Abstract
Abstract: Background: In the management of non-ST-elevation acute coronary syndrome (NST-ACS) a gap between guideline-recommended care and actual practice has been reported. A systematic overview of the actual extent of this gap, its potential impact on patient-outcomes, and influential factors is lacking.
Objective: To examine the extent of guideline adherence, to study associations with the occurrence of adverse cardiac events, and to identify factors associated with guideline adherence.
Method: Systematic literature review, for which PUBMED, EMBASE, CINAHL, and the Cochrane library were searched until March 2016. Further, a manual search was performed using reference lists of included studies. Two reviewers independently performed quality-assessment and data extraction of the eligible studies.
Results: Adherence rates varied widely within and between 45 eligible studies, ranging from less than 5.0% to more than 95.0% for recommendations on acute and discharge pharmacological treatment, 34.3% - 93.0% for risk stratification, and 16.0% - 95.8% for performing coronary angiography. Seven studies indicated that higher adherence rates were associated with lower mortality. Several patient-related (e.g. age, gender, co-morbidities) and organization-related (e.g. teaching hospital) factors influencing adherence were identified.
Conclusion: This review showed wide variation in guideline adherence, with a substantial proportion of NST-ACS patients possibly not receiving guideline-recommended care. Consequently, lower adherence might be associated with a higher risk for poor prognosis. Future research should further investigate the complex nature of guideline adherence in NST-ACS, its impact on clinical care, and factors influencing adherence. This knowledge is essential to optimize clinical management of NST-ACS patients and could guide future quality improvement initiatives.
Keywords: Acute coronary syndrome [MeSH], unstable angina [MeSH], systematic review, guideline adherence [MeSH]
INTRODUCTION
Non-ST-Elevation Acute Coronary Syndromes (NST-ACS) comprise one of the most common types of ACS, encompassing the two sub-conditions Unstable Angina (UA) and Non-ST-Elevation Myocardial Infarction (NSTEMI). The proportion of patients diagnosed with these conditions has increased substantially in the past two decades, whereas the proportion of ST-Elevation Myocardial Infarction (STEMI) patients has decreased [1]. In addition, NST-ACS patients have a higher long-term risk of myocardial infarction and/or death as compared with STEMI patients [2-5]. In the management of NST-ACS clinical practice guidelines (CPG’s) have become increasingly important. CPG’s are developed to guide physicians in clinical decision-making and to decrease variability in treatment practices in order to enhance the quality of care [6-8]. For the management of NST-ACS, several guidelines exists, such as the National Institute for Health and Care Excellence (NICE) guidelines [9], the European Society of Cardiology (ESC) guidelines [10], and the American College of Cardiology/American Heart Association (ACC/AHA) guidelines [11]. The ESC and ACC/AHA are most known and comprise class I recommendations on acute in-hospital pharmacological treatment, risk stratification, performing coronary angiography (CA), and the prescription of discharge medications [10, 11]. A gap between evidence-based medicine incorporated in these guidelines and actual practice seems to exist, with various studies indicating that a substantial proportion of NST-ACS patients does not receive care according to the guidelines [12, 13]. Up until now, only two literature reviews reported on potential guideline-practice gaps in the management of ACS patients. One review summarized literature on guideline adherence in ACS patients in general [14], whereas the second focused on adherence in the management of NST-ACS patients specifically [15]. This latter review, however, only included studies from a single registry (i.e., CRUSADE) conducted primarily in the USA. In addition, previous research concluded that the extent of adherence to clinical guidelines can be influenced by factors related to the patient, the health care provider or the organization [16-18]. Several studies showed a wide variety of factors that were associated with (under)utilization of evidence-based therapies, but an overview of potential factors associated with guideline adherence in NST-ACS patients is lacking. Given that in a previous study low guideline adherence in NST-ACS patients was associated with adverse cardiac events, such as death and myocardial infarction (MI) [19], and NST-ACS prevalence rates are increasing [20], insight in the extent of guideline adherence, potential practice gaps and the impact on patient outcomes in this specific patient group is necessary. The results can be used to stress the importance of optimizing clinical management among policy-makers and clinicians. The aims of the current systematic literature review were to 1) examine the extent of adherence to international cardiac guideline recommendations, 2) study the association between guideline adherence and adverse cardiac events (i.e., death and/or MI), and 3) identify potential factors associated with guideline adherence in the management of patients with NST-ACS.
METHODS
A systematic review of the literature was conducted. In reporting the results of this study, the “Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA)” statement was used [21].
Search Strategy
A literature search was conducted in PUBMED (including MEDLINE), EMBASE, CINAHL, and the Cochrane library until March 2016. The search strategies were constructed in cooperation with an information specialist from the library of the VU University Amsterdam and included search terms related to adherence combined with terms related to guidelines or protocols, MI, and UA (Appendix A). No restrictions were applied. In addition to the electronic search, reference lists of the included studies were manually screened for additional relevant articles. When the full-text of a study was not available online, either the first author was approached to request a copy of the study or a full-text copy was ordered online. The Cochrane database for systematic reviews was searched for systematic literature reviews on adherence in NST-ACS care, but none were found.
Selection of Studies
Two reviewers (JE, ND) independently screened all studies identified in the initial search on title and abstract. Studies were selected for full-text screening if guideline adherence in NST-ACS patients was addressed in either the title or abstract. In case of disagreement between the reviewers, a third reviewer was consulted (IvdW). Subsequently, two reviewers (JE, ND) screened the full-text of these selected studies independently. Studies that met all of the following criteria were included in this systematic literature review:
The study focused on adherence in NST-ACS patients to either the American College of Cardiology (ACC/AHA) or the European Society of Cardiology (ESC) guidelines (versions developed since 2000);
The study reported on one or more of the following guideline recommendations: acute in-hospital pharmacological treatment, risk stratification to decide on the need for early invasive procedures (i.e. electrocardiogram (ECG), troponin assessment, or use of validated risk scores), performance of in-hospital CA in intermediate to high risk patients, and/or the prescription of discharge medications (Box 1);
The study sample included adults (≥18 years) with NST-ACS (i.e., UA and/or NSTEMI);
The study design was observational or (quasi-) experimental;
The study was conducted in a hospital setting.
Table 1. Methodological quality of the included studies based on the STROBE criteria.
| Reference | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
| Total score | 9 | 10 | 9.5 | 10.5 | 8.5 | 7.5 | 10 | 9 | 6.5 | 10.5 | 7 | 9.5 | 10 | 9.5 | 9 |
| Reference | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 |
| Total score | 8 | 10 | 10 | 7 | 10 | 9 | 5.5 | 10 | 10 | 9.5 | 9.5 | 10 | 8 | 10 | 9 |
| Reference | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | 43 | 44 | 45 |
| Total score | 9 | 9 | 8 | 9 | 8.5 | 6.5 | 10 | 9.5 | 7 | 6 | 8 | 7.5 | 9 | 9.5 | 9 |
| Methodological quality was assessed using a checklist based on the STROBE criteria, consisting of 11 items. Items were scored as following: 1 = described, ½ = partly described, 0 = not/insufficiently described. Total score ranged from 0-11, where scores between 0 - 6 reflected poor study quality, >6 - <8 moderate study quality, ≥8 - <10 good study quality and ≥10 excellent study quality. 1. Amsterdam et al. 2009, 2. Banihashemi et al. 2009, 3. Bhatt et al. 2004, 4. Chandra et al. 2009, 5. Cheng et al. 2010, 6. Diercks et al. 2006, 7. Diercks et al. 2007, 8. Dziewierz et al. 2007, 9. Ellis et al. 2004, 10. Engel et al. 2015, 11. Ferreira et al. 2004, 12. Goldberg et al. 2007, 13. Hoekstra et al. 2005, 14. Kassab et al. 2013, 15. Kassaian et al. 2015, 16. Lee et al. 2008, 17. Maddox et al. 2012, 18. Maier et al. 2008, 19. Mandelzweig et al. 2006, 20. Mehta et al. 2006, 21. Miller et al. 2007, 22. Nieuwlaat et al. 2004, 23. Olivari et al. 2012, 24. Peterson et al. 2003, 25. Peterson et al. 2006, 26. Peterson et al. 2008, 27. Polonski et al. 2007, 28. Rao et al. 2009, 29. Roe, Parsons, et al. 2005, 30. Roe, Peterson, et al. 2005, 31. Roe, Chen, et al. 2006, 32. Roe, Peterson, et al. 2006, 33. Roe et al. 2007, 34. Schiele et al. 2005, 35. Sherwood et al. 2014, 36. Sinon et al. 2014, 37. Somma et al. 2012, 38. Sonel et al. 2005, 39. Tang et al. 2005, 40. Tricoci et al. 2006, 41. Valli et al. 2014, 42. Vikman et al. 2003, 43. Yan et al. 2007, 44. Zeymer et al. 2014, 45. Zhang et al. 2009. | |||||||||||||||
Studies were excluded from this systematic literature review when:
Adherence to ACC/AHA and/or ESC guideline recommendations was studied in a subgroup of NST-ACS patients (e.g., NST-ACS patients with diabetes mellitus);
The study design was not observational or (quasi-) experimental (e.g., review, editorial, letter to the editor, opinion paper, conference abstract, qualitative study, or design article).
Methodological quality assessments
The methodological quality of the included studies was assessed by two reviewers independently (JE, ND), using a checklist based on the STROBE statement for observational studies [22]. The checklist comprised 11 items: title and abstract, introduction and objectives, study design, participant selection and sample size, variables, data sources and methods, data analyses, participant flow, descriptive data, main results, and discussion. Each item on the checklist was scored 0 in case an adequate description of the item in the paper was lacking or not reported, 0.5 in case an adequate description was given but minimal data were reported, or 1 in case both were adequate. Scores on the 11 items were summed and as a result, each study received a total score that ranged from 0 (poor study quality) to 11 (excellent study quality). Scores between 0-6 reflected poor study quality, scores >6 – <8 reflected moderate study quality, scores ≥8 – <10 reflected good study quality and scores ≥10 reflected excellent study quality. Agreement between the reviewers was considered substantial: in 87% of the assessed studies quality scores of both reviewers did not differ more than 0.5 point and there were no studies of which the scores of both reviewers differed more than one point.
Data extraction
Data of the included studies were extracted by one reviewer (JE) and thoroughly checked by a second reviewer (ND). Using a standardized data extraction form, the following characteristics were extracted: first author, year of publication, country of data collection, study design, data collection methods, study sample, type of guideline(s) evaluated (i.e., ACC/AHA and/or ESC), type of recommendation(s) evaluated, and main results.
In the data extraction process, the following criteria were applied:
When included studies focused on the management of both STEMI and NST-ACS patients, only the results for NST-ACS patients were extracted;
When data of the included studies were collected at different time points (e.g., cohort studies), only details of the latest measurement were reported as these provided the most recent information;
When studies had a pretest-posttest design in which the effect of an intervention was assessed, only details from the pretest measurement were extracted, as we did not aim to evaluate intervention effects;
Of the studies focusing on potential factors associated with guideline adherence, only the statistically significant associations from multivariable analyses were extracted (p ≤ 0.05).
RESULTS
Description of the studies
The final selection of studies consisted of 45 studies (Fig. 1). Of the included studies, 21 studies were conducted in the USA [12, 13, 19, 23-40], 12 in Europe [41-52], four in Canada [53-56], five in Asia [57-61], two in New-Zealand [62, 63], and one study was conducted in multiple countries [64]. The majority of studies had an observational study design, with the exception of three studies who respectively concerned a pilot study [52], a descriptive study [61], and a before-after study [47]. Sample sizes of the included studies ranged from 121 to 2,515,106 patient admissions. Two studies were single-center studies [58, 63], while the other studies were multicenter studies.
Fig. (1).
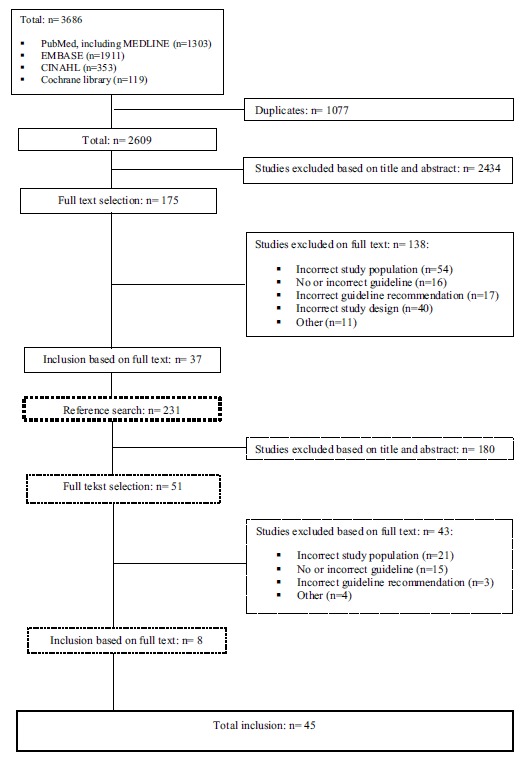
Flow chart of article selection.
Methodological Quality
The methodological quality assessment indicated that the quality of 36 included studies was excellent or good [12, 13, 19, 23-25, 27-38, 40, 41, 44, 45, 47, 48, 50-60, 64], whereas the quality of seven studies was scored moderate [26, 42, 46, 49, 61, 62, 63] and two studies were scored poor [39, 43] (Table 1)). Most studies lacked a detailed description of primary and secondary outcomes and related measurement sources, the handling of missing data, and/or the adjustment for confounders in multivariable analyses. With regard to the description of the study design, the majority of studies referred to a previously reported design paper.
Main Results
Results were categorized into (1) the extent of adherence to ACC/AHA and/or ESC guideline recommendations; (2) the association between guideline adherence and adverse cardiac events (i.e., death and/or MI); and/or (3) potential factors associated with guideline adherence. Given that guideline recommendations were overall comparable, in this categorization no distinction between the ACC/AHA and ESC guidelines was made. Also different versions of both guidelines, published over the years, were highly comparable in class and level of evidence (Box 1).
The Extent of Adherence to Cardiac Guideline Recommendations
Acute in-Hospital Pharmacological Treatment
Thirty-four studies reported on the extent of adherence to guideline recommendations on acute in-hospital pharmacological treatment, including the prescription of aspirin, beta-blockers, platelet aggregation inhibitors (e.g., clopidogrel), glycoprotein IIb/IIIa inhibitors, and/or heparin [12, 13, 19, 23, 25, 26, 28, 29, 31-38, 40-46, 48, 49, 51-54, 59-63]. Overall, adherence rates in these studies varied from 0.5% [61] to 98.3% [60]. The three lowest adherence rates were related to recommendations regarding the early prescription of glycoprotein IIb/IIIa inhibitors (0.5% [61], 0.6% [62], and 1.8% [59], whereas the three highest adherence rates were related to recommendations on the early prescription of aspirin (97.0% [41], 97.1% [13], and 98.3% [60]) (Table 2)).
