] [Google Scholar]
- 58.Zhang, J. X., and H. J. Flint. 1992. A bifunctional xylanase encoded by the xynA gene of the rumen cellulolytic bacterium Ruminococcus flavefaciens 17 comprises two dissimilar domains linked by an asparagine/glutamine-rich sequence. Mol. Microbiol. 6:1013-1023. [DOI] [PubMed] [Google Scholar]
- 59.Zverlov, V. V., K. Piotukh, O. Dakhova, G. Velikodvorskaya, and R. Borriss. 1996. The multidomain xylanase A of the hyperthermophilic bacterium Thermotoga neapolitana is extremely thermoresistant. Appl. Microbiol. Biotechnol. 45:245-247. [DOI] [PubMed] [Google Scholar]