Abstract
We report identification of the Escherichia coli ybhE gene as the pgl gene that encodes 6-phosphogluconolactonase. A tentative identification was first made based on the known approximate location of the pgl gene and the similarity of the presumptive ybhE-encoded protein sequence to a known Pgl enzyme. To test this notion, the ybhE gene was deleted and replaced with a drug marker. Like previously characterized pgl mutants, the ybhE deletion mutant had a Blu− phenotype (dark-blue staining with iodine due to accumulation of starch after growth on minimal maltose) and demonstrated impaired growth on minimal glucose medium when combined with a pgi mutation. Biochemical assay of crude extracts for 6-phosphogluconolactonase enzymatic activity showed that ybhE encodes this activity. The ybhE gene was transferred from the E. coli chromosome to an expression vector. This ybhE clone complemented both the precise deletion of the ybhE gene and a larger deletion, pglΔ8, for the Blu− phenotype and for phosphogluconolactonase activity, confirming that ybhE is the pgl gene. A newly observed phenotype of pgl strains is a lowered frequency of appearance of Bgl+ mutants that can utilize the β-glucoside salicin. This is likely due to poor growth of Bgl+ pgl strains on salicin due to the accumulation of 6-phosphogluconolactone.
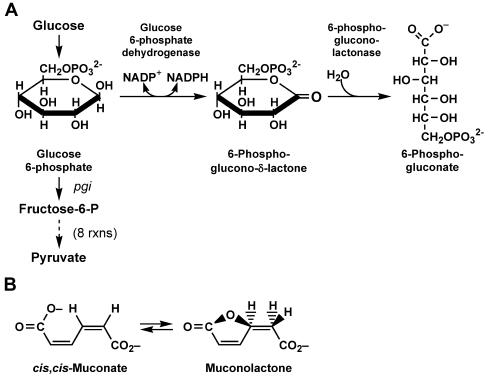
In Escherichia coli, the pentose phosphate (pentose-P) pathway is used to create ribose molecules for biosynthesis, to increase reducing power in the cell, and to metabolize some sugars. It can also be used to metabolize glucose if the primary glycolytic pathway, the Embden-Meyerhof pathway, is blocked, e.g., by mutation of the phosphoglucoisomerase (pgi) gene (for a review, see reference 10). A key enzyme of the pentose-P pathway is the product of the pgl gene, 6-phosphogluconolactonase (6-P-gluconolactonase, or Pgl), which catalyzes the hydrolysis of 6-phosphogluconolactone (6-P-gluconolactone, the product of glucose 6-phosphate dehydrogenase [G6PD], encoded by zwf) to 6-phospho-gluconate (6-P-gluconate) (Fig. 1A). Though this reaction can proceed spontaneously in vitro, cells carrying both pgi and pgl mutations grow very poorly on glucose as a sole carbon source, indicating a requirement for pgl in vivo. Although Pgl enzymes are predicted to be present in many organisms, a pgl gene has not been identified in most microbial genomes (7). In both mice and paramecia, G6PD and Pgl activities are contained in a single bifunctional protein (5, 6).
FIG. 1.
(A) Early steps of glycolysis and the pentose-P pathway, detailing reactions catalyzed by the G6PD and Pgl enzymes. G6PD and Pgl are encoded by the zwf and pgl genes, respectively. (B) The predicted activity of the YbhE/Pgl enzyme based on its annotation as a 3-carboxy-cis,cis-muconate lactonizing enzyme.
In E. coli, pgl mutations have been localized by conjugation experiments and deletion analysis to the region between attλ and modC (previously known as chlD), at approximately 17 min on the E. coli genetic map (9, 15). For reasons that are not entirely clear, pgl mutants accumulate starch when grown on maltose and are stained dark blue by iodine (1, 15). The maltose blue or Blu− phenotype (1) has been used as an indicator of the pgl phenotype.
In this paper, we report the identification of the E. coli ybhE gene as pgl. This identification is confirmed by the Blu− phenotype and loss of 6-P-gluconolactonase activity in crude extracts of ybhE mutants. Interestingly, the predicted Pgl/YbhE protein has been categorized as a 3-carboxy-cis,cis-muconate lactonizing enzyme (14), members of which catalyze the reversible formation of a lactone from 3-carboxy-cis,cis-muconates (Fig. 1B). Unexpectedly, we observed that Pgl mutants are deficient in growth on the β-glucoside salicin, perhaps because of the accumulation of 6-P-gluconolactone.
MATERIALS AND METHODS
Bacteria and plasmids.
