Abstract
Background:
The clinical significance of acute vasoreactivity testing (AVT) in patients with chronic thromboembolic pulmonary hypertension (CTEPH) remains unclear. We analyzed changes in hemodynamics and oxygenation dynamics indices after AVT in patients with CTEPH using patients with pulmonary arterial hypertension (PAH) as controls.
Methods:
We analyzed retrospectively the results of AVT in 80 patients with PAH and 175 patients with CTEPH registered in the research database of Beijing Chao-Yang Hospital between October 2005 and August 2014. Demographic variables, cardiopulmonary indicators, and laboratory findings were compared in these two subgroups. A long-term follow-up was conducted in patients with CTEPH. Between-group comparisons were performed using the independent-sample t-test or the rank sum test, within-group comparisons were conducted using the paired t-test or the Wilcoxon signed-rank test, and count data were analyzed using the Chi-squared test. Survival was estimated using the Kaplan-Meier method and log-rank test.
Results:
The rates of positive response to AVT were similar in the CTEPH (25/175, 14.3%) and PAH (9/80, 11.3%) groups (P > 0.05). Factors significantly associated a positive response to AVT in the CTEPH group were level of N-terminal pro-brain natriuretic peptide (≤1131.000 ng/L), mean pulmonary arterial pressure (mPAP, ≤44.500 mmHg), pulmonary vascular resistance (PVR, ≤846.500 dyn·s−1·m−5), cardiac output (CO, ≥3.475 L/min), and mixed venous oxygen partial pressure (PvO2, ≥35.150 mmHg). Inhalation of iloprost resulted in similar changes in mean blood pressure, mPAP, PVR, systemic vascular resistance, CO, arterial oxygen saturation (SaO2), mixed venous oxygen saturation, partial pressure of oxygen in arterial blood (PaO2), PvO2, and intrapulmonary shunt (Qs/Qt) in the PAH and CTEPH groups (all P > 0.05). The survival time in patients with CTEPH with a negative response to AVT was somewhat shorter than that in AVT-responders although the difference was not statistically significant (χ2 = 3.613, P = 0.057). The survival time of patients with CTEPH who received calcium channel blockers (CCBs) was longer than that in the group with only basic treatment and not shorter than that of patients who receiving targeted drugs or underwent pulmonary endarterectomy (PEA) although there was no significant difference between the four different treatment regimens (χ2 = 3.069, P = 0.381).
Conclusions:
The rates of positive response to AVT were similar in the CTEPH and PAH groups, and iloprost inhalation induced similar changes in hemodynamics and oxygenation dynamics indices. A positive response to AVT in the CTEPH group was significantly correlated with milder disease and better survival. Patients with CTEPH who cannot undergo PEA or receive targeted therapy but have a positive response to AVT might benefit from CCB treatment.
Keywords: Acute Vasoreactivity Testing, Calcium Channel Blocker Treatment, Chronic Thromboembolic Pulmonary Hypertension, Iloprost
Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) is a disease, in which an organized thrombus in the proximal pulmonary artery accompanied by distal vascular remodeling causes obstruction of the pulmonary artery and arterioles. Pulmonary vascular resistance (PVR) and pulmonary arterial pressure (PAP) increase, ultimately leading to hypertrophy and dilation of the right ventricle, which accompanies progressive aggravation of right-sided heart failure.[1,2] The incidence of CTEPH after acute pulmonary embolism (PE) has been reported to be between 0.4% and 8.8%.[3,4,5,6,7] Invasive pulmonary angiography and right heart catheterization (RHC) are the gold standards for establishing the diagnosis of CTEPH, while a pulmonary ventilation/perfusion scan, echocardiography, computed tomography pulmonary angiography (CTPA), and magnetic resonance imaging can also be used as diagnostic methods.[8,9,10] Although pulmonary endarterectomy (PEA) is a potentially curative treatment, not all patients with CTEPH are eligible for PEA.[11,12] Recent evidence shows that the use of pulmonary arterial hypertension (PAH) targeted drugs is also effective in the treatment of inoperable CTEPH.[13,14,15,16,17] Unfortunately, the high cost of such targeted drugs precludes their use in many patients with CTEPH in China. CTEPH and PAH are both classified as precapillary pulmonary hypertension (PH) and have similar histopathological characteristics.[1,18] Guidelines recommend that acute vasoreactivity testing (AVT) should be conducted before treating PAH to predict the effectiveness of calcium channel blockers (CCBs).[2,19] However, research on whether AVT can be performed in patients with CTEPH, especially in those who cannot be treated surgically or receive targeted treatment, is inconclusive.
The aim of this study was to analyze the results of AVT in patients with CTEPH by observing changes in hemodynamics and oxygenation dynamics before and after iloprost inhalation, with patients with PAH used as the controls. In addition, the effects of AVT and different treatment strategies on the prognosis of patients with CTEPH were evaluated.
Methods
Research subjects
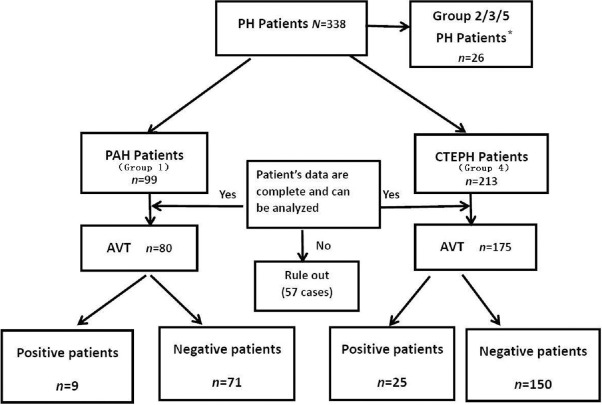
This study was approved by the Medical Ethics Committee of Beijing Chao-Yang Hospital. Included in the study were 338 patients with PH who were registered in the research database of Beijing Chao-Yang Hospital between October 2005 and August 2014, There were 26 patients belong to Group 2/3/5, which included that due to left heart disease (group 2), due to lung diseases and/or hypoxia (group 3) and PH with unclear and/or multifactorial mechanisms (group 5). Included in this study were 312 patients, specifically including 99 patients with PAH (Group 1) and 213 patients with CTEPH (Group 4). The diagnosis was established by echocardiography, a V/Q scan, CTPA, and RHC examination. The diagnostic criteria of PH, PAH, and CTEPH were in accordance with the following 2015 European Society of Cardiology/European Respiratory Society guidelines:[2](1) resting mean PAP (mPAP) measured through right cardiac catheterization ≥25 mmHg (1 mmHg = 0.133 kPa), and (2) pulmonary artery wedge pressure (PAWP) ≤15 mmHg. Additional criteria for CTEPH were (1) pulmonary angiography or radionuclide V/Q imaging confirming the presence of chronic thromboembolism after 3 months of anticoagulation treatment and (2) exclusion of other reasons for PH, such as PH related to left heart disease and lung disease. Finally, a PVR of >3 Wood units had to be present for a diagnosis of PAH. The PAH group included 53 patients with idiopathic pulmonary hypertension, six patients with heritable pulmonary hypertension, and 40 patients with disease-related pulmonary hypertension. A total of 57 patients were excluded because their data were incomplete and could not be analyzed. Of 80 patients who underwent AVT in the PAH group (80/99), nine had a positive and 71 had a negative response. Of 175 patients who underwent AVT in the CTEPH group (175/213), 25 had a positive and 150 had a negative response [Figure 1].
Figure 1.
Flow chart of inclusion criteria for clinical patients. A total of 338 patients with PH registered in the research database of Beijing Chao-Yang Hospital between October 2005 and August 2014, except 26 patients belonging to Group 2/3/5, included in this study were 312 patients, specifically including 99 patients with PAH and 213 patients with CTEPH. A total of 57 patients were excluded because their data were incomplete or could not be analyzed. Of 80 patients who underwent AVT in the PAH group (80/99), nine had a positive and 71 had a negative response. Of 175 patients who underwent AVT in the CTEPH group (175/213), 25 had a positive and 150 had a negative response. *Group 2/3/5 PH include that due to left heart disease (group 2), due to lung diseases and/or hypoxia (group 3) and PH with unclear and/or multifactorial mechanisms (group 5). AVT:Acute vasoreactivity testing; CTEPH:Chronic thromboembolic pulmonary hypertension;PAH:Pulmonary arterial hypertension;PH:Pulmonary hypertension.
