Abstract
Toxocariasis is the clinical term used to describe human infection with either the dog ascarid Toxocara canis or the feline ascarid Toxocara cati. As with other helminths zoonoses, the infective larvae of these Toxocara species cannot mature into adults in the human host. Instead, the worms wander through organs and tissues, mainly the liver, lungs, myocardium, kidney and central nervous system, in a vain attempt to find that, which they need to mature into adults. The migration of these immature nematode larvae causes local and systemic inflammation, resulting in the “larva migrans” syndrome. The clinical manifestations of toxocariasis are divided into visceral larva migrans, ocular larva migrans and neurotoxocariasis. Subclinical infection is often referred to as covert toxocariasis. One of the primary causes of death all around the world is cardiovascular disease that accounted for up to 30 percent of all-cause mortality. Cardiovascular disease and more precisely atherosclerotic cardiovascular disease, is predicted to remain the single leading cause of death (23.3 million deaths by 2030). A-quarter of people presenting the disease does not show any of the known cardiovascular risk factors. Therefore, there is considerable interest in looking for novel components affecting cardiovascular health, especially for those that could improve global cardiovascular risk prediction. This review endeavours to summarize the clinical aspects, new diagnostic and therapeutic perspectives of toxocaral disease with cardiovascular manifestations.
Keywords: Toxocariasis, cardiovascular, visceral larva migrans, epidemiology
INTRODUCTION
Toxocara canis (T. canis) and Toxocara cati (T. cati), roundworms of dogs and cats, are probably the most common gastrointestinal zoonotic helminths of domestic canids and felids word-wide [1]. Natural hosts as well as paratenic hosts, including the human become infected by ingesting either embryonated eggs containing third -stage larvae, from soil (geophagia, pica), dirty hands or raw vegetables, or larvae from undercooked giblets, meat or offals [2]. Toxocara larvae hatch in the stomach and migrate into the mucosa of upper small intestine and penetrate via blood and lymphatic vessels throughout the body, resulting in somatic in many types of tissues. The liver is an important site for controlling the migration of Toxocara larvae. Dissemination occurs via the blood-borne rout, through tissues and body cavities [3]. The classical clinical syndromes of Toxocara infection are divided into visceral larva migrans (VLM) and ocular larva migrans (OLM). VLM syndrome most commonly affects the liver, skin, and lungs, but involvement of the central nervous system and myocardium are rare. In OLM, migrating larvae can induce granulomatous retinal lesion, which are characterized by complaints of loss of visual acuity, squint and seeing lights. A third clinical syndrome, called covert toxocariasis (CT), was found in patients with clinical symptoms that are non-specific by itself and do not fall within the categories VLM or OLM, but form together a vague complex of symptoms [4]. The prevalence of patent Toxocara infections is highest in dogs and cats and in much less common in adult animals in many countries all over the world [5]. Several epidemiological and clinical studies have reported that regular contact to dog and cats, rural residence, geophagia or dementia has been identified as risk factors for the acquisition of human Toxocara infection [6-10].
One of the primary causes of death all around the world is cardiovascular disease that accounted for up to 30 percent of all-cause mortality [11]. Cardiovascular disease and more precisely atherosclerotic cardiovascular disease, is predicted to remain the single leading cause of death (23.3 million deaths by 2030) [12]. Despite the fact that major risk factors for cardiovascular disease have been firmly established, twenty-five percent of people presenting the disease do not show any of the known cardiovascular risk factors [13]. Therefore, there is considerable interest in looking for novel components affecting cardiovascular health, especially for those that could improve global cardiovascular risk prediction.
Based upon the immuno-inflammatory response elicited by infectious disease, the parasitological contribution to atherosclerosis, the pathological basis of the vast majority of cardiovascular disease, represents a long-standing controversy [14, 15].
Previous studies have suggests that some parasitic agents such as Trypanosoma cruzi, cause Chagas disease, might contribute to atherosclerotic vascular diseases [16]. In human toxocariasis (VLM syndrome) the larvae can enter the liver, the lung, the kidney, brain or the heart. The association between Toxocara spp infection and vasculitis manifestations is unusual but has been previously reported [17, 18].
To improve knowledge regarding the role of Toxocara in contributing to the disease, the present review conducted to the clinical manifestation cardiovascular consequences, diagnostic and treatment perspectives in human toxocariasis.
AGENTS
Toxocara species are nematodes, taxonomically included within the order and family Ascaridida.
Our concept is T. canis is more important than T. cati in causing human infection and disease because cats, not dogs, bury Toxocara ova contamination faeces, making infectious ova less accessible to susceptible individuals. Further reasons include the fact that morphometry of larvae in histological sections and early serology, using cross- reactive antigens, failed to implicate T. cati over T. canis in human cases and also the lesser tendency for brain involvement in the murine model of T. cati toxocariasis [19]. Based on generalized exclusion, T. canis was deemed to be the more important aetiological agent of human toxocariasis.
There are reports on the location of T. cati larvae in paratenic hosts as a causative agent of Toxocara infection in human [20]. Both visceral toxocariasis and ocular larva migrans, caused by migrating larvae, as well as adult T. cati infections of humans have been described [20]. T. cati has been reported in case of decreased visual acuity caused by a macular lesion of the eye, where the patient’s serum was positive reactive to T. cati antigens [21]. The studies by Zibaei et al. showed the role of T. cati in Toxocara infections of animal model [22, 23].
LIFE CYCLE
A wide variety of animals, including humans can become infected with Toxocara species by ingesting eggs contain larvae from contaminated soil and the consumption of contaminated raw meat or liver. In the paratenic or transport hosts, the larvae do not develop to maturity, but migrate for months throughout host tissues before lodging within host tissues in state of arrested development. The larvae do not encyst but remain exposed to the host environment, absorbing nutrients across the nematode cuticle. The larvae can survive in tissue for several years despite vigorous host immunologic responses to parasite antigens (Fig. 1).
