Abstract
Subclinical left ventricular (LV) dysfunction refers to subtle abnormalities in LV function which typically precede a reduction in the left ventricular ejection fraction (LVEF). The assessment of myocardial function using LVEF, a radial metric of systolic function, is subject to load dependence, intra-observer and inter-observer variability. Reductions in LVEF typically manifest late in the disease process thus compromising the ability to intervene before irreversible impairment of systolic perfor-mance sets in. 2-Dimensional speckle tracking echocardiography (2D-STE), a novel strain imaging modality has shown promise as a sensitive indicator of myocardial contractility. It arms the clinician with a powerful and practical tool to rapidly quantify cardiac mechanics, circumventing several inherent limitations of conventional echocardiography. This article highlights the incremental utility of 2D-STE in the detection of subclinical LV dysfunction.
Keywords: Subclinical left ventricular dysfunction, 2-dimensional speckle tracking echocardiography, Global longitudinal strain
INTRODUCTION
Strain refers to the change in the length of a segment of the myocardium relative to its original length and strain rate refers to the net change in strain per unit time. 2-Dimensional speckle tracking echocardiography (2D-STE), an angle-independent technique, employs automated algorithms to analyze temporal variations (frame to frame) in the mobility of acoustic speckle markers of 20-40 pixels size along different spatial orientations such as longitudinal, circumferential and radial planes to then quantitatively derive respective strain or deformational parameters. The endocardium contributes maximally to longitudinal strain (LS). Location of the endocardium farthest away from the epicardial coronary arteries and its exquisite sensitivity to ischemia makes it vulnerable to a variety of insults thereby making LS a highly sensitive indicator of LV dysfunction. In contrast, radial strain (RS), circumferential strain (CS) and torsional strains (TS) are affected later in the disease and are widely acknowledged to have lower inter- and intra-observer reproducibility [1].
One of the utilities of myocardial strain might be timely identification of subclinical left ventricular (LV) dysfunction, which could provide the clinician with a wider therapeutic window to potentially arrest or delay progression to clinically evident cardiovascular disease or impairment of myocardial performance. In this article, subclinical LV dysfunction refers to a compromise in LV function prior to reduction in LVEF ≤ 50%. The focus of this article is to highlight: 1. The clinical entities where subclinical LV dysfunction has been described by 2D-STE and 2. The potential incremental value of 2D-STE over conventional 2D-echocardiography in the management of these patients.
APPLICATIONS OF STRAIN
i). Valvular Heart Disease
A clear consensus on the timing of valve repair or replacement in asymptomatic patients with preserved LVEF is currently lacking [2]. In addition to the detection of subclinical LV dysfunction, strain parameters have shown promise for the prediction of post-surgical LV dysfunction. Myocardial strain could plausibly assist in the identification of high-risk patients who might benefit from early surgical intervention prior to a decline in the LVEF below guideline recommended threshold levels for surgical intervention (Table 1). Adaptive remodeling in early aortic regurgitation (AR) preserves LVEF by a compensatory rise in RS and CS for the
Table 1. Studies evaluating strain in valvular heart disease with speckle tracking echocardiography.
| Study | Sample size with description | Imaging modality (vendor name) | Objective | Outcomes | ||||
|---|---|---|---|---|---|---|---|---|
| Aortic Regurgitation | ||||||||
| Mizariene et al. [41] | Chronic AR:NT-proBNP<400 (n=44) vs. >400 vs. controls (n=64) | 2D-STE (EchoPac-GE) | Effect of LV strain on NT-proBNP | GLS>-16% predicts levels>400 | ||||
| Olsen et al. [5] | Chronic AR: Surgical (n=29) vs. conservative (n=35) | 2D-STE (EchoPAC-GE) | Strain indices in AR progression | Progression correlates with reduced systolic (-1.04 vs. -1.19s-1), early diastolic (1.2 vs. 1.6s-1) and myocardial SR (-16.3 vs. -19%) | ||||
| Smedsrud et al. [6] | Chronic AR (n=47) vs. controls (n=31) | 2D-STE (EchoPAC-GE) | GLS impairment and early LV dysfunction | Reduced GLS (-17.5 vs.-22.1%) identifies subclinical LV dysfunction; GLS and not LVEF correlates with post-operative LV function | ||||
| Kaneko et al. [37] | Chronic AR (n=36) vs. controls (n=15) | 2D-STE (Toshiba) | GRS decline-marker of myocardial dysfunction? | Endocardial GRS loss (28.9 vs. 37.1%) predicts myocardial dysfunction | ||||
| Di Salvo et al. [4] | Stable (n=17) vs. progressive AR (n=9) | 2D-STE (EchoPAC) | Strain parameters and AR progression | LV LS (cut-off >-19.5%) - only predictor for AR Progression | ||||
| Aortic Stenosis | ||||||||
| Van Dalen et al. [11] | AS (n=60) vs. controls (n=30) | 2D-STE (QLAB-Philips) | Twist indices and severity of AS, subendocardial ischemia | Elevated peak systolic LV twist (13.6 vs. 11.4) and endocardial twist-shortening ratio (0.6vs.0.4) correlates with ischemia and AS severity | ||||
| Ng et al. [9] | Aortic sclerosis (n=118) vs. Mild (n=81) vs. moderate (n=109) vs. severe (n=112) AS | 2D-STE (EchoPAC-GE) | Strain and severity of AS | Sequential deterioration in LS, CS, RS and SR correlates with progressive reduction in valve area | ||||
| Levy et al. [42] | Severe symptomatic AS | 2D-STE (EchoPAC-GE) | GLS on post-surgical atrial fibrillation rates | GLS>-15% predicts post-surgical atrial fibrillation | ||||
| Delgado et al. [10] | Severe AS (n=73) vs. controls (n=40) | 2D-STE (EchoPAC-GE) | Changes in LV strain pre and post-surgery | GLS (-14.6 vs. -20.3%) and SR-impaired despite pEF. LS, CS and RS improved following valve replacement | ||||
| Mitral Stenosis | ||||||||
| Ozdemir et al. [12] | Mild-moderate MS (n=60) vs. controls (n=52) | 2D-ECHO, STE (EchoPAC-GE) | LV dysfunction in MS with pEF | GLS (-17 vs.-19%) and GLS rate compromised in MS | ||||
| Mitral Regurgitation | ||||||||