Table 2. Characteristics of studies on the extent of adherence to pharmacological therapies recommended by the ACC/AHA and/or ESC NST-ACS guidelines.
|
First author, year
(country) [PMID] |
Study design | Sample |
Main results
I = acute pharmacological care (<24 h after admission) II = discharge medications |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amsterdam, 2009 [23] (USA) [PMID: 1985369] |
Prospective, multi-center, observational registry (CRUSADE) † |
138,714 NST-ACS patients, enrolled from 547 hospitals |
I. aspirin 96.0%, BB 90.8%, clopidogrel 57.8%, GP IIb/IIIa inhibitors 47.2%, any heparin 87.1% II. ACE/ARB 69.4%, aspirin 94.7%, BB 92.4%, clopidogrel 73.6%, statin 88.8% (Based on last measurement 2005, n=138.714 NSTEMI patients) |
|||||||||
| Banihashemi, 2009 [53] (Canada) [PMID: 19958875] | Prospective, multi-center, observational registry (GRACE)† | 5,806 NST-ACS patients, enrolled from 53 hospitals | I. Overall, 67.1% of patients received clopidogrel and/or GP IIb/IIIa inhibitors ≤ 24h: 97.8% of these patients received clopidogrel, 2.2% received GP IIb/IIIa inhibitors. | |||||||||
| Chandra, 2009 [25] (USA) [PMID: 19282062] |
Prospective, multi-center, observational registry (CRUSADE) † |
33,238 NST-ACS patients, enrolled from 344 hospitals | I. aspirin 96.0%, BB 90.9%, clopidogrel 56.2%, GP IIb/IIIa inhibitor 48.0%, any heparin 88.2% | |||||||||
| Cheng, 2010 [57] (Taiwan) [PMID: 20552592] |
Prospective, multi-center, observational registry (T-ACCORD) |
1,331 NST-ACS patients, enrolled from 27 hospitals | II. ACE or ARB 60.0%, aspirin only 16.0%, clopidogrel only 17.3%, aspirin and clopidogrel 61.8%, BB 50.2%, statin 68.8% | |||||||||
| Diercks, 2006 [26] (USA) [PMID: 16824844] |
Prospective, multi-center, observational registry (CRUSADE) † |
80,845 NST-ACS patients (Number of hospitals unknown) |
I. aspirin 92.2%, BB 80.1%, clopidogrel 41.3%, GP IIb/IIIa inhibitor 37.9%, any heparin 84.3% II. ACE/ARB 56.9%, aspirin 90.4%, BB 84.3%, clopidogrel 56.1%, statin 68.1% |
|||||||||
| Dziewierz, 2007 [45] (Poland) [PMID: 17496494] |
Prospective, multi-center, observational registry (Malopolska registry of ACS) | 807 NSTEMI patients, enrolled from 29 hospitals | I. mean pharmacotherapy index: 4.3 (range 0-7, one point for each medication received, including ACE/ARB, aspirin, BB, clopidogrel, GP IIb/IIIa inhibitor, LMW Heparin, and statin). Per medicine: ACE/ARB 76.6%, aspirin 94.9%, BB 83%, clopidogrel 9.9%, GP IIb/IIIa inhibitor 2.9%, LMW Heparin 73.9%, statin 84.4% | |||||||||
| Ellis, 2004 [62] (New Zealand) [PMID: 15326506] |
Prospective, multi-center, observational audit | 930 ACS patients, of which 333 UA and 287 NSTEMI, enrolled from 36 hospitals |
I. aspirin 79.0% NSTEMI / 81.0% UA, clopidogrel 13.0% NSTEMI / 6.2% UA, GP IIb/IIIa inhibitor 2.8% NSTEMI / 0.6% UA, LMW heparin 64.0% NSTEMI / 51.0% UA, UF heparin 8.8% NSTEMI / 6.6% UA II. ACE 45.0% NSTEMI / 39.0% UA, aspirin 83.0% NSTEMI / 80.0% UA, BB 63.0% NSTEMI / 59.0% UA, clopidogrel 9.5% NSTEMI / 5.1% UA, statin 55.0% NSTEMI / 52.0%UA |
|||||||||
| Ferreira, 2004 [46] (Portugal) [PMID: 15641292] |
Prospective, multi-center, observational registry (National Registry of ACS) | 7,348 ACS patients, of which 2,858 NSTEMI and 1,154 UA, enrolled from 44 hospitals |
I. aspirin 96.0% NSTEMI / 96.0% UA, BB 67.0% NSTEMI / 76.0% UA, GP IIb/IIIa inhibitor 37.0% NSTEMI / 26.0% UA, any heparin 97.0% NSTEMI / 95.0% UA II. ACE 66.0% NSTEMI / 53.0% UA, aspirin 91.0% NSTEMI / 91.0% UA, BB 64.0% NSTEMI / 71.0% UA, statins 77.0% NSTEMI / 78.0% UA |
|||||||||
|
First author, year (country) [PMID] |
Study design | Sample |
Main results I = acute pharmacological care (<24 h after admission) II = discharge medications |
|||||||||
| Goldberg, 2007 [64] (14 countries in North and South America, Europe, Australia and New- Zealand) [PMID:17846396] |
Prospective, multi-center, observational registry (GRACE) † |
26,413 ACS patients, of which 12,444 NSTEMI, enrolled from 113 hospitals |
II. ACE 73.0%, aspirin 95.0%, BB 90.0%, statin 83.0% (Based on latest measurement in 2005) |
|||||||||
| Hoekstra, 2005 [28] (USA) [PMID: 15863399] |
Prospective, multi-center, observational registry (CRUSADE) † |
56,804 NST-ACS patients, enrolled from 443 hospitals | I. GP IIb/IIIa inhibitors were provided in 35.5% of patients | |||||||||
| Kassab, 2013 [58] (Malaysia) [PMID: 22845427] |
Retrospective, cross-sectional, single center study | 380 ACS patients, of which 215 UA and 76 NSTEMI, enrolled from 1 hospital | II. ACE 69.7% NSTEMI / 60.5% UA, ARB 7.9% NSTEMI / 4.2% UA, aspirin 92.1% NSTEMI / 85.6% UA, BB 82.9% NSTEMI / 81.4% UA, clopidogrel 90.8% NSTEMI / 78.6% UA, statin 94.7% NSTEMI / 94.0% UA | |||||||||
| Kassaian, 2015 [60] (Iran) [26671947] |
Prospective, multi-center, observational registry | 1226 NST-ACS patients, enrolled from 11 hospitals | I. aspirin 98.3%, BB 88.7%, clopidogrel 89.7%, any heparin 93.9% | |||||||||
| Maddox, 2012 [30] (USA) [PMID: 22570355] |
Prospective, multi-center, observational registry (GWTG-CAD) † |
23,186 NSTEMI patients, enrolled from 382 hospitals | II. 53.9% had clopidogrel prescribed at discharge. | |||||||||
| Maier, 2008 [41] (Germany) [PMID: 18061689] |
Prospective, multi-center observational registry (BMIR) |
6,080 ACS patients, of which 1,766 NSTEMI, enrolled from 22 hospitals |
I. aspirin 97.0%, BB 90.8%, GP IIb/IIIa inhibitors 43.7% II. ACE 80.5%, aspirin 96%, BB 93.6%, statins 84.1% (Based on last measurement 2004, n=1087 NSTEMI patients) |
|||||||||
| Mandelzweig, 2006 [42] (32 countries in Europe and Mediterranean basin) [PMID: 16908490] |
Prospective, multi-center, observational survey (EHS-ACS-II) |
6,358 ACS patients, of which 3,063 NST-ACS, enrolled from 190 hospitals |
I. aspirin 94.5%, BB 82.8%, clopidogrel 67.4%, GP IIb/IIIa 20.8%, any heparin 90.0% II. ACE or ARB 67.0%, BB 78.0%, aspirin 88.0%, clopidogrel or other 59.0%, statins 76.0% |
|||||||||
| Mehta, 2006 [31] (USA) [PMID: 17030838] |
Prospective, multi-center, observational registry (CRUSADE) † |
113,595 NST-ACS patients, enrolled from 434 hospitals |
I. aspirin 95.3%, BB 86.8%, clopidogrel 51.5%, GP IIb/IIIa inhibitor 44.6%, any heparin 87.4% II. ACE 63.7%, aspirin 93.2%, BB 88.6%, clopidogrel 68.7%, statin 86.8% (Based on last quarter measurement n=11.111) |
|||||||||
| Miller, 2007 [29] (USA) [PMID: 17679127] |
Prospective, multi-center, observational registry (CRUSADE) † |
72,054 NST-ACS patients, enrolled from 509 hospitals | I. 82.5% of patients received beta blockers | |||||||||
|
First author, year (country) [PMID] |
Study design | Sample |
Main results I = acute pharmacological care (<24 h after admission) II = discharge medications |
|||||||||
| Nieuwlaat, 2004 [43] (The Netherlands) [PMID: 15497784] |
Prospective, multi-center, observational survey | 421 ACS patients, of which 198 NST-ACS, enrolled from 6 hospitals. |
I. aspirin 91.0%, BB 81.0%, clopidogrel 25.0%, any heparin 89.0% I1. ACE 36.0%, aspirin 84.0%, BB 79.0%, clopidogrel 25.0%, statin 69.0% |
|||||||||
| Peterson, 2003 [32] (USA) [PMID: 12849658] |
Prospective, multi-center, observational registry (NRMI) † |
60,770 NSTEM patients, enrolled from 1189 hospitals | I. 25.0% of patients received GP IIb/IIIa inhibitors | |||||||||
| Peterson, 2006 [19] (USA) [PMID: 16639050] |
Prospective, multi-center, observational registry (CRUSADE) † |
64,775 NST-ACS patients, enrolled from 350 hospitals |
Overall adherence rate: 74.0% (range 63.0% for lowest quartile to 82.0% for highest quartile) I. aspirin 92.0%, BB 79.0%, clopidogrel 41.0%, GP IIb/IIIa inhibitor 36.0%, any heparin 82.0% II. ACE 61.0%, aspirin 90.0%, BB 84.0%, clopidogrel 54.0%, statin 76.0% |
|||||||||
| Peterson, 2008 [33] (USA) [PMID: 19032998] |
Prospective, multi-center, observational registry (NRMI) † |
2,515,106 ACS patients, of which 1,368,497 NSTEMI, enrolled from 2157 hospitals |
I. aspirin 88.0%, BB 79.0%, any heparin 74.0% II. ACE/ARB 65.0%, aspirin 90.0%, BB 88.0%, statin 82.0% (Based on data last cohort 2003-2006, n=227.845 NSTEMI patients) |
|||||||||
| Polonski, 2007 [44] (Poland) [PMID: 17853315] |
Prospective, multi-center observational registry (Polish registry of ACS) | 100,193 ACS patients, of which ±42,281 UA and ±26,651 NSTEMI, enrolled from 417 hospitals |
I. aspirin 92.0% NSTEMI / 92.0% UA, BB 78.0% NSTEMI, 82.0% UA, thienopyridine 43.0% NSTEMI / 36.0% UA, LMW heparin 76.0% NSTEMI / 65.0% UA II. ACE 75.0% NSTEMI / 76.0% UA, aspirin 85.0% NSTEMI / 86.0% UA, BB 77.0% NSTEMI / 80.0% UA, thienopyridine 38.0% NSTEMI / 30.0% UA, statins 81.0% NSTEMI / 82.0% UA |
|||||||||
| Rao, 2009 [54] (Canada) [PMID: 19332190] |
Prospective, multi-center, observational registries (GRACE) † |
11,177 ACS patients, of which 5,194 NSTEMI and 2,892 UA, enrolled from 53 hospitals |
I. Clopidogrel 73.6% NSTEMI / 64.6% UA (Based on latest measurement in 2007, n=3063 NST-ACS) |
|||||||||
| Roe, 2005 [37] (USA) [PMID: 16157831] |
Prospective, multi-center, observational registry (CRUSADE) † |
23,298 NST-ACS patients (number of hospitals unknown) | I. aspirin 90.8%, BB 76.9%, clopidogrel 37.8%, GP IIb/IIIa inhibitor 31.6%, any heparin 83.2% | |||||||||
| Roe, 2005 [12] (USA) [PMID: 16043682] |
Prospective, multi-center, observational registry (NRMI) † |
185,968 ACS patients, of which 132,551 NSTEMI, enrolled from 1247 hospitals |
I. aspirin 84.9%, BB 72.2% II. ACE 51.2%, aspirin 83.8%, BB 78.3%, statin 85.7% |
|||||||||
| Roe, 2006 [35] (USA) [PMID: 16765118] |
Prospective, multi-center, observational registry (CRUSADE) † |
45,744 NST-ACS, enrolled from 424 hospitals | I. aspirin 91.2%, BB 77.8%, clopidogrel 40.0%, GP IIb/IIIa inhibitor 35.2%, any heparin 82.4% | |||||||||
|
First author, year (country) [PMID] |
Study design | Sample |
Main results I = acute pharmacological care (<24 h after admission) II = discharge medications |
|||||||||
| Roe, 2006 [34] (USA) [PMID: 16781220] |
Prospective, multi-center, observational registry (CRUSADE) † |
77,760 NST-ACS patients, enrolled from 457 hospitals. |
I. aspirin 91.5%, BB 78.8%, GP IIb/IIIa inhibitor 54.2%, any heparin 83.1% II. ACE/ARB 60.6%, aspirin 89.7%, BB 83.4%, clopidogrel 53.5%, statin 79.7% |
|||||||||
| Roe, 2007 [36] (USA) [PMID: 17709638] |
Prospective, multi-center, observational registry (CRUSADE) † |
55,994 NST-ACS, enrolled from 301 hospitals |
I. aspirin 91.8%, BB 78.5%, clopidogrel 42.5%, GP IIb/IIIa inhibitor 37.7%, any heparin 83.4% II. ACE 60.7%, aspirin 90.8%, BB 83.9%, clopidogrel 56.3%, statin 80.7% |
|||||||||
| Schiele, 2005 [48] (France) [PMID: 15681575] |
Prospective, multi-center, observational registry | 754 ACS patients, of which 421 NSTEMI patients, enrolled from 12 hospitals | Median compliance index: 0.66∞ I. aspirin 92.0%, BB 61.0%, GP IIb/IIIa inhibitors 31.0%, any heparin 94.0% |
|||||||||
| Sherwood, 2014 [40] (USA) [24732921] |
Prospective, multi-center, observational registry (GWTG-CAD) † |
158,492 NSTEMI patients, enrolled from 548 hospitals |
I. thienopyridine 54.9% II. thienopyridine 73.9% (Based on latest measurement in 2012) |
|||||||||
| Sinon, 2014 [61] (Philippines) [not availiable] |
Descriptive multi-center study | 1068 NST-ACS patients, enrolled from 39 hospitals | I. aspirin 75.3%, BB 53.9%, clopidogrel 78.0%, GP IIb/IIIa inhibitor 0.47%, any heparin 85.7% | |||||||||
| Somma, 2012 [13] (USA) [PMID: 22949493] |
Prospective, multi-center, observational registry (GWTG-CAD) † |
72,352 ACS patients, of which 48,966 NSTEMI, enrolled from 237 hospitals |
I. aspirin 97.1%, BB 90.8% II. ACE/ARB 77.4%, aspirin 97.3%, BB 97.0%, clopidogrel 67.0%, statin 88.0% |
|||||||||
| Sonel, 2005 [38] (USA) [PMID: 15769762] |
Prospective, multi-center, observational registry (CRUSADE) † |
43,317 NST-ACS patients, enrolled from 400 hospitals. |
I. aspirin 91.0%, BB 77.6%, clopidogrel 39.5%, GP IIb/IIIa inhibitor 34.9%, any heparin 82.5% II. ACE/ARB 60.1%, aspirin 89.5%, BB 82.8%, clopidogrel 52.2%, statin 74.4% |
|||||||||
| Tang, 2005 [63] (New-Zealand) [PMID: 16224502] |
Retrospective, cross-sectional, single-center, observational study | 577 ACS patients, of which 239 NSTEMI and 143 UA, enrolled from 1 hospital | I. clopidogrel 59.0% NSTEMI, GP IIb/IIIa inhibitors 37.0% NSTEMI, any heparin 93.0% UA/NSTEMI | |||||||||
| Valli, 2014 [52] (Italy) [26562982] |
Pilot study | 121 NSTEMI patients, enrolled from 7 Emergency departments | I. aspirin 58.7%, thienopyridine 48.8%, any heparin 64.5% | |||||||||
|
First author, year (country) [PMID] |
Study design | Sample |
Main results I = acute pharmacological care (<24 h after admission) II = discharge medications |
|||||||||
| Vikman, 2003 [49] (Finland) [PMID: 12944205] |
Prospective multi-center, observational registry (FINACS I) |
501 NST-ACS, enrolled from 9 hospitals |
I. aspirin 87.0%, BB 92.0%, clopidogrel 16.0%, heparin LMW 76.0%, GP IIb/IIIa inhibitor 18.0% II. statin 58.0% |
|||||||||
| Zeymer, 2014 [51] (Germany) [PMID: 25374386] |
Prospective, multi-center, observational registry (EPICOR) | 333 NST-ACS patients, enrolled from 29 hospitals |
I. aspirin 96.1%, BB 94.6%, thienopyridine 95.5% (73.0% clopidogrel / 22.5% prasugrel), GP IIb/IIIa inhibitors 18.9%, any heparin 96.7% II. ACE 89.5%, aspirin 95.2%, BB 91.3%, thienopyridine 83.2% (62.8% clopidogrel / 20.4% prasugrel), statin 92.2% |
|||||||||
| Zhang, 2009 [59] (China) [PMID: 19323898] |
Prospective, multi-center observational registry (GRACE) † |
618 NST-ACS, enrolled from 12 hospitals. | I. aspirin 95.6%, thienopyridine 85.9%, GP IIb/IIIa inhibitors 1.8%, any heparin 90.6% | |||||||||
Abbreviations: ACE, angiotensin-converting-enzyme inhibitor; ACS, acute coronary syndrome; ARB, angiotensin II AT1 receptor blockers; BB, beta-blocker; BMIR, Berlin Myocardial Infarction Registry; CRUSADE, Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the ACC/AHA Guidelines; EHS-ACS-II, Second Euro Heart Survey on Acute Coronary Syndrome; GP IIb/IIIa, Glycoprotein IIb/IIIa receptor inhibitors; GRACE, Global Registry of Acute Coronary Events; GWTG-CAD, Get With the Guidelines - Coronary Artery Disease; LMW, low molecular weight; NRMI, National Registry of Myocardial Infarction; NST-ACS, non-ST-elevation acute coronary syndrome; NSTEMI, non-ST-elevation myocardial infarction; PMID, PubMed ID; UA, unstable angina; UF, unfractioned.