Fig. (1).
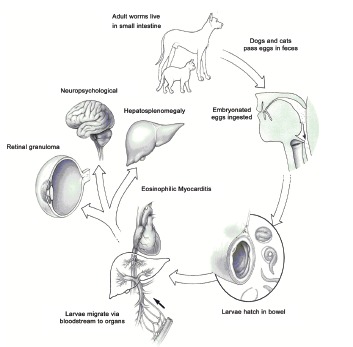
Life cycle of Toxocara spp. Although VLM is characteristic for Toxocara canis, it may occur with Toxocara cati from cats.
EPIDEMIOLOGY
T. canis and T. cati are nematode, which infects canines and felines worldwide, respectively, and as widespread environmental results dissemination of them ova in hosts faeces, other unnatural or paratenic hosts contains earthworms, ants, poultry, cockroach, and mammalians are contacted to the parasite. These helminths appear to have caused disease for millennia [24]. Toxocariasis in man is presumed to be contracted through the ingestion of eggs from contaminated soil (sapro-zoonosis); children with pica and contact to puppies appear to be particularly vulnerable [25]. Recent date suggests that dogs affected with T. canis may cause people infected through direct contact [26]. Additional risk factors include mental retardation requiring institutionalization and ingestion meat from paratenic hosts such as chicken and lamb [27-30]. Cats and other felines act as definitive hosts for the cat roundworm, T. cati, which is responsible for toxocariasis. The majority of reported human cases of toxocariasis in the past have been associated with T. canis not with T. cati. The habit of cats covering their faeces in soil tends to prevent the spread of eggs, give further support to this preference for T. canis infection [31]. For religious reasons, dogs are avoided in the more of Islamic regions, whereas cats are favorite pet. Worldwide studies of T. canis and T. cati have demonstrated a distribution between 1-45% and 0.8-59.3% in dogs and cats, respectively [32, 33].
Because toxocariasis does not become patent in humans, estimates of prevalence of humans depend on immunosurveys. These sero-epidemiologic surveys revealed wide difference depending on the man tested. The seroprevalence of most adults were 3.1% in Spain and 7.5% in Australia (developed countries), but the higher seroprevalence of Toxocara infection was found in 30.4% in Nigeria and 20.5% in Brazil (developing countries and tropical regions) [34-37]. Serological studies demonstrated primarily among children in developed countries have indicated sero-prevalence levels of T. canis in New Zealand (0.7%), Japan (1.6%), Denmark (2.4%), Australia (7.5%), the United States (14%), and 15% in Poland [32, 38, 39]. By contrast, the higher values of seroprevalence have been revealed in tropical and tropical regions or developing countries including Nigeria (30%), Swaziland (45%), La Reunion (93%), Nepal (81%), Indonesia (63.2%), Malaysia (58%), Brazil (36%) and 37% in Peru [40-42].
PATHOGENESIS
The compounds surface coat of Toxocara larvae is a dynamic structure that turns over quite rapidly and generates large quantities of glycosylated excretory-secretory proteins [43]. Majority of these proteins are formed a family of highly antigenic mucins that induce the cellular immunity, which determined by the secretion of cytokines that increasing of eosinophilia and the production of IgE antibodies [44]. The recruited eosinophils adhere to a membranous layer that is frequently detached from larval epicuticle, allowing the antigens [45]. Toxocara larva elicits humoral and cellular immune responses in humans. Due to host inflammatory responses tissues damage is more than the infection itself. In Toxocara infection, larval antigens induce granulomatous inflammation with eosinophils, histocystes and fibrous tissue. The granulomatous are found most often in liver and less in the lungs, eyes, brain, and the other organs. These eosinophil-mediated granulomatous responses are almost exclusively responsible for the clinical manifestation of Toxocara infection including overt toxocariasis (VLM and OLM), and covert toxocariasis (Table 1).
Table 1. Clinical forms of Toxocara infection and logical reasons for clinical treatment and prevention.
| Clinical characteristics of patients | Treatment reasons | ||||
|---|---|---|---|---|---|
| Clinical forms | Signs and Symptoms | Eosinophilia | Serology | Clinical | Prevention/Control |
| Visceral Larva Migrans | High/Mild | High | High/Mild | Yes* | Yes** |
| Ocular Larva Migrans | High | Doubtful | Mild | Yes | Yes |
| Neurotoxocariasis | Mild | Doubtful | Mild | Yes | Yes |
| Covert toxocariasis | Doubtful | Doubtful | Mild | Yes | Doubtful |
| Asymtomatic toxocariasis | None | Doubtful | Mild | No | Doubtful |
* In cases of clinical toxocariasis, thiabendazole (15 mg/kg/body weight/day) for 5 days.
** Positive results for eosinophilia and serological testes.
CHRONIC HELMINTHIC INFECTION AND ATHEROSCLEROSIS
The causative agents and processes, which may be involved in the pathogenesis of atherosclerosis, are being interested. The occurrence rate of atherosclerosis and cardiovascular diseases are much lower in developing countries. Due to the present knowledge of immune and infectious mechanisms related to atherosclerosis, it is proposed that chronic helminthic infections can have a significant bearing on the epidemiology of cardiovascular diseases.
Helminth infection can be increased the risk of atherosclerosis: (1) helminths evade or suppress host immune responses, by producing anti-inflammatory and other immunomodulatory molecules; (2) helminths induce chronic Th2 activation, which can modify cytokine profiles and immunological responses to heat shock proteins (HSP); (3) The chronic Th2 profile may modulate monocyte and macrophage activation and chemotaxis to inflammatory sites (atherosclerotic plaques); (4) Chronic Th2 activation may lead to a cytokine profile that could be beneficial for attenuation of atherosclerosis development (involved in producing IL-4, IL-10 and IL-13 and inhibition of proinflammatory cytokines); and (5) helminthic infections may reduce plasma LDL level not only by affecting the host nutrition, but also via modulation of naturally occurring antibodies to cholesterol [46].
