Abstract
The Bacillus subtilis Pho signal transduction network, which regulates the cellular response to phosphate starvation, integrates the activity of three signal transduction systems to regulate the level of the Pho response. This signal transduction network includes a positive feedback loop between the PhoP/PhoR and ResD/ResE two-component systems. Within this network, ResD is responsible for 80% of the Pho response. To date, the role of ResD in the generation of the Pho response has not been understood. Expression of two terminal oxidases requires ResD function, and expression of at least one terminal oxidase is needed for the wild-type Pho response. Previously, our investigators have shown that strains bearing mutations in resD are impaired for growth and acquire secondary mutations which compensate for the loss of the a-type terminal oxidases by allowing production of cytochrome bd. We report here that the expression of cytochrome bd in a ΔresDE background is sufficient to compensate for the loss of ResD for full Pho induction. A ctaA mutant strain, deficient in the production of heme A, has the same Pho induction phenotype as a ΔresDE strain. This demonstrates that the production of a-type terminal oxidases is the basis for the role of ResD in Pho induction. Terminal oxidases affect the redox state of the quinone pool. Reduced quinones inhibit PhoR autophosphorylation in vitro, consistent with a requirement for terminal oxidases for full Pho induction in vivo.
The Bacillus subtilis phosphate starvation response (Pho response) is under the control of a complex regulatory network that allows the cell to respond to the level of inorganic phosphate (Pi) in the environment. This system is critical to survival because phosphate is the limiting nutrient in soil (33), the natural environment for B. subtilis.
Central to the B. subtilis Pho response is the PhoP/PhoR two-component signal transduction system. The phoPR operon (23, 43) is subject to activation by PhoP under phosphate starvation conditions (34). PhoP/PhoR directly regulates the expression of genes involved in the cellular response to phosphate starvation. The histidine kinase, PhoR, is autophosphorylated in response to an environmental signal and then phosphorylates its cognate response regulator, PhoP. PhoP∼P activates the transcription of the alkaline phosphatases (19), phoA (formerly phoAIV) (20), and phoB (formerly phoAIII) (7); phosphodiesterases, phoD (11), and glpQ (1); a high-affinity phosphate transport system, pstS (37); teichuronic acid synthetic genes (teichuronic acid is a cell wall polymer lacking phosphate), tuaABCDEFGH (27, 46); and a gene encoding a 60-residue peptide of unknown function, ykoL (38). PhoP∼P has been shown to repress the expression of the tagAB and tagDEF genes responsible for the production of teichoic acid (a cell wall polymer containing phosphate) (26). The collective action of these products allows the cell to scavenge extracellular phosphate and to release additional Pi from the cell wall.
The regulation of the Pho response integrates the activity of three two-component signal transduction systems into a regulatory network. These systems are the PhoP/PhoR two-component system, the ResD/ResE two-component system, and the sporulation initiation phospho-relay (6). Previous work (49) has shown that both ResD and AbrB are required for full Pho induction. A ΔresDE strain lacks 80% of the wild-type Pho response, while a ΔabrB strain lacks 20% of the wild-type Pho response. A resD abrB double mutant shows no Pho induction, the same phenotype as a phoPR mutant strain (20), demonstrating that both ResD and AbrB are required upstream of PhoP and PhoR for full Pho induction. A spoOA mutation in the background of either the resD or abrB mutations leads to hyperinduction of the Pho regulon genes (49). A spoOA mutation in the resD abrB double mutant background does not alter the Pho-negative phenotype, indicating that only two upstream pathways, ResD and AbrB, regulate Pho induction, each of which is negatively regulated by SpoOA (3). Previous work has demonstrated that PhoP is an essential transcriptional activator of the promoter upstream of the resABCDE operon under phosphate starvation conditions (3). In addition, PhoP represses transcription from a minor internal promoter upstream of resD (3). These data together with the fact that ResD is required for 80% of the wild-type Pho response (49) demonstrate that a feedback loop exists between the ResD/ResE system and the PhoP/PhoR system. Both ResD and PhoP activate transcription from their own promoters under phosphate starvation conditions, adding to the feedback amplification (3, 25, 34). The question addressed here is how ResD generates 80% of the wild-type Pho response.
The primary role of the ResD/ResE two-component system is in the cellular response to respiratory conditions. ResD activates genes involved in both aerobic and anaerobic respiration, including fnr (32), hmp (32), nasDEF (32), hemN (17), hemZ (17), and the sbo-alb operon (31) under anaerobic conditions and ctaA (35, 56), ctaBCDEF (28), resABCDE (50), and petCBD (50) under aerobic conditions. ResD plays a critical role in the production of a-type terminal oxidases during aerobic respiration. CtaB catalyzes the conversion of heme B to heme O (28), which is then converted to heme A by CtaA (51). Strains lacking heme A cannot produce terminal oxidases aa3 or caa3 (30). In addition, ctaCDEF are the structural genes for cytochrome caa3 (2). Because ResD is required for ctaA and ctaBCDEF transcription, a resD mutant lacks both a-type terminal oxidases (49).
Three terminal oxidases have been described in B. subtilis. These include two a-type heme-copper oxidases, cytochromes aa3 (40) and caa3 (10). The third terminal oxidase is a member of the cytochrome bd family (54). A putative fourth terminal oxidase, YthAB, has been suggested from sequence homology and is proposed to be a member of the cytochrome bd family (53).
Previously, we demonstrated that ΔresDE strains spontaneously acquire secondary mutations which bypass some ΔresDE phenotypes, including restoring Pho induction to wild-type levels (41). These secondary mutations are loss-of-function mutations in ydiH, which encodes a negative regulator of the cydABCD operon, which encodes cytochrome bd in B. subtilis (41). The expression of cytochrome bd in a ΔresDE background is sufficient to bypass those resDE mutant phenotypes that are not directly associated with the loss of ResD as a transcription activator (41). These data led to the hypothesis that the activity of the terminal oxidases is involved in the role of ResD in the generation of the Pho response as examined here.
MATERIALS AND METHODS
Strains and plasmids.