†Concern large registries that provide access to quality improvement tools, e.g. quarterly feedback reports/benchmarks.
Risk Stratification
Six studies reported on guideline adherence regarding risk stratification to decide on the need for early invasive procedures [25, 27, 43, 47, 50, 61]. Adherence rates of 34.3% [27], 35.6% [25], and 82.0% [47] for the performance of an ECG within 10 min after arrival at the hospital were reported. In addition, two studies, one with poor and another with moderate methodological quality, indicated that in respectively 92.0% and 93.0% of NST-ACS patients troponin assessment was used as a risk stratification method [43, 61]. One study reported on the use of validated risk-scoring instruments in practice, such as the Global Registry of Acute Coronary Events (GRACE) or the Thrombolysis In Myocardial Infarction (TIMI) risk scores. In 57% of NST-ACS patients a validated risk score outcome was documented in their medical chart, with scores ranging between hospitals from 16.7% to 87.0% [50].
Performing in-Hospital CA
Twenty-four studies reported on adherence to guideline recommendations on the performance of in-hospital CA in intermediate to high-risk patients [24-27, 31, 33-39, 42-44, 46, 48, 49, 51, 55, 56, 60, 62, 63]. Overall, CA was performed in 16.0% [62] to 95.8% [51] of NST-ACS patients. More specifically, in 22.7% [27] to 47.5% [25] of patients in-hospital CA was performed within 24 h after admission, whereas in 42.5% [34] to 65.8% [25] CA was performed in-hospital within 48 h after admission. In four studies CA-adherence rates were stratified by patients’ risk status, with results being mixed. In three of these studies high-risk patients were less likely to receive in-hospital CA as compared with low-risk patients [38, 55, 56], while in one study 25.0% of low-risk patients received in-hospital CA versus 56.0% of high-risk patients [43] (Table 3)). However, methodological quality of this latter study was scored poor (Table 1)).
Table 3. Characteristics of studies on adherence to ACC/AHA and ESC NST-ACS guideline recommendations regarding performing coronary angiography.
|
First author, year
(country) [PMID] |
Study design | Sample | Main results |
|---|---|---|---|
| Bhatt, 2004 [24] (USA) [PMID: 15523070] |
Prospective, multi-center, observational registry (CRUSADE) † | 17,926 NST-ACS patients, enrolled from 248 hospitals | 62.2% CA in-hospital 44.8% CA <48 h |
| Chandra, 2009 [25] (USA) [PMID: 19282062] |
Prospective, multi-center, observational registry (CRUSADE) † | 33,238 NST-ACS patients, enrolled from 344 hospitals | 83.2% CA in-hospital 47.5% CA ≤24 h 65.8% CA ≤48 h |
| Diercks, 2006 [26] (USA) [PMID: 16824844] |
Prospective, multi-center, observational registry (CRUSADE) † | 80,845 NST-ACS patients (Number of hospitals unknown) | 70.4% CA in-hospital 49.4% CA ≤48 h |
| Diercks, 2007 [27] (USA) [PMID: 17496494] |
Prospective, multi-center, observational registry (CRUSADE) † | 42,780 NST-ACS patients, enrolled from 550 hospitals. | 74.5% CA in-hospital 22.7% CA ≤24 h 47.8% CA ≤48 h |
| Ellis, 2004 [62] (New Zealand) [PMID: 15326506] |
Prospective, multi-center, observational audit | 930 ACS patients, of which 333 UA and 287 NSTEMI, enrolled from 36 hospitals | 35.0% CA in-hospital (NSTEMI patients) 16.0% CA in-hospital (UA patients) |
| Ferreira, 2004 [46] (Portugal) [PMID: 15641292] |
Prospective, multi-center, observational registry (National Registry of ACS) | 7,348 ACS patients, of which 2,858 NSTEMI and 1,154 UA, enrolled from 44 hospitals | 51.0% CA in-hospital (NSTEMI patients) 60.0% CA in-hospital (UA patients) |
| Kassaian, 2015 [60] (Iran) [26671947] |
Prospective, multi-center, observational registry | 1226 NST-ACS patients, enrolled from 11 hospitals | 64.7% CA in-hospital |
|
First author, year (country) [PMID] |
Study design | Sample | Main results |
| Lee, 2008 [55] (Canada) [PMID: 18268170] |
Prospective, multi-center, observational registry (Canadian ACS II) | 2,136 NST-ACS patients, enrolled from 36 hospitals | 64.7% CA in-hospital. Of patients not referred for CA: 59.1% were found to be at intermediate to high risk according to their TIMI risk score and 70.2% according to their GRACE risk score. According to their level of risk, 73.7% of low risk, 73.7% of intermediate and 54.9% of high risk patients were referred for CA. |
| Mandelzweig, 2006 [42] (32 countries in Europe and Mediterranean basin) [PMID: 16908490] |
Prospective, multi-center, observational survey (EHS-ACS-II) |
6,358 ACS patients, of which 3,063 NST-ACS, enrolled from 190 hospitals | 62.9% CA in-hospital |
| Mehta, 2006 [31] (USA) [PMID: 17030838] |
Prospective, multi-center, observational registry (CRUSADE) † | 113,595 NST-ACS patients, enrolled from 434 hospitals | 67.3% CA in-hospital 34.6% CA ≤24 h 50.1% CA ≤48 h |
| Nieuwlaat, 2004 [43] (The Netherlands) [PMID: 15497784] |
Prospective, multi-center, observational survey | 421 ACS patients, of which 198 NST-ACS, enrolled from 6 hospitals. | 56.0% CA in high risk patients 25.0% CA in low risk patients |
| Peterson, 2008 [33] (USA) [PMID: 19032998] |
Prospective, multi-center, observational registry (NRMI) † | 2,515,106 ACS patients, of which 1,368,497 NSTEMI, enrolled from 2157 hospitals | 70.0% CA in-hospital |
| Polonski, 2007 [44] (Poland) [PMID: 17853315] |
Prospective, multi-center observational registry (Polish registry of ACS) | 100,193 ACS patients, of which ±42,281 UA and ±26,651 NSTEMI, enrolled from 417 hospitals | 31.7% CA in-hospital (NSTEMI patients) 29.4% CA in-hospital (UA patients) |
| Roe, 2005 [37] (USA) [PMID: 16157831] |
Prospective, multi-center, observational registry (CRUSADE) † |
23,298 NST-ACS patients (number of hospitals unknown) | 66.1% CA in-hospital 29.8% CA ≤24 h 44.9% CA ≤48 h |
| Roe, 2006 [35] (USA) [PMID: 16765118] |
Prospective, multi-center, observational registry (CRUSADE) † |
45,744 NST-ACS, enrolled from 424 hospitals | 66.3% CA in-hospital 46.0% CA ≤48 h |
| Roe, 2006 [34] (USA) [PMID: 16781220] |
Prospective, multi-center, observational registry (CRUSADE) † |
77,760 NST-ACS patients, enrolled from 457 hospitals. | 61.9% CA in-hospital 42.5% CA ≤48 h |
| Roe, 2007 [36] (USA) [PMID: 17709638] |
Prospective, multi-center, observational registry (CRUSADE) † |
55,994 NST-ACS, enrolled from 301 hospitals | 72.7% CA in-hospital 51.5% CA ≤48 h |
| Schiele, 2005 [48] (France) [PMID: 15681575] |
Prospective, multi-center, observational registry | 754 ACS patients, of which 421 NSTEMI patients, enrolled from 12 hospitals | 64.0% CA in-hospital |
| Sonel, 2005 [38] (USA) [PMID: 15769762] |
Prospective, multi-center, observational registry (CRUSADE) † |
43,317 NST-ACS patients, enrolled from 400 hospitals. | 66.1% CA in-hospital, of which 81.5% of low risk patients and 53.8% of high risk patients received CA. 47.4% CA ≤48 h, of which 62.7% of low risk patients and 33.7% of high risk patients received CA. |
|
First author, year (country) [PMID] |
Study design | Sample | Main results |
| Tang, 2005 [63] (New-Zealand) [PMID: 16224502] |
Retrospective, cross-sectional, single-center, observational study | 577 ACS patients, of which 239 NSTEMI and 143 UA, enrolled from 1 hospital | 73.0% CA in-hospital |
| Tricoci, 2006 [39] (USA) [PMID: 17056321] |
Prospective, multi-center, observational registry (CRUSADE) † |
87,640 NST-ACS patients, enrolled from 338 hospitals | 61.0% CA ≤48 h (Based on last measurement, n=29.586 NSTEMI patients) |
| Vikman, 2003 [49] (Finland) [PMID: 12944205] |
Prospective multi-center, observational registry (FINACS I) |
501 NST-ACS, enrolled from 9 hospitals | 41.2% CA in-hospital |
| Yan, 2007 [56] (Canada) [PMID: 17533203] |
Prospective, multi-center, observational registry (Canadian ACS 1 and 2) |
4,414 NST-ACS patients, enrolled from 51 (ACS1) and 36 hospitals (ACS2) | 63.5% CA in-hospital, of which 73.8% of low risk patients, 66.9% of intermediate patients and 49.7% of high risk patients received CA. (Based on ACS 2 data, n=1580 NSTEMI patients) |
| Zeymer, 2014 [51] (Germany) [PMID: 25374386] |
Prospective, multi-center, observational registry (EPICOR) | 333 NST-ACS patients, enrolled from 29 hospitals | 95.8% CA in-hospital |
| Abbreviations: ACS, acute coronary syndrome; CA, coronary angiography; CRUSADE, Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the ACC/AHA Guidelines; NST-ACS, non-ST-elevation acute coronary syndrome; EHS-ACS-II, Second Euro Heart Survey on Acute Coronary Syndrome; NRMI, National Registry of Myocardial Infarction; NSTEMI, non-ST-elevation myocardial infarction; PMID, PubMed ID; UA, unstable angina; †Concern large registries that provide access to quality improvement tools, e.g. quarterly feedback reports/benchmarks. | |||
Discharge Medications
Twenty-three studies reported on guideline adherence with regard to recommended discharge medications, including angiotensin-converting-enzyme (ACE) inhibitors /angiotensin II AT1 receptor blockers (ARBs), aspirin, beta-blockers, platelet aggregation inhibitors (e.g., clopidogrel), and/or statins [12, 13, 19, 23, 26, 30, 31, 33, 34, 36, 38, 40-44, 46, 49, 51, 57, 58, 62, 64]. Overall, adherence rates in these studies varied from 4.2% [58] to 97.3% [13]. The three lowest adherence rates were related to recommendations regarding the prescription of ARBs (4.2%) [58], clopidogrel (9.5% for NSTEMI and 5.1% for UA) [62], and aspirin (16.0%) [57] at discharge. Hence, all three studies had relatively small sample sizes (ranging from 380-1,331). Although in the majority of studies low adherence rates were reported for the prescription of clopidogrel at discharge (<59.0%), in six studies adherence rates were found ranging from 67.0% to 90.8% [13, 23, 31, 40, 51, 58]. The study with the highest adherence score, however, concerned a single center study with a small sample size (n=380).
The three highest adherence rates were related to recommendations regarding the prescription of aspirin (96.0% [41] and 97.3% [13], respectively) and beta-blockers (97.0% [13]) at discharge. Overall, adherence rates for the prescription of aspirin at discharge were higher than 90.0%, but in one study only 16.0% of NST-ACS patients were prescribed this type of medication at discharge [57]. However, combined with the administration of clopidogrel 61.8% also received aspirin (Table 2)).