Since the Toxocara species can be cause chronic parasitic infection and considering that a link between atherosclerosis and helminth infections has not been investigated, studies are needed to clarify these suggestions.
TOXOCARA INFECTION AND CARDIOVASCULAR DISEASE
Human toxocariasis is widespread as evidenced by seropositivity. Ocular toxocariasis is almost certainly an organ specific subdivision of visceral toxocariasis. Visceral toxocariasis may be overt or covert. Covert toxocariasis is much more common than overt toxocariasis. Initial reports of overt toxocariasis were of severe cases in children under the age of four years [47]. Extreme eosinophilia (50% or more of the total white blood cell count) was regarded as an essential criterion for diagnosis with varying accompanying features of increasing the body temperature (fever), pulmonary infiltration, hepatosplenomegaly, muscle and joint pains, abdominal pain, and cardiac involvement rarely occurs but can be serious [48, 49].
Eosinophilic Myocarditis
Myocardial damage and endomyocardial fibrosis has been associated clinically with chronic eosinophilia [50]. Chronic eosinophilia can be due to helminthic infection such as toxocariasis, drug reactions, allergic disorders, or other causes. Dramatic eosinophil counts (up to 90 percent), may occur in patients as VLM, who often present with leukocytosis. Visceral larva migrans syndrome results from the ingestion of embryonated eggs of Toxocara spp. After ingestion, hatching occurs in duodenum (upper small intestine). The larvae penetrate into the mucosa and via the portal circulation migrate to the liver, heart, lungs and enter systemic circulation. Because the larvae size is larger than diameter blood vessels, it may bore through the vessel wall of the host, migrate within the tissues, and elicit an immune response. Eventually, a larva becomes enmeshed in a fibrotic encapsulated granuloma [51-53]. Severe myocarditis ensues when the third-stage larvae (L3) invade cardiac tissue (Fig. 2). Several cases of myocarditis associated with VLM syndrome have been reported [54]. Myocarditis may occur in up to 15 percent in patients with visceral larva migrans and the myocarditis is accompanied by significantly increased levels of circulating eosinophils in all of patients. Brockington and Olsen [55] described that eosinophilic endomyocardial disease occurs in three stages: I) the acute necrotic stage, the thrombotic stage, and the late fibrotic or end stage. The acute necrotic stage is seen in patients ill for only a few weeks, and is characterized by areas of acute necrosis and inflammatory cell infiltrates into the endomyocardium, with focal areas of damage to the subendocardium, II) the second stage, after several months, begins when a layer of granulation tissue develops between the endocardium and subendocardium, accompanied by intraventricular thrombi and thrombi in small and medium sized blood vessels, and III) the end stage occurs when dense endomyocardial fibrosis is seen that produces a restrictive myocardiopathy. Friedman and Hervada in 1960 described a child with strong evidence of toxocaral infection who suffered from severe myocarditis that resolved on treatment [56]. Spry et al. described myocardial lesions in patients with eosinophilic endomyocarditis and tropical endocardia fibrosis [57]. In a case of Toxocara infection associated with myocarditis has previously been showed in which elevated C-reactive protein (1.4 mg/dl), creatinine phosphokinase (679 IU/l), aspartate aminotransferase (124 IU/l), alanine aminotransferase (182 IU/l) and lactic dehydrogenase (733 IU/l) [58].
Fig. (2).
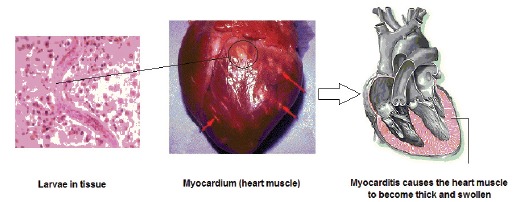
Helminths infection and cardiovascular diseases.
Pericarditis and Pleural Effusion
The clinical spectrum of Toxocara infection is broad, producing pulmonary effusion, cardiac tamponade, and ascites [59-61]. Less commonly, toxocariasis can be self-limiting with few clinical manifestations. A case report revealed systematic effusion in a woman cardiomyositis with congestive heart failure, serositis with collagen vascular disorders. Elevation in with blood cell count (13,900/μl) with 5% eosinophils, serum brain natriuretic peptide to 340 pg/ml (normal < 18.4 pg/ml), and chest roentgenogram with chest computed tomography has been observed in patient diagnosed with toxocariasis- associated pericarditis [62]. Bachmeyer et al. in a case found fever, night sweats, weight loss, hilar and mediastinal lymphadenopathy, bilateral pleural effusion, and eosinophilia- mimicking lymphoma related to T. canis infection [59]. The patient appeared pneumonia with eosinophilia that responsible for acute respiratory disorders. The patient was healthy (physical examination) and her medical history was unremarkable. Laboratory examinations of the peripheral blood showed the white blood cells count 10.8 × 103/μl with eosinophilia (26%). Results of serum electrophoresis revealed an albumin level of 32.8 g/l (normal range more than 39 g/l); α2-globulin, 9.8 g/l (normal range more than 7 g/l); and polyclonal γ-globulinemia, 16.9 g/l (normal range more than 10 g/l). After diagnosis the disease, the patient treated with oral albendazole (400 mg daily for 10 days). Unusually, in a case of life-threatening Toxocara infection, Henry et al. demonstrated that the chest roentgenograms was revealed bilateral pleural effusion with normal parenchyma but an enlarged cardiac image [63]. Laboratory tests showed a leukocyte count of 15.4 × 103/mm3 with 2.2 × 103 eosinophils/mm3. On pericardiotomy of the patient, more than 800 ml of pericardial fluid with a cellular component of 20% eosinophils evacuated. Serology was positive for T. canis antibodies. On basis of the clinical pictures and the conducted tests a diagnosis of acute toxocariasis was established; ivermectin 200 μg/kg/day at day 10 and day 25 was applied in treatment.