Table 1 lists the strains and plasmids used in this study. Escherichia coli DH5α was the host for all plasmid constructions. B. subtilis JH642 was the host for all strain constructions. The construction of strains MH5878, MH5879, MH5880, MH5884, and MH5885 and of plasmids pMS34, pMS35, pMS37, pMS38, pMS40, and pMS45 was described previously (41). Chromosomal DNA from JH12586 (ΔabrB) was transformed into MH5888 (ΔresDE ydiH), creating MH7131. Transformation of chromosomal DNA from MH5124 (ΔphoR) into MH5878 created MH6303.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| E. coli strain | ||
| DH5α | Lab stock | |
| B. subtilis strains | ||
| JH12586 | trpC2 ΔabrB::Cmr | J. A. Hoch |
| JH642 | pheA1 trpC2 | J. A. Hoch |
| MH5124 | pheA1 trpC2 phoRΔBalI::Tetr | 44 |
| MH5202 | pheA1 trpC2ΔresDE::Tetr | 50 |
| MH5857 | pheA1 trpC2ΔresDE::TetrydiH amyE::phoD-lacZ Cmr | 41 |
| MH5878 | pheA1 trpC2 amyE::cydA-lacZ Cmr | 41 |
| MH5879 | pheA1 trpC2 ΔresDE::TetrydiH amyE::cydA-lacZ Cmr | 41 |
| MH5880 | pheA1 trpC2 ΔresDE::TetramyE::cydA-lacZ Cmr | 41 |
| MH5884 | pheA1 trpC2 Pspac-cydABCD Cmr | 41 |
| MH5885 | pheA1 trpC2 Pspac-cydABCD Cmr ΔresDE::Tetr | 41 |
| MH5887 | pheA1 trpC2ΔresDE:TetrydiH | Guofu Sun |
| MH5888 | pheA1 trpC2ΔresDE:TetrydiH | Ruth Chestnut |
| MH6303 | pheA1 trpC2 phoRΔBal1::TetramyE::cydA-lacZ Cmr | This study |
| MH7124 | pheA1 trpC2 ctaAΑΩpAE25 Spcr | This study |
| MH7128 | pheA1 trpcC2 ctaAΑΩpAE25 SpcrPspac-cydABCD Cmr | This study |
| MH7131 | pheA1 trpC2ΔresDE::TetrydiH ΔabrB::Cmr | This study |
| Plasmids | ||
| pCR2.1 | Vector for cloning PCR products | Invitrogen |
| pDH32 | Vector for construction of promoter-lacZ fusions, Ampr Cmr | 45 |
| pDG1727 | Vector for mutant generation, Spcr | 14 |
| pDH88 | Vector containing the Pspac promoter for creation on an IPTG-inducible promoter | 16 |
| ECE 75 | Vector for antibiotic conversion, Cmr to Tetr | 47 |
| pMS34 | Fragment of cydA promoter in pCR 2.1, Ampr | 41 |
| pMS35 | cydA-lacZ fusion in pDH32, Ampr Cmr | 41 |
| pMS37 | Fragment of cydA in pCR2.1, Ampr | 41 |
| pMS38 | Pspac-cydABCD in pDH88, Ampr Cmr | 41 |
| pMS40 | Internal fragment of ydiH in pCR2.1, Ampr | 41 |
| pMS45 | Internal fragment of ydiH in pDG1727, Ampr Spcr | 41 |
| pAE24 | Internal fragment of ctaA in pCR2.1, Ampr | This study |
| pAE25 | Internal fragment of ctaA in pDG1727, Ampr Spcr | This study |
To create an insertion duplication mutation in ctaA, we cloned an internal fragment into pDG1727. Primers FMH831 (5′-AGAAGCTT91TCCGGCCAAGGATGCGGCAGACAGTG116-3′; HindIII site underlined) and FMH832 (5′-GGTCTAGA820AGCCCAGAGCCAGTTCAGAGTATACG795-3′; XbaI site underlined) were used to amplify a 729-bp internal fragment of ctaA, using JH642 as the template DNA. This fragment was cloned to pCR2.1 (Invitrogen) to create pAE24. The ctaA fragment was released by digestion with EcoRV and BamHI and cloned into the complementary sites in pDG1727 to create pAE25. pAE25 was transformed to JH642 to create MH7124 (ΩctaA). The disruption of the ctaA gene was confirmed by PCR. The strain exhibited growth phenotypes associated with ctaA mutant strains on tryptose blood agar base plates (TBAB) and TBAB plates with 0.5% glucose (TBABG) (30). It also showed the phenotypes associated with a ctaA mutant strain with respect to acid accumulation on purification agar and sporulation (30). Due to the stress placed on the cell by the ctaA mutation, these strains were grown in the presence of spectinomycin to prevent loss of the plasmid insertion by recombination. To examine the effect of expressing cytochrome bd in a ctaA mutant background, the strain MH7128 (ΩctaA Pspac-cydABCD) was made by transformation of chromosomal DNA from MH7124 (ΩctaA) into MH5884 (Pspac-cydABCD).
Genetic manipulations.
Transformation of B. subtilis was done by the two-step transformation method of Cutting and Vander Horn (9). Transformants were selected on TBABG with the appropriate antibiotic. Antibiotics were added for selection of B. subtilis transformants at the following concentrations: chloramphenicol (5 μg/ml), spectinomycin (100 μg/ml), and tetracycline (10 μg/ml). Transformation of E. coli was done according to the method of Hanahan (15). Transformants were selected on Luria-Bertani (LB) plates containing ampicillin (100 μg/ml).
Growth conditions and enzyme assays.
Total alkaline phosphatase (APase) activity (APase expression from phoA and phoB) was measured in cells that had been grown in low-phosphate defined medium (LPDM) as described previously (18) or high-phosphate defined medium (HPDM). LPDM contains 0.4 mM Pi; HPDM contains 5.0 mM Pi. One unit of APase activity was defined as 1 μM para-nitrophenol produced min−1, and specific activity was calculated as units of APase per milliliter. β-Galactosidase activity was detected using the method of Ferrari et al. (12). The activity unit was defined as 0.33 nmol of ortho-nitrophenol produced min−1, and the specific activity was calculated as activity per milligram of protein. When appropriate, isopropyl-β-d-thiogalactosidase (IPTG) was added at a final concentration of 1 mM throughout growth. Culture density and APase activity were measured hourly from cells grown under culture conditions described previously (7).
Preparation and spectrophotometry of solubilized membrane vesicles.