Bacterial strains and plasmids are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Source or reference | Relevant genotype | Background |
|---|---|---|---|
| DY378 | Court laboratory | (λcI857Δcro-bio) | W3110 |
| MG1655 | B. Bochner | Wild type | E. coli K-12 |
| M1924 | This study | ybhE<>tet (λcI857Δcro-bio) | MG1655 |
| M1925-29 | This study | Spontaneous Bgl+ | MG1655 |
| M1951-55 | This study | ybhE<>tet transductants | M1925-29 |
| M1971 | This study | ybhE<>tet | MG1655 |
| M2523 | This study | ybhE<>amp | MG1655 |
| M2524 | This study | ybhE<>tet (λcI857Δcro-bio) [pJL6], Ampr | M1924 |
| M2526 | This study | ybhE<>tet (λcI857Δcro-bio) [pJL6-ybhE/pgl], Ampr | M1924 |
| M2527 | This study | cI857 allele at lac [pJL6] Ampr | M2537 |
| M2528 | This study | cI857 allele at lac [pJL6-ybhE/pgl] Ampr | M2537 |
| M2537 | This study | cI857 allele at lac | MG1655 |
| M2531 | This study | ybhE<>amp pgi::Tn10 | MG1655 |
| M2532 | This study | ybhE<>amp pgi::Tn10 zwf<>kan | MG1655 |
| M2534 | This study | zwf<>kan pgi::Tn10 | MG1655 |
| M2536 | This study | ybhE<>amp zwf<>kan | MG1655 |
| M2537 | Court laboratory | cI857 allele at lac | MG1655 |
| pJL6 | 20 | pL expression vector under cI857 control; Ampr | |
| pJL6-ybhE/pgl | This study | pJL6 expressing ybhE/pgl, Ampr |
Primers.
Sequences of primers used to make and confirm mutations are available upon request.
Construction of a ybhE deletion, ybhE<>tet and other mutant strains.
A “precise” deletion of the ybhE open reading frame was constructed with recombineering (in vivo genetic engineering) technology (22). Briefly, hybrid primers with approximately 50 bases of 5′ homology to DNA sequences flanking the ybhE gene were used with the PCR to amplify a drug cassette encoding tetracycline resistance. This PCR product was transformed into electrocompetent DY378 cells induced for the phage λ Red recombination system. Samples of the electroporation mixture were plated on Luria-Bertani plates containing 12.5 μg of tetracycline/ml. Drug-resistant colonies were purified, and the novel junctions between the drug marker and the ybhE-flanking DNA were confirmed by a secondary PCR analysis that could also confirm that the wild-type copy of ybhE was absent (see reference 23 for details). The cojoined symbols (<>) indicate the removal of a gene and its replacement with a drug marker by recombineering. The ybhE<>tet and other mutations generated by recombineering were introduced into MG1655 by P1 transduction. To ensure that the tightly linked defective λ prophage used for recombineering was not cotransduced, the transductants were plated at 42°C to select against the temperature-sensitive λ lysogens. Loss of λ immunity and the ability to plate the T4rII phage, both being indicators of the absence of the prophage, were confirmed. A ybhE<>amp construct was also generated from the ybhE<>tet cassette for some experiments. A similar recombineering strategy was used to delete the zwf gene and replace it with a chloramphenicol (cam) or kanamycin (kan) cassette and to replace the yieK gene with a cam cassette. All of the deletions made by recombineering removed the entire structural gene.
Assay of the Blu phenotype.
Bacteria were streaked on minimal maltose agar petri plates and incubated at 32 or 37°C until colonies appeared. The plates were then inverted over plastic 100- by 15-mm petri dishes containing solid I2 crystals for 2 min, allowing iodine vapor to stain the colonies. Mutant pgl colonies stain dark blue-black, whereas wild-type colonies are not stained (1).
Isolation of Bgl+ mutants.
Strain MG1655 (like other K-12 strains) cannot use salicin as a C source and is designated Bglo (cryptic). When spread on MacConkey plates containing 1% salicin (Mac-SLC) and incubated at 30°C, MG1655 forms pale colonies but red papillae eventually appear, due to spontaneous mutations to a Bgl+ phenotype. Papillae were picked and purified on Mac-SLC plates. The frequency of Bgl+ mutations was estimated from Mac-SLC plates spread with ∼50 to 500 cells and incubated at 30°C. After 2 to 4 days, the colonies were examined under a binocular microscope and the number of salicin-fermenting papillae per colony was recorded. The Poisson distribution was used to estimate the average number of papillae per colony (8).
Retrieval of the ybhE gene in a gap repair reaction using recombineering.