LABORATORY DIAGNOSIS
The diagnosis of toxocariasis, like most parasitic infection, is made initially on clinical signs such as hepatomegaly, fever or wheezing, with confirmatory testing playing a secondary role. Blood cells count always showed the presence of blood eosinophilia, which was usually marked and was sometimes massive: approximately 1 × 103 cells /mm3 [64-66]. In the absence of hepatomegaly, the symptoms and sings of toxocariasis mimic tropical pulmonary eosinophilia.
Diagnosis of human toxocariasis is confirmed by visual methods (as called the gold standard). Microscopic observation of biopsies or organ fragments can find larval sections or debris [67, 68]. Other laboratory results include increase in immunoglobulin and elevated isomemagglutinin titers to A and B antigens (blood group antigens) [69]. Radiographic imaging of involved organs using ultrasound, computed tomography (CT) scan, and magnetic resonance imaging (MRI) can describe focal lesions [70]. Advance in serological techniques was obtained due to the difficulties and uncertainties of direct visual detection. The diagnosis is most established by confirmatory serological testing using an ELISA (enzyme linked-immunosorbent assay) that detecting IgG antibodies against TES (Toxocara excretory-secretory) antigens (TES-ELISA). ES proteins from second stage T. canis or T. cati larvae maintained in vitro form the substrate for ELISA [71, 72]. The sensitivity and specifity have been estimated to be approximately 78.0% and 92.0% for T. canis and 97.0% and 96.7% for T. cati, respectively [73-75]. Limited data indicate no significant cross-reactivity in individuals infected with common helminthic infections [76, 77]. A Western blotting assay using TES antigens is more specific [75, 78]. In the past decade, immunoblot assays have been developed using recombinant ES proteins [79, 80]. Antigens capture testes using monoclonal antibody to toxocaral ES proteins offers the ability to distinguish current infection [81].
Molecular diagnostic methods have proven to be useful to investigate of parasite in tissue fragments from surgical operations or dissections and in cerebrospinal fluid, but this approach are currently not used practically [23, 82-84].
TREATMENT AND PROGNOSIS
The majority of patients with Toxocara infection do not require treatment, since toxocaral infections are usually subclinical and self-limited, nevertheless, the disease can be fatal with severe clinical manifestation. Treatment is generally reserved for cases with severe toxocariasis, although this remains a controversial point. Usually, acute toxocariasis is treated either symptomatic or with specific anti-parasitic treatment, depending on the severity of clinical disease. Patients with either peripheral eosinophilia or mild toxocariasis are often treated cautiously. Asymptomatic cases with increased eosinophils and those with mild Toxocara infection in the absence of increased eosinophils normally do not need any special treatment. Mebendazole, albendazole, thiabendazole, ivermectin and diethylcarbamazine have been reported as treatment for human toxocariasis. In cases of clinical toxocariasis, thiabendazole (15 mg/kg/day) appeared to be effective; however, a similar course of albendazole resulted in more frequented clinical cure and a measurable decrease in eosinophils count. A dose of 400 mg of albendazole twice a day for 5 days is the currently recommended therapy [84]. A twenty-one days course of mebendazole with dose of 20-25 mg/kg/day has been shown to be effective, alternatively [85]. Severe symptomatic treatments, including either albendazole or mebendazole, and corticosteroids (prednisone) with dose of 0.5-1.0 mg/kg daily are often administrated concomitantly.
Abe et al. in a case report demonstrated that a 4-weeks course of oral albendazole and prednisolone results in resolution of clinical symptoms in myocarditis-associated with Toxocara infection [58]. Enko and colleagues reported a case of fulminant eosinophilic myocarditis associated with toxocariasis that treated with both oral albendazole for four weeks and intravenous methylprednisolone (~500 mg/d) for 3 days, followed by oral medications of prednisolone (50 mg/d) for 2 weeks. Following treatment with those drugs, significantly improved ventricular function and quickly reduced the number of eosinophils and normalized [86].
CONCLUDING REMARKS
Toxocara canis and Toxocara cati, roundworms of dogs and cats respectively, are zoonotic parasites that cause widespread and common human toxocariasis. The infective larvae both Toxocara species cannot mature into adults in the human host. The worms wander through tissues and organs in a vain attempt to find that which they need to mature into adults. A variety of disease states are described but symptoms are not well understood and require further elucidation. The migration of immature larvae causes local and systematic inflammation, resulting in the “larval migrans syndrome”. Visceral larva migrans is defined not only by chronic increased eosinophils and increased γ-globulin in the blood, but also by involvement of the liver, lungs, eyes, the cerebral nervous system, skin and the heart as a result of migration the local larval. In toxocariasis inflammation of the heart muscle (myocarditis) may be caused by direct larval invasion to the myocardium and hypersensitivity reactions to the parasite. Several cases of pleural effusion and pericardial associated with Toxocara infection have been reported. With the exception of overwhelming infections, widespread of small numbers of tissue-dwelling larvae dictates that serology tool of choice for diagnosing human infection. Strategies for the treatment of this disease in human, is use of mebendazole, albendazole, thiabendazole, ivermectin, diethylcarbamazine with corticosteroids.
ACKNOWLEDGEMENT
Declared none.
CONFLICT OF INTEREST
The author confirms that this article content has no conflict of interest.