Membrane vesicles were prepared as described by Bisschop and Konings (4) with the following modifications. Cells were collected from Pho-induced stationary-phase cultures grown in LPDM. DNase and RNase were omitted from the lysis procedure. Solubilization of cytochromes and analysis of difference absorption spectra were performed as described by Mueller and Taber (30). Difference absorption spectra (dithionite reduced minus ferricyanide oxidized) were recorded at room temperature at a scan speed of 5 nm/s with a Hitachi U-2000 spectrophotometer. Reduction and oxidation were performed as previously described (30).
Purification of PhoR and phosphorylation assays.
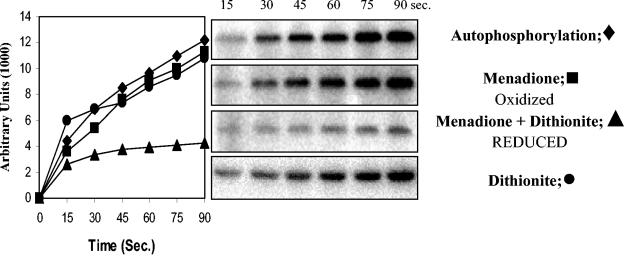
Overexpression and purification of the truncated histidine kinase *PhoR231-589 was performed as described previously (44). Purified protein *PhoR231-589 (3 μM) was incubated with 5 μCi of [γ-32P]ATP (specific activity, 6,000 ci/mmol; 10 mCi/ml; Amersham Biosciences) at room temperature in P-buffer (50 mM HEPES [pH 8.0], 50 mM KCl, and 5 mM MgCl2) in the presence or absence of menadione (MK3; 2 mM; Sigma-Aldrich) and/or dithionite (2 mM; Sigma-Aldrich). The reaction was started by the addition of [γ-32P]ATP. Samples were taken at the indicated time intervals (see Fig. 6), and the reaction was terminated by adding an equal volume of 4× sodium dodecyl sulfate (SDS) loading sample buffer. Samples were subjected to SDS-polyacrylamide gel electrophoresis on 12.5% polyacrylamide gels (22). Radioactivity of the PhoR∼P protein on the dried gels was detected with a PhosphorImager (Molecular Dynamics) and was quantitated by using ImageQuant (version 5.1).
FIG. 6.
Effect of MK3 on the rate of autophosphorylation of the *PhoR231-589 histidine kinase. The purified protein *PhoR231-589 was incubated with [γ-32P]ATP in the presence or absence of MK3 and/or dithionite. The right panel shows the autoradiograms of the different SDS-polyacrylamide gel electrophoresis gels. In the left panel, quantification of the radioactive PhoR∼P with time was measured with ImageQuant, and the results are expressed in arbitrary units.
RESULTS
ΔresDE mutant strains bearing ydiH mutations have wild-type levels of Pho induction.
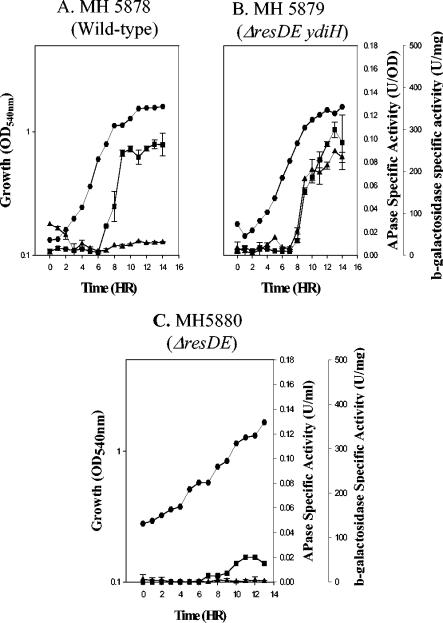
Our investigators have previously shown that B. subtilis ΔresDE strains that express cytochrome bd during growth in LB plus 0.5% glucose are complemented for those resD mutant phenotypes that are not directly associated with the role of ResD as a transcriptional activator (41). Strains bearing mutations in resD have approximately 20% of the wild-type Pho response during phosphate deprivation (49). We asked if the ydiH mutation which suppresses the ΔresDE phenotype is sufficient to bypass the requirement of ResD for full Pho induction. The APase specific activities in the parental strain (MH5857), the ΔresDE strain (MH5880), and the ΔresDE ydiH strain (MH5879) grown in LPDM showed that the ydiH mutant strain had wild-type levels of Pho induction despite the fact that resD is not functional (Fig. 1).
FIG. 1.
cydA-lacZ and APase expression in strains cultured in LPDM. •, growth; ▪, APase production; ▴, cydA-lacZ expression. (A) MH5878 (wild-type cydA-lacZ); (B) MH5879 (ΔresDE ydiH cydA-lacZ); (C) MH5880 (ΔresDE cydA-lacZ).
Cytochrome bd is expressed in a ΔresDE ydiH mutant strain under phosphate starvation conditions.
If cytochrome bd were essential to Pho induction in a ΔresDE ydiH strain, we would expect to see aberrant expression of cytochrome bd under phosphate starvation conditions. Expression of a cydA-lacZ fusion and APase induction was assayed in a wild-type background, a ydiH ΔresDE background, and a ΔresDE background (Fig. 1) from cells grown in LPDM. The cydA-lacZ fusion was expressed at low levels in the wild-type strain (Fig. 1A) and was not expressed in a ΔresDE strain (Fig. 1C) under phosphate starvation conditions. This suggests that cytochrome bd is a minor terminal oxidase under these growth conditions. In the suppressed ΔresDE strain bearing the ydiH mutation (MH5879) (Fig. 1B), the cydA-lacZ fusion was highly expressed during stationary phase, and APase was restored to wild-type levels. This expression was 10-fold higher than that seen in the wild-type background (MH5878) (Fig. 1A). These data suggest that elevated expression of cytochrome bd (and perhaps other terminal oxidases) is able to restore full Pho induction in a ΔresDE mutant strain.
Additional evidence for the increased expression of cytochrome bd in a ΔresDE ydiH strain was shown by the light absorption difference (dithionite-reduced minus ferricyanide-oxidized) spectra from a strain bearing the ydiH ΔresDE mutation grown to stationary phase in LPDM. Strains bearing a ΔresDE mutation lack a-type cytochromes and therefore do not produce the spectral peak at 600 nm (50) that is observed in wild-type membranes (Fig. 2A). Differential spectra of membranes from a ΔresDE ydiH strain grown in LPDM showed the expected pattern for cytochrome bd expression (Fig. 2B), namely, a trough at 650 nm and peaks at 622 and 595 nm (54). The characteristic cytochrome bd pattern was not observed in a strain bearing a ΔresDE mutation (Fig. 2C) or in membranes from a wild-type strain grown under the same conditions (Fig. 2A). These data confirm that cytochrome bd is expressed during Pho induction in LPDM in the suppressed ΔresDE ydiH double mutant.