The expression vector pJL6 is an ampicillin-resistant pBR322 derivative containing the phage λ pL promoter for use in gene expression (20). A portion of the plasmid containing the origin of replication, the bla gene, and the pL promoter was amplified by PCR with primers with flanking homology to the ybhE/pgl gene, such that recombination of the gene onto the plasmid would result in its expression from the pL promoter (17). The PCR-derived linear vector was used in a recombineering reaction in strain DY378, and colonies resistant to 100 μg of ampicillin/ml were selected. When the prophage in DY378 was not induced for recombineering, no Ampr colonies appeared, because the linear DNA cannot circularize. Induction of the recombination system generated approximately 160 Ampr colonies from one electroporation mixture. Sixteen drug-resistant colonies were purified and plasmid DNA was isolated; 14 of 16 colonies contained the correct insert as defined by restriction analysis. The plasmid from one correct clone was designated pJL6-ybhE/pgl.
6-P-gluconolactonase activity assay.
The assay of Kupor and Fraenkel (15) was used. The substrate, 6-P-gluconolactone, is unstable and thus not commercially available but was made by lyophilization of 6-P-gluconic acid (Sigma P-7627) with a predicted yield of approximately 25% (15). Prior to lyophilization, 200 mg of 6-P-gluconic acid was dissolved in 100 ml of distilled water, equilibrated with 5 g of AG 50W-X8 Resin Hydrogen form (Bio-Rad) for 1 h at room temperature, and filtered to remove the resin. Bacteria to be assayed were grown overnight at 37°C in 5 ml of Luria-Bertani broth with aeration (30°C for temperature-sensitive strains). Cells were collected by centrifugation, washed once with 0.85% NaCl, and resuspended in 2 ml of 0.1 M potassium phosphate buffer (pH 7.0) containing 1 mM dithiothreitol. Cells were broken by sonication, the debris was pelleted by centrifugation, and the supernatant (∼2.5 mg/ml protein) was assayed for Pgl activity. The lactone (2.0 μmol in ∼40 μl of lyophilized product resuspended in 5 ml of H2O) was added to 1.25 mg of protein extract in 1 ml of 0.1 M K-phosphate buffer (pH 7.0) and incubated at 30°C. At 0, 15, and 30 min, 0.2-ml portions were transferred to microcentrifuge tubes containing 0.2 ml of 3.5 M NaOH and 0.2 ml of 3 M NH2OH. After 2 min, 0.2 ml of 4 M HCl was added, the tubes were mixed and centrifuged to remove precipitated protein, and 0.2 ml of 0.37 M FeCl3 in 0.1 M HCl was added to each supernatant fraction. The absorbance of the ferric hydroxamate was read at 540 nm.
RESULTS
Tentative identification of ybhE as pgl.
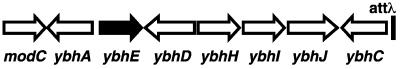
The pgl gene was previously mapped between modC (formerly chlD) and the bacteriophage λ attachment site (attλ); mutation of pgl gives a Blu− phenotype (9, 15). According to the annotated E. coli K-12 sequence (4), there are seven genes between modC and attλ (Fig. 2). The predicted protein sequences of these seven genes were subjected to BLAST analysis (2, 3). The only sequence in this region that showed significant homology to known Pgl enzymes was the product of the ybhE gene, annotated as a putative isomerase with a predicted length of 331 amino acids. A BLAST comparison of the YbhE protein sequence against the entire nonredundant database indicated homologs in a number of other bacteria and produced one alignment to a putative 6-P-gluconolactonase from Bacillus cereus (12). The alignment shows 27% identical residues and 43% conserved residues over a region of 294 amino acids. The BLAST analysis also identified a conserved domain, COG2706, defining a 3-carboxymuconate cyclase enzyme. The proposed activity of this enzyme is conversion of cis,cis muconates to muconolactones (14) (Fig. 1B). This activity is not that expected for the Pgl reaction but the similarity encouraged us to analyze the ybhE gene.
FIG. 2.
Genetic map of the E. coli chromosome in the region between modC and attλ. The genes and putative gene products listed in the annotated E. coli genome sequence are as follows: ybhA, phosphatase; ybhE (shown here to be pgl), isomerase; ybhD, LysR-type transcriptional regulator; ybhH, unknown; ybhI, membrane pump protein; ybhJ, unknown; and ybhC, pectinesterase.