Right cardiac catheterization
A 7.5-F Swan-Ganz Catheter (Edwards Inc., USA) was placed from the right internal jugular vein to the right lower pulmonary artery. After a 30-min stabilization period, hemodynamic parameters were collected, including mPAP, right atrial pressure (RAP), and PAWP. Blood from the femoral artery and mixed venous blood was collected for blood gas analysis. Parameters, such as partial pressure of oxygen in arterial blood (PaO2), arterial oxygen saturation, and mixed venous oxygen saturation (SvO2), were recorded. A continuous cardiac output monitor (Vigilance I, Edwards Inc., USA) was used to measure cardiac output (CO). An M1165A multifunction patient monitor (Hewlett-Packard Company, USA) was employed to monitor the mean blood pressure (mBP) and heart rate. The measured hemodynamics and oxygenation dynamics parameters were transferred to a Hewlett-Packard patient monitor, and PVR, systemic vascular resistance (SVR), and intrapulmonary shunt (Qs/Qt) were calculated.
Acute vasoreactivity testing
A total of 20 μg (2 ml) of iloprost (Bayer Schering Pharma, Germany) was mixed with 1 ml of saline and placed into a PARI BOY N-type air compressor nebulizer (PARI GmbH, Germany). Patients were asked to breathe for 10–15 min. The hemodynamics and oxygenation dynamics parameters listed above were measured again immediately after the inhalation. The following three conditions had to be met simultaneously for a positive response to AVT:[20,21] (1) a mPAP decreased of ≥10 mmHg compared to the preinhalation value, (2) mPAP ≤40 mmHg after inhalation, and (3) no change or an increase in CO. Indications for test termination were (1) systemic hypotension (typically systolic BP <90 mmHg, although some patients were able to tolerate lower BP), (2) an increased in RAP by 20–50%, or a decrease in cardiac index >10% compared to the level before inhalation, or (3) moderately or severely intolerable adverse effects, such as nausea or headache.
Follow-up
Regular telephone follow-up was performed in 175 patients with CTEPH until July 2016 at 3 months, 6 months, 12 months, 18 months, 2 years, 3 years, 4 years, 5 years, 6 years, 7 years, 8 years, 9 years, and 10 years after enrollment. The follow-up included questions about regular symptoms, quality of life, medications, and time of death.
Statistical analysis
Statistical analysis was performed with the SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). The quantitative data followed a normal distribution were expressed as mean ± standard deviation (SD). The quantitative data of nonnormal distribution were presented as medians (quartile range). Categorical variables were expressed as numbers of cases or percentages. Between-group comparisons were performed using the independent-sample t-test or the rank sum test (Mann-Whitney U-test). Within-group comparisons were conducted using the paired t-test or the Wilcoxon signed-rank test. Count data were tested using the Chi-squared test. Logistic regression analysis was used to screen for factors predicting the AVT-positive rate in patients with CTEPH. Prediction efficiency of the equation was evaluated using receiver operating characteristic (ROC) curves. Survival was estimated by the Kaplan-Meier method and the log-rank test. Multivariable analyses with the Cox proportional-hazards model were used to estimate the simultaneous effects of prognostic factors on survival. A value of P < 0.05 was considered a statistically significant difference.
Results
Comparison of baseline data in the chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension groups
The average age of patients in the CTEPH group was higher than that in the PAH group. In addition, CTEPH patients were more likely to be male (61.7% vs. 22.5%), to have a higher body mass index, Qs/Qt and levels of N-terminal pro-brain natriuretic peptide (NT-proBNP), and to have significantly lower values of PaO2, mixed venous oxygen partial pressure (PvO2), SvO2, and SaO2 [Table 1]. A positive response to AVT was observed in 25 patients with CTEPH (14.3%) and nine patients with PAH (11.3%) (P > 0.05). In the CTEPH group, AVT-positive patients had significantly lower levels of NT-proBNP, mPAP, PVR, and SVR and significantly higher levels of SvO2, PvO2, and CO than AVT-negative patients (P < 0.05; Table 2). In the PAH group, there were no significant difference in above variables between AVT-positive and AVT-negative patients (all P > 0.05, Supplementary Table 1).
Table 1.
Comparison of baseline data between patients with CTEPH and PAH before acute vasoreactivity testing
| Factors | PAH (n = 80) | CTEPH (n = 175) | Statistics | P |
|---|---|---|---|---|
| Gender (n) | ||||
| Female | 62 | 67 | 33.775† | <0.010 |
| Male | 18 | 108* | ||
| Age (years) | 45.19 ± 15.19 | 56.98 ± 11.67* | 6.098‡ | <0.010 |
| Body mass index (kg/m2) | 22.93 ± 3.44 | 24.68 ± 3.89* | 3.425‡ | 0.001 |
| WHO-FC (n) | ||||
| I | 8 | 10 | 1.882† | 0.597 |
| II | 37 | 78 | ||
| III | 32 | 79 | ||
| IV | 3 | 8 | ||
| 6MWD (m) | 349.12 ± 113.76 | 333.09 ± 110.53 | −0.938‡ | 0.350 |
| NT-proBNP (ng/L) | 810.90 (1680.9) | 1476.00 (2373.20)* | −2.778§ | 0.006 |
| mBP (mmHg) | 89.73 ± 12.18 | 92.40 ± 13.83 | 1.485‡ | 0.139 |
| mPAP (mmHg) | 53.50 (22.52) | 51.00 (12.00) | −1.737§ | 0.082 |
| PVR (dyn·s−1·m−5) | 1105.50 (1031.25) | 990.00 (583.00) | −1.295§ | 0.195 |
| SVR (dyn·s−1·m−5) | 2088.00 (1139.00) | 2063.50 (891.50) | −0.841§ | 0.400 |
| CO (L/min) | 3.44 ± 1.55 | 3.39 ± 1.03 | −0.191‡ | 0.849 |
| SaO2 (%) | 94.50 (3.70) | 91.50 (5.20)* | −5.160§ | <0.001 |
| SvO2 (%) | 66.20 ± 10.34 | 62.93 ± 11.79* | −2.173‡ | 0.031 |
| PaO2 (mmHg) | 75.69 ± 18.05 | 63.89 ± 12.78* | −5.412‡ | <0.001 |
| PvO2 (mmHg) | 39.30 (7.80) | 36.35 (9.60)* | −2.415§ | 0.016 |
| Qs/Qt (%) | 18.84 ± 9.65 | 24.08 ± 7.79* | 3.231‡ | 0.001 |
Data are presented as mean ± SD or median (interquartile range). *Means the data in CTEPH group versus that in PAH group, P<0.05; †χ2 value; ‡t value; §Z value. The independent-sample t-test was used to analyze the date of normal distribution. The rank sum test of two independent samples (Mann-Whitney U-test) was used to analyze the date of nonnormal distribution. Categorical data on gender and WHO-FC were expressed as number of cases and analyzed using the Chi-square test. 6MWD: 6-min walking distance; CO: Cardiac output; CTEPH: Chronic thromboembolic pulmonary hypertension; mBP: Mean blood pressure; mPAP: Mean pulmonary arterial pressure; NT-proBNP: N-terminal pro-brain natriuretic peptide; PAH: Pulmonary arterial hypertension; PaO2: Partial pressure of oxygen in arterial blood; PvO2: Mixed venous oxygen partial pressure; PVR: Pulmonary vascular resistance; Qs/Qt: Intrapulmonary shunt; SaO2: Arterial oxygen saturation; SvO2: Mixed venous oxygen saturation; SVR: Systemic vascular resistance; WHO-FC: World Health Organization functional class; SD: Standard deviation. 1 mmHg = 0.133 kPa.
Table 2.