FIG. 2.
Light absorption difference (dithionite-reduced minus ferricyanide-oxidized) spectra of membranes from B. subtilis strains. JH642 (wild type), MH5202 (ΔresDE), and MH5202 (ΔresDE ydiH) were grown in LPDM and harvested during Pho induction. A representative result is shown for each strain. (A) JH642; (B) MH5857; (C) MH5202.
Mutations in ctaA mimic the ΔresDE Pho-defective phenotype.
To test whether the loss of terminal oxidases was responsible for the role of ResD in Pho induction, we determined the level of Pho induction in a ctaA mutant strain. ResD is a direct transcriptional activator of ctaA (35, 56). A ΔresDE strain lacks CtaA and cannot synthesize heme A (50), leading to a deficiency in the a-type terminal oxidases aa3 and caa3. A strain bearing a ctaA mutation has some of the growth phenotypes associated with the loss of the a-type terminal oxidases that are seen in a ΔresDE strain (50) Compared to the parental strain, a ctaA mutant showed decreased Pho induction similar to that of a ΔresDE strain (compare Fig. 3A and B). Thus, the lack of a-type terminal oxidases, owing to a mutation in either resD or ctaA, resulted in decreased Pho induction. Interestingly, the ctaA mutant strain grew at a similar rate as the wild-type strain in LPDM (Fig. 3). Further, this suggests that the depression of Pho induction in a ΔresDE strain is associated with the lack of terminal oxidases, rather than the poor growth phenotype of the strain.
FIG. 3.
Effect of ctaA on growth and APase production in strains cultured in LPDM. Solid symbols, growth; open symbols, APase specific activity. (A) JH642 (wild type); (B) MH7124 (ΩctaA). The results for MH7124 (ΩctaA) are the average of readings from five ctaA mutant clones.
Expression of the cydABCD operon is sufficient to bypass the requirement for either ResD or CtaA for full Pho induction.
To confirm that cytochrome bd expression is responsible for the restoration of growth and Pho induction in a ydiH strain, we used strain MH5885 (ΔresDE Pspac-cydABCD), which allowed control of the production of cytochrome bd in a ΔresDE background. If the role of ResD in Pho induction were not the result of direct transcriptional activation of the phoPR operon, then the expression of cytochrome bd might be sufficient to restore Pho induction to wild-type levels, because our group previously showed (41) that induction of cytochrome bd in a ΔresDE background was sufficient to restore other phenotypes resulting from the absence of terminal oxidase production in a resDE mutant.
Strain MH5885 (ΔresDE Pspac-cydABCD), when grown in LPDM in the presence of IPTG (Fig. 4A), showed growth and Pho induction similar to that of a ΔresDE ydiH strain (MH5887) (Fig. 4A), indicating that expression of cytochrome bd was sufficient to bypass the requirement for ResD for full Pho induction. Further, because IPTG was present at the time of inoculation of MH5885 and APase induction in that strain is coincident with APase expression in the ydiH resDE strain, a strain shown to induce cydA postexponentially (Fig. 1B), this suggests that Pho induction is not dependent on when bd is made, only that bd is there when phosphate deficiency occurs. When IPTG was absent (Fig. 4A), MH5885 (ΔresDE Pspac-cydABCD) showed the reduced growth rate associated with a resD strain, which resulted in APase specific activity reaching 0.02 after 14 h, a similar APase specific activity as expressed in the ΔresDE strain upon phosphate starvation (data not shown).
FIG. 4.
Constitutive expression of cydABCD restores full Pho induction in resDE or ctaA strains. (A) Induction of cytochrome bd in a ΔresDE background is sufficient to restore Pho induction. Solid symbols, growth; open symbols, APase; diamonds, MH5857 (ΔresDE ydiH) with IPTG; triangles, MH5885 (ΔresDE Pspac-cydABCD) with IPTG; circles, MH5885 (ΔresDE Pspac-cydABCD) without IPTG. (B) Induction of cytochrome bd in a ctaA mutant background is sufficient to restore Pho induction. The results from each strain are the average APase specific activity from 20 cultures. Lane 1, JH642 (wild type) without IPTG; lane 2, MH7124 (ΩctaA) without IPTG; lane 3, MH7128 (ΩctaA Pspac-cydABCD) with IPTG; lane 4, MH7128 (ΩctaA Pspac-cydABCD) without IPTG.
To determine if expression of cytochrome bd in a ctaA mutant background could restore a wild-type level of Pho induction, we combined a ctaA mutation with the IPTG-inducible cydABCD operon (MH7128). Expression of APase in MH7128 (ΩctaA Pspac-cydABCD) grown in LPDM in the presence of IPTG (Fig. 4B, lane 3) showed a wild-type level of induction (JH642) (lane 1), whereas the same strain grown without IPTG (lane 4) had a low Pho induction level, similar to that of a ctaA mutant strain (lane 2). The fact that the ctaA mutant strain containing Pspac-cydABCD (MH7128) had a higher level than the parental strain (ctaA) is likely due to low expression from the Pspac promoter in the absence of IPTG. These data, taken together with the results of similar assays in a ΔresDE background (Fig. 4A), confirm that expression of a terminal oxidase in either a ctaA or a resD mutant background is sufficient to restore Pho induction to wild-type levels. This demonstrates that the presence of a terminal oxidase is needed for full Pho induction.
Activation of Pho induction by bd expression does not require AbrB but does require reduced Pi levels and PhoR.
To test whether bd expression in the resD ydiH mutant acts on Pho induction through the ResD-independent AbrB-dependent parallel pathway, we examined the effect of an abrB mutation on Pho induction in the ΔresDE ydiH background. A ΔresDE ydiH abrB triple mutant (MH7131) (Fig. 5A) retained the growth and Pho induction phenotypes of a ΔresDE ydiH double mutant (MH5887) (Fig. 5A), providing evidence that the AbrB pathway is not involved in suppression of the ΔresD phenotype on Pho induction in ΔresDE ydiH strains.
FIG. 5.