| Isla et al. [7] | Chronic severe MR (n=38) | 2D, 3D-ECHO, Doppler ECHO and 2D-STE (Q-Lab) | Preoperative strain on post-operative LV dysfunction | Longitudinal SR at mid interventricular septal level <-0.8s-1 -best predictors of LVEF reduction > 10% | ||||
| Kim et al. [43] | Chronic severe MR (n=59) vs. controls (n=34) | 2D-ECHO,angiography and 2D-STE (EchoPac-GE) | Strain assessment of latent LV dysfunction | Peak systolic radial SR of 2.0 s-1 best correlates with peak dP/dt; short axis function offers better prediction | ||||
| Study | Sample size with description | Imaging modality (vendor name) | Objective | Outcomes | ||||
| Florescu et al. [8] | Severe primary MR (n=28) vs. controls (n=10) | 2D-ECHO, TVI and STE (EchoPac-GE) | Pre-op strain and post-valve replacement LV function | Systolic TVI and the combination of systolic TVI and LS are main independent predictors of post-operative LVEF drop >10% | ||||
| Pandis et al. [44] | Degenerative MR (n=40) | 2D, 3D-ECHO, and 2D-STE (TomTec) | Predictors of recurrent MR post-surgery | Mid-lateral RS≤-27 and apical lateral RS ≤-25-significant predictors | ||||
| Mascle et al. [45] | Severe degenerative MR: post op LVEF≥50% (n=73) + LVEF<50% (n=15) | 2D-ECHO, 2D-STE and TDI (EchoPAC-GE) | Pre-operative GLS on post-operative LV dysfunction | GLS >-18% predicts post-operative LVEF drop to <50% | ||||
2D, 2 dimensional; 3D, 3 dimensional; AS, aortic stenosis; AR, aortic regurgitation; CS, circumferential strain; ECHO, echocardiography; GLS, global longitudinal strain; GRS, global radial strain; LS, longitudinal strain; LV, left ventricle; LVEF, left ventricular ejection fraction; MS, mitral stenosis; MR, mitral regurgitation; NT-proBNP, N terminal-pro brain natriuretic peptide; pEF, preserved ejection fraction; RS, radial strain; SR, strain rate; TDI, tissue Doppler imaging; TVI, tissue velocity imaging.
early loss of LS with additional compensation provided by LV hypertrophy and increase in LV volume [3]. Reduction in LVEF to <50% in asymptomatic AR heralds an increase in the rate of progression to symptoms, a surgical indication, from 4.3% to 25% per year [2]. Amongst patients with preserved ejection fraction (pEF), LS attenuation >-19.5% is 77% sensitive and 94% specific for symptomatic progression of asymptomatic moderate-severe AR [4]. Pre-operative GLS attenuation, but not LVEF, has been associated with post-operative LV dysfunction [5, 6]. Likewise, in mitral regurgitation, pre-operative GLS impairment (GLS<-18%) has been shown to identify subclinical LV dysfunction in patients with LVEF>60% and also predict post-operative LV dysfunction [7, 8].
In aortic stenosis (AS), the degree of strain deterioration correlates significantly with lower aortic valve (AV) area and severity of AS [9] while strain recovery has been noted post AV replacement [10], even prior to recovery of LVEF. Furthermore, altered twist mechanics in AS offers a non-invasive means of predicting subendocardial ischemia [11]. Similarly, identification of subclinical LV dysfunction by attenuated GLS and GLS rate in asymptomatic or minimally symptomatic mitral stenosis (MS) [12] might aid in optimal timing of mitral valve (MV) interventions prior to permanent myocardial remodeling [13, 14].
ii). Coronary Artery Disease
2D-STE is robust in terms of its prognostic utility and reproducibility in the assessment of subclinical CAD (Table 2) [15]. Various strain parameters such as early myocardial systolic lengthening (15), ratio of peak systolic LS difference to peak systolic GLS [16] and a combination of early systolic and early diastolic SR [17] provide incremental value over GLS for obstructive CAD. Strain assessment during dobutamine stress echocardiography (DSE) could enhance the sensitivity of conventional 2D-echo by minimizing inter-observer variability in delineating endocardial borders and interpreting wall motion abnormalities as demonstrated by using software such as automated functional imaging (AFI). Strain rate imaging offers incremental value over DSE derived wall motion scores in the prediction of mortality [18]. Additionally, a remarkable coronary territorial correlation between AFI derived strain and angiography with a sensitivity >90% in the anterior and >79% in the posterior circulations has been demonstrated [16, 18].
Table 2. Studies highlighting utility of speckle tracking echocardiography in subclinical coronary artery disease.
| Study | Sample size with description | Imaging modality with vendor name | Objective | Outcomes |
|---|---|---|---|---|
| Nucifora et al. [46] | Non-obstructive (n=60) vs. obstructive (n=63) vs. no CAD (n=59) | CT angiography and 2D-STE (EchoPAC-GE) | GLS prediction of obstructive CAD | GLS≥-17.4% predicts obstructive CAD better than diastolic dysfunction or Duke clinical scores |
| Hanekom et al. [47] | Significant CAD (150) | 2D-STE, TVI, DSE and coronary angiography (EchoPAC-GE) | Strain from STE vs. TVI during DSE in predicting obstructive CAD | STE-equally efficacious to TVI in anterior but not in posterior circulation |
| Liang et al. [17] | Obstructive (n=39) vs. non-obstructive or no CAD (n=15) | 2D-STE, coronary angiography (EchoPAC-GE) | Diastolic strain in obstructive CAD | Early diastolic SR impairment is superior to systolic strain: 77% sensitive, 93% specific |
| Tsai et al. [16] | CAD (n=75) vs. no CAD (n=77) | STE, coronary angiography (EchoPAC-GE) | LS in prediction of obstructive CAD | Sensitivity, specificity of GLS>-19%: 75%, 81%; peak segmental LS difference/peak systolic GLS ratio>1: 77%, 79% |
| Smedsrud et al. [15] | Significant (n=43) vs. non-significant CAD (n=43) | 2D-STE, coronary angiography (EchoPac-GE) | Does duration of early systolic lengthening predict significant CAD? | Early systolic lengthening (cutoff 58 milliseconds) predicts significant CAD better than peak systolic LS attenuation |
2D-STE, 2 dimensional-speckle tracking echocardiography; CAD, coronary artery disease; CT, computerized tomography; DSE, dobutamine stress echocardiography; GLS, global longitudinal strain; LS, longitudinal strain; pEF, preserved ejection fraction; SR, strain rate; TVI, tissue velocity imaging.