Baseline data of patients with chronic thromboembolic pulmonary hypertension before acute vasoreactivity testing
| Factors | Negative group (n = 150) | Positive group (n = 25) | Statistics | P |
|---|---|---|---|---|
| Gender (n) | ||||
| Female | 57 | 10 | 0.036† | 0.820 |
| Male | 93 | 15 | ||
| Age (years) | 56.75 ± 11.89 | 58.36 ± 10.31 | 0.636‡ | 0.520 |
| Body mass index (kg/m2) | 24.43 ± 3.73 | 26.17 ± 4.55* | 2.047‡ | 0.040 |
| WHO-FC (n) | ||||
| I–II | 72 | 16 | 2.194† | 0.130 |
| III–IV | 78 | 9 | ||
| 6MWD (m) | 330.13 ± 101.04 | 347.65 ± 150.77 | 0.534‡ | 0.600 |
| NT-proBNP (ng/L) | 1690.00 (2702.80) | 569.25 (1011.39)* | −3.712§ | <0.010 |
| mBP (mmHg) | 91.66 ± 13.34 | 97.04 ± 16.11 | 1.762‡ | 0.070 |
| mPAP (mmHg) | 52.02 ± 11.31 | 46.52 ± 10.15* | −2.285‡ | 0.020 |
| PVR (dyn·s−1·m−5) | 1163.81 ± 513.96 | 738.12 ± 234.03* | −6.731‡ | <0.010 |
| VR (dyn·s−1·m−5) | 2097.00 (981.50) | 1742.00 (667.00)* | −2.347§ | 0.020 |
| CO (L/min) | 3.28 ± 1.01 | 4.06 ± 0.85* | 3.530‡ | <0.010 |
| SaO2 (%) | 91.55 (5.03) | 91.00 (4.55) | −0.017§ | 0.970 |
| SvO2 (%) | 62.00 ± 12.12 | 68.16 ± 8.05* | 3.274‡ | <0.010 |
| PaO2 (mmHg) | 64.31 ± 12.80 | 61.18 ± 12.65 | −0.706‡ | 0.300 |
| PvO2 (mmHg) | 35.80 (9.80) | 39.80 (13.95)* | −3.652§ | <0.010 |
| Qs/Qt (%) | 23.83 ± 9.93 | 25.72 ± 8.98 | 0.745‡ | 0.490 |
Data are presented as mean ± SD or median (interquartile range). *Means the data in positive group versus that in negative group, P<0.05; †χ2 value; ‡t value; §Z value. The independent-sample t-test was used to analyze the date of normal distribution. The rank sum test of two independent samples (Mann-Whitney U-test) was used to analyze the date of nonnormal distribution. Categorical data on gender and WHO-FC were expressed as number of cases and analyzed using the Chi-square test. 6MWD: 6-min walking distance; CO: Cardiac output; mBP: Mean blood pressure; mPAP: Mean pulmonary arterial pressure; NT-proBNP: N-terminal pro-brain natriuretic peptide; PaO2: Partial pressure of oxygen in arterial blood; PvO2: Mixed venous oxygen partial pressure; PVR: Pulmonary vascular resistance; Qs/Qt: Intrapulmonary shunt; SaO2: Arterial oxygen saturation; SvO2: Mixed venous oxygen saturation; SVR: Systemic vascular resistance; WHO-FC: World Health Organization functional class; SD: Standard deviation. 1 mmHg = 0.133 kPa.
Supplementary Table 1.
Baseline data of patients with pulmonary arterial hypertension before acute vasoreactivity testing
| Factors | Negative response (n = 71) | Positive response (n = 9) | Statistics | P |
|---|---|---|---|---|
| Gender (n) | ||||
| Female | 56 | 6 | 0.683† | 0.409 |
| Male | 15 | 3 | ||
| Age (years) | 44.14 ± 14.70 | 53.22 ± 17.43 | 1.706‡ | 0.092 |
| Body mass index (kg/m2) | 22.68 ± 3.38 | 24.89 ± 3.44 | 1.837‡ | 0.070 |
| WHO-FC (n) | ||||
| I–II | 39 | 6 | 0.447† | 0.504 |
| III–IV | 32 | 3 | ||
| 6MWD (m) | 369.00 (144.00) | 360.00 (183.00) | −0.078§ | 0.948 |
| NT-proBNP (ng/L) | 872.00 (1623.00) | 439.10 (1537.38) | −0.608§ | 0.546 |
| mBP (mmHg) | 89.23 ± 12.22 | 93.67 ± 11.79 | 1.027‡ | 0.308 |
| mPAP (mmHg) | 56.38 ± 17.89 | 52.67 ± 14.65 | −0.597‡ | 0.552 |
| PVR (dyn·s−1·m−5) | 1156.00 (1068.00) | 800.00 (717.50) | −1.622§ | 0.105 |
| SVR (dyn·s−1·m−5) | 2122.00 (1209.00) | 1790.00 (1249.00) | −1.038§ | 0.299 |
| CO (L/min) | 3.37 ± 1.59 | 4.04 ± 1.07 | 1.229‡ | 0.223 |
| SaO2 (%) | 93.96 ± 3.52 | 92.35 ± 3.16 | −1.301‡ | 0.197 |
| SvO2 (%) | 65.87 ± 10.03 | 68.77 ± 12.88 | 0.789‡ | 0.433 |
| PaO2 (mmHg) | 76.25 ± 18.50 | 71.35 ± 14.10 | −0.764‡ | 0.447 |
| PvO2 (mmHg) | 40.38 ± 9.48 | 46.57 ± 13.62 | 1.751‡ | 0.084 |
| Qs/Qt (%) | 17.60 (13.05) | 19.90 (29.90) | −1.013§ | 0.332 |
Data are presented as mean ± SD or median (interquartile range). †χ2 value; ‡t value; §Z value. The independent-sample t-test was used to analyze the date of normal distribution. The rank sum test of two independent samples (Mann-Whitney U-test) was used to analyze the date of non-normal distribution. Categorical data on gender and WHO cardiac function were expressed as number of cases and analyzed using the Chi-square test. 6MWD: 6-min walking distance; CO: Cardiac output; mBP: Mean blood pressure; mPAP: Mean pulmonary arterial pressure; NT-proBNP: N-terminal pro-brain natriuretic peptide; PaO2: Partial pressure of oxygen in arterial blood; PvO2: Mixed venous oxygen partial pressure; PVR: Pulmonary vascular resistance; Qs/Qt: Intrapulmonary shunt; SaO2: Arterial oxygen saturation; SvO2: Mixed venous oxygen saturation; SVR: Systemic vascular resistance; WHO-FC: World Health Organization functional class; SD: Standard deviation. 1 mmHg = 0.133 kPa.
Comparison of hemodynamics and oxygenation dynamics parameters before and after inhalation in the chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension groups
In the CTEPH group, mPAP, mBP, SVR, and PVR decreased after inhalation of iloprost (all P < 0.01). The inhalation also reduced the levels of SaO2 and PaO2 (both P < 0.05) and increased the levels of SvO2 (P < 0.01), Qs/Qt, and CO (all P < 0.01). There were no significant changes in PvO2 (P > 0.05; Table 3). Subgroup analysis of the CTEPH group showed that iloprost inhalation resulted in significant decreases in the levels of mPAP, PVR, mBP, and SVR in both AVT-positive and AVT-negative subgroups (P < 0.01) and significant increases in the levels of CO (P < 0.01; Table 3.1). In addition, inhalation of iloprost was associated with decreased SaO2 and PaO2 (P < 0.01) in the AVT-positive group in the absence of changes in Qs/Qt and SvO2. In the AVT-negative group, inhalation was associated with significant decreases in PaO2 and SaO2 (P < 0.01) and increases in Qs/Qt (P < 0.01; Table 3.2).
Table 3.