Restoration of full Pho induction in a resDE ydiH strain does not require AbrB but does depend on reduced Pi levels and PhoR. (A) Pho induction in a resDE ydiH strain does not require AbrB. Solid symbols, growth; open symbols, APase specific activity; circles, MH5888 (resDE ydiH); squares, MH7131 (resDE ydiH abrB). (B) Pho induction in a resDE ydiH strain depends on reduced Pi levels and PhoR. The APase specific activities shown are the averages of duplicate readings from two cultures. Lane 1, MH5857 (resDE ydiH) grown in LPDM; lane 2, MH5857 (resDE ydiH) grown in HPDM; lane 3, MH6303 (phoR Pspac-cydABCD) grown in LPDM with IPTG; lane 4, MH5124 (ΔphoR) grown in LPDM with IPTG; lane 5, JH642 grown in LPDM.
To confirm that Pho induction in a resD ydiH strain was phosphate starvation dependent, APase expression was assayed in strain MH5857 (resD ydiH) grown in LPDM and HPDM. MH5857 APase specific activity (Fig. 5B, lane 1) was comparable to that of the WT control (JH642) (lane 5) when grown in LPDM but failed to induce APase in the high-phosphate medium (HPDM) (Fig. 5B, lane 2), as did the phoR-negative control (MH5124) (lane 4) grown in LPDM, indicating Pho induction in a resD ydiH strain was phosphate starvation dependent.
To rule out the possibility that bd expression-dependent Pho induction was PhoR independent, we constructed a phoR Pspac-cydABCD strain, MH6303. APase was not induced in MH6303 (Fig. 5B, lane 3) grown in LPDM containing IPTG, indicating that expression of cytochrome bd from an IPTG-inducible promoter did not restore the Pho response in the absence of PhoR via activation of another kinase capable of PhoP phosphorylation.
The redox state of quinones affects PhoR autophosphorylation.
The function of terminal oxidases in the cell is to oxidize the reduced menaquinone pool. The redox state of quinones has been reported to affect autophosphorylation of the histidine kinase of three signaling systems: ArcB (E. coli) (13), BvgS (Bordetella pertussis), and EvgS (E. coli) (5). The major form of quinones in B. subtilis is the menaquinone MK-7 (8). When the soluble analog, MK3, was incubated with or without dithionite in the presence of [γ-32P]ATP and purified *PhoR231-589, a soluble NH2-terminal truncation of PhoR (44), autophosphorylation of *PhoR was inhibited in the presence of dithionite-reduced MK3 (Fig. 6) but not in the presence of MK3 or dithionite alone compared to PhoR autophosphorylation in the absence of quinones. These in vitro data suggest that reduced quinones inhibit PhoR autophosphorylation. The finding that the addition of the membrane-penetrating reductant dithiothreitol (DTT) to low-Pi agar medium resulted in colonies with decreased expression of reporters of Pho induction (phoA-lacZ, phoD-lacZ, or phoP-lacZ fusions) compared to the same strains on the same medium without DTT is consistent with our in vitro data (data not shown).
DISCUSSION
In this report, we have shown that the expression of a terminal oxidase (cytochrome bd) in a ΔresDE background can bypass the requirement for ResD for full Pho induction. Previous reports (49) have demonstrated that ResD is required for 80% of the wild-type B. subtilis Pho response. To understand this mechanism, we studied ΔresDE strains bearing secondary mutations in ydiH. These strains exhibited wild-type levels of Pho induction despite the presence of a resD mutation (41). ydiH encodes a DNA-binding protein that acts as a negative regulator for the production of cytochrome bd (41). Our group had previously shown that a resD mutant did not produce terminal oxidases aa3 and caa3 (50) because ResD directly activates ctaA expression (35), which is required for conversion of heme O to heme A (51, 52). The absence of cydA expression in a resDE mutant compared to low-level expression during growth of the parental strain in LPDM (JH642) (Fig. 1) and the dependency of cydA expression on ResD (unpublished data) in the same strain grown in NSMPG (nutrient sporulation medium with phosphate buffer and fucose) indicate that a resDE strain is also deficient in cytochrome bd synthesis. Importance of terminal oxidases in Pho induction was supported by the observation that a ctaA strain also has reduced Pho induction and that expression of cytochrome bd in either a ΔresDE background or a ctaA mutant background restored Pho induction to wild-type levels (Fig. 5).
The observation that the ctaA strain had reduced Pho induction is also important because it separated the Pho regulon dependency on terminal oxidases from the poor growth in LPDM. While there may be a number of reasons for the improved growth of a ctaA mutant strain compared to that of resDE, the most simple may be that all Res regulon genes except ctaA remain functional in a ctaA strain.
Among the Res regulon genes, cydA expression is likely important. Figure 2 showed that low-level expression of cytochrome bd takes place in the wild-type background but not in the ΔresDE background. Assuming the level of cytochrome bd production in the ctaA background is similar to that in the wild-type background, this difference may contribute to the difference in the growth phenotypes between the resD and ctaA strains. A role for ResD in control of other possible terminal oxidases remains untested, such as that with the ythA ythB genes (53).
Data presented here lead to the question of why ResD and the terminal oxidases are required for 80% of the wild-type Pho response. One possible role for ResD is in activation of transcription of the phoPR operon. However, previous work (Y. Chen and F. M. Hulett, unpublished data) showed that ResD does not footprint on the phoPR promoter at protein concentrations 20-fold higher than needed (56) for ResD binding on the ctaA promoter, leading us to believe that the role of ResD is not in transcriptional activation of phoPR. Analysis of an abrB resDE ydiH triple mutant that maintained the restored resDE ydiH Pho phenotype (Fig. 5A) ruled out the possibility that the bypass of the ResD role in Pho induction by terminal oxidase expression was via the upstream parallel AbrB pathway. Because constitutive bd expression does not induce the Pho response in a phoR mutant or in elevated Pi medium, the terminal oxidase ResD bypass for Pho induction retained dependency on PhoR and the requirement for a low-phosphate signal. The latter result indicates that the role of the terminal oxidases does not control the on-off signal received by PhoR. Rather, our data suggest that the role is likely modulation of the signal. Firstly, a ΔresDE strain showed 20% of the wild-type Pho induction upon phosphate depletion, indicating PhoR received the Pi deficiency signal but was incapable of full Pho induction. Secondly, constitutive expression of cydABCD encoding bd oxidase failed to induce Pho regulon genes during phosphate-sufficient growth, but upon Pi depletion supported full Pho induction similar to a resDE YdiH strain (Fig. 4A).