Association Between Guideline Adherence and Adverse Cardiac Events
Seven of the included studies reported on the association between guideline adherence and occurrence of adverse cardiac events (i.e., death and/or MI) in NST-ACS patients [19, 24, 28, 29, 32, 45, 55] (Table 4). Overall, in all studies, higher adherence to guideline recommendations was significantly associated with a lower occurrence of death or the composite endpoint of death/MI. For example, patients who received early treatment with glycoprotein IIb/IIIa inhibitors [28] or underwent in-hospital CA [24] had lower mortality rates than patients who did not receive such therapies. Mixed results were found for the association between guideline adherence and the occurrence of myocardial infarction (MI). In one study higher guideline adherence was associated with lower rates of MI [29], whereas in two studies higher guideline adherence was associated with higher rates of MI [32, 55]. In two other studies, no significant association between guideline adherence and MI was found [24, 28].
Table 4. Overview of included studies on the association between guideline adherence and adverse cardiac events.
|
First author, year
(country) |
Study design | Sample | Guideline recommendations† | Univariate associations with occurrence of adverse cardiac events‡ Significance level: p≤0.05 | ||||
|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | |||||
| Bhatt, 2004 [24] (USA) [PMID: 15523070] |
Prospective, multi-center, observational registry (CRUSADE)§ | 17,926 NST-ACS patients, enrolled from 248 hospitals | X | Patients who underwent early CA (<48 h after hospital admission) (vs. not receiving early CA) had significantly: ▪ lower in-hospital mortality (2.0% versus 6.2%, AOR 0.63; 95%CI 0.52-0.77); ▪ lower composite endpoint of death/MI (4.7% versus 8.9%, AOR 0.79; 95%CI 0.69-0.90) |
||||
| Dziewierz, 2007 [45] (Poland) [PMID: 17496494] |
Prospective, multi-center, observational registry (Malopolska registry of ACS) | 807 NSTEMI patients, enrolled from 29 hospitals | X | Being prescribed aspirin, clopidogrel, BB, ACE/ARB and statins (vs. not receiving such therapies) was significantly associated with: ▪ a lower risk of in-hospital death, as for every unit of increase on the pharmacotherapy index∞ the risk of death decreased by 46.0% |
||||
| Hoekstra, 2005 [28] (USA) [PMID: 15863399] |
Prospective, multi-center, observational registry (CRUSADE)§ | 56,804 NST-ACS patients, enrolled from 443 hospitals | X | Being prescribed with early GP IIb/IIIa inhibitors (vs. not receiving early GP IIb/IIIa inhibitors) was significantly associated with: ▪ lower in-hospital mortality (2.7% versus 4.7%) ▪ lower composite endpoint of death/MI (5.7% versus 7.7%) |
||||
|
First author, year (country) |
Study design | Sample | Guideline recommendations† | Univariate associations with occurrence of adverse cardiac events‡ Significance level: p≤0.05 | ||||
| I | II | III | IV | |||||
| Lee, 2008 [55] (Canada) [PMID: 18268170] |
Prospective, multi-center, observational registry (Canadian ACS II) | 2,136 NST-ACS patients, enrolled from 36 hospitals | X | Patients who underwent in-hospital CA (vs. patients not receiving in-hospital CA) had significantly: ▪ lower in-hospital mortality (0.8% versus 3.7%) and lower 1-year mortality (4.0% versus 10.9%). ▪ higher rates of MI (6.8% versus 2.4%) ▪ higher composite endpoint of death/MI (7.1% versus 5.0%). However 1 year after discharge patients had lower rates of death/MI (12.5% versus 16.4%). |
||||
| Miller, 2007 [29] (USA) [PMID: 17679127] |
Prospective, multi-center, observational registry (CRUSADE)§ | 72,054 NST-ACS patients, enrolled from 509 hospitals | X | Being prescribed acute BB <24 h after admission (vs. not receiving acute BB) was significantly associated with: ▪ lower in-hospital mortality (3.9% versus 6.9%, AOR 0.66; 95%CI 0.60-0.72) ▪ lower MI (3.0% versus 3.6%, AOR 0.80, 95%CI 0.72-0.89). |
||||
| Peterson, 2003 [32] (USA) [PMID: 12849658] |
Prospective, multi-center, observational registry (NRMI)§ | 60,770 NSTEM patients, enrolled from 1189 hospitals | X | Being prescribed with early GP IIb/IIIa inhibitors <24 h after admission (vs. not receiving early GP IIb/IIIa inhibitors) was significantly associated with: ▪ lower unadjusted mortality (3.3% versus 9.6%), lower adjusted mortality (AOR 0.88; CI95% 0.79-0.97) ▪ lower death/MI (4.5% versus 10.3%) ▪ higher rates of MI (1.5% versus 1.1%) |
||||
| Peterson, 2006 [19] (USA) [PMID: 16639050] |
Prospective, multi-center, observational registry (CRUSADE)§ | 64,775 NST-ACS patients, enrolled from 350 hospitals |
X | X | Hospitals with higher guideline adherence rates had significantly: ▪ lower in-hospital mortality rates (4.15% for highest adherence quartile versus 6.31% for lowest adherence quartile, AOR 0.81; 95%CI 0.68-0.97) ▪ Every 10% increase in composite adherence score = 10% reduction in mortality rate (AOR 0.90; 95%CI 0.84-0.97) |
|||
|
Abbreviations: ACE, angiotensin-converting-enzyme inhibitor; ACS; acute coronary syndromes; ARB, angiotensin II AT1 receptor blockers; BB, beta-blocker; CA, coronary angiography; CRUSADE, Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the ACC/AHA Guidelines; GP IIb/IIIa, Glycoprotein IIb/IIIa receptor inhibitors; MI, myocardial infarction; NST-ACS, Non-ST-Elevation Acute Coronary Syndromes; NSTEMI, non-ST-elevation myocardial infarction; NRMI, National Registry of Myocardial Infarction. †class I guideline recommendation: I = acute pharmacological care (<24 h after admission), II = risk stratification, III = invasive procedures, IV = discharge medications. ‡Only significant associations are presented, and where possible adjusted odds ratios (AOR) and their 95% confidence intervals (CI) are provided. §Concern large registries that provide access to quality improvement tools, e.g. quarterly feedback reports/benchmarks. ∞Pharmacotherapy index: range from 0-7, one point for each medication received, ASA, clopidogrel, GB IIa/IIIb inhibitor, LMW Heparin, BB, ACE/ARB and statin. | ||||||||
Potential Factors Associated with Guideline Adherence
Fifteen of the included studies examined potential factors that were associated with lower or higher guideline adherence [19, 24, 25, 28-30, 32, 34, 37, 49, 50, 53, 56, 57, 64] (Fig. 2, Table 2). Of these, eight studies reported on factors associated with adherence to guideline recommendations on acute in-hospital pharmacological treatment [19, 25, 28, 29, 32, 34, 53, 56]. In addition, four studies reported on potential factors influencing adherence to the performance of in-hospital CA [24, 37, 49, 56], whereas seven studies reported on potential factors related to the prescription of discharge medications [19, 28, 30, 34, 56, 57, 64]. One study reported on potential factors associated with adherence to recommendations on risk stratification [50]. Overall, these factors could be categorized in either patient-related or organization-related factors.
Fig. (2).
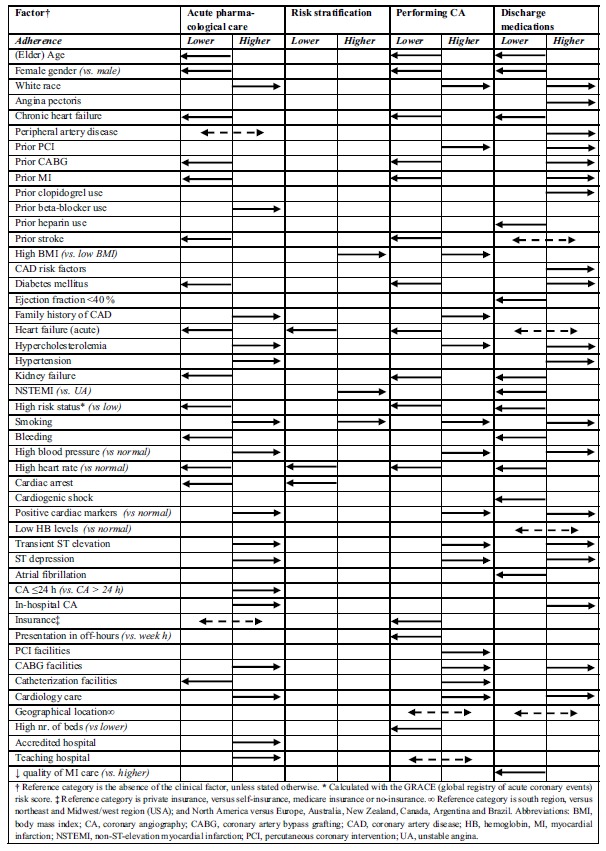
Factors significantly (p<0.05) associated with lower or higher guideline adherence.
Acute in-Hospital Pharmacological Treatment
The following patient-related factors were associated with higher prescription rates of acute in-hospital pharmacological treatment: white race [28, 32], hypercholesterolemia [28, 29], (recent) smoker [28, 32], hypertension [28], family history of coronary artery disease [28, 29], prior beta-blocker use [29], high admission blood pressure [29], positive cardiac markers (e.g. troponin, CK-MB, CK) [28, 34], transient ST-elevation or ST-depression on the ECG [28, 29, 34], and receiving CA in-hospital or within 24 h after admission [53]. On the contrary, the following patient-related factors were related to lower prescription of acute in-hospital pharmacological treatment: older age [28, 29, 32, 34], female gender [28, 29, 32], high admission heart rate [28, 29], chronic heart failure [28, 29, 53], prior stroke [28], prior MI [28] prior CABG [28], diabetes mellitus [34], acute in-hospital heart failure [28, 29, 34], kidney failure [28, 29, 34], bleeding [53], high GRACE risk status [53, 56], presentation at the hospital with cardiac arrest [53]. Mixed results were found for factors prior percutaneous coronary intervention (PCI) and health-insurance, which were in some studies associated with higher prescription rates of acute in-hospital pharmacological treatment [29, 32, 53], whereas in other studies they were related to lower prescription rates [28, 29].
On an organizational level, patients with a cardiologist as their primary care provider [19, 28, 29, 34], patients treated at hospitals accredited by the Society of Cardiovascular Patient Accreditation (SCPC) [25], and patients treated at hospitals with a teaching status [29] or cardiac surgery facilities (e.g., facilities for coronary artery bypass grafting (CABG) surgery) [19] were more likely to receive acute in-hospital pharmacological treatment. Patients treated at hospitals with catheterization, but no cardiac surgery, facilities were less likely to receive such treatment [53].
Performing in-Hospital CA
Patient-related factors, including white race [24], high admission blood pressure [24], hypercholesterolemia [24], (recent) smoking [24], high body mass index [24], positive family history of CAD [24], prior PCI [24], positive cardiac markers (e.g. troponin, CK-MB, CK) [24, 37, 49], and transient ST-elevation or ST-depression on the ECG [24, 49], were associated with higher performance rates of in-hospital CA. On the other hand, older age [24, 49], female gender [24, 56], high admission heart rate [24], chronic heart failure [24], diabetes mellitus [24, 49], in-hospital heart failure [24], prior stroke [24], kidney failure [24], high GRACE risk status [56], prior CABG [24], prior MI [24], presenting in-2 3 4
hospital during off-hours [24], and having no insurance or a Medicare insurance [24] were related to lower performance rates of in-hospital CA.
On an organizational level, factors such as, patients treated at hospitals with catheterization [56], PCI [24], or cardiac surgery facilities [24], patients form the Midwest/west region (USA) (geographical location) [24] and patients with a cardiologist as their primary care provider [24, 56] were more likely to receive in-hospital CA. However, patients admitted at larger size hospitals (i.e., higher number of hospital beds) [24], and patients from Northeast region (USA) (geographical location) [24] were less likely to receive in-hospital CA. Mixed results were found on an organizational level with regard to a hospital’s teaching status, with in one study this factor being associated with higher performance rates of in-hospital CA [49], whereas in another study this factor was associated with lower CA-rates [24].
Risk Stratification
The following patient-related factors were associated with higher cardiac risk score use: obesity and former smoker, whereas a diagnosis of unstable angina (versus NSTEMI), being resuscitated in-hospital, acute heart failure and tachycardia were associated with lower cardiac risk score use [50].
Discharge Medications
The following patient-related factors were associated with higher prescription rates of discharge medications: white race [30], high admission blood pressure [30], hypercholesterolemia [30], (recent) smoking [30], angina pectoris [64], peripheral artery disease [30], prior PCI [30], prior CABG [30], prior MI [30, 64], diabetes mellitus [30], hypertension [64], prior clopidogrel use [30, 57], risk factors for 2 coronary artery disease [57], positive cardiac markers (e.g. troponin, CK-MB, CK) [30, 34], transient ST-elevation or ST-depression on the ECG [34], and receiving in-hospital CA [30]. On the contrary, older age [34, 64], female gender [64], high admission heart rate [30], chronic heart failure [64], high GRACE risk status [56], diagnosis of NSTEMI [57], prior heparin use [30], kidney failure [34], ejection fraction of less than 40% [30], bleeding [30], atrial fibrillation [64], and in-hospital cardiogenic shock [64] were associated with lower prescription of discharge medications. Mixed results were found for in-hospital heart failure, prior stroke, and low hemoglobin levels with in some studies these factors being associated with higher prescription rates of discharge medications [57], whereas in other studies opposite associations were found [30, 64].