Comparison of hemodynamics and oxygenation dynamics parameters after acute vasoreactivity testing in patients with chronic thromboembolic pulmonary hypertension
| Factors | Before AVT (n = 175) | After AVT (n = 175) | Statistics | P |
|---|---|---|---|---|
| mPAP (mmHg) | 51.00 (12.00) | 45.00 (14.00)* | −10.360† | <0.001 |
| mBP (mmHg) | 91.00 (17.00) | 88.00 (16.00)* | −6.440† | <0.001 |
| PVR (dyn·s−1·m−5) | 990.00 (583.00) | 807.00 (500.50)* | −9.887† | <0.001 |
| SVR (dyn·s−1·m−5) | 2063.50 (891.50) | 1762.00 (714.00)* | −8.215† | <0.001 |
| CO (L/min) | 3.40 ± 1.02 | 3.81 ± 1.15* | −7.969‡ | <0.001 |
| SaO2 (%) | 91.50 (5.00) | 89.80 (5.40)* | −6.210† | <0.001 |
| SvO2 (%) | 64.20 (13.35) | 65.50 (13.28)* | −4.163† | <0.001 |
| PaO2 (mmHg) | 62.50 (14.30) | 56.60 (11.90)* | −7.051† | <0.001 |
| PvO2 (mmHg) | 36.35 (9.60) | 35.95 (8.12) | −0.848† | 0.396 |
| Qs/Qt (%) | 24.10 ± 9.91 | 29.79 ± 12.68* | −5.909‡ | <0.001 |
Data are presented as mean ± SD or median (interquartile range). *Means the data after AVT versus that before AVT, P<0.05; †Z value; ‡t value. The paired-sample t-test was used to analyze the date of normal distribution. The Wilcoxon signed-rank test of two related samples was used to analyze the date of nonnormal distribution. AVT: Acute vasoreactivity testing; CO: Cardiac output; mBP: Mean blood pressure; mPAP: Mean pulmonary arterial pressure; PaO2: Partial pressure of oxygen in arterial blood; PvO2: Mixed venous oxygen partial pressure; PVR: Pulmonary vascular resistance; Qs/Qt: Intrapulmonary shunt; SaO2: Arterial oxygen saturation; SvO2: Mixed venous oxygen saturation; SVR: Systemic vascular resistance; SD: Standard deviation. 1 mmHg = 0.133 kPa.
Table 3.1.
Comparison of hemodynamics parameter after iloprost inhalation between patients with a positive and negative response to acute vasoreactivity testing in the chronic thromboembolic pulmonary hypertension group
| Factors | Negative group (n = 150) | Positive group (n = 25) | ||||||
|---|---|---|---|---|---|---|---|---|
| Before AVT | After AVT | Statistics | P | Before AVT | After AVT | t | P | |
| mBP (mmHg) | 91.00 (16.25) | 87.50 (14.50) | −5.603† | <0.001 | 96.88 ± 15.80 | 92.20 ± 14.57* | 4.138 | <0.001 |
| mPAP (mmHg) | 52.03 ± 11.31 | 47.47 ± 11.32* | 12.322‡ | <0.001 | 46.52 ± 10.16 | 31.88 ± 7.43* | 15.050 | <0.001 |
| PVR (dyn·s−1·m−5) | 1071.00 (555.50) | 880.00 (487.75)* | −8.842† | <0.001 | 738.12 ± 234.03 | 444.32 ± 160.96* | 10.450 | <0.001 |
| SVR (dyn·s−1·m−5) | 2097.00 (981.50) | 1783.00 (739.00)* | −7.541† | <0.001 | 1836.87 ± 454.41 | 1568.26 ± 359.50* | 3.862 | 0.001 |
| CO (L/min) | 3.30 ± 1.01 | 3.67 ± 1.12* | −7.141‡ | <0.001 | 4.06 ± 0.85 | 4.62 ± 1.05* | −3.566 | 0.002 |
Data are presented as mean ± SD or median (interquartile range). *Means the data after AVT versus that before AVT, P<0.05; †Z value; ‡t value. The paired-sample t-test was used to analyze the date of normal distribution. The Wilcoxon signed-rank test of two related samples was used to analyze the date of nonnormal distribution. AVT: Acute vasoreactivity testing; CO: Cardiac output; mBP: Mean blood pressure; mPAP: Mean pulmonary arterial pressure; PVR: Pulmonary vascular resistance; SVR: Systemic vascular resistance; SD: Standard deviation. 1 mmHg = 0.133 kPa.
Table 3.2.
Comparison of oxygenation dynamics parameter after iloprost inhalation between patients with a positive and negative response to acute vasoreactivity testing in the chronic thromboembolic pulmonary hypertension group
| Factors | Negative group (n = 150) | Positive group (n = 25) | ||||||
|---|---|---|---|---|---|---|---|---|
| Before AVT | After AVT | Statistics | P | Before AVT | After AVT | t | P | |
| SaO2 (%) | 91.55 (5.03) | 89.85 (5.12)* | −5.392† | <0.001 | 91.54 ± 3.58 | 88.60 ± 4.58* | 3.406 | 0.002 |
| SvO2 (%) | 62.90 (14.50) | 65.50 (13.55)* | −4.996† | <0.001 | 68.16 ± 8.06 | 66.67 ± 8.08 | 1.131 | 0.269 |
| PaO2 (mmHg) | 62.70 (14.03) | 56.65 (12.65)* | −6.178† | <0.001 | 61.98 ± 12.74 | 55.23 ± 11.17* | 4.367 | <0.010 |
| PvO2 (mmHg) | 35.80 (9.80) | 35.50 (7.25) | −0.037† | 0.971 | 44.46 ± 9.70 | 40.94 ± 9.44* | 2.119 | 0.045 |
| Qs/Qt (%) | 23.68 ± 10.00 | 29.31 ± 11.97* | −5.818‡ | <0.001 | 25.72 ± 8.98 | 30.85 ± 16.61 | −1.538 | 0.146 |
Data are presented as mean ± SD or median (interquartile range). *Means the data after AVT versus that before AVT, P<0.05; †Z value; ‡t value. The paired-sample t-test was used to analyze the date of normal distribution. The Wilcoxon signed-rank test of two related samples was used to analyze the date of nonnormal distribution. AVT: Acute vasoreactivity testing; PaO2: Partial pressure of oxygen in arterial blood; PvO2: Mixed venous oxygen partial pressure; Qs/Qt: Intrapulmonary shunt; SaO2: Arterial oxygen saturation; SvO2: Mixed venous oxygen saturation; SD: Standard deviation. 1 mmHg = 0.133 kPa.
Iloprost inhalation led to significant declines in mPAP, PVR, mBP, SVR, SaO2, and PaO2 and increase in CO, SvO2, and Qs/Qt in patients with PAH. In contrast, there was no significant change in PvO2 [Supplementary Table 2].
Supplementary Table 2.
Comparison of hemodynamics and oxygenation dynamics parameters before and after acute vasoreactivity testing in patients with pulmonary arterial hypertension
| Factors | Before AVT (n = 80) | After AVT (n = 80) | Statistics | P |
|---|---|---|---|---|
| mPAP (mmHg) | 55.96 ± 17.51 | 48.07 ± 17.81* | 8.052† | <0.001 |
| mBP (mmHg) | 89.73 ± 12.18 | 83.88 ± 14.91* | 4.354† | <0.001 |
| PVR (dyn·s−1·m−5) | 1105.50 (1031.25) | 896.50 (801.50)* | −6.585‡ | <0.001 |
| SVR (dyn·s−1·m−5) | 2088.00 (1139.00) | 1815.00 (1011.00)* | −5.379‡ | <0.001 |
| CO (L/min) | 3.44 ± 1.55 | 3.73 ± 1.52* | −4.091† | <0.001 |
| SaO2 (%) | 94.50 (3.70) | 93.85 (4.72)* | −2.390‡ | 0.017 |
| SvO2 (%) | 66.20 ± 10.34 | 69.53 ± 9.44* | −3.578† | 0.001 |
| PaO2 (mmHg) | 75.69 ± 18.05 | 70.58 ± 16.53* | 3.456† | 0.001 |
| PvO2 (mmHg) | 39.30 (7.80) | 38.40 (8.60) | −0.638‡ | 0.524 |
| Qs/Qt (%) | 17.70 (13.13) | 28.85 (17.65)* | −3.775‡ | <0.001 |
Data are presented as mean ± SD or median (interquartile range). *Means the data after AVT versus that before AVT, P<0.05; †t value; ‡Z value. The paired-sample t-test was used to analyze the date of normal distribution. The Wilcoxon signed-rank test of two related samples was used to analyze the date of nonnormal distribution. AVT: Acute vasoreactivity testing; CO: Cardiac output; mBP: Mean blood pressure; mPAP: Mean pulmonary arterial pressure; PaO2: Partial pressure of oxygen in arterial blood; PvO2: Mixed venous oxygen partial pressure; PVR: Pulmonary vascular resistance; Qs/Qt: Intrapulmonary shunt; SaO2: Arterial oxygen saturation; SvO2: Mixed venous oxygen saturation; SVR: Systemic vascular resistance; SD: Standard deviation. 1 mmHg = 0.133 kPa.