Postexponential expression of the cydA operon in the resDE ydiH mutant or wild-type strain has been observed in all media examined in which expression occurs (LB containing 0.5% glucose [41] and NSMPG [54; Schau et al., unpublished]) and LPDM (Fig. 1B), suggesting that there is regulation on the cydA promoter in addition to the YdiH repression, since the cydA promoter identified in a wild-type strain grown in NSMPG (54) was a putative σA promoter. A candidate regulator for the late induction of a σA promoter is a transition state regulator (48) that holds cydA silent until the transition from exponential to stationary growth, as has been shown or suggested for the bc complex (55) and caa3 (53). Because LPDM was designed so that cells would enter stationary growth due to phosphate deficiency and cydA was expressed postexponentially in medium containing high levels of phosphate, it is reasonable that cydA would also induce postexponentially in LPDM, not in response to phosphate starvation but rather the same postexponential regulation exhibited in other media. Further work is required to determine the regulator(s) responsible for cydA postexponential expression.
That terminal oxidases are important for the level of Pho induction in B. subtilis provides an explanation for previous work which showed that a strain bearing a mutation in nhaC, which encodes a Na+/H+ antiporter, hyperinduces Pho regulon genes during phosphate deprivation (36). Other reports (24, 39, 42) had shown that K+/H+ and Na+/H+ antiporters, as well as protonophores, inhibit respiration in B. subtilis. If the Na+/H+ antiporter inhibits respiration, which results in reduction of terminal oxidase activity, which in turn inhibits Pho induction, then deletion of a gene responsible for the production of an Na+/H+ antiporter should improve respiration, leading to increased activity of the terminal oxidases causing hyperinduction of Pho induction, as was reported in the nhaC deletion strain.
Our data suggest that reduced quinones inhibit PhoR autophosphorylation. The mechanism of inhibition is currently under examination. Possible mechanisms include conformational changes in PhoR upon quinone binding or reduction of four potentially redox-sensitive cysteine residues in PhoR. Oxidized quinones have been shown to inhibit autophosphorylation in three systems that are involved in anaerobic energy production: ArcB (E. coli) (13), BvgS (B. pertussis), and EvgS (E. coli) (5). In contrast, PhoR is inhibited by reduced quinones and is involved in a positive feedback loop with the two-component regulators of aerobic respiration, ResD and ResE, under growth-limiting phosphate conditions (49). In the ArcB system it was recently reported (29) that the kinase inhibition involves two cytosol-located redox-active cysteine residues that participate in intermolecular disulfide bond formation, with the oxidative power for the reaction dependent on the redox state of the quinone pool. Because accumulation of reduced quinones, either by deletion of the E. coli terminal oxidases (21) or addition of DTT to the growth medium, led to activation of ArcA∼P-activated reporter genes during aerobic growth, the redox state of quinones is believed to control the ArcB signal. In contrast, our current understanding of the B. subtilis Pho system suggests quinones control modulation of the phosphate deficiency signal. Our working hypothesis envisions that aberrant expression of cytochrome bd in the ΔresDE ydiH strain restores terminal oxidase function to a level that allows PhoR to autophosphorylate at wild-type levels and that the level of Pho induction ultimately depends on the level of PhoR autophosphorylation (Fig. 6).
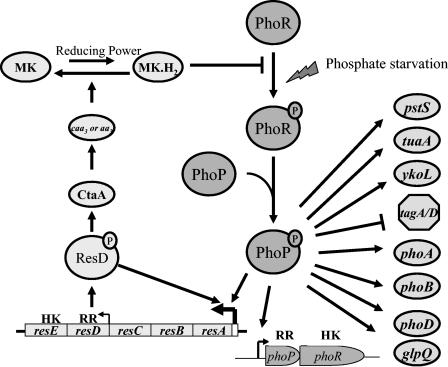
Figure 7 illustrates our present understanding of the signal transduction loop between the PhoP/R system and the ResD/E system. PhoR autophosphorylates in response to a phosphate deficiency signal when phosphate levels in the medium decrease below 0.1 mM Pi. PhoP is phosphorylated in a PhoR-dependent manner, and low-level induction of PhoP regulon genes results. The phoPR operon is autoinduced, and the low levels of PhoP∼P plus ResD (from the weak internal resDE promoter) are sufficient to activate the stronger resA promoter, thereby increasing cellular concentrations of ResD and ResE. ResD∼P activates transcription of ctaA and ctaB, which are required for heme A biosynthesis, and thus a-type terminal oxidases aa3 and caa3. The terminal oxidases oxidize the reduced quinones to relieve inhibition of PhoR autophosphorylation to promote full Pho induction, completing the positive feedback loop between the Pho and Res systems under phosphate-limiting conditions.
FIG. 7.
Model depicting the present understanding of the feedback amplification loop between the ResD-ResE two-component signal transduction system and the PhoP-PhoR two-component system. Solid lines indicate that direct interaction has been demonstrated. Positive regulation is labeled with an arrow, while repression is labeled with a ⊥.
Acknowledgments
This work was supported by National Institutes of Health grant GM-33471 to F.M.H.