iii). Hypertension and Diabetes Mellitus
Attenuation of 2D-STE derived GLS presents a vital differentiating parameter between hypertensive heart disease and an athlete’s heart while providing excellent correlation with the magnitude of diastolic dysfunction in hypertensives [19]. Detection of LS impairment by 2D-STE precedes detection by tissue Doppler imaging (TDI) in hypertensives with preserved LVEF even prior to the development of left ventricular hypertrophy (LVH) [20] thus providing an early opportunity to arrest progression to LVH. Indeed, preserved CS and consequential wall thickening despite reduction in LS has been posited to explain preservation of LVEF in patients with systemic hypertension [21].
2D-STE derived GLS attenuation might be useful for risk stratification in asymptomatic diabetics via prediction of subclinical systolic LV dysfunction with the decrease in LS being proportional to diabetes duration [22]. Furthermore, impairment of GLS has been associated with higher coronary artery calcium scores in diabetics, a surrogate for subclinical coronary atherosclerosis [23].
iv). Heart Failure with Preserved Ejection Fraction (HFpEF)
Although diastolic dysfunction is believed to be the underlying pathophysiology in HFpEF, recent studies have indicated abnormalities of systolic function in these patients [24]. Indeed, traditional diastolic dysfunction parameters were absent in approximately one-third of the HFpEF trial cohorts [25, 26]. Systolic impairment of strain parameters (LS, CS) has been demonstrated in HFpEF patients, with LS attenuation being associated with higher NT-pro BNP levels [27]. 2D-STE derived increases in LS delay index and time to achieve peak longitudinal velocity unveil the dysfunctional systolic component of HFpEF indicating dyssynchronous and ineffective ventricular contraction [28] (Table 3). Previous studies have hypothesized that reduction of dyssynchrony by cardiac resynchronization therapy could translate into energy efficient contractility and mitigate progression to systolic failure [28].
Table 3. Evaluation of heart failure with preserved ejection fraction with speckle tracking echocardiography.
| Study | Sample size with description | Imaging modality with vendor name | Objective | Outcomes |
|---|---|---|---|---|
| Phan et al. [48] | HfPEF (n=47) vs. young (n=27) vs. old controls (n=26) | 2D-ECHO, 2D-STE (EchoPAC-GE) | Comparing strain profiles: HfPEF vs. age related changes | Increased TS seen with ageing. CS increase (-24.7% vs. -20%) differentiates HfPEF from old controls |
| Phan et al. [28] | HfPEF (n=38) vs. controls (n=33) | 2D-ECHO and 2D-STE (EchoPAC-GE) | Dyssynchrony assessment in HfPEF | Systolic and diastolic dyssynchrony observed. LS impairment (-17.6% vs. -19.9%) and higher LV dyssynchrony (LS delay index-14.4% vs.-10.7%) noted |
| Donal et al. [39] | HfPEF (n=21) vs. controls (n=15) | 2D-ECHO, exercise stress testing and 2D-STE (EchoPAC-GE) | To characterize resting and exercise induced strain changes | GLS impaired at rest [Rest: (-16% vs. -20%) and aggravated by stress: (-17% vs. -23%); similar trend with global CS |
| Kraigher-Krainer et al. [27] | HfPEF (n=219) vs. controls (n=50) and Hypertensive Heart Disease (n=44) | Vendor independent STE Software | Strain parameters in HfPEF | Lower LS and CS in HfPEF (-14.6±3.3 vs.. 20.0±2.1 in controls); Lower LS values co-related with higher NT-ProBNP |
2D, 2 dimensional; CS, circumferential strain; ECHO, echocardiography; GLS, global longitudinal strain; HfPEF, heart failure with preserved ejection fraction; LS, longitudinal strain; RS, radial strain; STE, speckle tracking echocardiography; TS: torsional strain; BNP: Brain Natiuretic Peptide.
v). Perimyocarditis
Incipient myocardial dysfunction has been demonstrated in acute perimyocarditis with compromise in GLS and twist angle preceding reduction of LVEF [29] (Fig. 1). Impairment of CS and TS with intact LS in constrictive pericarditis as opposed to impaired LS and intact CS in restrictive cardiomyopathy effectively differentiates these conditions [30].
Fig. (1).
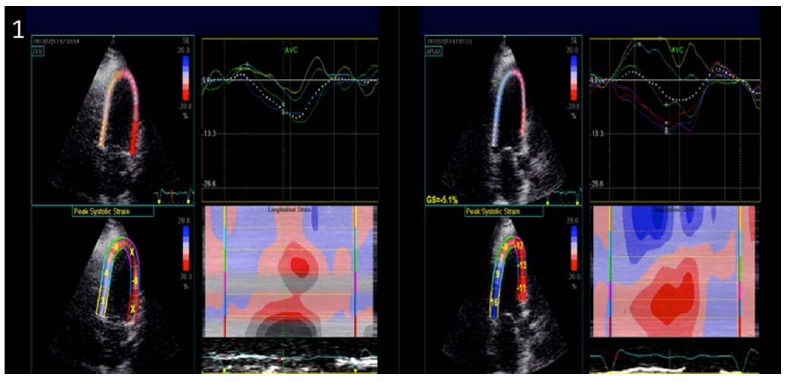
Paradoxical longitudinal strain in inferior and inferolateral walls in a patient admitted with uremic pericarditis and found to have newly reduced systolic function without any wall motion abnormalities, suggestive of perimyocarditis. The systolic function recovered following treatment for pericarditis in an echocardiogram performed 2 weeks later.
vi). Cancer Chemotherapy
Routine serial assessment of LV strain parameters could aid in earlier identification of chemotherapy-induced cardiotoxicity prior to conventional changes in LVEF (Table 4). Attenuation of 2D-STE derived LV apical cap peak systolic strain [31], GLS, torsion, twisting and untwisting rates occur as early as 1 month after anthracycline (AT) therapy, preceding impairment in LVEF and Doppler parameters and also offers excellent correlation with cumulative AT dose [32]. AT therapy has been associated with a 16% prevalence of higher LV systolic dyssynchrony index reflective of energy inefficient contraction, positing a role for cardiac resynchronization therapy in these patients. Likewise, strain imaging has been shown to recognize pre-clinical changes in systolic function in prospectively followed breast cancer patients on adjuvant trastuzumab therapy [31].