A comparison of hemodynamics and oxygenation dynamics parameters before and after inhalation between the PAH and CTEPH groups showed no significant changes in delta (Δ)mBP, ΔmPAP, ΔPVR, ΔSVR, ΔCO, ΔSaO2,ΔSvO2, ΔPaO2, ΔPvO2 and ΔQs/Qt (“Δ” indicates the change in the index after iloprost inhalation, all P > 0.05; Table 4).
Table 4.
Comparison of hemodynamics and oxygenation dynamics parameters after acute vasoreactivity testing inpatients with CTEPH and PAH
| Factors | CTEPH (n = 175) | PAH (n = 80) | Statistics | P |
|---|---|---|---|---|
| ΔmBP (mmHg) | −4.00 (7.00) | −4.00 (9.00) | −0.860* | 0.390 |
| ΔmPAP (mmHg) | −5.00 (7.00) | −7.00 (8.75) | −1.224* | 0.221 |
| ΔPVR (dyn·s−1·m−5) | −233.00 (226.50) | −212.50 (319.00) | −0.094* | 0.925 |
| ΔSVR (dyn·s−1·m−5) | −309.00 (537.50) | −292.00 (516.00) | −0.253* | 0.801 |
| ΔCO (L/min) | 0.39 ± 0.65 | 0.28 ± 0.62 | 1.239† | 0.217 |
| ΔPaO2 (mmHg) | −5.00 (10.50) | −5.25 (12.88) | −0.145* | 0.885 |
| ΔPvO2 (mmHg) | 0 (5.08) | −0.20 (5.90) | −0.085* | 0.932 |
| ΔQs/Qt (%) | 5.57 ± 10.07 | 6.18 ± 11.68 | −0.360† | 0.719 |
Data are presented as mean ± SD or median (interquartile range). “Δ”: The change in the index after iloprost inhalation. *Z value; †t value. The independent-sample t-test was used to analyze the date of normal distribution. The rank sum test of two independent samples (Mann-Whitney U-test) was used to analyze the date of nonnormal distribution. CTEPH: Chronic thromboembolic pulmonary hypertension; PAH: Pulmonary arterial hypertension; AVT: Acute vasoreactivity testing; CO: Cardiac output; mBP: Mean blood pressure; mPAP: Mean pulmonary arterial pressure; PaO2: Partial pressure of oxygen in arterial blood; PvO2: Mixed venous oxygen partial pressure; PVR: Pulmonary vascular resistance; Qs/Qt: Intrapulmonary shunt; SaO2: Arterial oxygen saturation; SvO2: Mixed venous oxygen saturation; SVR: Systemic vascular resistance; SD: Standard deviation. 1 mmHg = 0.133 kPa.
Logistic regression analysis of the relationships between the baseline data and rates of positive response to acute vasoreactivity testing in the two groups
The rate of positive response to AVT in the CTEPH group was 14.3%, and the baseline data of AVT-positive patients significantly differed from those of the AVT-negative patients. For example, the levels of NT-proBNP, mPAP, PVR, and SVR were significantly lower among AVT-positive patients, while the levels of SvO2, PvO2, and CO were significantly higher [Table 2]. Regression analysis of the baseline data of the two subgroups showed that a positive response to AVT in the CTEPH group was significantly correlated with levels of NT-proBNP, mPAP, PVR, CO, and PvO2 (P < 0.05; Table 5). In contrast, in the PAH group, a positive response to AVT was not correlated with any of these factors (P > 0.05; Supplementary Table 3).
Table 5.
Logistic regression analysis of relationships between a positive response to acute vasoreactivity testing in patients with chronic thromboembolic pulmonary hypertension and baseline index
| Factors | Regression coefficient | OR | 95% CI | P |
|---|---|---|---|---|
| Body mass index (kg/m2) | 0.017 | 1.017 | 0.828–1.250 | 0.869 |
| NT-proBNP (ng/L) | 0.001 | 1.001 | 1.000–1.003 | 0.036 |
| mPAP (mmHg) | −0.200 | 0.819 | 0.687–0.975 | 0.025 |
| PVR (dyn·s−1·m−5) | 0.018 | 1.018 | 1.006–1.030 | 0.003 |
| SVR (dyn·s−1·m−5) | <0.001 | 0.999 | 0.997–1.002 | 0.554 |
| CO (L/min) | 2.563 | 12.972 | 1.096–153.528 | 0.042 |
| SvO2 (%) | 0.049 | 1.050 | 0.930–1.186 | 0.428 |
| PvO2 (mmHg) | −0.187 | 0.829 | 0.734–0.937 | 0.003 |
CO: Cardiac output; mPAP: Mean pulmonary arterial pressure; NT-proBNP: N-terminal pro-brain natriuretic peptide; OR: Odds ratio; PvO2: Mixed venous oxygen partial pressure; PVR: Pulmonary vascular resistance; SVR: Systemic vascular resistance; SvO2: Mixed venous oxygen saturation; CI: Confidence interval. 1 mmHg = 0.133 kPa.
Supplementary Table 3.
Logistic regression analysis of relationships between a positive response to AVT in patients with PAH and each index in baseline data
| Factors | Regression coefficient | OR | 95% CI | P |
|---|---|---|---|---|
| Body mass index (kg/m2) | −1.340 | 0.875 | 0.680–1.124 | 0.296 |
| NT-proBNP (ng/L) | <0.010 | 1.000 | 0.999–1.001 | 0.793 |
| mPAP (mmHg) | −0.100 | 0.990 | 0.792–1.062 | 0.782 |
| PVR (dyn·s−1·m−5) | <0.010 | 1.000 | 0.997–1.004 | 0.840 |
| SVR (dyn·s−1·m−5) | 0.001 | 1.001 | 0.997–1.003 | 0.531 |
| CO (L/min) | 0.307 | 1.359 | 0.422–4.373 | 0.607 |
| SvO2 (%) | <0.010 | 0.999 | 0.904–1.105 | 0.989 |
| PvO2 (mmHg) | −0.020 | 0.980 | 0.908–1.057 | 0.597 |
CO: Cardiac output; mPAP: Mean pulmonary arterial pressure; NT-proBNP: N-terminal pro-brain natriuretic peptide; OR: Odds ratio; PvO2: Mixed venous oxygen partial pressure; PVR: Pulmonary vascular resistance; SvO2: Mixed venous oxygen saturation; SVR: Systemic vascular resistance; CI: Confidence interval; AVT: Acute vasoreactivity testing; PAH: Pulmonary arterial hypertension. 1 mmHg = 0.133 kPa.
Predictive ability and cutoff value of the acute vasoreactivity testing-positive model in patients with chronic thromboembolic pulmonary hypertension
Levels of NT-proBNP, mPAP, PVR, CO, and PvO2 were used to predict the rates of positive response to AVT in patients with CTEPH using ROC curve analysis. The areas under the curve for these factors were 0.754, 0.644, 0.814, 0.745, and 0.730, respectively. The sensitivity and specificity for predicting a positive response to AVT in patients with CTEPH was higher when the cutoff values of NT-proBNP, mPAP, PVR, CO, and PvO2 were 1131.000 ng/L, 44.500 mmHg, 846.500 dyn·s−1·m−5, 3.475 L/min, and 35.150 mmHg, respectively [Table 6 and Figures 2, 3].
Table 6.