Another E. coli protein gave repeated hits when the E. coli K-12 database was searched with some known protein sequences annotated as 6-P-gluconolactonase. This was the product of the yieK gene, also a putative isomerase. The YieK sequence, used in a BLAST search against the entire nonredundant database, gave a conserved domain hit to NagB, a glucosamine-6-phosphate isomerase/deaminase, and highly significant alignments to several enzymes indicated to be both 6-P-gluconolactonase and glucosamine-6-phosphate isomerase/deaminase, such as those from Thermoanaerobacter tengcongensis and Vibrio vulnificus CMCP6.
Deletion of ybhE and other genes by recombineering.
Phage λ Red recombination (22) was used to remove the ybhE open reading frame and replace it with a tetracycline resistance cassette. This recombineering was also used to delete the yieK and the zwf genes and replace them with drug cassettes. The zwf gene encodes glucose-6-phosphate dehydrogenase, which acts prior to 6-P-gluconolactonase to create the 6-P-gluconolactone hydrolyzed by Pgl (14) (Fig. 1A). All mutations generated by recombineering were moved into MG1655 by P1 transduction; the absence of the λ prophage from the final constructs was confirmed.
Blu− phenotype of the ybhE mutant strain.
Bacteria deleted for ybhE were incubated on minimal maltose agar plates and tested for the Blu phenotype. The ybhE deletion strains stained dark blue and thus were Blu−, a phenotype diagnostic of pgl mutants. In contrast, wild-type MG1655 colonies turned slightly yellow when subjected to the same regimen. The presence or absence of the closely linked λ prophage did not affect the Blu phenotype. Strains defective for both the ybhE and zwf genes turned brown but not blue-black; this indicates that zwf is required for the Blu− phenotype. Mutants that lacked only the yieK or zwf genes had a Blu+ phenotype.
Growth of ybhE mutants on minimal glucose plates.
We assayed growth of the ybhE mutant on minimal glucose agar at 37°C, alone and in combination with mutations in zwf and/or in pgi, which encodes phosphoglucose isomerase. Since Pgi is required in the primary trunk of the Embden Meyerhoff glycolysis pathway just beyond the pentose-P branch pathway, mutants blocked in both the pentose-P and Embden-Myerhoff pathways should not grow on glucose as a sole carbon source; the growth of such mutants is actually inhibited due to accumulated high levels of glucose-6-P (13). We also assayed a pgi zwf double mutant. Whereas the ybhE and zwf single mutants grew well on minimal glucose, the pgi single mutant grew more slowly and never achieved the same large colony size. The pgi zwf double mutant was largely unable to grow using glucose as a sole carbon source, with only a faint suggestion of colony formation, whereas the pgi ybhE double mutant grew slowly, forming very small colonies after several days. The ybhE zwf pgi triple mutant did not grow on minimal glucose, forming only microcolonies after several days. Our observations confirm those of Kupor and Fraenkel (15, 16) and suggest that elimination of only the ybhE/pgl gene by mutation is not sufficient to eliminate carbon flow through the pentose-P pathway. The growth of strains with both pgi and zwf mutations may also be inhibited by glucose-6-P accumulation.
Retrieval of the ybhE gene onto an expression vector.
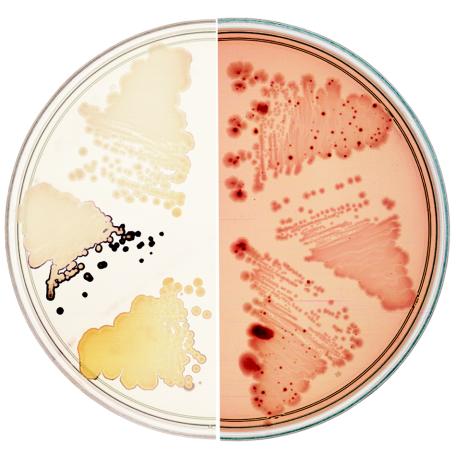
The ybhE gene was directly cloned from the E. coli chromosome to the expression vector, pJL6 (20), under the control of the phage λ pL promoter using λ Red to catalyze the recombination (17). The ybhE+ clone and the vector lacking an insert were introduced by electroporation into M1924 and M2537. These strains carry a chromosomal copy of the phage λ cI857 encoding a temperature-sensitive repressor in order to repress the pL promoter. Purified transformants were grown on minimal maltose plates at 30°C, and the Blu phenotype was tested. The pL promoter on the pJL6 plasmid is somewhat derepressed even at 30°C (20), and the pJL6 plasmid with the ybhE insert (pJL6-ybhE/pgl) was found to complement the Blu− phenotype at this temperature (Fig. 3). The ybhE clone also complemented the other Pgl phenotypes we monitored: 6-P-gluconolactonase activity and Bgl+ papillation (see below).