Table 4. Strain imaging in the assessment of chemotherapy associated cardio toxicity.
| Study | Sample size with description | Imaging modality with vendor name | Objective | Outcomes |
|---|---|---|---|---|
| Tsai et al. [40] | Hodgkin's lymphoma and radiotherapy (n=47) [AT (n=27), No AT (n=20)] vs. controls (n=20) | 2D-ECHO and 2D-STE (EchoPAC-GE) | LV dysfunction with anthracycline (AT) and radiotherapy (RT) | Higher GLS impairment in RT+AT (-16.1%) than RT and no AT (-17.5%); CS-similar trend; pEF-all groups |
| Poterucha et al. [31] | AT (n=19) vs. controls (n=19) | 2D-ECHO and 2D-STE (EchoPAC-GE) | Temporal changes in strain and LVEF | Longitudinal PSS reduction started at 4 months (8.7%) and worsened by 8 months (9.2%) with AT. LVEF decreased by 4.3% only at 8 months. |
| Motoki et al. [32] | AT therapy (n=25) | 2D-ECHO, TDI and 2D-STE (EchoPAC-GE) | Torsional strain and subclinical LV dysfunction | Impairment in GLS, TS, twisting and untwisting rates occur as early as 1 month; LVEF, TDI indices-preserved |
| Ho et al. [49] | AT (n=19) vs. AT+TZ (n=51) vs. controls (n=50) | 2D-ECHO, Doppler ECHO and 2D-STE (EchoPAC-GE) | Subclinical LV dysfunction in combination therapy (AT+TZ) | Reduced GLS in AT (-17.7) vs. AT+TZ (-19.2) vs. controls (-19.6). No additive cardiotoxicity with TZ |
| Fallah-Rad et al. [50] | No toxicity (n=10) vs. TZ toxicity (n=32) | TVI, CMR and 2D-STE (EchoPac-GE) | Detection of TZ cardiotoxicity with various techniques | 2D-STE impairment in GLS (-19.9 ± 1.8% to. -16.4 ± 1.1%) and GRS (42.4 ± 10.5% to. 32.5 ± 15.2%) occur earliest (3 months). LVEF preserved until 6 months |
2D-ECHO, 2 dimensional echocardiography; 2D-STE, 2 dimensional speckle tracking echocardiography; AT, anthracycline; CMR, cardiac magnetic resonance; CS, circumferential strain; GLS, global longitudinal strain; GRS, global radial strain; LV, left ventricle; LVEF, left ventricular ejection fraction; pEF, preserved ejection fraction; PSS, peak systolic strain; RS, radial strain; RT, radiotherapy; TS, torsional strain; TDI, tissue Doppler imaging; TZ, trastuzumab.
Miscellaneous Conditions
Apical sparing, evidenced by relative apical LS>1 (mean apical LS/mean basal LS+ mean mid-LS) discriminates cardiac amyloidosis from AS or hypertrophic cardiomyopathy induced LVH with a sensitivity>90% and specificity>80% [32] (Fig. 2). LS impairment is associated with elevated NT-proBNP in Behcet’s disease (Table 5). In Fabry’s disease, systolic strain attenuation in the basal postero-lateral segments offers a sensitivity and specificity of 90% and 97% respectively, for the identification of MRI confirmed fibrotic arrhythmogenic foci [33]. Systemic Sclerosis is characterized by impairment of global and LV longitudinal peak systolic strain (PSS) with higher attenuation in the basal segments and sparing of the apical and medial segments [34]. Moreover, reduced GLS and global CS were independently predictive of ventricular arrhythmias in systemic sclerosis patients [35].
Fig. (2).
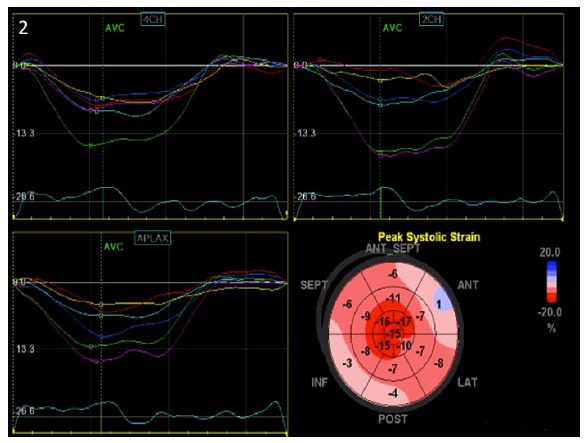
Reduced basal and mid-segmental strain values with apical sparing suggestive of amyloidosis in the presence of normal systolic function.