REFERENCES
- 1.Despommier D. Toxocariasis: clinical aspects, epidemiological, medical ecology and molecular aspects. Clin. Microbiol. Rev. 2005;16:265–272. doi: 10.1128/CMR.16.2.265-272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schantz P.M. Toxocara larva migrans now. Am. J. Trop. Med. Hyg. 1989;41:21–34. doi: 10.4269/ajtmh.1989.41.21. [DOI] [PubMed] [Google Scholar]
- 3.Rubinsky-Elefant G., Hirata C.E., Yamamoto J.H., Ferreira M.U. Human toxocariasis: diagnosis, worldwide seroprevalences and clinical expression of the systemic and ocular forms. Ann. Trop. Med. Parasitol. 2010;104:3–23. doi: 10.1179/136485910X12607012373957. [DOI] [PubMed] [Google Scholar]
- 4.Glickman L.T., Schantz P.M. Epidemiology and pathogenesis of zoonotic toxocariasis. Epidemiol. Rev. 1981;3:230–250. doi: 10.1093/oxfordjournals.epirev.a036235. [DOI] [PubMed] [Google Scholar]
- 5.Castillo D., Parades C., Zanartu C. Environmental contamination with Toxocara spp. eggs in public squares and parks from Santiago, Chile. Bol. Chil. Parasitol. 2000;55:86–91. [PubMed] [Google Scholar]
- 6.Dubinsky P., Havasivoa-Reitterova K., Petko B. Role of small mammals in the epidemiology of toxocariasis. Parasitology. 1995;110:187–193. doi: 10.1017/s0031182000063952. [DOI] [PubMed] [Google Scholar]
- 7.Giacometti A., Cirioni O., Fortuna M. Environmental and serological evidence for the presence of toxocariasis in the urban area of Ancona, Italy. Eur. J. Epidemiol. 2000;16:1023–1026. doi: 10.1023/a:1010853124085. [DOI] [PubMed] [Google Scholar]
- 8.Zibaei M., Sadjjadi S.M., Sarkari B. Prevalence of Toxocara cati and other intestinal helminths in stray cats in Shiraz, Iran. Trop. Biomed. 2007;24:39–43. [PubMed] [Google Scholar]
- 9.Mizgajska H. Eggs of Toxocara spp. in the environment and their public health implications. J. Helminthol. 2001;75:147–151. [PubMed] [Google Scholar]
- 10.Uga S. Prevalence of Toxocara eggs and number of fecal deposits from dogs and cats in sandpits of public parks in Japan. J. Helminthol. 1993;67:78–82. doi: 10.1017/s0022149x0001289x. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization . Global status report on noncommunicable diseases 2010. Geneva: World Health Organization; 2011. [Google Scholar]
- 12.Mathers C.D., Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magnus P., Beaglehole R. The real contribution of the major risk factors to the coronary epidemics: time to end the only 50% myth. Arch. Intern. Med. 2001;161:2657–2660. doi: 10.1001/archinte.161.22.2657. [DOI] [PubMed] [Google Scholar]
- 14.Nieto F.J. Infective agents and cardiovascular disease. Semin. Vasc. Med. 2002;2:401–415. doi: 10.1055/s-2002-36769. [DOI] [PubMed] [Google Scholar]
- 15.Rosenfeld M.E., Campbell L.A. Pathogens and atherosclerosis: update on the potential contribution of multiple infectious organisms to the pathogenesis of atherosclerosis. Thromb. Haemost. 2011;106:858–867. doi: 10.1160/TH11-06-0392. [DOI] [PubMed] [Google Scholar]
- 16.Frustaci M., Francone N. Petrosillo, Chimenti C. High prevalence of myocarditis in patients with hypertensive heart disease and cardiac deterioration. Eur. J. Heart Fail. 2013;15:284–291. doi: 10.1093/eurjhf/hfs169. [DOI] [PubMed] [Google Scholar]
- 17.Hamidou M.A., Gueglio B., Cassagneau E., Trewick D., Grolleau J.Y. Henoch-Schönlein purpura associated with Toxocara canis infection. J. Rheumatol. 1999;26:443–445. [PubMed] [Google Scholar]
- 18.Pawlowska-Kamieniak A., Mroczkowska-Juchkiewicz A., Papierkowski A. Henoch-Schönlein purpura and toxocariasis. Pol. Merkuriusz Lek. 1998;4:217–218. [PubMed] [Google Scholar]
- 19.Havasiova-Reiterova K., Tomasovicova O., Dubinsky O. Effect of various doses of infective Toxocara canis and Toxocara cati eggs on the humoral response and distribution of larvae in mice. Parasitol. Res. 1995;81:13–17. doi: 10.1007/BF00932411. [DOI] [PubMed] [Google Scholar]
- 20.Fisher M. Toxocara cati: zoonosis or not zoonosis. Trends Parasitol. 2003;19:167–170. doi: 10.1016/s1471-4922(03)00027-8. [DOI] [PubMed] [Google Scholar]
- 21.Zibaei M., Sadjjadi S.M., Jahadi-Hosseini S.H. Toxocara cati larvae in the eye of a child: a case report. Asian Pac. J. Trop. Biomed. 2014;4(Suppl. 1):S53–S55. doi: 10.12980/APJTB.4.2014C1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zibaei M., Sadjjadi S.M., Uga S. Experimental Toxocara cati infection in gerbils and rats. Korean J. Parasitol. •••;48(4):331–333. doi: 10.3347/kjp.2010.48.4.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zibaei M, Sadjjadi SM, Karamian M, Uga S, Oryan A, Jahadi-Hosseini SH. 2013. [DOI] [PMC free article] [PubMed]
- 24.Bouchet F., Araujo A., Harter S., et al. Toxocara canis (Werner, 17820) eggs in the Pleistocene site of Menez-Dregan, France (300,000-500,000 years before present). Mem. Inst. Oswaldo Cruz. 2003;98(Suppl.):137–139. doi: 10.1590/s0074-02762003000900020. [DOI] [PubMed] [Google Scholar]