FIG. 3.
Two phenotypes of E. coli pgl mutants. (Left) pgl mutants accumulate starch and stain blue-black with iodine vapors when grown on minimal maltose plates, not seen for wild-type strains. (Right) Wild-type strains cannot utilize salicin as a C source but give rise to Bgl+ mutants that appear as dark red papillae on MacConkey-salicin plates, not seen in pgl mutants. Streaks of three different strains are shown on each plate. From the top and proceeding clockwise: wt (MG1655), a pgl mutant containing the control plasmid pJL6 (M2524), the pgl mutant containing the pgl+ plasmid pJL6-ybhE/pgl (M2526), MG1655, M2524, and M2526.
Assay for 6-P-gluconolactonase activity.
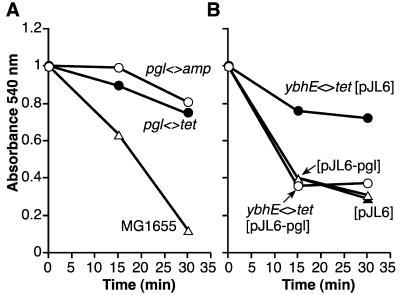
Extracts of overnight cultures of ybhE mutants and wild-type cells were assayed for 6-P-gluconolactonase activity (Fig. 4A) as described in Materials and Methods. The assay is not entirely quantitative as the initial concentration of the substrate, 6-P-gluconolactone, is unknown. However, the assay demonstrates that the absorbance (monitored at 540 nm) of the ferric hydroxamate compound formed by conjugation with the lactone ester decreased substantially over the time monitored for ybhE+ strains, but displayed only a slight decrease over the same time period for ybhE mutant strains. Extracts of the pJL6-ybhE/pgl plasmid-containing strain expressing YbhE/Pgl function complemented the ybhE<>tet strain in the 6-P-gluconolactonase activity assay (Fig. 4B).
FIG. 4.
6-P-gluconolactonase assays of ybhE/pgl mutant and wild type E. coli, and of bacteria containing the YbhE/Pgl expression vector and control plasmid. (A) ▵, Assay of MG1655 containing an intact ybhE/pgl gene; ○ and •, M2523 (ybhE<>amp) and M1971(ybhE<>tet), respectively. (B) •, M2524 (ybhE<>tet [pJL6]); ○, M2526 (ybhE<>tet with ybhE expression plasmid [pJL6-ybhE/pgl]); ▴, M2527 (cI857 [pJL6]); ▵, M2528 (cI857 [pJL6-ybhE/pgl]). Details of the assay are described in Materials and Methods.
Feeding of pgi zwf and pgi zwf pgl mutant strains by pgl mutants.
To minimize confusion, we will henceforth refer to the ybhE open reading frame as pgl and the YbhE enzyme as Pgl. The pentose-P pathway intermediate 6-P-gluconolactone accumulates in pgl mutants, especially when pgi-defective. Kupor and Fraenkel (16) have characterized a bypass reaction for metabolism of this compound in pgl mutants in which 6-P-gluconolactone is converted to gluconate 6-P. We tested whether bacteria which cannot grow on glucose due to mutations in both pgi and zwf or pgi zwf and pgl would be “fed” by pgl mutants when spread side by side on minimal glucose plates. Figure 5 shows a composite picture from three minimal glucose plates, illustrating that the triple-mutant strain pgl pgi zwf (on top in all cases) can be fed by a pgl pgi double mutant, less well by a pgl single mutant, and not at all by a pgl zwf double mutant. The pgi zwf double mutant is fed in a similar fashion by the pgl pgi double mutant and the pgl single mutant but not by the pgl zwf double mutant (Table 2). We interpret these results to mean that 6-P-gluconolactone (or its breakdown product) accumulates in a pgl mutant (and even more so when pgi is also defective) and that this compound can be used to feed the pgi zwf cells, in accordance with the model of Kupor and Fraenkel (16).
FIG. 5.
Detail of strains grown side by side on minimal glucose agar demonstrating feeding of M2532, the pgl<>amp pgi::Tn10 zwf<>kan triple mutant, by both M2523, the pgl<>amp single mutant (at lower left) and M2531, the double mutant pgl<>amp pgi::Tn10 (lower center). In all cases, the upper strain is the triple mutant and is being fed. M2536, the pgl<>amp zwf<>kan strain (lower right), cannot feed the triple mutant, presumably because 6-P-gluconolactone is not made in the absence of G6PD. The arrow indicates the edge of the streak of the triple mutant, where small colonies arise due to feeding. See Table 2 for interpretations.