On an organizational level, NST-ACS patients treated at hospitals with cardiac surgery facilities [19], as well as 5
Table 5. Potential factors associated with guideline adherence.
| Type of factor | Factor | Main results† | Guideline recommendations‡∞ | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | |||||||||||||||||||||||
| Patient | Demographics | |||||||||||||||||||||||||
| Age |
Elderly patients were less likely to receive acute aspirin, BB, heparin [34] and GP IIb/IIIa inhibitors [28,32], CA ≤48 h or in-hospital [24,49], statin [34], and all guideline recommended therapies (i.e. ACE, aspirin, BB, statin) [64] at discharge than younger patients Patients’ aged between 55 years and 74 years were less likely to receive acute BB [29] than patients below 55 years or of 75 years and older |
↓ ↓ |
↓ | ↓ | ||||||||||||||||||||||
| Gender | Female patients were less likely to receive acute BB [29] and GP IIb/IIIa inhibitors [28,32], to receive CA ≤48 h or in-hospital [24,56], and to receive all guideline recommended discharge therapies (i.e. ACE, aspirin, BB, statin) [64] than male patients | ↓ | ↓ | ↓ | ||||||||||||||||||||||
| Race | Patients of white race were more likely to receive acute GPIIb/IIIa inhibitors [28,32], CA ≤48 h [24], and clopidogrel at discharge [30] than patients of a non-white race | ↑ | ↑ | ↑ | ||||||||||||||||||||||
| Clinical factors | ||||||||||||||||||||||||||
| Angina pectoris | Patients with a history of angina pectoris were more likely to receive all guideline recommended discharge therapies (i.e. ACE, aspirin, BB, statin), than patients without a history of angina pectoris [64] | ↑ | ||||||||||||||||||||||||
| CHF | Patients with chronic heart failure were less likely to receive acute antiplatelet therapy (e.g. clopidogrel) [53], BB [29] and GPIIb/IIIa inhibitors [28], to receive CA ≤48 h [24], and all guideline recommended discharge therapies (i.e. ACE, aspirin, BB, statin) [64], than patients without chronic heart failure | ↓ | ↓ | ↓ | ||||||||||||||||||||||
| PAD | Patients with PAD were more likely to be prescribed with clopidogrel at discharge, than patients without PAD [30] | ↑ | ||||||||||||||||||||||||
| Prior PCI |
Patients with a prior PCI were more likely to receive acute antiplatelet therapy (e.g. clopidogrel) [53] and GPIIb/IIIa inhibitors [32], to receive CA ≤48 h [24], and to receive clopidogrel at discharge [30], than patients without a PCI in their medical history Patients with a prior PCI were less likely to be treated with acute BB, than patients without a PCI in their medical history [29] |
↑ ↓ |
↑ | ↑ | ||||||||||||||||||||||
| Prior CABG |
Patients with a prior CABG were less likely to receive acute GP IIb/IIIa inhibitors [28], and to receive CA ≤48 h [24], than patients without a CABG in their medical history Patients with a prior CABG were more likely to be prescribed with clopidogrel at discharge, than patients without a CABG in their medical history [30] |
↓ | ↓ | ↑ | ||||||||||||||||||||||
| Prior MI |
Patients with a prior MI were more likely to receive clopidogrel [30] and all guideline recommended therapies (i.e. ACE, aspirin, BB, statin) [64] at discharge, than patients without a MI in their medical history Patients who had a prior MI were less likely to receive acute GPIIb/IIIa inhibitors [28], and CA ≤48 h, than patients without a MI in their medical history [24] |
↓ | ↓ | ↑ | ||||||||||||||||||||||
| Prior clopidogrel use | Patients who used clopidogrel before hospitalization were more likely to receive clopidogrel at discharge, than patients who did not use clopidogrel before hospitalization [30, 57] | ↑ | ||||||||||||||||||||||||
| Prior BB use | Patients who used BB before hospitalization were more likely to receive acute BB, than patients who did not use BB before hospitalization [29] | ↑ | ||||||||||||||||||||||||
| Prior heparin use | Patients who used heparin before hospitalization were less likely to be prescribed with clopidogrel at discharge, than patients who did not use heparin before hospitalization [30] | ↓ | ||||||||||||||||||||||||
| Prior stroke |
Patients with a prior stroke were less likely to receive acute GP IIb/IIIa inhibitors [28], to receive CA ≤48 h [24], and all guideline recommended therapies (i.e. ACE, aspirin, BB, statin) [64] at discharge, than patients without a stroke in their medical history Patients with a prior stroke were more likely to receive clopidogrel at discharge [57], than patients without a stroke in their medical history |
↓ | ↓ | ↓ ↑ |
||||||||||||||||||||||
| BMI | Patients with a high BMI were more likely to receive CA ≤48 h [24], and more likely to have a risk score documented in their medical chart [50], than patients with a normal BMI | ↑ | ↑ | |||||||||||||||||||||||
| CAD risk factors | Patients with two or more risk factors for CAD were more likely to receive clopidogrel at discharge, than patients with one or no risk factors for CAD [57] | ↑ | ||||||||||||||||||||||||
| Type of factor | Factor | Main results† | Guideline recommendations‡∞ | |||||||||||||||||||||||
| I | II | III | IV | |||||||||||||||||||||||
| Patient | Clinical factors | |||||||||||||||||||||||||
| Diabetes mellitus |
Patients with diabetes mellitus were less likely to receive acute aspirin [34], and to receive CA ≤48 h or in-hospital [24,49], than patients without diabetes mellitus Patients with diabetes mellitus were more likely to receive clopidogrel [30] and all guideline recommended therapies (i.e. ACE, aspirin, BB, statin) [64] at discharge, than patients without diabetes mellitus |
↓ | ↓ | ↑ | ||||||||||||||||||||||
| EF <40% | Patients with an EF <40% were less likely to be prescribed with clopidogrel at discharge, than patients without an EF <40% [30] | ↓ | ||||||||||||||||||||||||
| Family history of CAD | Patients with a positive family history for CAD were more likely to receive acute BB [29] and GP IIb/IIIa inhibitors [28], and CA ≤48 h [24] than patients with a negative family history of CAD | ↑ | ↑ | |||||||||||||||||||||||
| Heart failure (acute) |
Patients with acute heart failure were less likely to receive acute aspirin, heparin [34], GP IIb/IIIa inhibitors [28] and BB [29,34], to receive CA ≤48 h [24], and less likely to receive all guideline recommended discharge therapies (i.e. ACE, aspirin, BB, statin)[64], than patients without acute heart failure. They were also less likely to have a risk score documented in their medical chart [50] Patients with acute heart failure were more likely to be prescribed with ACE at discharge, than patients without acute heart failure [34] |
↓ | ↓ | ↓ | ↓ ↑ |
|||||||||||||||||||||
| Hypercholesterolemia | Patients with hypercholesterolemia were more likely to receive acute BB [29] and GPIIb/IIIa inhibitors [28], to receive CA ≤48 h [24], and to receive clopidogrel at discharge [30], than patients without hypercholesterolemia | ↑ | ↑ | ↑ | ||||||||||||||||||||||
| Hypertension | Patients with a history of hypertension were more likely to receive acute GPIIb/IIIa inhibitors [28], and to receive all guideline recommended discharge therapies (i.e. ACE, aspirin, BB, statin) [64], than patients without a history of hypertension | ↑ | ↑ | |||||||||||||||||||||||
| Kidney failure | Patients with kidney failure were less likely to receive acute aspirin, heparin [34], BB [29] and GPIIb/IIIa inhibitors [28], to receive CA ≤48 h [24], and to receive aspirin and ACE at discharge [34], than patients without kidney failure | ↓ | ↓ | ↓ | ||||||||||||||||||||||
| NSTEMI | NSTEMI patients were less likely to receive clopidogrel at discharge than patients with UA [57], but were more likely to have a risk score documented in their medical chart [50] | ↑ | ↓ | |||||||||||||||||||||||
| Risk status (GRACE) | Patients with a high risk status are less likely to receive acute antiplatelet therapy [53] and other acute medications [56], to receive CA, and appropriate discharge medications [56] compared to patients with a low risk status | ↓ | ↓ | ↓ | ||||||||||||||||||||||
| Smoking |
(Recent) smokers were more likely to receive acute GPIIb/IIIa inhibitors [28,32], CA ≤48 h [24], and clopidogrel at discharge [30], than non-smokers (Recent) smokers were also more likely to have a risk score documented in their medical chart than non-smokers [50] |
↑ | ↑ | ↑ | ↑ | |||||||||||||||||||||
| Bleeding | Patients with a major bleeding in their medical history were less likely to be treated with antiplatelet therapy (e.g. clopidogrel) [53] or to receive clopidogrel at discharge [30], than patients without a major bleeding | ↓ | ↓ | |||||||||||||||||||||||
| Hemodynamics | ||||||||||||||||||||||||||
| Blood pressure | Patients with a high blood pressure at admission were more likely to receive acute BB [29], CA ≤48 h [24], and clopidogrel at discharge [30] than patients with a normal blood pressure at admission | ↑ | ↑ | ↑ | ||||||||||||||||||||||
| Heart rate | Patients with a high heart rate were less likely to receive acute BB [29] and GP IIb/IIIa inhibitors [28], to receive CA ≤48 h [24], and to receive clopidogrel at discharge [30] than patients with a normal heart rate at admission. They were also less likely to have a risk score documented in their medical chart [50] | ↓ | ↓ | ↓ | ↓ | |||||||||||||||||||||
| Cardiac arrest / resuscitation | Patients presenting with cardiac arrest or who were resuscitated at hospital-admission were less likely to be treated with acute antiplatelet therapy (e.g. clopidogrel) [53], and less likely to have a risk score documented in their medical chart [50], than patients not presenting with cardiac arrest or being resuscitated in hospital | ↓ | ↓ | |||||||||||||||||||||||
| Cardiogenic shock | Patients presenting with cardiogenic shock were less likely to receive all guideline recommended discharge therapies (i.e. ACE, aspirin, BB, statin), than patients without cardiogenic shock [64] | ↓ | ||||||||||||||||||||||||
| Type of factor | Factor | Main results† | Guideline recommendations‡∞ | |||||||||||||||||||||||
| I | II | III | IV | |||||||||||||||||||||||
| Patient | Laboratory results | |||||||||||||||||||||||||
| Cardiac markers (e.g. troponin, CK-MB, CK) | Patients with positive cardiac markers were more likely to receive acute aspirin, BB, heparin [34] and GP IIb/IIIa inhibitors [28], to receive CA ≤48 h or in-hospital [24,37,49], and ACE, aspirin, BB, Statin [34], clopidogrel at discharge [30] than patients with normal cardiac markers levels | ↑ | ↑ | ↑ | ||||||||||||||||||||||
| HB | Patients with HB levels of 9g/dL or lower were either less likely to receive clopidogrel at discharge [30] or more likely to receive clopidogrel at discharge [57] than patients with normal HB levels | ↑ ↓ |
||||||||||||||||||||||||
| Electrocardiogram findings | ||||||||||||||||||||||||||
| Transient ST elevation | Patients with transient ST elevation were more likely to receive acute aspirin [34], BB [29,34] and heparin [34], to receive CA ≤48 h [24], and to be discharged with aspirin, BB and ACE [34] than patients without such deviations on the electrocardiogram | ↑ | ↑ | ↑ | ||||||||||||||||||||||
| ST depression | Patients with ST depression were more likely to receive acute aspirin [34], BB [29,34], heparin [34] and GP IIb/IIIa inhibitors [28], and to receive CA ≤48 h or in-hospital [24, 49] and to be discharged with ACE, aspirin, and BB [34] than patients without such deviations on the electrocardiogram | ↑ | ↑ | ↑ | ||||||||||||||||||||||
| Atrial fibrillation | Patients with atrial fibrillation were less likely to receive all guideline recommended discharge therapies (i.e. ACE, aspirin, BB, statin), than patients without such deviation on the electrocardiogram [64] | ↓ | ||||||||||||||||||||||||
| Invasive diagnostic procedures | ||||||||||||||||||||||||||
| CA ≤24 h | Patients catheterized within the first 24 h after admission were more likely to be treated with antiplatelet therapy (e.g. clopidogrel), than patients that were not catheterized within the first 24 h after admission [53] | ↑ | ||||||||||||||||||||||||
| In-hospital CA | Patients receiving CA in-hospital were more likely to receive antiplatelet therapy (e.g. clopidogrel) [53], and to receive clopidogrel at discharge [30] than patients not receiving CA in-hospital | ↑ | ↑ | |||||||||||||||||||||||
| Other | ||||||||||||||||||||||||||
| Insurance |
Patients with medicare or no insurance were less likely to receive acute BB [29] and GP IIb/IIIa inhibitors [28,32], and to receive CA ≤48 h than patients with private insurance [24] Patients with self-insurance were more likely to receive acute BB [29], but less likely to receive acute GP IIb/IIIa inhibitors [28], than patients with private insurance |
↓ ↑ |
↓ | |||||||||||||||||||||||
| Time of presentation | Patients presenting at hospital during off-hours (i.e. between 5 pm to 7 am or in weekends) were less likely to receive CA ≤48 h, than patients presenting between during the week hours between 7 am to 5 pm [24] | ↓ | ||||||||||||||||||||||||
| Organization | PCI facilities | Patients treated at hospitals with PCI facilities were more likely to receive CA ≤48 h, than patients treated in hospitals without such facilities [24] | ↑ | |||||||||||||||||||||||
| CABG facilities | Patients treated at hospitals with surgical facilities were more likely to receive CA ≤48 h [24], and be among centers with the highest adherence rates regarding acute and discharge therapies [19] than patients treated at hospitals without surgical facilities | ↑ | ↑ | ↑ | ||||||||||||||||||||||
| Catheterization facilities |
Patients admitted to hospitals with onsite catheterization facilities were less likely to be treated with antiplatelet therapy (e.g. clopidogrel), than patients admitted to hospitals without such facilities [53] Patients admitted to hospitals with onsite catheterization facilities were more likely to receive CA, than patients treated in hospitals without such facilities [56] |
↓ | ↑ | |||||||||||||||||||||||
| Cardiology care | Patients cared for by cardiologists were more likely to receive acute aspirin [34], BB [29,34], heparin [34] and GP IIb/IIIa inhibitors [28], to receive CA ≤48 h or in-hospital [24,56], and ACE, aspirin, BB, statin at discharge [34], and to be among centers with the highest adherence rates regarding acute and discharge therapies [19], than patients treated by other specialists | ↑ | ↑ | ↑ | ||||||||||||||||||||||
| Type of factor | Factor | Main results† | Guideline recommendations‡∞ | |||||||||||||||||||||||
| I | II | III | IV | |||||||||||||||||||||||
| Organization | Geographical location |
Patients from the Northeast region (USA) were less likely to receive CA ≤48 h than patients in the south region [24] Patients from the Midwest/west region (USA) were more likely to receive CA ≤48 h than patients in the south region [24] Patients treated in Europe, Australia, New-Zealand and Canada were more likely to receive all guideline recommended discharge therapies (i.e. ACE, aspirin, BB, statin) than patients treated in North America [64] Patients treated in Argentina and Brazil were less likely to receive all guideline recommended discharge therapies (i.e. ACE, aspirin, BB, statin) than patients treated in the North America [64] |
↓ ↑ |
↑ ↓ |
||||||||||||||||||||||
| Nr. of beds | Patients treated in hospitals with higher numbers of hospital beds were less likely to receive CA ≤48 h, than patients treated in hospital with lower number of hospital beds [24] | ↓ | ||||||||||||||||||||||||
| Accreditation | Patients treated at SCPC accredited hospitals were more likely to receive acute aspirin and BB, than patients not treated in such hospitals [25] | ↑ | ||||||||||||||||||||||||
| Hospitals’ teaching status |
Patients treated at teaching hospitals were more likely to receive acute BB [29] and to receive CA in-hospital [49], than patients treated in non-teaching hospitals Patients treated at teaching hospitals were less likely to receive CA ≤48 h, than patients treated in non-teaching hospitals [24] |
↑ | ↑ ↓ |
|||||||||||||||||||||||
| Quality of MI care | Patients treated at hospitals with lower quality measures of MI care were less likely to receive clopidogrel at discharge, than patients treated at hospitals with higher quality of care measures of MI care [30] | ↓ | ||||||||||||||||||||||||
| Abbreviations: ACE, angiotensin-converting-enzyme inhibitor; BB, beta-blocker; BMI, body mass index; CA, coronary angiography; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CHF, chronic heart failure; EF, ejection fraction; GP IIb/IIIa, Glycoprotein IIb/IIIa receptor inhibitors; GRACE, global registry of acute coronary events; HB, hemoglobin; MI, myocardial infraction; NSTEMI, non-ST-elevation myocardial infarction; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; SCPC accreditation, society of cardiovascular patient care accreditation; UA, unstable angina. †Factors significantly (p≤0.05) associated with guideline adherence in multivariable analysis. ‡class I guideline recommendation: I = acute pharmacological care (<24 h after admission), II = risk stratification, III = invasive procedures, IV = discharge pharmacological care. ↑= higher adherence, ↓ = lower adherence. ∞ All factors are derived from studies studying adherence to the ACC/AHA guidelines, except Vikman 2003 (49) & Engel 2015 (50) who studied adherence to the ESC guidelines. | ||||||||||||||||||||||||||
patients with a cardiologist as their primary care provider [19, 34] were more likely to receive recommended discharge medications, whereas patients admitted to hospitals with lower quality measures on MI-care [30] were less likely to receive guideline recommended pharmacological discharge care. Regarding the factor geographical location, the extent of adherence depended on the type of country where treatment was provided [64].