- 25.Marmor M., Glickman L., Shofer F., et al. Toxocara canis infection of children: Epidemiologic and neuropsychologic findings. Am. J. Public Health. 1987;77:554–559. doi: 10.2105/ajph.77.5.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolfe A., Wright I.P. Human toxocariasis and direct contact with dogs. Vet. Rec. 2003;152:419–422. doi: 10.1136/vr.152.14.419. [DOI] [PubMed] [Google Scholar]
- 27.Huminer D., Symon K., Groskopf I., et al. Seroepidemiologic study of toxocariasis and Strongyloidiasis in institutionalized mentally retarded adults. Am. J. Trop. Med. Hyg. 1992;46:278–281. doi: 10.4269/ajtmh.1992.46.278. [DOI] [PubMed] [Google Scholar]
- 28.Sturchler D., Weiss N., Gassner M. Transmission of toxocariasis. J. Infect. Dis. 1990;162:571. doi: 10.1093/infdis/162.2.571. [DOI] [PubMed] [Google Scholar]
- 29.Salem G., Schantz P. Toxocara visceral larva migrans after ingestion of lamb liver. Clin. Infect. Dis. 1992;15:743–744. doi: 10.1093/clind/15.4.743. [DOI] [PubMed] [Google Scholar]
- 30.Nagakura K., Tachibana H., Kaneda Y., Kato Y. Toxocariasis possibly caused by ingesting raw chicken. J. Infect. Dis. 1989;160:735–736. doi: 10.1093/infdis/160.4.735. [DOI] [PubMed] [Google Scholar]
- 31.Zibaei M., Uga S. Contamination by Toxocara spp. Eggs in sandpits in Kobe, Japan. J Environ Cont Tech. 2008;26:32–37. [Google Scholar]
- 32.Fan C.K., Liao C.W., Cheng Y.C. Factors affecting disease manifestation of toxocariasis in humans: genetics and environment. Vet. Parasitol. 2013;193:342–352. doi: 10.1016/j.vetpar.2012.12.030. [DOI] [PubMed] [Google Scholar]
- 33.Mikaeili F., Mirhendi H., Hosseini M., Asgari Q., Kia E.B. Toxocara nematodes in stray cats from Shiraz, southern Iran: intensity of infection and molecular identification of the isolates. Iran. J. Parasitol. 2013;8:593–600. [PMC free article] [PubMed] [Google Scholar]
- 34.Nicholas W.L., Stewart A.C., Walker J.C. Toxocariasis: a serological survey of blood donors in the Australian Capital Territory together with observations on the risks of infection. Trans. R. Soc. Trop. Med. Hyg. 1986;80:217–221. doi: 10.1016/0035-9203(86)90015-5. [DOI] [PubMed] [Google Scholar]
- 35.Jimenez J.F., Valladares B., Fernandez-Palacios J.M., de Armas D., del Castillo A. A serologic study of human toxocariasis in the Canary Islands (Spain): environmental influences. Am. J. Trop. Med. Hyg. 1997;56:113–115. doi: 10.4269/ajtmh.1997.56.113. [DOI] [PubMed] [Google Scholar]
- 36.Anaruma F.F., Chieffi P.P., Correa C.R., et al. Human toxocariasis: a seroepidemiological survey in the municipality of Campinas (SP), Brazil. Rev. Inst. Med. Trop. Sao Paulo. 2002;44:303–307. doi: 10.1590/s0036-46652002000600002. [DOI] [PubMed] [Google Scholar]
- 37.Ajayi O.O., Dunhlinska D.D., Agwale S.M., Njoku M. Frequency of human toxocariasis in Jos, Plateau State, Nigeria. Mem. Inst. Oswaldo Cruz. 2000;95:147–149. doi: 10.1590/S0074-02762000000200002. [DOI] [PubMed] [Google Scholar]
- 38.Macpherson C.N. The epidemiology and public health importance of toxocariasis: a zoonosis of global importance. Int. J. Parasitol. 2013;43:999–1008. doi: 10.1016/j.ijpara.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 39.Jarosz W., Mizgajska-Wiktor H., Kirwan P., Konarski J., Rychlicki W., Wawrzyniak G. Developmental age, physical fitness and Toxocara seroprevalence amongst lower- secondary students living in rural areas contaminated with Toxocara eggs. Parasitology. 2010;137:53–63. doi: 10.1017/S0031182009990874. [DOI] [PubMed] [Google Scholar]
- 40.Liao C.W., Sukati H., D'Lamini P., et al. Seroprevalence of Toxocara canis infection among children in the Kingdom of Swaziland, Southern Africa. Ann. Trop. Med. Parasitol. 2010;104:73–80. doi: 10.1179/136485910X12607012373795. [DOI] [PubMed] [Google Scholar]
- 41.Roldan W.H., Espinoza Y.A., Huapaya P.E., Huiza A.F., Sevilla C.R., Jimenez S. Frequency of human toxocariasis in a rural population from Cajamarca, Peru determined by DOT-ELISA test. Rev. Inst. Med. Trop. Sao Paulo. 2009;51:67–71. doi: 10.1590/s0036-46652009000200002. [DOI] [PubMed] [Google Scholar]
- 42.Schoenardie E.R., Scaini C.J., Brod C.S., et al. Seroprevalence of Toxocara infection in children from southern Brazil. J. Parasitol. 2013;99:537–539. doi: 10.1645/GE-3182. [DOI] [PubMed] [Google Scholar]
- 43.Page A.P., Rudin W., Fluri E., Blaxter M.L., Maizels R.M. Toxocara canis: A labile antigenic coat overlying the epicuticle of infective larvae. Exp. Parasitol. 2000;75:72–86. doi: 10.1016/0014-4894(92)90123-r. [DOI] [PubMed] [Google Scholar]