TABLE 2.
Ability of pgl mutant strains to feed various glycolysis mutants on minimal glucose
| Feeder strain | Strain being fed | Effect and interpretation |
|---|---|---|
| pgl | pgi zwf | +; Confirms that the pgl mutant supplies either the 6-P-gluconolactone or other metabolite that can feed the pgi zwf strain |
| pgl pgi | pgi zwf | ++; Enhanced ability to feed the pgi zwf strain results from more carbon being forced through the pentose-P pathway of the feeder strain |
| pgl zwf | pgi zwf | −; Confirms that 6-P-gluconolactone is needed for feeding |
| pgl pgi | pgl pgi zwf | ++; Because this strain can feed the triple mutant, the 6-P-gluconolactone must be converted to some other compound, presumably gluconate-6-P, either enzymatically or by spontaneous hydrolysis |
Fewer Bgl+ mutants from pgl strains.
E. coli does not normally ferment β-glucosides such as salicin because of silencing by H-NS and transcription termination within the bgl operon (Bglo cryptic phenotype). However, spontaneous Bgl+ variants arise frequently, mostly due to transposition of IS1 or IS5 to the bgl region (19). Colonies of wild-type cells spread on MacConkey-salicin (Mac-SLC) plates are initially pale, since the salicin is not utilized. After 2 days at 30°C, however, red papillae of salicin-utilizing (Bgl+ phenotype) mutants are readily detected within many colonies. We noticed that very few Bgl+ mutants arose from the pgl<>tet mutant and these only after additional days of incubation at 30°C (Fig. 3). We considered two possibilities: either the pgl defect interferes with the mutational event itself or it interferes with the ability of the Bgl+ mutants to utilize salicin. To distinguish between these possibilities, five independent Bgl+ (M1925-M1929) mutants were isolated from wild-type MG1655 cells and then transduced to pgl with P1 grown on pgl<>tet donors (M1951-M1955). We reasoned that if the defect was in the production of the mutants, these Bgl+ pgl cells should now normally express the Bgl+ phenotype. We observed, however, that the Bgl+ pgl cells did not express the Bgl+ phenotype fully. When Bgl+ cells were spread on Mac-SLC plates at 30°C, the red color due to salicin-utilization was much fainter and the colony size was smaller for the pgl transductants than for the parental strains. Furthermore, when growing in log-phase on minimal medium with salicin as sole C source, the doubling time of Bgl+ pgl+ cells was 150 to 165 min, whereas that of the Bgl+ pgl<>tet transductants was 270 min. Similar results were obtained for the rare Bgl+ mutants isolated directly from pgl cells; when plated on either Mac-SLC plates or minimal-salicin plates, the growth on salicin was reduced. These results show that pgl cells grow poorly on salicin.
The normal substrate for Pgl, 6-P-gluconolactone, accumulates in pgl mutants. Others have indicated (21) that the lactone may inhibit Bgl+ activity. Therefore, we tested a strain that carried a mutation for both pgl and zwf. The zwf mutation prevents 6-P-gluconolactone production. If 6-P-gluconolactone accumulation is the basis for the poor expression of the bgl operon, a doubly mutant pgl zwf strain should express bgl normally. Indeed, this was observed. The frequency of salicin papillation in a pgl zwf strain was similar to that of the wild type, as was the color reaction on Mac-SLC plates and the growth on minimal-salicin plates. Thus, the accumulation of 6-P-gluconolactone appears to be the basis for the poor growth of Bgl+ pgl cells on salicin.
Thompson and coworkers (21) have shown that the β-glucosidase of Fusobacterium mortiferum is active on salicin and similar β-glucosides and have observed that 6-P-gluconolactone binds this β-glucosidase and severely inhibits its activity. We examined the possibility that 6-P-gluconolactone inhibited BglB by assaying BglB (18) in Bgl+ cells growing on salicin as a sole C source. No significant difference was observed in BglB activity for Bgl+ strains whether pgl+ or lacking pgl (data not shown). Thus, the accumulation of 6-P-gluconolactone does not seem to affect β-glucosidase activity and must contribute to the poor utilization of salicin in some other manner.