Table 5. Miscellaneous conditions underscoring the clinical applicability of speckle tracking echocardiography.
| Study | Sample size with description | Imaging modality with vendor name | Objective | Outcomes |
|---|---|---|---|---|
| Yagmur et al. | Behcets disease (n=32) vs. controls (n=27) | 2D-ECHO and 2D-STE (QLab-Philips) | Detection of subclinical LV dysfunction | Reduced LS (-17.8 ± 2.7%) in disease vs. controls (-20.5 ± 1.8%); NT-proBNP independent corelate of mean LS |
| Liu et al. [51] | Hemodialysis patients (n=102) | 2D-ECHO, TDI and 2D-STE (EchoPac-GE) | Predictors of significant CAD despite pEF in hemodialysis patients | Reduction in LS ≥-15% in ≥6 myocardial segments predicts CAD |
| Dedobbeleer et al. [52] | Friedreich’s ataxia (n=20) vs. controls (n=20) | 2D-ECHO and 2D-STE (Qlab-Philips) | Stain profiling, detection of subclinical LV dysfunction | Reduced GLS (-15.3 vs. -17.5%), peak LV twist and untwisting rates |
| Caputo et al. [53] | At least 1 cardiovascular risk factor (n=70) | 2D-ECHO, TDI and 2D-STE (EchoPAC-GE) | Abnormal LV strain in overweight (BMI) despite pEF | Peak LS of LV-reduced in overweight vs. normal BMI (-17.2% vs. -18.7%) |
| Takamura et al. [54] | Acute PE (n=25) vs. controls (n=25) | 2D-ECHO and 2D-STE (EchoPAC-GE) | Impact of acute RV pressure overload on LV strain | Global LS (-16 vs. -20), CS (-17 vs. -24) and RS (44 vs. 59) reduced in acute PE, recover with the resolution of pressure overload |
| Shahul et al. [55] | Preeclampsia (n=11) vs. non-proteinuric hypertension (n=11) vs. normotensives (n=17) | 2D-ECHO and 2D-STE (TomTec) | Is subclinical LV dysfunction inherent to preeclampsia? | Impaired GLS (-13.7 vs. -15.9 vs. -20.1), GRS (22.4 vs. 40.7 vs. 39.8) and GCS (-17.9 vs. -28.2 vs. -21.6) in preeclampsia. pEF in all groups |
| Inoue et al. [56] | RV apical pacing (n=51)+ RV septal pacing (n=52) vs. controls (n=50) | 2D-ECHO and 2D-STE (EchoPAC-GE) | Subclinical LV dysfunction with RV apical pacing | Maximal impairment of GLS with RV apical pacing [-14.3 vs. -16.8 vs. -18.2] |
| Miszalski-Jamka et al. [57-59] | Wegener's granulomatosis (n=22) vs. controls (n=22) | 2D-ECHO and 2D-STE (EchoPAC-GE) | Subclinical LV dysfunction identification | Global LS (-17.9 vs.-19.7), CS (-18.4 vs. -21.6) and RS (38.8 vs. 50.1) impairment noted, correlate with disease severity. |
2D-ECHO, 2 dimensional echocardiography; 2D-STE, 2 dimensional speckle tracking echocardiography; BMI, body mass index; CAD, coronary artery disease; CS, circumferential strain; GCS, global circumferential strain, GRS, global radial strain; LS, longitudinal strain; LV, left ventricle; PE, pulmonary embolism; pEF, preserved ejection fraction; RS, radial strain; RV, right ventricle; TDI, tissue Doppler imaging.
Strain derived radial LV wall motion discoordination was shown to be an independent predictor of decline in cardiac output in acute pulmonary embolism [54]. Subclinical LV dysfunction inherent to preeclampsia has been identified by a higher decline in global LS, RS and CS than in non-proteinuric hypertension [55]. Sickle cell crisis results in transient LV dysfunction with reversal of regional LS impairment on crisis resolution [38].
Obligatory Need for Standardization in Speckle Tracking Echocardiography
Marwick et al. have described discordant strain values from different vendors to result from three components: 1) Image procurement and storage resolution; 2) Processing of image data for strain quantification and 3) Hemodynamic status [35]. Image acquisition with vendor specific ultrasound machines, heterogeneity in frame rates used for compression (30 frames/sec for vendor neutral software), differences in the methodology of algorithms used to derive strain, types of strain used (natural or Lagrangian strain), anatomical regions studied to derive strain (endocardial, epicardial or both), phase of the cardiac cycle in which images are acquired and blood pressure variations during image procurement are all sources of discrepancies [35]. Head-to-head comparisons between different combinations of ultrasound machines and vendor specific versus vendor neutral post-processing software has depicted higher variations from post-processing and not image acquisition [36]. When vendor neutral software is used, standardization of methods to derive strain has been shown to minimize GLS variations even at frame rates as low as 30 frames/second [39, 40].
A concerted effort to standardize strain software between a conglomerate of vendors supported by the American Society of Echocardiography and the European Society of Echocardiography is afoot. It is hoped that this endeavor will eventually culminate into standardization of postprocessing software to minimize variability between vendor offerings.
Future Directions
Barriers to use of strain imaging in routine practice include physician reluctance to adopt innovative technology with perceived complexity, lack of standardization and reimbursement limitations. Whether interventions following identification of subclinical LV dysfunction could translate into reduced cardiovascular morbidity and mortality needs further appraisal in prospective randomized controlled trials. Further technologic refinements targeting these limitations could transform 2D-STE into a robust modality for the routine detection of subclinical myocardial dysfunction, given its increasing ease of use and wide applicability.
ACKNOWLEDGEMENTS
Declared none.