- 44.Del Prete G.F., De Carli M., Mastromauro C., et al. Purified protein derivative of Mycobacterium tuberculosis and excretory-secretory antigen(s) of Toxocara canis expand in vitro human T cell with stable and opposite (type 1 T helper or type 2 T helper) profile of cytokine production. J. Clin. Invest. 1991;88:346–350. doi: 10.1172/JCI115300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Badley J.E., Grieve R.B., Rockey J.H., Glickman L.T. Immune-mediated adherence of eosinophils to Toxocara canis infective larvae: The role of excretory-secretory antigens. Parasite Immunol. 1987;9:133–143. doi: 10.1111/j.1365-3024.1987.tb00494.x. [DOI] [PubMed] [Google Scholar]
- 46.Magen E., Borkow G., Bentwich Z., Mishal J., Scharf S. Can worms defend our hearts? Chronic helminthic infections may attenuate the development of cardiovascular diseases. Med. Hypotheses. 2005;64:904–909. doi: 10.1016/j.mehy.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 47.Zibaei M., Sadjjadi S.M., Geramizadeh B., Firoozeh F. Hepatic toxocariasis in a child: A case report from Shiraz, Southern Iran. Hepat. Mon. 2008;8:310–312. [Google Scholar]
- 48.Boschetti A., Kasznica J. Visceral larva migrans induced eosinophilic cardiac pseudotumor. A case of sudden death in a child. J. Forensic Sci. 1995;40:1097–1099. [PubMed] [Google Scholar]
- 49.De Cock C., Lemaitre J., Deuvaret E.F. Löeffler endomyocarditis: a clinical presentation as right ventricular tumor. J. Heart Valve Dis. 1998;7:668–671. [PubMed] [Google Scholar]
- 50.Spry C.J. In: Eosinophils and endomyocardial fibrosis: A review of clinical and experimental studies, 1980-86, Pathogenesis of Myocarditis and Cardiomyopathy. Recent Experimental and Clinical Studies. Kawai C., Abelmann W.H., editors. Tokyo: University of Tokyo Press; 1987. [Google Scholar]
- 51.Glickman L.T., Schantz P.M. Epidemiology and pathogenesis of zoonotic toxocariasis. Epidemiol. Rev. 1981;3:230–250. doi: 10.1093/oxfordjournals.epirev.a036235. [DOI] [PubMed] [Google Scholar]
- 52.Schantz P.M., Glickman L.T. Roundworms in dogs and cats: Veterinary and public health considerations. Cont Educ Prac Vet. 1981;3:773–784. [Google Scholar]
- 53.Schantz P.M., Glickman L.T. Toxocaral visceral larva migrans. N. Engl. J. Med. 1978;298:436–439. doi: 10.1056/NEJM197802232980806. [DOI] [PubMed] [Google Scholar]
- 54.Vargo T.A., Singer D.B., Gillette P.C., Fernbach D.J. Myocarditis due to visceral larva migrans. J. Pediatr. 1977;90:322–323. doi: 10.1016/s0022-3476(77)80666-5. [DOI] [PubMed] [Google Scholar]
- 55.Brockington I.F., Olsen E.G. Loffler's endocarditis and Davies' endomyocardial fibrosis. Am. Heart J. 1973;85:308–322. [Google Scholar]
- 56.Friedman S., Hervada A.R. Severe myocarditis with recovery in a child with visceral larva migrans. J. Pediatr. 1960;56:91–96. doi: 10.1016/s0022-3476(60)80292-2. [DOI] [PubMed] [Google Scholar]
- 57.Spry C.J., Davies J., Tai P-C., Fattah D. In: The pathogenesis of eosinophilic myocardial disease, Immunobiology of the Eosinophil. Yoshida T., Torisu M., editors. New York: Elsevier Science Publishing Company; 1983. [Google Scholar]
- 58.Abe K., Shimokawa H., Kubota T., Nawa Y., Takeshita A. Myocarditis associated with visceral larva migrans due to Toxocara canis. Intern. Med. 2002;41:706–708. doi: 10.2169/internalmedicine.41.706. [DOI] [PubMed] [Google Scholar]
- 59.Bachmeyer C., Lamarque G., Morariu R., Molina T., Bouree P., Delmer A. Visceral larva migrans mimicking lymphoma. Chest. 2003;123:1296–1297. doi: 10.1378/chest.123.4.1296. [DOI] [PubMed] [Google Scholar]
- 60.Herry I., Phillipe B., Hennequin C., Danel C., Lejeunne C., Meyer G. Acute life-threatening toxocaral tamponade. Chest. 1997;112:1692–1693. doi: 10.1378/chest.112.6.1692. [DOI] [PubMed] [Google Scholar]
- 61.Chira O., Badea R., Dumitrascu D., et al. Eosinophilic ascites in patient with Toxocara canis infection. A case report. Rom. J. Gastroenterol. 2005;14:397–400. [PubMed] [Google Scholar]
- 62.Matsuki Y., Fujii T., Nakamura-Uchiyama F., et al. Toxocariasis presenting with multiple effusions in the pericardial space, thoracic cavity, and Morrison’s Pouch. Int Med. 2007;46:913–914. doi: 10.2169/internalmedicine.46.6427. [DOI] [PubMed] [Google Scholar]
- 63.Herry I., Philippe B., Hennequin C., Danel C., Lejeunne C., Meyer G. Acute life-threatening toxocaral tamponade. Chest. 1997;112:1692–1693. doi: 10.1378/chest.112.6.1692. [DOI] [PubMed] [Google Scholar]
- 64.Altcheh J., Nallar M., Conca M., Biancardi M., Freilij H. Toxocariasis: clinical and laboratory features in 54 patients. An. Pediatr. (Barc.) 2003;58:425–431. doi: 10.1016/s1695-4033(03)78088-6. [DOI] [PubMed] [Google Scholar]
- 65.Ehrhard T., Kernbaum S. Toxocara canis and human toxocariasis. Bull. Inst. Pasteur. 1979;77:225–287. [Google Scholar]
- 66.Lassmann B., Tsigrelis C., Virk A. 33-years-old woman with marked eosinophilia. Mayo Clin. Proc. 2007;82:103–106. doi: 10.4065/82.1.103. [DOI] [PubMed] [Google Scholar]