DISCUSSION
The availability of completely sequenced and annotated genomes of many organisms has expedited comparative and functional genomic analyses. Using the approximate location of the E. coli pgl gene and an easily scored phenotype (Blu), we have demonstrated that the E. coli ybhE gene, predicted to encode an isomerase, actually encodes the 6-P-gluconolactonase enzyme and thus is pgl. The identification of ybhE as pgl was confirmed by three independent tests: (i) the Blu− phenotype of the ybhE mutant and its complementation by the cloned ybhE gene, (ii) the behavior of the mutant strain on minimal glucose when combined with a pgi mutation, and (iii) a biochemical assay for 6-P-gluconolactonase activity. Our functional analysis of pgl was greatly facilitated by another emerging technology, recombineering (22). Recombineering was used to create most of the mutations described in this paper, and was also used to retrieve the pgl gene from the E. coli chromosome into an expression vector without the necessity of amplifying the gene with PCR.
6-P-gluconolactonase, encoded by pgl, is the second enzyme in the pentose-P pathway and converts 6-P-gluconolactone to gluconate-6-P. Gluconate-6-P is used as a substrate for both the pentose-P pathway and the inducible pathway for catabolism of gluconate, the Entner-Doudoroff pathway. Thus, the Pgl enzyme has the potential to contribute a metabolite to two pathways. Double mutants in pgl and pgi form small colonies on minimal glucose agar, however, demonstrating that Pgl is not absolutely required for some carbon flow through either the pentose-P pathway or the Entner-Doudoroff pathway utilizing gluconate. This leakiness may be due to the reaction characterized by Kupor and Fraenkel (16) in which 6-P-gluconolactone is dephosphorylated, secreted, converted to gluconate, and phosphorylated upon uptake, yielding gluconate-6-P. However, spontaneous hydrolysis of 6-P-gluconolactone can also account for these results, and either interpretation would explain the feeding results described here.
The secondary structure of Pgl is apparently not well conserved among different organisms. The E. coli Pgl/YbhE protein was previously categorized as a 3-carboxy-cis,cis-muconate lactonizing enzyme (Fig. 1B) and predicted to have a beta propeller cycloisomerase structure (14). We have not checked this enzymatic activity, since our results demonstrate a 6-P-gluconolactonase activity for Pgl (Fig. 1A and 4). It remains theoretically possible that Pgl is a bifunctional enzyme. BLAST alignment of the Pseudomonas aeruginosa Pgl enzyme (11) with the E. coli Pgl protein reveals no significant homology, although the P. aeruginosa Pgl has significant homology to NagB, a glucosamine-6-phosphate deaminase, as does the E. coli YieK protein. YieK shows homology to some Pgl enzymes but the yieK mutant did not display a Blu− phenotype in our assays, did not reduce growth on minimal glucose when combined with both pgl and pgi mutations, and did not decrease the frequency of appearance of spontaneous Bgl+ mutants on Mac-SLC plates. In mice and malarial parasites, Pgl and G6PD activities are both present in a single bifunctional enzyme (5, 6). The gene encoding Pgl remains unidentified in a number of microbes (7). Our results suggest that some genes annotated as encoding a 3-carboxy-cis,cis-muconate lactonizing enzyme may actually encode a 6-P-gluconolactonase.
Transposition of IS1 or IS5 to the bgl region of the chromosome is mainly responsible for the genesis of Bgl+ mutants (19). It was previously observed that rho mutants of E. coli give rise to Bgl+ mutants at a lower frequency than the wild type, and this was traced to defective transposition in rho mutants (8). However, the lower frequency of appearance of Bgl+ mutants from pgl parents observed here has a different explanation, since the Bgl+ mutants that arose in wild-type cells (and found to be due to IS1 and IS5 transpositions) displayed poor growth on salicin after they were transduced to pgl<>tet. This seems related to the accumulation of 6-P-gluconolactone, since the frequency of appearance of Bgl+ mutants from zwf pgl mutants is like that from wild-type cells. Reconstruction experiments showed that when pgl cells were mixed with a very low proportion of Bgl+ pgl<>tet cells and streaked on Mac-SLC plates, the Bgl+ cells did not produce the red color due to fermentation of salicin that is seen when they are streaked by themselves (data not shown). However, the precise defect that is responsible for this remains unknown.
Acknowledgments
We thank Rick Wolf for the pgi::Tn10 strain and Sankar Adhya for helpful suggestions. Suggestions from anonymous reviewers improved the manuscript.