List of Abbreviations
- 2D-ECHO
2-dimensional echocardiography
- 2D-STE
2-dimensional speckle tracking echocardiography
- AF
Atrial fibrillation
- AFI
Automated functional imaging
- AR
Aortic regurgitation
- AS
Aortic stenosis
- AT
Anthracycline
- AV
Aortic valve
- CAD
Coronary artery disease
- CS
Circumferential strain
- DSE
Dobutamine stress echocardiography
- GLS
Global longitudinal stain
- HFpEf
Heart failure with preserved ejection fraction
- LS
Longitudinal strain
- LV
Left ventricle
- LVEF
Left ventricular ejection fraction
- MS
Mitral stenosis
- MV
Mitral valve
- NT-proBNP
N terminal-pro brain natriuretic peptide
- pEF
Preserved ejection fraction
- PSS
Peak systolic strain
- RS
Radial strain
- RV
Right ventricle
- SR
Strain rate
- TS
Torsional strain
- TDI
Tissue doppler imaging
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Leischik R., Dworrak B., Hensel K. Intraobserver and interobserver reproducibility for radial, circumferential and longitudinal strain echocardiography. Open Cardiovasc. Med. J. 2014;8:102–109. doi: 10.2174/1874192401408010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonow R.O., Carabello B.A., Chatterjee K., et al. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2008;118(15):e523–e661. doi: 10.1161/CIRCULATIONAHA.108.190748. [DOI] [PubMed] [Google Scholar]
- 3.Iida N., Seo Y., Ishizu T., et al. Transmural compensation of myocardial deformation to preserve left ventricular ejection performance in chronic aortic regurgitation. J. Am. Soc. Echocardiogr. 2012;25(6):620–628. doi: 10.1016/j.echo.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Di Salvo G., Rea A., Mormile A., et al. Usefulness of bidimensional strain imaging for predicting outcome in asymptomatic patients aged </= 16 years with isolated moderate to severe aortic regurgitation. Am. J. Cardiol. 2012;110(7):1051–1055. doi: 10.1016/j.amjcard.2012.05.039. [DOI] [PubMed] [Google Scholar]
- 5.Olsen N.T., Sogaard P., Larsson H.B., et al. Speckle-tracking echocardiography for predicting outcome in chronic aortic regurgitation during conservative management and after surgery. JACC Cardiovasc. Imaging. 2011;4(3):223–230. doi: 10.1016/j.jcmg.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Smedsrud M.K., Pettersen E., Gjesdal O., et al. Detection of left ventricular dysfunction by global longitudinal systolic strain in patients with chronic aortic regurgitation. J. Am. Soc. Echocardiogr. 2011;24(11):1253–1259. doi: 10.1016/j.echo.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 7.de Isla L.P., de Agustin A., Rodrigo J.L., et al. Chronic mitral regurgitation: a pilot study to assess preoperative left ventricular contractile function using speckle-tracking echocardiography. J. Am. Soc. Echocardiogr. 2009;22(7):831–838. doi: 10.1016/j.echo.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 8.Florescu M., Benea D.C., Rimbas R.C., et al. Myocardial systolic velocities and deformation assessed by speckle tracking for early detection of left ventricular dysfunction in asymptomatic patients with severe primary mitral regurgitation. Echocardiography. 2012;29(3):326–333. doi: 10.1111/j.1540-8175.2011.01563.x. [DOI] [PubMed] [Google Scholar]
- 9.Ng A.C., Delgado V., Bertini M., et al. Alterations in multidirectional myocardial functions in patients with aortic stenosis and preserved ejection fraction: a two-dimensional speckle tracking analysis. Eur. Heart J. 2011;32(12):1542–1550. doi: 10.1093/eurheartj/ehr084. [DOI] [PubMed] [Google Scholar]
- 10.Delgado V., Tops L.F., van Bommel R.J., et al. Strain analysis in patients with severe aortic stenosis and preserved left ventricular ejection fraction undergoing surgical valve replacement. Eur. Heart J. 2009;30(24):3037–3047. doi: 10.1093/eurheartj/ehp351. [DOI] [PubMed] [Google Scholar]
- 11.van Dalen B.M., Tzikas A., Soliman O.I., et al. Assessment of subendocardial contractile function in aortic stenosis: a study using speckle tracking echocardiography. Echocardiography. 2013;30(3):293–300. doi: 10.1111/echo.12051. [DOI] [PubMed] [Google Scholar]
- 12.Ozdemir A.O., Kaya C.T., Ozcan O.U., et al. Prediction of subclinical left ventricular dysfunction with longitudinal two-dimensional strain and strain rate imaging in patients with mitral stenosis. Int. J. Cardiovasc. Imaging. 2010;26(4):397–404. doi: 10.1007/s10554-009-9550-2. [DOI] [PubMed] [Google Scholar]
- 13.Wang B., Xu Z.Y., Han L., et al. Impact of preoperative atrial fibrillation on mortality and cardiovascular outcomes of mechanical mitral valve replacement for rheumatic mitral valve disease. Eur. J. Cardiothorac. Surg. 2013;43(3):513–519. doi: 10.1093/ejcts/ezs213. [DOI] [PubMed] [Google Scholar]
- 14.Ancona R., Comenale Pinto S., Caso P., et al. Two-dimensional atrial systolic strain imaging predicts atrial fibrillation at 4-year follow-up in asymptomatic rheumatic mitral stenosis. J. Am. Soc. Echocardiogr. 2013;26(3):270–277. doi: 10.1016/j.echo.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 15.Smedsrud M.K., Sarvari S., Haugaa K.H., et al. Duration of myocardial early systolic lengthening predicts the presence of significant coronary artery disease. J. Am. Coll. Cardiol. 2012;60(12):1086–1093. doi: 10.1016/j.jacc.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 16.Tsai W.C., Liu Y.W., Huang Y.Y., et al. Diagnostic value of segmental longitudinal strain by automated function imaging in coronary artery disease without left ventricular dysfunction. J. Am. Soc. Echocardiogr. 2010;23(11):1183–1189. doi: 10.1016/j.echo.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Liang H.Y., Cauduro S., Pellikka P., et al. Usefulness of two-dimensional speckle strain for evaluation of left ventricular diastolic deformation in patients with coronary artery disease. Am. J. Cardiol. 2006;98(12):1581–1586. doi: 10.1016/j.amjcard.2006.07.038. [DOI] [PubMed] [Google Scholar]
- 18.Bjork Ingul C., Rozis E., Slordahl S.A., Marwick T.H. Incremental value of strain rate imaging to wall motion analysis for prediction of outcome in patients undergoing dobutamine stress echocardiography. Circulation. 2007;115(10):1252–1259. doi: 10.1161/CIRCULATIONAHA.106.640334. [DOI] [PubMed] [Google Scholar]