REFERENCES
- 1.Antelmann, H., C. Scharf, and M. Hecker. 2000. Phosphate starvation-inducible proteins of Bacillus subtilis: proteomics and transcriptional analysis. J. Bacteriol. 182:4478-4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bengtsson, J., H. Tjalsma, C. Rivolta, and L. Hederstedt. 1999. Subunit II of Bacillus subtilis cytochrome c oxidase is a lipoprotein. J. Bacteriol. 181:685-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birkey, S. M., W. Liu, X. Zhang, M. F. Duggan, and F. M. Hulett. 1998. Pho signal transduction network reveals direct transcriptional regulation of one two-component system by another two-component regulator: Bacillus subtilis PhoP directly regulates production of ResD. Mol. Microbiol. 30:943-953. [DOI] [PubMed] [Google Scholar]
- 4.Bisschop, A., and W. N. Konings. 1976. Reconstitution of reduced nicotinamide adenine dinucleotide oxidase activity with menadione in membrane vesicles from the menaquinone-deficient Bacillus subtilis aroD. Relation between electron transfer and active transport. Eur. J. Biochem. 67:357-365. [DOI] [PubMed] [Google Scholar]
- 5.Bock, A., and R. Gross. 2002. The unorthodox histidine kinases BvgS and EvgS are responsive to the oxidation status of a quinone electron carrier. Eur. J. Biochem. 269:3479-3484. [DOI] [PubMed] [Google Scholar]
- 6.Burbulys, D., K. A. Trach, and J. A. Hoch. 1991. Initiation of sporulation in B. subtilis is controlled by a multicomponent phosphorelay. Cell 64:545-552. [DOI] [PubMed] [Google Scholar]
- 7.Chesnut, R. S., C. Bookstein, and F. M. Hulett. 1991. Separate promoters direct expression of phoAIII, a member of the Bacillus subtilis alkaline phosphatase multigene family, during phosphate starvation and sporulation. Mol. Microbiol. 5:2181-2190. [DOI] [PubMed] [Google Scholar]
- 8.Collins, M. D., and D. Jones. 1981. Distribution of isoprenoid quinone structural types in bacteria and their taxonomic implications. Microbiol. Rev. 45:316-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cutting, S. M., and P. B. Vander Horn. 1990. Genetic analysis, p. 24-74. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley & Sons, Inc., New York, N.Y.
- 10.de Vrij, W., B. van den Burg, and W. N. Konings. 1987. Spectral and potentiometric analysis of cytochromes from Bacillus subtilis. Eur. J. Biochem. 166:589-595. [DOI] [PubMed] [Google Scholar]
- 11.Eder, S., L. Shi, K. Jensen, K. Yamane, and F. M. Hulett. 1996. A Bacillus subtilis secreted phosphodiesterase/alkaline phosphatase is the product of a Pho regulon gene, phoD. Microbiology 142:2041-2047. [DOI] [PubMed] [Google Scholar]
- 12.Ferrari, E., S. M. Howard, and J. A. Hoch. 1986. Effect of stage 0 sporulation mutations on subtilisin expression. J. Bacteriol. 166:173-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Georgellis, D., O. Kwon, and E. C. Lin. 2001. Quinones as the redox signal for the Arc two-component system of bacteria. Science 292:2314-2316. [DOI] [PubMed] [Google Scholar]
- 14.Guerout-Fleury, A. M., K. Shazand, N. Frandsen, and P. Stragier. 1995. Antibiotic-resistance cassettes for Bacillus subtilis. Gene 167:335-336. [DOI] [PubMed] [Google Scholar]
- 15.Hanahan, D. 1985. Techniques for transformation of E. coli, p. 109-135. In D. M. Glover (ed.), DNA cloning II: a practical approach. IRL Press, Washington, D.C.
- 16.Henner, D. J. 1990. Inducible expression of regulatory genes in Bacillus subtilis. Methods Enzymol. 185:223-228. [DOI] [PubMed] [Google Scholar]
- 17.Homuth, G., A. Rompf, W. Schumann, and D. Jahn. 1999. Transcriptional control of Bacillus subtilis hemN and hemZ. J. Bacteriol. 181:5922-5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hulett, F. M., C. Bookstein, and K. Jensen. 1990. Evidence for two structural genes for alkaline phosphatase in Bacillus subtilis. J. Bacteriol. 172:735-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hulett, F. M., E. E. Kim, C. Bookstein, N. V. Kapp, C. W. Edwards, and H. W. Wyckoff. 1991. Bacillus subtilis alkaline phosphatases III and IV. Cloning, sequencing, and comparisons of deduced amino acid sequence with Escherichia coli alkaline phosphatase three-dimensional structure. J. Biol. Chem. 266:1077-1084. [PubMed] [Google Scholar]
- 20.Hulett, F. M., J. Lee, L. Shi, G. Sun, R. Chesnut, E. Sharkova, M. F. Duggan, and N. Kapp. 1994. Sequential action of two-component genetic switches regulates the Pho regulon in Bacillus subtilis. J. Bacteriol. 176:1348-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iuchi, S., V. Chepuri, H. A. Fu, R. B. Gennis, and E. C. Lin. 1990. Requirement for terminal cytochromes in generation of the aerobic signal for the arc regulatory system in Escherichia coli: study utilizing deletions and lac fusions of cyo and cyd. J. Bacteriol. 172:6020-6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 23.Lee, J. W., and F. M. Hulett. 1992. Nucleotide sequence of the phoP gene encoding PhoP, the response regulator of the phosphate regulon of Bacillus subtilis. Nucleic Acids Res. 20:5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lemma, E., G. Unden, and A. Kroger. 1990. Menaquinone is an obligatory component of the chain catalyzing succinate respiration in Bacillus subtilis. Arch. Microbiol. 155:62-67. [DOI] [PubMed] [Google Scholar]
- 25.Liu, W. 1997. Biochemical and genetic analyses establish a dual role for PhoP in Bacillus subtilis Pho regulation. Ph.D. dissertation. University of Illinois at Chicago, Chicago.
- 26.Liu, W., S. Eder, and F. M. Hulett. 1998. Analysis of Bacillus subtilis tagAB and tagDEF expression during phosphate starvation identifies a repressor role for PhoP-P. J. Bacteriol. 180:753-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu, W., and F. M. Hulett. 1998. Comparison of PhoP binding to the tuaA promoter with PhoP binding to other Pho-regulon promoters establishes a Bacillus subtilis Pho core binding site. Microbiology 144:1443-1450. [DOI] [PubMed] [Google Scholar]
- 28.Liu, X., and H. W. Taber. 1998. Catabolite regulation of the Bacillus subtilis ctaBCDEF gene cluster. J. Bacteriol. 180:6154-6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malpica, R., B. Franco, C. Rodriguez, O. Kwon, and D. Georgellis. 2004. Identification of a quinone-sensitive redox switch in the ArcB sensor kinase. Proc. Natl. Acad. Sci. USA 101:13318-13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mueller, J. P., and H. W. Taber. 1989. Isolation and sequence of ctaA, a gene required for cytochrome aa3 biosynthesis and sporulation in Bacillus subtilis. J. Bacteriol. 171:4967-4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakano, M. M., G. Zheng, and P. Zuber. 2000. Dual control of sbo-alb operon expression by the Spo0 and ResDE systems of signal transduction under anaerobic conditions in Bacillus subtilis. J. Bacteriol. 182:3274-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakano, M. M., Y. Zhu, M. Lacelle, X. Zhang, and F. M. Hulett. 2000. Interaction of ResD with regulatory regions of anaerobically induced genes in Bacillus subtilis. Mol. Microbiol. 37:1198-1207. [DOI] [PubMed] [Google Scholar]
- 33.Ozanne, P. G. 1980. Phosphate nutrition of plants—a general treatise, p. 559-585. In E. Khasswenh (ed.), The role of phosphorus in agriculture. American Society of Agronomy, Madison, Wis.