Area under the ROC curve and cutoff values of patients with chronic thromboembolic pulmonary hypertension
| Factors | AUC | 95% CI | P | Cutoff value | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| NT-proBNP (ng/L) | 0.754 | 0.648–0.860 | <0.001 | 1131.000 | 0.621 | 0.800 |
| mPAP (mmHg) | 0.644 | 0.505–0.783 | 0.039 | 44.500 | 0.847 | 0.450 |
| PVR (dyn·s−1·m−5) | 0.814 | 0.725–0.903 | <0.001 | 846.500 | 0.798 | 0.700 |
| CO (L/min) | 0.745 | 0.650–0.839 | 0.001 | 3.475 | 0.842 | 0.661 |
| PvO2 (mmHg) | 0.730 | 0.626–0.834 | 0.001 | 35.150 | 0.947 | 0.512 |
AUC: Area under the curve; CO: Cardiac output; mPAP: Mean pulmonary arterial pressure; NT-proBNP: N-terminal pro-brain natriuretic peptide; PvO2: Mixed venous oxygen partial pressure; PVR: Pulmonary vascular resistance; CI: Confidence interval; ROC: Receiver operating characteristic. 1 mmHg = 0.133 kPa.
Figure 2.
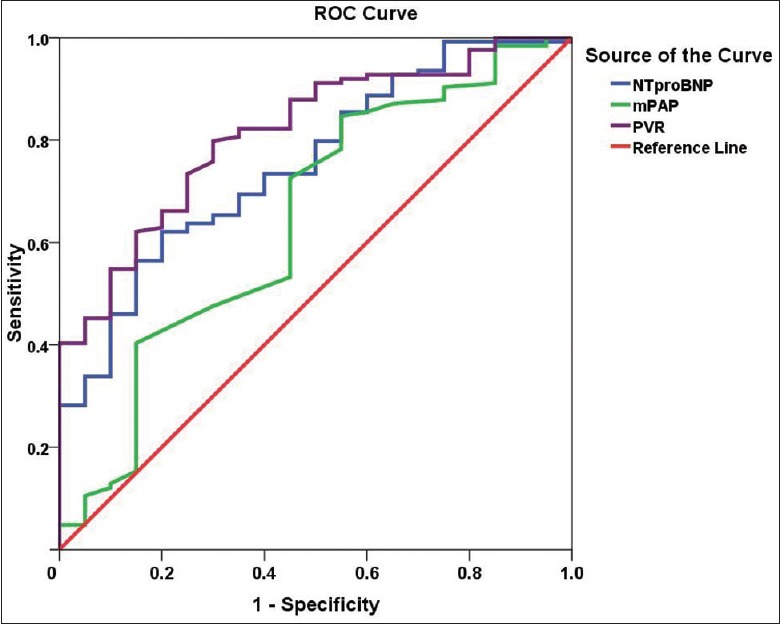
ROC curve derived from the logistic regression analysis of levels of NT-proBNP, mPAP, and PVR for predicting a positive response to AVT in patients with CTEPH. Note: the NT-proBNP curve is blue, the mPAP curve is green, and the PVR curve is purple. ROC analysis for NT-proBNP (P < 0.001): area under the ROC curve, 0.754; sensitivity, 62.1%; specificity, 80.0% for a cutoff of 1131.000 ng/L. ROC analysis for mPAP (P = 0.039): area under the ROC curve, 0.664; sensitivity, 84.7%; specificity, 45.0% for a cutoff of 44.500 mmHg. ROC analysis for PVR (P < 0.001): area under the ROC curve, 0.814; sensitivity, 79.8%; specificity, 70.0% for a cutoff of 846.500 dyn·s−1·m−5. AVT: Acute vasoreactivity testing; ROC: Receiver operating characteristic; CTEPH: Chronic thromboembolic pulmonary hypertension; mPAP: Mean pulmonary arterial pressure; NT-proBNP: N-terminal pro-brain natriuretic peptide; PVR: Pulmonary vascular resistance.
Figure 3.
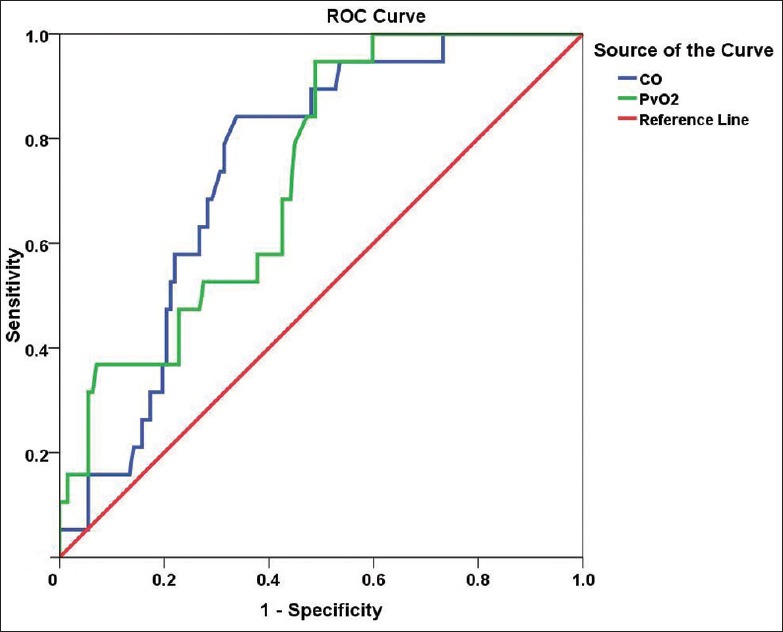
ROC curve derived from the logistic regression analysis of levels of CO and PvO2 for predicting a positive response to AVT in patients with CTEPH. Note: the CO curve is blue and the PvO2 curve is green. ROC analysis for CO (P = 0.001): area under the ROC curve, 0.745; sensitivity, 84.2%; specificity, 66.1% for a cutoff of 3.475 L/min. ROC analysis for PvO2 (P = 0.001): area under the ROC curve, 0.730; sensitivity, 94.7%; specificity, 51.2% for a cutoff of 35.150 mmHg. AVT: Acute vasoreactivity testing; CO: Cardiac output; CTEPH: Chronic thromboembolic pulmonary hypertension; ROC: Receiver operating characteristic; PvO2: Mixed venous oxygen partial pressure.
Treatment and follow-up
Regular telephone follow-up was conducted for 175 patients with CTEPH. As of July 2016, 57 patients had died, six were lost to follow-up, and the rest were alive. The median survival time was 9 years. Of the 25 patients with a positive response to AVT, seven received only basic therapy (warfarin anticoagulation, diuretics, or digoxin). In addition to the basic treatment, two patients also received targeted drug therapy (riociguat in the first case, sildenafil and bosentan in the second case). The remaining 14 patients also received CCBs (diltiazem in 13 cases and nifedipine in one case), while two patients additionally underwent PEA surgery. Of the 150 patients with a negative response to AVT, 78 received only basic treatment, while 45 also received targeted drugs (sildenafil in 22 cases, tadalafil in nine cases, bosentan in six cases, ambrisentan in five cases, beraprost in three cases, riociguat in six cases, and double-targeted drug therapy in six cases). Twenty-seven patients also underwent PEA surgery, and no patients were treated with CCBs [Table 7]. The follow-up data were analyzed using Kaplan-Meier survival curves and the log-rank test. The survival time in patients with a negative response to AVT was somewhat shorter than that in AVT-responders although the difference was not statistically significant (χ2 = 3.613, P = 0.057, Figure 4). The follow-up revealed that about 67% of patients who underwent PEA survived and had mostly normal physical recovery and excellent quality of life. The survival time of patients with CTEPH who received CCBs was longer than that in the group with only basic treatment and not shorter than that of patients who received targeted drugs or underwent PEA, although there was no significant difference in survival between the four different treatment regimens (χ2 = 3.069, P = 0.381, Figure 5). A Cox regression model was used for multivariate analysis of the survival time of patients with CTEPH, which included age (≥60 and <60 years), sex, mPAP level (26–35, 36–45, ≥46), 6-min walking distance level (≥400 m and <400 m), World Health Organization functional class (WHO-FC I–II, III, and IV), response to AVT (positive versus negative), and treatment strategy (basic treatment, basic treatment + targeted drugs treatment, basic treatment + CCB treatment, basic treatment + PEA) [Supplementary Table 4]. The analysis revealed that WHO-FC was the strongest factor affecting survival time of patients with CTEPH, with the risk of death in patients with WHO FC I–II significantly lower than that in patients with WHO-FC III or IV (relative risk [RR] = 0.108, P < 0.001 and RR = 0.147, P < 0.001, respectively, Figure 6).
Table 7.