- 19.Galderisi M., Lomoriello V.S., Santoro A., et al. Differences of myocardial systolic deformation and correlates of diastolic function in competitive rowers and young hypertensives: a speckle-tracking echocardiography study. J. Am. Soc. Echocardiogr. 2010;23(11):1190–1198. doi: 10.1016/j.echo.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Imbalzano E., Zito C., Carerj S., et al. Left ventricular function in hypertension: new insight by speckle tracking echocardiography. Echocardiography. 2011;28(6):649–657. doi: 10.1111/j.1540-8175.2011.01410.x. [DOI] [PubMed] [Google Scholar]
- 21.Sengupta S.P., Caracciolo G., Thompson C., Abe H., Sengupta P.P. Early impairment of left ventricular function in patients with systemic hypertension: New insights with 2-dimensional speckle tracking echocardiography. Indian Heart J. 2013;65(1):48–52. doi: 10.1016/j.ihj.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakai H., Takeuchi M., Nishikage T., Lang R.M., Otsuji Y. Subclinical left ventricular dysfunction in asymptomatic diabetic patients assessed by two-dimensional speckle tracking echocardiography: correlation with diabetic duration. Eur. J. Echocardiogr. 2009;10(8):926–932. doi: 10.1093/ejechocard/jep097. [DOI] [PubMed] [Google Scholar]
- 23.Scholte A.J., Nucifora G., Delgado V., et al. Subclinical left ventricular dysfunction and coronary atherosclerosis in asymptomatic patients with type 2 diabetes. Eur. J. Echocardiogr. 2011;12(2):148–155. doi: 10.1093/ejechocard/jeq165. [DOI] [PubMed] [Google Scholar]
- 24.Borlaug B.A., Lam C.S., Roger V.L., Rodeheffer R.J., Redfield M.M. Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 2009;54(5):410–418. doi: 10.1016/j.jacc.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Persson H., Lonn E., Edner M., et al. Diastolic dysfunction in heart failure with preserved systolic function: need for objective evidence:results from the CHARM Echocardiographic Substudy-CHARMES. J. Am. Coll. Cardiol. 2007;49(6):687–694. doi: 10.1016/j.jacc.2006.08.062. [DOI] [PubMed] [Google Scholar]
- 26.Zile M.R., Gottdiener J.S., Hetzel S.J., et al. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation. 2011;124(23):2491–2501. doi: 10.1161/CIRCULATIONAHA.110.011031. [DOI] [PubMed] [Google Scholar]
- 27.Kraigher-Krainer E., Shah A.M., Gupta D.K., et al. Impaired Systolic Function by Strain Imaging in Heart Failure with Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2014;63(5):447–456. doi: 10.1016/j.jacc.2013.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phan T.T., Abozguia K., Shivu G.N., et al. Myocardial contractile inefficiency and dyssynchrony in heart failure with preserved ejection fraction and narrow QRS complex. J. Am. Soc. Echocardiogr. 2010;23(2):201–206. doi: 10.1016/j.echo.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Leitman M., Bachner-Hinenzon N., Adam D., et al. Speckle tracking imaging in acute inflammatory pericardial diseases. Echocardiography. 2011;28(5):548–555. doi: 10.1111/j.1540-8175.2010.01371.x. [DOI] [PubMed] [Google Scholar]
- 30.Sengupta P.P., Krishnamoorthy V.K., Abhayaratna W.P., et al. Disparate patterns of left ventricular mechanics differentiate constrictive pericarditis from restrictive cardiomyopathy. JACC Cardiovasc. Imaging. 2008;1(1):29–38. doi: 10.1016/j.jcmg.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Poterucha J.T., Kutty S., Lindquist R.K., Li L., Eidem B.W. Changes in left ventricular longitudinal strain with anthracycline chemotherapy in adolescents precede subsequent decreased left ventricular ejection fraction. J. Am. Soc. Echocardiogr. 2012;25(7):733–740. doi: 10.1016/j.echo.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 32.Phelan D., Collier P., Thavendiranathan P., et al. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart. 2012;98(19):1442–1448. doi: 10.1136/heartjnl-2012-302353. [DOI] [PubMed] [Google Scholar]
- 33.Kramer J., Niemann M., Liu D., et al. Two-dimensional speckle tracking as a non-invasive tool for identification of myocardial fibrosis in Fabry disease. Eur. Heart J. 2013;34(21):1587–1596. doi: 10.1093/eurheartj/eht098. [DOI] [PubMed] [Google Scholar]
- 34.Spethmann S., Dreger H., Schattke S., et al. Two-dimensional speckle tracking of the left ventricle in patients with systemic sclerosis for an early detection of myocardial involvement. Eur. Heart J. Cardiovasc. Imaging. 2012;13(10):863–870. doi: 10.1093/ehjci/jes047. [DOI] [PubMed] [Google Scholar]
- 35.Yiu K.H., Schouffoer A.A., Marsan N.A., et al. Left ventricular dysfunction assessed by speckle-tracking strain analysis in patients with systemic sclerosis: relationship to functional capacity and ventricular arrhythmias. Arthritis Rheum. 2011;63(12):3969–3978. doi: 10.1002/art.30614. [DOI] [PubMed] [Google Scholar]
- 36.Negishi K., Lucas S., Negishi T., Hamilton J., Marwick T.H. What is the primary source of discordance in strain measurement between vendors: imaging or analysis? Ultrasound Med. Biol. 2013;39(4):714–720. doi: 10.1016/j.ultrasmedbio.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 37.Kaneko A., Tanaka H., Onishi T., et al. Subendocardial dysfunction in patients with chronic severe aortic regurgitation and preserved ejection fraction detected with speckle-tracking strain imaging and transmural myocardial strain profile. Eur. Heart J. Cardiovasc. Imaging. 2013;14(4):339–346. doi: 10.1093/ehjci/jes160. [DOI] [PubMed] [Google Scholar]