All Guideline Recommendations
The following patient-related factors were associated with higher adherence to three or more guideline recommendations: white race, high blood pressure, hypercholesterolemia, (recent) smoker, positive cardiac markers (e.g. troponin, CK-MB, CK), transient ST elevation or ST depression on the electrocardiogram. On the contrary, elder age, female gender, high heart rate, chronic or acute heart failure, kidney failure, high GRACE risk status, were related to lower guideline adherence. On an organizational level, the presence of cardiac surgery facilities (e.g. CABG) and having a cardiologist as the primary care provider were associated with higher guideline adherence.
DISCUSSION
This systematic literature review examined the extent of adherence to ACC/AHA and ESC guideline recommendations on acute in-hospital pharmacological treatment, risk stratification, performing in-hospital CA, and the prescription of discharge medications in the management of NST-ACS patients. In addition, associations between guideline adherence and adverse cardiac events were examined and potential factors associated with lower or higher guideline adherence were identified.
Results of this systematic literature review showed a wide variation in guideline adherence rates to various cardiac recommendations, possibly reflecting a guideline-practice gap in the management of NST-ACS patients. Adherence rates for pharmacological therapies at admission or at discharge ranged from less than 5.0% to more than 95.0%, whereas adherence rates for the performance of in-hospital CA ranged between 16.0% and 95.8%, and between 34.3% and 93.0% for risk stratification. In addition, although the number of studies reporting on the association between adherence and adverse cardiac events was relatively small, lower guideline adherence was consistently found to be associated with poorer prognosis (i.e. higher rates of death, and the composite endpoint of death/MI). Finally, several patient-related (e.g. age, gender, presence of co-morbidities) and organization-related factors (e.g. teaching hospital, availability of PCI/CABG facilities) possibly influencing the extent of adherence to different guideline recommendations were identified.
The results of the current systematic literature review corroborate the findings of a previous literature review, in which suboptimal guideline adherence in the management of NST-ACS was demonstrated, with overall 25.0% of patients not receiving appropriate pharmacological treatment [15]. Our findings also confirm results of studies on guideline adherence in other cardiac patient groups. For example, the wide variation in adherence rates found in this systematic review is in line with previous studies in STEMI patients. In some of these studies rates of 0.0% to 2.0% were indicated for adherence to guideline recommendations on pharmacological treatment [65, 66], whereas in other studies rates of 98.5% or even higher were reported [13]. In addition, this wide variation in adherence rates has been demonstrated before in a systematic review comparing guideline adherence between patients with different diseases, including cardiovascular disease, in the pre-hospital and emergency care setting [67]. Overall, adherence to various medical guidelines ranged from 0.0% to 98.0% in this study, with the lowest rates found for adherence to recommendations of cardiac guidelines.
Previous studies mentioned several potential reasons for this practice variation, which should be taken into account in the interpretation of our results. First, the majority of included studies concerned registries in which information on guideline adherence was derived from patients’ medical records. This way, specific contra-indications providing a legit reason to deviate from the guidelines might be overlooked, as it is known that contra-indications are not always properly documented by attending physicians [68]. Consequently, guideline adherence rates reflected in these studies might be an underestimation of actual adherence rates in clinical practice. Second, it was suggested that physicians sometimes deviate from the guidelines because of inconclusive or insufficient evidence underlying guideline recommendations [16, 69]. In this review, low adherence rates were found for the early prescription of glycoprotein IIa/IIIb inhibitors and the early and discharge prescription of clopidogrel. However, at the time of publication of the majority of these studies these pharmacological therapies were relatively new, and therefore probably not yet routinely prescribed. Third, it has been shown that physicians sometimes deviate from the guidelines because of calculated complication risks. For example, cardiologists could argue that it would be better not to perform CA in high-risk patients, because of the risk of bleeding associated with this treatment. However, this kind of decision-making is in contrast with the guidelines, which state that especially high-risk patients should receive guideline-recommended therapies [10, 11].
Although over the past years there has been growing evidence regarding the effectiveness of risk stratification methods to guide clinical decision-making for the appropriate treatment, in this literature review only a minority of studies reported on this topic. Of these, three studies reported on the use of ECG findings for risk stratification and two studies reported on the use of troponin assessment. These latter studies were however of poor and moderate methodological quality, so results should be interpreted with caution. In addition, only one of the included studies reported on the use of validated risk-scoring instruments (i.e., GRACE and TIMI risk scores). The lack of studies on this topic could be explained by the fact that the use of these validated risk-scoring instruments in clinical decision making is a relatively new concept, which is mainly highlighted in the latest versions of the ACC/AHA and ESC guidelines. To further examine the actual use of validated risk scoring instruments and other risk stratification methods in clinical practice, and their effects on the quality of care, further research is needed.
Consistent with previous studies in MI and heart failure patients [70-73], in this systematic literature review lower guideline adherence was associated with adverse cardiac outcomes, including higher rates of mortality and death/MI. However, the association between adherence and the composite endpoint of death/MI should be interpreted with caution, as it has been reported before that the magnitude of the effect can differ across different components of a composite endpoint [74-77]. In other words, given that mixed results were found with regard to the association between guideline adherence and MI, the association between lower guideline adherence and higher rates of death/MI seems to be mainly driven by an impact of adherence on mortality rather than infarction. Furthermore, although all the included studies on the relationship between adherence and clinical outcomes had a prospective design, the causality of this relationship needs further investigation. One could argue that it could also be the case that severe progressing symptoms - a poorer prognosis - motivates healthcare professionals to deviate from the guidelines and apply career-based, rather than evidence-based procedures.
In this systematic review a distinction could be made between factors associated with specific guideline recommendations and factors associated with recommendations on all guideline recommendations. In previous studies, in addition to patient- and organization-related factors which were found in this systematic review, also health care provider-related factors were identified as potential associates of guideline adherence. For example, cardiologists’ awareness, familiarity, and personal agreement with guidelines and its recommendations have been linked to the extent of adherence to clinical practice guidelines, as well as high workload and accessibility of the guideline [16]. Furthermore, in a study on potential reasons for non-adherence in patients with ischemic heart disease, it was indicated that the inability of guidelines to directly manage the care of individual patients could be a reason for cardiologists to deviate from guideline recommendations [78]. Given that in our review results on the association between patient- and organization-related factors and guideline adherence were mixed and information on health care provider-related factors was lacking, future research focusing on the influence of patient-, organization-, as well as provider-related factors on guideline adherence in NST-ACS patients is warranted.
Given the large variation in adherence rates and lower guideline adherence being associated with adverse clinical outcomes in several studies, close monitoring of the extent of adherence to the latest ACC/AHA and ESC guidelines for NST-ACS is essential to maintain a high standard of care in this patient group [10, 11]. Previously, several quality improvement programs have been developed, aimed to fasten implementation of cardiac guidelines in clinical practice and increase adherence rates [71, 79, 80]. However, these programs often targeted the entire population of either ACS or NST-ACS patients, rather than focusing on NST-ACS patients in which treatment according to the guidelines have proven to be less likely. Two previous studies in ACS patients evaluated quality improvement initiatives in which implementation strategies were tailored to individual patient characteristics. These studies showed substantial improvements in adherence rates [81, 82]. Hence, knowledge on potential patient-, organization-, and provider-related factors influencing guideline adherence in NST-ACS could contribute to the identification of high-risk patients and the development of tailored implementation strategies aimed to increase adherence in this specific patient group [17, 83]. Additionally, previous quality improvement programs often focused on implementation of the guideline as a whole, rather than the improvement of adherence to specific guideline recommendations. It is suggested, however, that the latter more tailored approach is possibly more successful in improving adherence, as the current review and also previous studies show that adherence varies largely across individual recommendations [84].
Study limitations
In interpreting the results of this systematic literature review, several limitations should be taken into account. First, due to heterogeneity in study design (e.g., observational versus quasi-experimental, study sample (i.e., NST-ACS, NSTEMI, and/or UA patients), and type of guideline recommendations under study, a meta-analysis was not feasible. Generalizability of study results might therefore be hampered. In addition, study quality scores of the included studies ranged from poor to excellent, which could have distorted the interpretation of study results. However, the impact of these differences is expected to be limited, as the wide variation in adherence rates was prevalent in all different types of studies, including both poor and excellent quality studies.
A second limitation of the current literature review was that the majority of included studies derived their data from patients’ medical charts, which may incorporate a high risk of bias.
A third limitation is that only a few of the included studies reported on the latest versions of the ACC/AHA and ESC guidelines, published respectively in 2014 [11] and 2015 [10]. However, guideline recommendations described in the most recent versions of the guidelines are comparable to recommendations in the earlier versions of the ESC and ACC/AHA guidelines included in this review, except for the prescription of glycoprotein IIb/IIIa inhibitors, which degraded from a class 1 recommendation to a class II recommendation in both guidelines. It is recommended that future studies take the newest guidelines into account when studying the extent of adherence in the management of NST-ACS patients, and for instance explore any trends in guideline adherence.
The final limitation concerns the assessment of the methodological quality of the eligible studies by using a checklist based on the STROBE criteria. The STROBE is developed to assist authors in reporting their researcher, rather than assessing study quality. As a consequence bias can be introduced, with the methodological quality reported in this review being an overestimation or underestimation of the actual study quality. However, reliable and generally accepted tools to assess the quality of observational studies are lacking [85].
CONCLUSION
Despite NST-ACS being one of the most common types of ACS demanding urgent and guideline-recommended care, results of this systematic literature review indicated that there seems to exist a practice gap in the management of NST-ACS, with a substantial proportion of patients not receiving guideline-recommended care. Consequently, lower adherence might be associated with a higher risk for poor prognosis. Future research should further investigate the complex nature of guideline adherence in this patient group, its impact on clinical care, and potential patient-, organization-, and provider-related factors influencing adherence. This knowledge is essential to optimize clinical management of NST-ACS patients and could guide future quality improvement initiatives.
Box 1. Trends in class of evidence of guideline recommendations in NST-ACS patients.
| Cardiac guideline recommendations †‡ | ESC | ACC/AHA | ||||||
|---|---|---|---|---|---|---|---|---|
| Year of publication | 2015 | 2011 | 2007 | 2002∞/2000 | 2014 | 2011∞ | 2007 | 2002∞/2000 |
| Acute (<24 h) in-hospital pharmacological treatment | ||||||||
| • Prescription of aspirin | IA | IA | IA | IA | IA | IA | IA | IA |
| • Prescription of beta-blockers | IB | IB | IB | IB | IA | - | IB | IB |
| • Prescription of platelet aggregation inhibitors (e.g. thienopyridine) | IA | IA | IA | IB | IB | IB | IA | IB |
| • Prescription of glycoprotein IIb/IIIa inhibitors | IIb-A | IB | IB | IA | IIb-B | IB | IB | IA |
| • Prescription of anti-coagulant (e.g. heparin) | IB | IA | IA | IA | IA/IB | IA | IA/IB | IA |
| Risk stratification | ||||||||
| • ECG within 10 min after arrival in the hospital | IB | IB | IC | - | IC | - | IB | IC |
| • Troponin assessment | IA | IA | IA | IA | IA | - | IB | IB |
| • (Use of) validated risk scores for prognosis (e.g., GRACE) | IB | IB | IB | - | IA | - | IIa-B | - |
| Invasive procedure in intermediate to high risk patients | ||||||||
| • (Early) In-hospital coronary angiography (CA) | IA | IA | IA | IB | IA | IA | IA | IA |
| Discharge medications | ||||||||
| • Prescription of ACE inhibitor and/or ARB | IA | IA | IA | - | IA | - | IA | IA |
| • Prescription of aspirin | IA | IA | IA | IA | IA | IA | IA | IA |
| • Prescription of beta-blockers | IA | IA | IA | IA | IC | - | IB | IB |
| • Prescription of platelet aggregation inhibitors (e.g. thienopyridine) | IA | IA | IA | IB | IB | IB | IB | IB |
| • Prescription of statins | IA | IB | IB | - | IA | - | IA | IA |
|
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin II AT1 receptor blockers; CA, coronary angiography; ECG, electrocardiogram; GRACE, global registry of acute coronary events. †class of recommendation: class I refers to the condition in which there is evidence or general agreement that a certain procedure or treatment is beneficial, useful, effective, and thus recommended/should be performed; class II refers to the condition in which there is conflicting evidence about the usefulness or efficacy of a certain procedure or treatment, and thus should (class IIa) or may (class IIb) be considered. class III refers to the condition in which there is evidence or general agreement that a certain procedure or treatment is not useful or effective, and even in some cases be harmful, and is thus not recommended. †Level of evidence: Level A refers to data derived from multiple randomized clinical trials or meta-analyses; Level B refers to data derived from a single randomized clinical trial or large non-randomized studies; Level C refers to consensus of opinion of the experts and/or small studies, retrospective studies or registries. ‡In eligible patients according to the guidelines. ∞ Guideline update. | ||||||||
ACKNOWLEDGEMENT
All authors were involved in conception and design of the study. JE and ND were responsible for data collection, analysis, and quality assessment. JE and ND prepared the manuscript for publication. All authors contributed to interpretation of data and revision of manuscript for important intellectual content (JE, ND, IvdW, MdB, CW). All authors read and approved the final manuscript.
LIST OF ABBREVIATIONS
- ACC/AHA
American College of Cardiology/American Heart Association
- ACE
angiotensin-converting-enzyme
- ACS
acute coronary syndrome
- ARB
angiotensin II AT1 receptor blockers
- CA
coronary angiography
- CABG
coronary artery bypass grafting
- CPG
clinical practice guideline
- CRUSADE
Can Rapid risk stratification of Unstable angina patients Suppress Adverse outcomes with Early implementation of the ACC/AHA Guidelines
- ECG
electrocardiogram
- ESC
European Society of Cardiology
- GRACE
Global Registry of Acute Coronary Events
- MI
myocardial infarction
- NST-ACS
non-ST-elevation acute coronary syndrome
- NSTEMI
non-ST-elevation myocardial infarction
- PCI
percutaneous coronary intervention
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- SCPC
Society of Cardiovascular Patient Accreditation
- STEMI
ST-elevation myocardial infarction
- STROBE
STrengthening the Reporting of Observational studies in Epidemiology
- TIMI
Thrombolysis In Myocardial Infarction
- UA
unstable angina
Appendix A - Systematic review search strategies.