Summary of treatment and follow-up for patients with CTEPH
| Groups | Treatment | Total (n) | Died (n) | Survived (n) | Lost to follow-up (n) |
|---|---|---|---|---|---|
| AVT-positive | Basic treatment | 7 | 1 | 6 | 0 |
| Basic treatment + targeted drugs | 2 | 0 | 2 | 0 | |
| Basic treatment + CCBs | 14 | 3 | 10 | 1 | |
| Basic treatment + PEA | 2 | 0 | 2 | 0 | |
| AVT-negative | Basic treatment | 78 | 32 | 42 | 4 |
| Basic treatment + targeted drugs | 45 | 12 | 32 | 1 | |
| Basic treatment + CCBs | 0 | 0 | 0 | 0 | |
| Basic treatment + PEA | 27 | 9 | 18 | 0 | |
| Overall | 175 | 57 | 112 | 6 | |
AVT: Acute vasoreactivity testing; CCBs: Calcium channel blockers; CTEPH: Chronic thromboembolic pulmonary hypertension; PEA: Pulmonary endarterectomy.
Figure 4.
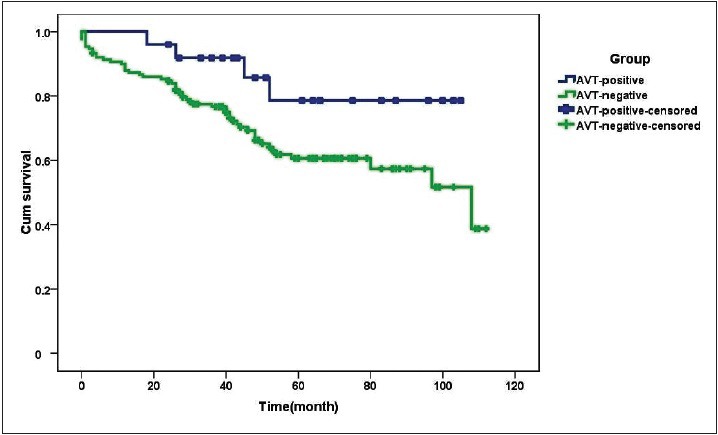
Survival curves of patients with CTEPH with a positive and negative response to AVT (n = 175). The curve for AVT responders is blue, whereas that for AVT-nonresponders is green. The follow-up data were analyzed using Kaplan-Meier survival curves and the log-rank test. The survival time in patients with a negative response to AVT was somewhat shorter than that in AVT-responders although the difference was not statistically significant (χ2 = 3.613, P = 0.057). AVT: Acute vasoreactivity testing; CTEPH: Chronic thromboembolic pulmonary hypertension.
Figure 5.
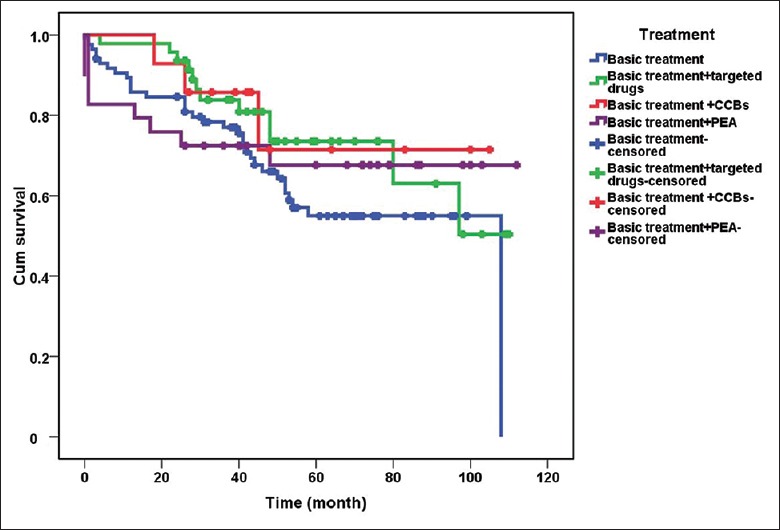
Survival curves of patients with CTEPH depending on treatment strategy (n = 175). The curves for basic treatment, targeted drugs treatment, CCB treatment, and PEA are blue, green, red and purple, respectively. About 67% of patients who underwent PEA survived and had mostly normal physical recovery and excellent quality of life. The survival time of patients with CTEPH who received CCBs was longer than that in the group with only basic treatment and not shorter than that of patients who received targeted drugs or underwent PEA, although there was no significant difference in survival between the four different treatment regimens (χ2 = 3.069, P = 0.381). CCB: Calcium channel blocker; CTEPH: Chronic thromboembolic pulmonary hypertension; PEA: Pulmonary endarterectomy.
Supplementary Table 4.
Cox’s regression model for multivariate analysis of the survival time of patients with CTEPH
| Factors | Exp(B) | 95% CI for Exp(B) | P |
|---|---|---|---|
| Age (years) | 1.597 | 0.914–2.790 | 0.100 |
| Gender | 1.805 | 0.991–3.286 | 0.053 |
| Treatment | 0.461 | ||
| Treatment (1) | 1.209 | 0.547–2.673 | 0.639 |
| Treatment (2) | 0.737 | 0.298–1.823 | 0.509 |
| Treatment (3) | 2.279 | 0.211–24.656 | 0.498 |
| Group | 0.207 | 0.028–1.541 | 0.124 |
| 6MWD | 1.049 | 0.521–2.111 | 0.894 |
| WHO-FC | <0.001 | ||
| WHO-FC (1)* | 0.126 | 0.049–0.319 | <0.001 |
| WHO-FC (2)* | 0.182 | 0.074–0.445 | <0.001 |
| mPAP | 0.540 | ||
| mPAP (1) | 0.460 | 0.061–3.470 | 0.452 |
| mPAP (2) | 1.266 | 0.675–2.375 | 0.463 |
*The difference is significant at the 0.05 level. Treatment, 1: Basic treatment, 2: Basic treatment + targeted drugs treatment, 3: Basic treatment + CCB treatment, WHO-FC, 1: WHO-FC I–II, 2: WHO-FC III, mPAP, 1: 26≤ mPAP ≤35, 2: 36≤ mPAP ≤45. The analysis revealed that WHO-FC was the strongest factor affecting survival time of patients with CTEPH, with the risk of death in patients with WHO FC I–II significantly lower than that in patients with WHO-FC III or IV, P < 0.001. 6MWD: 6-min walking distance; CTEPH: Chronic thromboembolic pulmonary hypertension; mPAP: Mean pulmonary arterial pressure; WHO-FC: World Health Organization functional class; RR: Relative risk; CI: Confidence interval; PEA: Pulmonary endarterectomy; CCB: Calcium channel blocker.
Figure 6.
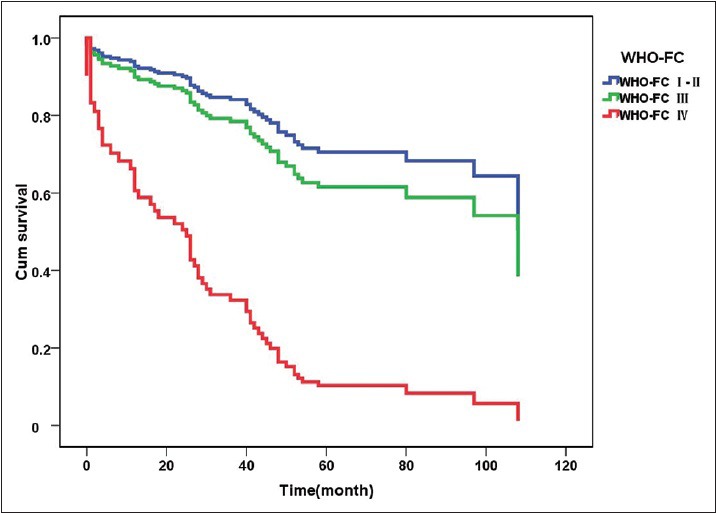
Survival curves of patients with CTEPH with different WHO-FC (n = 175). The curve for WHO-FC I–II is blue, the curve for WHO-FC III is green, and the curve for WHO-FC IV is red. A Cox regression model was used for multivariate analysis of survival time of patients with CTEPH. WHO-FC was the strongest factor affecting survival time of patients with CTEPH, with the risk of death in patients with WHO FC I–II significantly lower than that in patients with WHO-FC III or IV (WHO-FC I–II versus WHO-FC III: RR = 0.108, 95% CI: 0.045–0.258, P < 0.001; WHO-FC I–II versus WHO-FC IV: RR = 0.147, 95% CI: 0.062–0.347. P < 0.001). CTEPH: Chronic thromboembolic pulmonary hypertension; WHO-FC: World Health Organization functional class. RR: Relative risk; CI: Confidence interval.