REFERENCES
- 1.Adhya, S., and M. Schwartz. 1971. Phosphoglucomutase mutants of Escherichia coli K-12. J. Bacteriol. 108:621-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altschul, S. F., W. Gish, W. Miller, E. W., Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 4.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M., Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 5.Clarke, J. L., and P. J. Mason. 2003. Murine hexose-6-phosphate dehydrogenase: a bifunctional enzyme with broad substrate specificity and 6-phosphogluconolactonase activity. Arch. Biochem. Biophys. 415:229-234. [DOI] [PubMed] [Google Scholar]
- 6.Clarke, J. L., D. A. Scopes, O. Sodeinde, and P. J. Mason. 2001. Glucose-6-phosphate dehydrogenase-6-phosphogluconolactonase. A novel bifunctional enzyme in malaria parasites. Eur. J. Biochem. 268:2013-2019. [DOI] [PubMed] [Google Scholar]
- 7.Cordwell, S. J. 1999. Microbial genomes and “missing” enzymes: refining biochemical pathways. Arch. Microbiol. 172:269-279. [DOI] [PubMed] [Google Scholar]
- 8.Datta, A. R., and J. L. Rosner. 1987. Reduced transposition in rho mutants of Escherichia coli K-12. J. Bacteriol. 169:888-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feiss, M., S. Adhya, and D. L. Court. 1972. Isolation of plaque-forming, galactose-transducing strains of phage lambda. Genetics 71:189-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraenkel, D. G. 1996. Glycolysis, p. 189-198. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 11.Hager, P. W., M. W. Calfee, and P. V. Phibbs. 2000. The Pseudomonas aeruginosa devB/SOL homolog, pgl, is a member of the hex regulon and encodes 6-phosphogluconolactonase. J. Bacteriol. 182:3934-3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ivanova, N., A. Sorokin, I. Anderson, N. Galleron, B. Candelon, V. Kapatral, A. Bhattacharyya, G. Reznik, N. Mikhailova, A. Lapidus, L. Chu, M. Mazur, E. Goltsman, N. Larsen, M. D'Souza, T. Walunas, Y. Grechkin, G. Pusch, R. Haselkorn, M. Fonstein, D. S. D. Ehrlich, R. Overbeek, and N. Kyrpides. 2003. Genome sequence of Bacillus cereus and comparative analysis with Bacillus anthracis. Nature 423:87-91. [DOI] [PubMed] [Google Scholar]
- 13.Kadner, R. J., G. P. Murphy, and C. M. Stephens. 1992. Two mechanisms for growth inhibition by elevated transport of sugar phosphates in Escherichia coli. J. Gen. Microbiol. 138:2007-2014. [DOI] [PubMed] [Google Scholar]
- 14.Kajander, T., M. C. Merckel, A. Thompson, A. M. Deacon, P. Mazur, J. W. Kozarich, and A. Goldman. 2002. The structure of Neurospora crassa 3-carboxy-cis,cis-muconate lactonizing enzyme, a beta propeller cycloisomerase. Structure 10:483-492. [DOI] [PubMed] [Google Scholar]
- 15.Kupor, S. R., and D. G. Fraenkel. 1969. 6-Phosphogluconolactonase mutants of Escherichia coli and a maltose blue gene. J. Bacteriol. 100:1296-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kupor, S. R., and D. G. Fraenkel. 1972. Glucose metabolism in 6-phosphogluconolactonase mutants of Escherichia coli. J. Biol. Chem. 247:1904-1910. [PubMed] [Google Scholar]
- 17.Lee, E. C., D. Yu, J. Martinez de Velasco, L. Tessarollo, D. A. Swing, D. L. Court, N. A. Jenkins, and N. G. Copeland. 2001. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics 73:56-65. [DOI] [PubMed] [Google Scholar]
- 18.Prasad, I., and S. Schaefler. 1974. Regulation of the β-glucoside system in Escherichia coli K-12. J. Bacteriol. 120:638-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schnetz, K., and B. Rak. 1992. IS5: a mobile enhancer of transcription in Escherichia coli. Proc. Natl. Acad. Sci. USA 89:1244-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sisk, W. P., J. G. Chirikjian, J. Lautenberger, C. Jorcyk, T. S. Papas, M. L. Berman, R. Zagursky, and D. L. Court. 1986. A plasmid vector for cloning and expression of gene segments: expression of an HTLV-I envelope gene segment. Gene 48:183-193. [DOI] [PubMed] [Google Scholar]
- 21.Thompson, J., S. A. Robrish, C. L. Bouma, D. I. Freedberg, and J. E. Folk. 1997. Phospho-β-glucosidase from Fusobacterium mortiferum: purification, cloning, and inactivation by 6-phosphoglucono-δ-lactone. J. Bacteriol. 179:1636-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu, D., H. M. Ellis, E. C. Lee, N. A. Jenkins, N. G. Copeland, and D. L. Court. 2000. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. USA 97:5978-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]