- 38.Sengupta S.P., Jaju R., Nugurwar A., Caracciolo G., Sengupta P.P. Left ventricular myocardial performance assessed by 2-dimensional speckle tracking echocardiography in patients with sickle cell crisis. Indian Heart J. 2012;64(6):553–558. doi: 10.1016/j.ihj.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nelson M.R., Hurst R.T., Raslan S.F., et al. Echocardiographic measures of myocardial deformation by speckle-tracking technologies: the need for standardization? J. Am. Soc. Echocardiogr. 2012;25(11):1189–1194. doi: 10.1016/j.echo.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 40.Risum N., Ali S., Olsen N.T., et al. Variability of global left ventricular deformation analysis using vendor dependent and independent two-dimensional speckle-tracking software in adults. J. Am. Soc. Echocardiogr. 2012;25(11):1195–1203. doi: 10.1016/j.echo.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 41.Mizariene V., Grybauskiene R., Vaskelyte J., et al. Strain value in the assessment of left ventricular function and prediction of heart failure markers in aortic regurgitation. Echocardiography. 2011;28(9):983–992. doi: 10.1111/j.1540-8175.2011.01483.x. [DOI] [PubMed] [Google Scholar]
- 42.Levy F., Debry N., Labescat A.L., et al. Echocardiographic prediction of postoperative atrial fibrillation after aortic valve replacement for aortic stenosis: a two-dimensional speckle tracking left ventricular longitudinal strain multicentre pilot study. Arch. Cardiovasc. Dis. 2012;105(10):499–506. doi: 10.1016/j.acvd.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 43.Kim M.S., Kim Y.J., Kim H.K., et al. Evaluation of left ventricular short- and long-axis function in severe mitral regurgitation using 2-dimensional strain echocardiography. Am. Heart J. 2009;157(2):345–351. doi: 10.1016/j.ahj.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 44.Pandis D., Grapsa J., Athanasiou T., Punjabi P., Nihoyannopoulos P. Left ventricular remodeling and mitral valve surgery: prospective study with real-time 3-dimensional echocardiography and speckle tracking. J. Thorac. Cardiovasc. Surg. 2011;142(3):641–649. doi: 10.1016/j.jtcvs.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 45.Mascle S., Schnell F., Thebault C., et al. Predictive value of global longitudinal strain in a surgical population of organic mitral regurgitation. J. Am. Soc. Echocardiogr. 2012;25(7):766–772. doi: 10.1016/j.echo.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 46.Nucifora G., Schuijf J.D., Delgado V., et al. Incremental value of subclinical left ventricular systolic dysfunction for the identification of patients with obstructive coronary artery disease. Am. Heart J. 2010;159(1):148–157. doi: 10.1016/j.ahj.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 47.Hanekom L., Cho G.Y., Leano R., Jeffriess L., Marwick T.H. Comparison of two-dimensional speckle and tissue Doppler strain measurement during dobutamine stress echocardiography: an angiographic correlation. Eur. Heart J. 2007;28(14):1765–1772. doi: 10.1093/eurheartj/ehm188. [DOI] [PubMed] [Google Scholar]
- 48.Phan T.T., Shivu G.N., Abozguia K., et al. Left ventricular torsion and strain patterns in heart failure with normal ejection fraction are similar to age-related changes. Eur. J. Echocardiogr. 2009;10(6):793–800. doi: 10.1093/ejechocard/jep072. [DOI] [PubMed] [Google Scholar]
- 49.Ho E., Brown A., Barrett P., et al. Subclinical anthracycline- and trastuzumab-induced cardiotoxicity in the long-term follow-up of asymptomatic breast cancer survivors: a speckle tracking echocardiographic study. Heart. 2010;96(9):701–707. doi: 10.1136/hrt.2009.173997. [DOI] [PubMed] [Google Scholar]
- 50.Fallah-Rad N., Walker J.R., Wassef A., et al. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with adjuvant trastuzumab therapy. J. Am. Coll. Cardiol. 2011;57(22):2263–2270. doi: 10.1016/j.jacc.2010.11.063. [DOI] [PubMed] [Google Scholar]
- 51.Liu Y.W., Su C.T., Wang S.P., et al. Application of speckle-tracking echocardiography in detecting coronary artery disease in patients with maintenance hemodialysis. Blood Purif. 2011;32(1):38–42. doi: 10.1159/000323536. [DOI] [PubMed] [Google Scholar]
- 52.Dedobbeleer C., Rai M., Donal E., Pandolfo M., Unger P. Normal left ventricular ejection fraction and mass but subclinical myocardial dysfunction in patients with Friedreich's ataxia. Eur. Heart J. Cardiovasc. Imaging. 2012;13(4):346–352. doi: 10.1093/ejechocard/jer267. [DOI] [PubMed] [Google Scholar]
- 53.Caputo M., Urselli R., Zaca V., et al. Detection of Early Left Ventricular and Atrial Dysfunction in Overweight Patients with Preserved Ejection Fraction: A Speckle Tracking Analysis. Echocardiography. 2013;30(5):551–557. doi: 10.1111/echo.12102. [DOI] [PubMed] [Google Scholar]
- 54.Takamura T., Dohi K., Onishi K., et al. Reversible left ventricular regional non-uniformity quantified by speckle-tracking displacement and strain imaging in patients with acute pulmonary embolism. J. Am. Soc. Echocardiogr. 2011;24(7):792–802. doi: 10.1016/j.echo.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 55.Shahul S., Rhee J., Hacker M.R., et al. Subclinical left ventricular dysfunction in preeclamptic women with preserved left ventricular ejection fraction: a 2D speckle-tracking imaging study. Circ Cardiovasc Imaging. 2012;5(6):734–739. doi: 10.1161/CIRCIMAGING.112.973818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Inoue K., Okayama H., Nishimura K., et al. Right ventricular septal pacing preserves global left ventricular longitudinal function in comparison with apical pacing: analysis of speckle tracking echocardiography. Circ. J. 2011;75(7):1609–1615. doi: 10.1253/circj.cj-10-1138. [DOI] [PubMed] [Google Scholar]
- 57.Miszalski-Jamka T., Szczeklik W., Nycz K., et al. Two-dimensional speckle-tracking echocardiography reveals systolic abnormalities in granulomatosis with polyangiitis (Wegener's). Echocardiography. 2012;29(7):803–809. doi: 10.1111/j.1540-8175.2012.01699.x. [DOI] [PubMed] [Google Scholar]
- 58.Witkowski T.G., Thomas J.D., Debonnaire P.J., et al. Global longitudinal strain predicts left ventricular dysfunction after mitral valve repair. Eur. Heart J. Cardiovasc. Imaging. 2013;14:69–76. doi: 10.1093/ehjci/jes155. [DOI] [PubMed] [Google Scholar]
- 59.Ternacle J., Berry M., Alonso E., et al. Incremental value of global longitudinal strain for predicting early outcome after cardiac surgery. Eur. Heart J. Cardiovasc. Imaging. 2013;14:77–84. doi: 10.1093/ehjci/jes156. [DOI] [PubMed] [Google Scholar]