- 67.Kirchner T., Altmann H.W. Parasitic larva as a cause of circumscribed liver lesions. Morphology and differential diagnosis. Pathologe. 1987;8:31–36. [PubMed] [Google Scholar]
- 68.Marty A.M. Toxocariasis. In: Meyers W.M., Neafie R.C., Marty A.M., Wear D.J., editors. Pathology of infectious disease. Washington, DC: Armed Force Institute of Pathology; 2000. [Google Scholar]
- 69.Bratt D.E., Tikasingh E.S. Visceral larva migrans in seven members of one family in Trinidad. Trop. Geogr. Med. 1992;44:109–112. [PubMed] [Google Scholar]
- 70.Ota K.V., Dimaras H., Héon E., Gallie B.L., Chan H.S. Radiologic surveillance for retinoblastoma metastases unexpectedly showed disseminated toxocariasis in liver, lung, and spinal cord. Can. J. Ophthalmol. 2010;45:185–186. doi: 10.3129/i09-216. [DOI] [PubMed] [Google Scholar]
- 71.de Savigny D.H., Voller A., Woodruff A.W. Toxocariasis: Serological diagnosis by enzyme immunoassay. J. Clin. Pathol. 1979;32:284–288. doi: 10.1136/jcp.32.3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zibaei M., Sadjjadi S.M., Sarkari B., Oryan A., Uga S. In vitro cultivation of Toxocara cati adult worms for production of eggs and evaluation of oviposition. Helminthologia. 2009;1:28–30. [Google Scholar]
- 73.Glickman L., Schantz P., Dombroske R., Cypess R. Evaluation of serodiagnostic testes for visceral larval migrans. Am. J. Trop. Med. Hyg. 1978;27:492–498. doi: 10.4269/ajtmh.1978.27.492. [DOI] [PubMed] [Google Scholar]
- 74.Jacquier P., Gottstein B., Stingelin Y., Eckert J. Immunodiagnosis of toxocariasis in humans: evaluation of a new enzyme-linked immunosorbent assay kit. J. Clin. Microbiol. 1991;29:1831–1835. doi: 10.1128/jcm.29.9.1831-1835.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zibaei M., Sadjjadi S.M., Sarkari B., Uga S. Evaluation of Toxocara cati excretory–secretory larval antigens in serodiagnosis of human toxocariasis. J. Clin. Lab. Anal. 2016;30(3):248–253. doi: 10.1002/jcla.21844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thompson D.E., Bundy D.A., Cooper E.S., Schantz P.M. Epidemiological characteristics of Toxocara canis zoonotic infection of children in a Caribbean community. Bull. World Health Organ. 1986;64:283–290. [PMC free article] [PubMed] [Google Scholar]
- 77.Lynch N.R., Hagel I., Vargas V., et al. Comparable seropositivity for ascariasis and toxocariasis in tropical slum children. Parasitol. Res. 1993;79:547–550. doi: 10.1007/BF00932238. [DOI] [PubMed] [Google Scholar]
- 78.Magnaval J.F., Fabre R., Maurières P., Charlet J.P., de Larrard B. Application of the Western blotting procedure for the immunodiagnosis of human toxocariasis. Parasitol. Res. 1991;77:697–702. doi: 10.1007/BF00928685. [DOI] [PubMed] [Google Scholar]
- 79.Fong M.Y., Lau Y.L., Init I., Jamaiah I., Anuar A.K., Rahmah N. Recombinant expression of Toxocara canis excretory-secretory antigen TES-120 in Escherichia coli. Southeast Asian J. Trop. Med. Public Health. 2003;34:723–726. [PubMed] [Google Scholar]
- 80.Zahabiun F., Sadjjadi S.M., Yunus M.H., et al. Production of Toxocara cati TES-120 recombinant antigen and comparison with its T. canis homolog for serodiagnosis of toxocariasis. Am. J. Trop. Med. Hyg. 2015;93:319–325. doi: 10.4269/ajtmh.15-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zibaei M., Sadjjadi S.M., Ishiyama S., Sarkari B., Uga S. Production of monoclonal antibody against Toxocara cati second-stage larvae and it application for the detection of circulation antigens. Hybridoma (Larchmt) 2010;29:217–220. doi: 10.1089/hyb.2009.0108. [DOI] [PubMed] [Google Scholar]
- 82.Ishiwata K., Shinohara A., Yagi K., Horii Y., Tsuchiya K., Nawa Y. Identification of tissue embedded ascarid larvae by ribosomal DNA sequencing. Parasitol. Res. 2004;95:50–52. doi: 10.1007/s00436-003-1010-7. [DOI] [PubMed] [Google Scholar]
- 83.Caldera F., Burlone M.E., Genchi C., Pirisi M., Bartoli E. Toxocara encephalitis presenting with autonomous nervous system involvement. Infection. 2013;41:691–694. doi: 10.1007/s15010-012-0342-6. [DOI] [PubMed] [Google Scholar]
- 84.Stürchler D., Schubarth P., Gualzata M., Gottstein B., Oettli A. Thiabendazole vs. albendazole in treatment of toxocariasis: A clinical trial. Ann. Trop. Med. Parasitol. 1989;83:473–478. doi: 10.1080/00034983.1989.11812374. [DOI] [PubMed] [Google Scholar]
- 85.Magnaval J.F. Comparative efficacy of diethylcarbamazine and mebendazole for the treatment of human toxocariasis. Parasitology. 1995;110:529–533. doi: 10.1017/s0031182000065240. [DOI] [PubMed] [Google Scholar]
- 86.Enko K., Tada T., Ohgo K.O., et al. Fulminant eosinophilic myocarditis associated with visceral larva migrans caused by Toxocara canis infection. Circ. J. 2009;73:1344–1348. doi: 10.1253/circj.cj-08-0334. [DOI] [PubMed] [Google Scholar]