I. PUBMED (including MEDLINE)
| Search | Query | Nr. Of hits |
|---|---|---|
| #1 | Search (“Angina, Unstable”[Mesh] OR (Angina[tw] AND (unstable[tw]) | 17,138 |
| #2 | Search (“Myocardial Infarction”[Mesh] OR (Myocardial infarct*[tw] OR Myocardium infarct*[tw] OR heart infarct*[tw] OR cardiac infarct*[tw])) | 212,441 |
| #3 | Search (“Acute Coronary Syndrome”[Mesh] OR acute coronary syndrome*[tw]) | 24,181 |
| #4 | Search (#1 OR #2 OR #3) | 231,402 |
| #5 | Search (“Guideline Adherence”[Mesh] OR ((“Guidelines as Topic”[Mesh] OR guideline*[tw] OR protocol*[tw]) AND (adheren*[tw] OR complian*[tw]))) | 49,064 |
| #6 | Search (#4 AND #5) | 1303 |
II. EMBASE.
| Search | Query | Nr. Of hits |
|---|---|---|
| #1 | 'unstable angina pectoris'/exp OR (angina:de,ab,ti AND (unstable:de,ab,ti OR preinfarction:de,ab,ti)) | 24,792 |
| #2 | 'heart infarction'/exp OR (myocardial NEXT/1 infarct*):de,ab,ti OR (myocardium NEXT/1 infarct*):de,ab,ti OR (heart NEXT/1 infarct*):de,ab,ti OR (cardiac NEXT/1infarct*):de,ab,ti | 344,803 |
| #3 | 'acute coronary syndrome'/exp OR ('acute coronary' NEXT/1 syndrome*):de,ab,ti | 47,295 |
| #4 | #1 OR #2 OR #3 | 374,317 |
| #5 | 'protocol compliance'/exp OR 'practice guideline'/exp OR guideline*:de,ab,ti OR protocol*:de,ab,ti AND (adheren*:de,ab,ti OR complian*:de,ab,ti) | 56,329 |
| #6 | #4 AND #5 | 1911 |
III. CINAHL.
| Search | Query | Nr. Of hits |
|---|---|---|
| S1 | (MH “Angina, Unstable”) | 1,758 |
| S2 | TI angina OR AB angina OR SU angina | 7,817 |
| S3 | TI ((unstable OR preinfarction)) OR AB ((unstable OR preinfarction)) OR SU ((unstable OR preinfarction)) | 6,944 |
| S4 | (TI (unstable OR preinfarction) OR AB (unstable OR preinfarction) OR SU (unstable OR preinfarction)) AND (S2 AND S3) | 2,489 |
| S5 | S1 OR S4 | 3,248 |
| S6 | (MH “Myocardial Infarction+”) OR TI ((“Myocardial infarct*” OR “Myocardium infarct*” OR “heart infarct*” OR “cardiac infarct*”)) OR AB ((“Myocardial infarct*” OR “Myocardium infarct*” OR “heart infarct*” OR “cardiac infarct*”)) OR SU ((“Myocardial infarct*” OR “Myocardium infarct*” OR “heart infarct*” OR “cardiac infarct*”)) | 39,768 |
| S7 | (MH “Acute Coronary Syndrome”) OR TI “acute coronary syndrome*” OR AB “acute coronary syndrome*” OR SU “acute coronary syndrome*” | 6,654 |
| S8 | S5 OR S6 OR S7 | 46,962 |
| S9 | (MH “Guideline Adherence”) | 8,688 |
| S10 | TI ((guideline* OR protocol*)) OR AB ((guideline* OR protocol*)) OR SU ((guideline* OR protocol*)) | 159,823 |
| S11 | TI ((adheren* OR complian*)) OR AB ((adheren* OR complian*)) OR SU ((adheren* OR complian*)) | 76,633 |
| S12 | S10 AND S11 | 17,615 |
| S13 | S9 OR S12 | 9,290 |
| S14 | S8 AND S13 | 353 |
IV. Cochrane library.
| Search | Query | Nr. Of hits |
|---|---|---|
| #1 | Angina:ti,ab,kw AND (unstable:ti,ab,kw OR preinfarction:ti,ab,kw) | 2,445 |
| #2 | “Myocardial infarct*”:ti,ab,kw OR “Myocardium infarct*”:ti,ab,kw OR “heart infarct*”:ti,ab,kw OR “cardiac infarct*”:ti,ab,kw | 19,235 |
| #3 | acute coronary syndrome*:ti,ab,kw | 3,365 |
| #4 | #1 or #2 or #3 | 21,664 |
| #5 | (guideline*:ti,ab,kw OR protocol*:ti,ab,kw) AND (adheren*:ti,ab,kw OR complian*:ti,ab,kw) | 5920 |
| #6 | #4 and #5 | 119 |
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
This study was funded by the Dutch Ministry of Public health, Welfare and Sports. The study sponsor had no role in the study design, collection, analysis and interpretation of the data, or in writing of the manuscript and decision to submit the manuscript for publication.
REFERENCES
- 1.Rogers W.J., Frederick P.D., Stoehr E., et al. Trends in presenting characteristics and hospital mortality among patients with ST elevation and non-ST elevation myocardial infarction in the National Registry of Myocardial Infarction from 1990 to 2006. Am. Heart J. 2008;156:1026–1034. doi: 10.1016/j.ahj.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 2.McManus D.D., Gore J., Yarzebski J., Spencer F., Lessard D., Goldberg R.J. Recent trends in the incidence, treatment, and outcomes of patients with STEMI and NSTEMI. Am. J. Med. 2011;124:40–47. doi: 10.1016/j.amjmed.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darling C.E., Fisher K.A., McManus D.D., et al. Survival after hospital discharge for ST-segment elevation and non-ST-segment elevation acute myocardial infarction: a population-based study. Clin. Epidemiol. 2013;5:229–236. doi: 10.2147/CLEP.S45646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia-Garcia C., Subirana I., Sala J., et al. Long-term prognosis of first myocardial infarction according to the electrocardiographic pattern (ST elevation myocardial infarction, non-ST elevation myocardial infarction and non-classified myocardial infarction) and revascularization procedures. Am. J. Cardiol. 2011;108:1061–1067. doi: 10.1016/j.amjcard.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Terkelsen C.J., Lassen J.F., Norgaard B.L., et al. Mortality rates in patients with ST-elevation vs. non-ST-elevation acute myocardial infarction: observations from an unselected cohort. Eur. Heart J. 2005;26:18–26. doi: 10.1093/eurheartj/ehi002. [DOI] [PubMed] [Google Scholar]
- 6.Clinical Practice Guidelines: Directions for a new program. Washington DC: National Academy Press; 1990. Institute of medicine (US) committee to advise the public health service on clinical practice guidelines. [PubMed] [Google Scholar]
- 7.Grimshaw J.M., Russell I.T. Effect of clinical guidelines on medical practice: a systematic review of rigorous evaluations. Lancet. 1993;342:1317–1322. doi: 10.1016/0140-6736(93)92244-n. [DOI] [PubMed] [Google Scholar]
- 8.Field M.J. Practice guidelines and quality of care. J. Natl. Med. Assoc. 1994;86:255. [editorial]. [PMC free article] [PubMed] [Google Scholar]
- 9.National Clinical Guideline Centre (UK) Unstable Angina and NSTEMI. The early management of Unstable angina and Non-ST-Segment-Elevation Myocardial infarction. NICE clinical guidelines, No.94. Londen. Royal College of Physicians; 2010. [PubMed] [Google Scholar]
- 10.Roffi M., Patrono C., Collet J.P., et al. ESC Guidelines for the management of acute coronary syndromes in patienst presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2015;37:267–315. doi: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 11.Amsterdam E.A., Wenger N.K., Brindis R.G., et al. 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;64:2645–2687. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 12.Roe M.T., Parsons L.S., Pollack C.V., Jr, et al. Quality of care by classification of myocardial infarction: treatment patterns for ST-segment elevation vs non-ST-segment elevation myocardial infarction. Arch. Intern. Med. 2005;165:1630–1636. doi: 10.1001/archinte.165.14.1630. [DOI] [PubMed] [Google Scholar]
- 13.Somma K.A., Bhatt D.L., Fonarow G.C., et al. Guideline adherence after ST-segment elevation versus non-ST segment elevation myocardial infarction. Circ Cardiovasc Qual Outcomes. 2012;5:654–661. doi: 10.1161/CIRCOUTCOMES.111.963959. [DOI] [PubMed] [Google Scholar]
- 14.Van de Werf F., Ardissino D., Bueno H., et al. Acute coronary syndromes: considerations for improved acceptance and implementation of management guidelines. Expert Rev. Cardiovasc. Ther. 2012;10:489–503. doi: 10.1586/erc.12.20. [DOI] [PubMed] [Google Scholar]
- 15.Tricoci P., Peterson E.D., Roe M.T. Patterns of guideline adherence and care delivery for patients with unstable angina and non-ST-segment elevation myocardial infarction (from the CRUSADE Quality Improvement Initiative). Am. J. Cardiol. 2006;98:30Q–35Q. doi: 10.1016/j.amjcard.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 16.Cabana M.D., Rand C.S., Powe N.R., et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458–1465. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 17.Grol R., Grimshaw J. From best evidence to best practice: effective implementation of change in patients' care. Lancet. 2003;362:1225–1230. doi: 10.1016/S0140-6736(03)14546-1. [DOI] [PubMed] [Google Scholar]
- 18.Francke A.L., Smit M.C., de Veer A.J., Mistiaen P. Factors influencing the implementation of clinical guidelines for health care professionals: a systematic meta-review. BMC Med. Inform. Decis. Mak. 2008;8:38. doi: 10.1186/1472-6947-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peterson E.D., Roe M.T., Mulgund J., et al. Association between hospital process performance and outcomes among patients with acute coronary syndromes. JAMA. 2006;295:1912–1920. doi: 10.1001/jama.295.16.1912. [DOI] [PubMed] [Google Scholar]
- 20.Murray C.J., Lopez A.D. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet. 1997;349:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 21.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Elm E., Altman D.G., Egger M., et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J. Clin. Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Amsterdam E.A., Peterson E.D., Ou F.S., et al. Comparative trends in guidelines adherence among patients with non-ST-segment elevation acute coronary syndromes treated with invasive versus conservative management strategies: Results from the CRUSADE quality improvement initiative. Am. Heart J. 2009;158:748–754. doi: 10.1016/j.ahj.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Bhatt D.L., Roe M.T., Peterson E.D., et al. Utilization of early invasive management strategies for high-risk patients with non-ST-segment elevation acute coronary syndromes: results from the CRUSADE Quality Improvement Initiative. JAMA. 2004;292:2096–2104. doi: 10.1001/jama.292.17.2096. [DOI] [PubMed] [Google Scholar]
- 25.Chandra A., Glickman S.W., Ou F.S., et al. An analysis of the Association of Society of Chest Pain Centers Accreditation to American College of Cardiology/American Heart Association non-ST-segment elevation myocardial infarction guideline adherence. Ann. Emerg. Med. 2009;54:17–25. doi: 10.1016/j.annemergmed.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 26.Diercks D.B., Roe M.T., Mulgund J., et al. The obesity paradox in non-ST-segment elevation acute coronary syndromes: results from the Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the American College of Cardiology/American Heart Association Guidelines Quality Improvement Initiative. Am. Heart J. 2006;152:140–148. doi: 10.1016/j.ahj.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 27.Diercks D.B., Roe M.T., Chen A.Y., et al. Prolonged emergency department stays of non-ST-segment-elevation myocardial infarction patients are associated with worse adherence to the American College of Cardiology/American Heart Association guidelines for management and increased adverse events. Ann. Emerg. Med. 2007;50:489–496. doi: 10.1016/j.annemergmed.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 28.Hoekstra J.W., Roe M.T., Peterson E.D., et al. Early glycoprotein IIb/IIIa inhibitor use for non-ST-segment elevation acute coronary syndrome: patient selection and associated treatment patterns. Acad. Emerg. Med. 2005;12:431–438. doi: 10.1197/j.aem.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 29.Miller C.D., Roe M.T., Mulgund J., et al. Impact of acute beta-blocker therapy for patients with non-ST-segment elevation myocardial infarction. Am. J. Med. 2007;120:685–692. doi: 10.1016/j.amjmed.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 30.Maddox T.M., Ho P.M., Tsai T.T., et al. Clopidogrel use and hospital quality in medically managed patients with non-ST-segment-elevation myocardial infarction. Circ Cardiovasc Qual Outcomes. 2012;5:523–531. doi: 10.1161/CIRCOUTCOMES.112.965285. [DOI] [PubMed] [Google Scholar]
- 31.Mehta R.H., Roe M.T., Chen A.Y., et al. Recent trends in the care of patients with non-ST-segment elevation acute coronary syndromes: insights from the CRUSADE initiative. Arch. Intern. Med. 2006;166:2027–2034. doi: 10.1001/archinte.166.18.2027. [DOI] [PubMed] [Google Scholar]
- 32.Peterson E.D., Pollack C.V., Roe M.T., et al. Early use of glycoprotein IIb/IIIa inhibitors in non-ST-elevation acute myocardial infarction: observations from the National Registry of Myocardial Infarction 4. J. Am. Coll. Cardiol. 2003;42:45–53. doi: 10.1016/s0735-1097(03)00514-x. [DOI] [PubMed] [Google Scholar]
- 33.Peterson E.D., Shah B.R., Parsons L., et al. Trends in quality of care for patients with acute myocardial infarction in the National Registry of Myocardial Infarction from 1990 to 2006. Am. Heart J. 2008;156:1045–1055. doi: 10.1016/j.ahj.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 34.Roe M.T., Peterson E.D., Newby L.K., et al. The influence of risk status on guideline adherence for patients with non-ST-segment elevation acute coronary syndromes. Am. Heart J. 2006;151:1205–1213. doi: 10.1016/j.ahj.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 35.Roe M.T., Chen A.Y., Riba A.L., et al. Impact of congestive heart failure in patients with non-ST-segment elevation acute coronary syndromes. Am. J. Cardiol. 2006;97:1707–1712. doi: 10.1016/j.amjcard.2005.12.068. [DOI] [PubMed] [Google Scholar]
- 36.Roe M.T., Chen A.Y., Mehta R.H., et al. Influence of inpatient service specialty on care processes and outcomes for patients with non ST-segment elevation acute coronary syndromes. Circulation. 2007;116:1153–1161. doi: 10.1161/CIRCULATIONAHA.107.697003. [DOI] [PubMed] [Google Scholar]
- 37.Roe M.T., Peterson E.D., Li Y., et al. Relationship between risk stratification by cardiac troponin level and adherence to guidelines for non-ST-segment elevation acute coronary syndromes. Arch. Intern. Med. 2005;165:1870–1876. doi: 10.1001/archinte.165.16.1870. [DOI] [PubMed] [Google Scholar]
- 38.Sonel A.F., Good C.B., Mulgund J., et al. Racial variations in treatment and outcomes of black and white patients with high-risk non-ST-elevation acute coronary syndromes: insights from CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines?). Circulation. 2005;111:1225–1232. doi: 10.1161/01.CIR.0000157732.03358.64. [DOI] [PubMed] [Google Scholar]