- 34.Paul, S., S. Birkey, W. Liu, and F. M. Hulett. 2004. Autoinduction of Bacillus subtilis phoPR operon transcription results from enhanced transcription from EσA- and EσE-responsive promoters by phosphorylated PhoP. J. Bacteriol. 186:4262-4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paul, S., X. Zhang, and F. M. Hulett. 2001. Two ResD-controlled promoters regulate ctaA expression in Bacillus subtilis. J. Bacteriol. 183:3237-3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pragai, Z., C. Eschevins, S. Bron, and C. R. Harwood. 2001. Bacillus subtilis NhaC, an Na+/H+ antiporter, influences expression of the phoPR operon and production of alkaline phosphatases. J. Bacteriol. 183:2505-2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qi, Y., Y. Kobayashi, and F. M. Hulett. 1997. The pst operon of Bacillus subtilis has a phosphate-regulated promoter and is involved in phosphate transport but not in regulation of the Pho regulon. J. Bacteriol. 179:2534-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robichon, D., M. Arnaud, R. Gardan, Z. Pragai, M. O'Reilly, G. Rapoport, and M. Debarbouille. 2000. Expression of a new operon from Bacillus subtilis, ykzB-ykoL, under the control of the TnrA and PhoP-PhoR global regulators. J. Bacteriol. 182:1226-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samuilov, V. D., and S. A. Khakimov. 1991. Dependence of Bacillus subtilis cell respiration on monovalent cations. Biokhimiia 56:1209-1214. [PubMed] [Google Scholar]
- 40.Santana, M., F. Kunst, M. F. Hullo, G. Rapoport, A. Danchin, and P. Glaser. 1992. Molecular cloning, sequencing, and physiological characterization of the qox operon from Bacillus subtilis encoding the aa3-600 quinol oxidase. J. Biol. Chem. 267:10225-10231. [PubMed] [Google Scholar]
- 41.Schau, M., Y. Chen, and F. M. Hulett. 2004. Bacillus subtilis YdiH is a direct negative regulator of the cydABCD operon. J. Bacteriol. 186:4585-4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schirawski, J., and G. Unden. 1998. Menaquinone-dependent succinate dehydrogenase of bacteria catalyzes reversed electron transport driven by the proton potential. Eur. J. Biochem. 257:210-215. [DOI] [PubMed] [Google Scholar]
- 43.Seki, T., H. Yoshikawa, H. Takahashi, and H. Saito. 1987. Cloning and nucleotide sequence of phoP, the regulatory gene for alkaline phosphatase and phosphodiesterase in Bacillus subtilis. J. Bacteriol. 169:2913-2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi, L., and F. M. Hulett. 1999. The cytoplasmic kinase domain of PhoR is sufficient for the low phosphate-inducible expression of Pho regulon genes in Bacillus subtilis. Mol. Microbiol. 31:211-222. [DOI] [PubMed] [Google Scholar]
- 45.Shimotsu, H., and D. J. Henner. 1986. Construction of a single-copy integration vector and its use in analysis of regulation of the trp operon of Bacillus subtilis. Gene 43:85-94. [DOI] [PubMed] [Google Scholar]
- 46.Soldo, B., V. Lazarevic, M. Pagni, and D. Karamata. 1999. Teichuronic acid operon of Bacillus subtilis 168. Mol. Microbiol. 31:795-805. [DOI] [PubMed] [Google Scholar]
- 47.Steinmetz, M., and R. Richter. 1994. Plasmids designed to alter the antibiotic resistance expressed by insertion mutations in Bacillus subtilis, through in vivo recombination. Gene 142:79-83. [DOI] [PubMed] [Google Scholar]
- 48.Strauch, M. A., and J. A. Hoch. 1993. Transition-state regulators: sentinels of Bacillus subtilis post-exponential gene expression. Mol. Microbiol. 7:337-342. [DOI] [PubMed] [Google Scholar]
- 49.Sun, G., S. M. Birkey, and F. M. Hulett. 1996. Three two-component signal-transduction systems interact for Pho regulation in Bacillus subtilis. Mol. Microbiol. 19:941-948. [DOI] [PubMed] [Google Scholar]
- 50.Sun, G., E. Sharkova, R. Chesnut, S. Birkey, M. F. Duggan, A. Sorokin, P. Pujic, S. D. Ehrlich, and F. M. Hulett. 1996. Regulators of aerobic and anaerobic respiration in Bacillus subtilis. J. Bacteriol. 178:1374-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Svensson, B., and L. Hederstedt. 1994. Bacillus subtilis CtaA is a heme-containing membrane protein involved in heme A biosynthesis. J. Bacteriol. 176:6663-6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Svensson, B., M. Lubben, and L. Hederstedt. 1993. Bacillus subtilis CtaA and CtaB function in haem A biosynthesis. Mol. Microbiol. 10:193-201. [DOI] [PubMed] [Google Scholar]
- 53.Winstedt, L., and C. von Wachenfeldt. 2000. Terminal oxidases of Bacillus subtilis strain 168: one quinol oxidase, cytochrome aa3 or cytochrome bd, is required for aerobic growth. J. Bacteriol. 182:6557-6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Winstedt, L., K. Yoshida, Y. Fujita, and C. von Wachenfeldt. 1998. Cytochrome bd biosynthesis in Bacillus subtilis: characterization of the cydABCD operon. J. Bacteriol. 180:6571-6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu, J., L. Hederstedt, and P. Piggot. 1995. The cytochrome bc complex (menaquinone:cytochrome c reductase) in Bacillus subtilis has a nontraditional subunit organization. J. Bacteriol. 177:6751-6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang, X., and F. M. Hulett. 2000. ResD signal transduction regulator of aerobic respiration in Bacillus subtilis: ctaA promoter regulation. Mol. Microbiol. 37:1208-1219. [DOI] [PubMed] [Google Scholar]