- 39.Tricoci P., Peterson E.D., Mulgund J., et al. Temporal trends in the use of early cardiac catheterization in patients with non-ST-segment elevation acute coronary syndromes (results from CRUSADE). Am. J. Cardiol. 2006;98:1172–1176. doi: 10.1016/j.amjcard.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 40.Sherwood M.W., Wiviott S.D., Peng S.A., et al. Early clopidogrel versus prasugrel use among contemporary STEMI and NSTEMI patients in the US: insights from the National Cardiovascular Data Registry. J. Am. Heart Assoc. 2014;3:e000849. doi: 10.1161/JAHA.114.000849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maier B., Thimme W., Schoeller R., Fried A., Behrens S., Theres H., Berlin Myocardial Infarction Registry Improved therapy and outcome for patients with acute myocardial infarction--data of the Berlin Myocardial Infarction Registry from 1999 to 2004. Int. J. Cardiol. 2008;130:211–219. doi: 10.1016/j.ijcard.2007.08.043. [DOI] [PubMed] [Google Scholar]
- 42.Mandelzweig L., Battler A., Boyko V., et al. The second Euro Heart Survey on acute coronary syndromes: Characteristics, treatment, and outcome of patients with ACS in Europe and the Mediterranean Basin in 2004. Eur. Heart J. 2006;27:2285–2293. doi: 10.1093/eurheartj/ehl196. [DOI] [PubMed] [Google Scholar]
- 43.Nieuwlaat R., Vermeer F., Scholte Op Reimer W.J., Boersma E., van het Hof A.W., Simoons M.L. Treatment of patients with acute coronary syndromes in the Netherlands in 2000-2001; a comparison with other European countries and with the guidelines. Ned. Tijdschr. Geneeskd. 2004;148:1878–1882. [PubMed] [Google Scholar]
- 44.Polonski L., Gasior M., Gierlotka M., et al. Polish Registry of Acute Coronary Syndromes (PL-ACS). Characteristics, treatments and outcomes of patients with acute coronary syndromes in Poland. Kardiol. Pol. 2007;65:861–872. [PubMed] [Google Scholar]
- 45.Dziewierz A., Siudak Z., Rakowski T., et al. More aggressive pharmacological treatment may improve clinical outcome in patients with non-ST-elevation acute coronary syndromes treated conservatively. Coron. Artery Dis. 2007;18:299–303. doi: 10.1097/MCA.0b013e32812cb91c. [DOI] [PubMed] [Google Scholar]
- 46.Ferreira J., Monteiro P., Mimoso J. National Registry of Acute Coronary Syndromes: results of the hospital phase in 2002. Rev. Port. Cardiol. 2004;23:1251–1272. [PubMed] [Google Scholar]
- 47.Olivari Z., Steffenino G., Savonitto S., et al. The management of acute myocardial infarction in the cardiological intensive care units in Italy: the 'BLITZ 4 Qualita' campaign for performance measurement and quality improvement. Eur. Heart J. Acute Cardiovasc. Care. 2012;1:143–152. doi: 10.1177/2048872612450520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schiele F., Meneveau N., Seronde M.F., et al. Compliance with guidelines and 1-year mortality in patients with acute myocardial infarction: a prospective study. Eur. Heart J. 2005;26:873–880. doi: 10.1093/eurheartj/ehi107. [DOI] [PubMed] [Google Scholar]
- 49.Vikman S., Airaksinen K.E., Peuhkurinen K., et al. Gap between guidelines and management of patients with acute coronary syndrome without persistent ST elevation. Finnish prospective follow-up survey. Scand. Cardiovasc. J. 2003;37:187–192. doi: 10.1080/14017430310014919. [DOI] [PubMed] [Google Scholar]
- 50.Engel J., Van der Wulp I., De Bruijne M., Wagner C. A cross-sectional multicentre study of cardiac risk score use in the management of unstable angina and non-ST-elevation myocardial infarction. BMJ Open. 2015;5:e008523. doi: 10.1136/bmjopen-2015-008523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeymer U., Heuer H., Schwimmbeck P., Genth-Zotz S., Wolff K., Nienaber C.A. Guideline-adherence therapy in patients with acute coronary syndromes. The EPICOR registry in Germany. Herz. 2015;40:27–35. doi: 10.1007/s00059-014-4161-7. [DOI] [PubMed] [Google Scholar]
- 52.Valli G., De Marco F., Spina M.T., et al. A pilot study on the application of the current European guidelines for the management of acute coronary syndrome without elevation of ST segment (NSTEMI) in the emergency department setting in the Italian region Lazio. Monaldi Arch. Chest Dis. 2014;82:175–182. doi: 10.4081/monaldi.2014.61. [DOI] [PubMed] [Google Scholar]
- 53.Banihashemi B., Goodman S.G., Yan R.T., et al. Underutilization of clopidogrel and glycoprotein IIb/IIIa inhibitors in non-ST-elevation acute coronary syndrome patients: the Canadian global registry of acute coronary events (GRACE) experience. Am. Heart J. 2009;158:917–924. doi: 10.1016/j.ahj.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 54.Rao R.V., Goodman S.G., Yan R.T., et al. Temporal trends and patterns of early clopidogrel use across the spectrum of acute coronary syndromes. Am. Heart J. 2009;157:642–650. doi: 10.1016/j.ahj.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 55.Lee C.H., Tan M., Yan A.T., et al. Use of cardiac catheterization for non-ST-segment elevation acute coronary syndromes according to initial risk: reasons why physicians choose not to refer their patients. Arch. Intern. Med. 2008;168:291–296. doi: 10.1001/archinternmed.2007.78. [DOI] [PubMed] [Google Scholar]
- 56.Yan A.T., Yan R.T., Tan M., et al. Management patterns in relation to risk stratification among patients with non-ST elevation acute coronary syndromes. Arch. Intern. Med. 2007;167:1009–1016. doi: 10.1001/archinte.167.10.1009. [DOI] [PubMed] [Google Scholar]
- 57.Cheng C.I., Chen C.P., Kuan P.L., et al. The causes and outcomes of inadequate implementation of existing guidelines for antiplatelet treatment in patients with acute coronary syndrome: the experience from Taiwan Acute Coronary Syndrome Descriptive Registry (T-ACCORD Registry). Clin. Cardiol. 2010;33:E40–E48. doi: 10.1002/clc.20730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kassab Y.W., Hassan Y., Aziz N.A., Akram H., Ismail O. Use of evidence-based therapy for the secondary prevention of acute coronary syndromes in Malaysian practice. J. Eval. Clin. Pract. 2013;19:658–663. doi: 10.1111/j.1365-2753.2012.01894.x. [DOI] [PubMed] [Google Scholar]
- 59.Zhang L.J., Chen Y.D., Song X.T., Zhao F.H., Lu S.Z. Antithrombotic and antiplatelet therapies in relation to risk stratification in patients with non-ST elevation acute coronary syndrome: insights from the Sino-Global Registry of Acute Coronary Events. Chin. Med. J. (Engl.) 2009;122:502–508. [PubMed] [Google Scholar]
- 60.Kassaian SE, Masoudkabir F. Clinical characteristics, management and 1-year outcomes of patienst with acute coronary synrome in Iran: the Iranian project for assessment of coronary events 2 (IPACE2). BMJ Open. 2015;5:e007786. doi: 10.1136/bmjopen-2015-007786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sinon J.B., Dorado E.D., Lelis M.A., Barrameda M.C. A descriptive study on the initial management of patients with acute coronary syndrome in emergency roos of Philippine hospitals. Philipp. J. Intern. Med. 2014;52:1–9. [Google Scholar]
- 62.Ellis C., Gamble G., French J., et al. Management of patients admitted with an Acute Coronary Syndrome in New Zealand: results of a comprehensive nationwide audit. N. Z. Med. J. 2004;117:U953. [PubMed] [Google Scholar]
- 63.Tang E., Wong C.K., Wilkins G., et al. Use of evidence-based management for acute coronary syndrome. N. Z. Med. J. 2005;118:U1678. [PubMed] [Google Scholar]
- 64.Goldberg R.J., Spencer F.A., Steg P.G., et al. Increasing use of single and combination medical therapy in patients hospitalized for acute myocardial infarction in the 21st century: a multinational perspective. Arch. Intern. Med. 2007;167:1766–1773. doi: 10.1001/archinte.167.16.1766. [DOI] [PubMed] [Google Scholar]
- 65.Jernberg T., Johanson P., Held C., Svennblad B., Lindback J., Wallentin L. SWEDEHEART/RIKS-HIA. Association between adoption of evidence-based treatment and survival for patients with ST-elevation myocardial infarction. JAMA. 2011;305:1677–1684. doi: 10.1001/jama.2011.522. [DOI] [PubMed] [Google Scholar]
- 66.Carlhed R., Bojestig M., Wallentin L., et al. Improved adherence to Swedish national guidelines for acute myocardial infarction: the Quality Improvement in Coronary Care (QUICC) study. Am. Heart J. 2006;152:1175–1181. doi: 10.1016/j.ahj.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 67.Ebben R.H., Vloet L.C., Verhofstad M.H., Meijer S., Mintjes-de Groot J.A., van Achterberg T. Adherence to guidelines and protocols in the prehospital and emergency care setting: a systematic review. Scand. J. Trauma Resusc. Emerg. Med. 2013;21:9. doi: 10.1186/1757-7241-21-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Atwater B.D., Dai D., Allen-Lapointe N.M., et al. Is heart failure guideline adherence being underestimated? The impact of therapeutic contraindications. Am. Heart J. 2012;164:750–755. doi: 10.1016/j.ahj.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 69.Grol R., Dalhuijsen J., Thomas S., In het Veld C., Rutten G., Mokkink H. Attributes of clinical guidelines that influence use of guidelines in general practice: observational study. BMJ. 1998;317:858–861. doi: 10.1136/bmj.317.7162.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krumholz H.M., Radford M.J., Wang Y., Chen J., Heiat A., Marciniak T.A. National use and effectiveness of beta-blockers for the treatment of elderly patients after acute myocardial infarction: National Cooperative Cardiovascular Project. JAMA. 1998;280:623–629. doi: 10.1001/jama.280.7.623. [DOI] [PubMed] [Google Scholar]
- 71.Eagle K.A., Montoye C.K., Riba A.L., et al. Guideline-based standardized care is associated with substantially lower mortality in medicare patients with acute myocardial infarction: the American College of Cardiology's Guidelines Applied in Practice (GAP) Projects in Michigan. J. Am. Coll. Cardiol. 2005;46:1242–1248. doi: 10.1016/j.jacc.2004.12.083. [DOI] [PubMed] [Google Scholar]
- 72.Stukel T.A., Alter D.A., Schull M.J., Ko D.T., Li P. Association between hospital cardiac management and outcomes for acute myocardial infarction patients. Med. Care. 2010;48:157–165. doi: 10.1097/MLR.0b013e3181bd4da7. [DOI] [PubMed] [Google Scholar]
- 73.Wang T.Y., Dai D., Hernandez A.F., et al. The importance of consistent, high-quality acute myocardial infarction and heart failure care results from the American Heart Association's Get with the Guidelines Program. J. Am. Coll. Cardiol. 2011;58:637–644. doi: 10.1016/j.jacc.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 74.Freemantle N., Calvert M., Wood J., Eastaugh J., Griffin C. Composite outcomes in randomized trials: greater precision but with greater uncertainty? JAMA. 2003;289:2554–2559. doi: 10.1001/jama.289.19.2554. [DOI] [PubMed] [Google Scholar]
- 75.Cordoba G., Schwartz L., Woloshin S., Bae H., Gotzsche P.C. Definition, reporting, and interpretation of composite outcomes in clinical trials: systematic review. BMJ. 2010;341:c3920. doi: 10.1136/bmj.c3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Neaton J.D., Gray G., Zuckerman B.D., Konstam M.A. Key issues in end point selection for heart failure trials: composite end points. J. Card. Fail. 2005;11:567–575. doi: 10.1016/j.cardfail.2005.08.350. [DOI] [PubMed] [Google Scholar]
- 77.Lim E., Brown A., Helmy A., Mussa S., Altman D.G. Composite outcomes in cardiovascular research: a survey of randomized trials. Ann. Intern. Med. 2008;149:612–617. doi: 10.7326/0003-4819-149-9-200811040-00004. [DOI] [PubMed] [Google Scholar]
- 78.Powell-Cope G.M., Luther S., Neugaard B., Vara J., Nelson A. Provider-perceived barriers and facilitators for ischaemic heart disease (IHD) guideline adherence. J. Eval. Clin. Pract. 2004;10:227–239. doi: 10.1111/j.1365-2753.2003.00450.x. [DOI] [PubMed] [Google Scholar]
- 79.Hoekstra J.W., Pollack C.V., Roe M.T., et al. Improving the care of patients with non-ST-elevation acute coronary syndromes in the emergency department: the CRUSADE initiative. Acad. Emerg. Med. 2002;9:1146–1155. doi: 10.1111/j.1553-2712.2002.tb01569.x. [DOI] [PubMed] [Google Scholar]
- 80.Flather M.D., Babalis D., Booth J., et al. Cluster-randomized trial to evaluate the effects of a quality improvement program on management of non-ST-elevation acute coronary syndromes: The European Quality Improvement Programme for Acute Coronary Syndromes (EQUIP-ACS). Am. Heart J. 2011;162:700–707. doi: 10.1016/j.ahj.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 81.Daudelin D.H., Sayah A.J., Kwong M., et al. Improving use of prehospital 12-lead ECG for early identification and treatment of acute coronary syndrome and ST-elevation myocardial infarction. Circ Cardiovasc Qual Outcomes. 2010;3:316–323. doi: 10.1161/CIRCOUTCOMES.109.895045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cannon C.P., Hoekstra J.W., Larson D.M., et al. A report of quality improvement in the care of patients with acute coronary syndromes. Crit. Pathw. Cardiol. 2011;10:29–34. doi: 10.1097/HPC.0b013e318204eb8b. [DOI] [PubMed] [Google Scholar]
- 83.Baker R., Camosso-Stefinovic J., Gillies C., et al. Tailored interventions to overcome identified barriers to change: effects on professional practice and health care outcomes. Cochrane Database Syst. Rev. 2010;3:CD005470. doi: 10.1002/14651858.CD005470.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lugtenberg M., Burgers J.S., Westert G.P. Effects of evidence-based clinical practice guidelines on quality of care: a systematic review. Qual. Saf. Health Care. 2009;18:385–392. doi: 10.1136/qshc.2008.028043. [DOI] [PubMed] [Google Scholar]
- 85.Sanderson S., Tatt I.D., Higgins J.P. Tools for assessing quality and susceptibility to bias in observation studies in epidemiology: a systematic review and annotated bibliography. Int. J. Epidemiol. 2007;36:666–676. doi: 10.1093/ije/dym018. [DOI] [PubMed] [Google Scholar]