Discussion
AVT is based on the theory of reversible pulmonary vasoconstriction. The agents for AVT should act selectively on the pulmonary artery, have a short half-life, and have only a minor effect on the systemic circulation. Currently, there is no preferred agent for AVT, with intravenous epoprostenol, adenosine, and inhaled nitric oxide being widely used.[21] However, in China, the above three drugs are difficult to obtain or inconvenient to use. Iloprost is a stable prostacyclin analog which, when nebulized, decreases PVR and PAP and increases CO. Following recommendations in the literature,[22,23,24,25] inhaled iloprost was used for AVT in the present study. In the subgroups with a positive response to AVT, mPAP, PVR, mBP, and SVR decreased significantly while CO increased significantly immediately after iloprost inhalation, which was consistent with published results.[26] The present study demonstrated that inhalation of iloprost was followed by a transient deterioration of Qs/Qt in both groups. However, this might have been caused by a significant increase in CO after inhalation, which to some extent compensated for the effects of decreased oxygenation on the body's oxygen supply, and might be the reason for the observed increase of SvO2. Therefore, iloprost inhalation appears to be safe for AVT.
AVT plays a very important role in predicting the efficiency of CCB treatment in patients with PH. Patients with PAH who respond to AVT appear to have a considerable number of pulmonary arteries in the spastic state and may benefit from CCB treatment. In contrast, nonresponders will receive no benefit from CCB treatment and might even experience serious, potentially life-threatening adverse reactions in the process.[27,28,29]
In this study, patients with CTEPH had Δ mBP, ΔmPAP, ΔPVR, ΔSVR, ΔCO, ΔSaO2, ΔSvO2, ΔPaO2, ΔPvO2, and ΔQs/Qt after inhalation of iloprost similar to those of patients with PAH. This finding suggests similarities between the two groups in terms of reversible pulmonary artery contraction. In the cohort, in which all patients inhaled iloprost, the rate of positive response to AVT among patients with CTEPH (14.3%) was higher than that among patients with PAH (11.3%), but the difference was not statistically significant. Our criteria for positive response to AVT were consistent with those of Ulrich et al.,[25] who conducted AVT in 57 patients with PH (22 with CTEPH and 35 with PAH) and obtained rates of positive response to AVT of 17% in individuals with PAH and only 5% in individuals with CTEPH after inhalation of iloprost. A much higher rate of positive response to AVT in patients with CTEPH obtained in this study may be a consequence of differences in patient selection since the study included more patients with mild symptoms or better laboratory findings. Kramm et al.[30,31] demonstrated that inhalation of iloprost reduced residual PH after surgery and reduced the load in the right ventricle. A positive response to AVT indicates that the elevated pulmonary vascular tone contributes to the pathophysiological mechanism of CTEPH, and therefore, CCB treatment may be effective. The results also showed that a positive response to AVT in patients with CTEPH was significantly correlated with lower levels of NT-proBNP, mPAP, and PVR, and higher levels of PvO2 and CO, which declared the AVT responders in the CTEPH group significantly correlated with milder disease. Therefore, it was presumed that the pathological basis of patients with milder CTEPH mainly involved pulmonary vasoconstriction.
In this study, survival was better in patients with CTEPH who had a positive response to AVT than in those with a negative response although the difference was not statistically significant. Of the 25 patients with a positive response to AVT, 14 received CCBs, of which three died, one dropped out, and the rest were alive. The follow-up revealed that these patients had a better quality of life than patients receiving only basic treatment. Survival analysis of the 175 patients with CTEPH showed that the survival time of patients receiving CCBs was greater than that of patients receiving basic treatment only and not lower than that of patients receiving targeted drugs or PEA, although no significant differences were found between the four groups. Both the literature and guidelines[2,11,12] recommend that patients with CTEPH should be evaluated as early as possible because PEA is a potentially curative treatment for CTEPH. In this study, 29 patients underwent PEA. One-third of them died within half a year, whereas the rest were almost entirely cured, with two patients with a positive response to AVT reaching a complete recovery. The high short-term mortality in patients with CTEPH after PEA surgery may be a consequence of low survival of patients with a WHO-FC III or IV. In this regard, the Cox survival analysis suggested that the prognosis was related to WHO-FC, with a WHO-FC of III or IV being an independent risk factor for decreased survival time in patients with CTEPH.
The results of the current study should be interpreted within the constraint of several potential limitations. There were only 14 patients with a positive response to AVT who were treated with CCBs and showed better survival than individuals who received basic treatment only, and their survival time was not different from that of patients receiving targeted drug treatment or PEA. Therefore, further research, such as a randomized controlled study, is necessary to validate the results.
In conclusion, it was demonstrated that the rates of positive response to AVT were similar in patients with CTEPH and PAH. There were common trends in the changes of hemodynamics and oxygenation dynamics indices in the PAH and CTEPH groups after iloprost inhalation. A positive response to AVT in the CTEPH group was significantly correlated with milder disease and tended to be correlated with better survival. Patients with CTEPH who cannot undergo PEA or receive targeted therapy might benefit from CCB treatment if they have a positive response to AVT.
Supplementary information is linked to the online version of the paper on the Chinese Medical Journal website.
Financial support and sponsorship
This study was supported by grants from the China Key Research Projects of the 12th National Five-Year Development Plan (No. 2011BAI11B17, and No. 2013BAI09B00), and the Key Project of Natural Science Research of Colleges and Universities in Anhui Province in 2015 (No. KJ2015A159).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Peng Lyu
References
- 1.Tuder RM, Archer SL, Dorfmüller P, Erzurum SC, Guignabert C, Michelakis E, et al. Relevant issues in the pathology and pathobiology of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D4–12. doi: 10.1016/j.jacc.2013.10.025. doi: 10.1016/j.jacc.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Respir J. 2015;46:903–75. doi: 10.1183/13993003.01032-2015. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 3.Pengo V, Lensing AW, Prins MH, Marchiori A, Davidson BL, Tiozzo F, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350:2257–64. doi: 10.1056/NEJMoa032274. doi: 10.1056/NEJMoa032274. [DOI] [PubMed] [Google Scholar]
- 4.Becattini C, Agnelli G, Pesavento R, Silingardi M, Poggio R, Taliani MR, et al. Incidence of chronic thromboembolic pulmonary hypertension after a first episode of pulmonary embolism. Chest. 2006;130:172–5. doi: 10.1378/chest.130.1.172. doi: 10.1378/chest.130.1.172. [DOI] [PubMed] [Google Scholar]
- 5.Dentali F, Donadini M, Gianni M, Bertolini A, Squizzato A, Venco A, et al. Incidence of chronic pulmonary hypertension in patients with previous pulmonary embolism. Thromb Res. 2009;124:256–8. doi: 10.1016/j.thromres.2009.01.003. doi: 10.1016/j.thromres.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Poli D, Grifoni E, Antonucci E, Arcangeli C, Prisco D, Abbate R, et al. Incidence of recurrent venous thromboembolism and of chronic thromboembolic pulmonary hypertension in patients after a first episode of pulmonary embolism. J Thromb Thrombolysis. 2010;30:294–9. doi: 10.1007/s11239-010-0452-x. doi: 10.1007/s11239-010-0452-x. [DOI] [PubMed] [Google Scholar]
- 7.den Exter PL, van der Hulle T, Lankeit M, Huisman MV, Klok FA. Long-term clinical course of acute pulmonary embolism. Blood Rev. 2013;27:185–92. doi: 10.1016/j.blre.2013.06.003. doi: 10.1016/j.blre.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Ley S. Imaging pulmonary arterial thromboembolism: Challenges and opportunities. Magn Reson Imaging Clin N Am. 2015;23:261–71. doi: 10.1016/j.mric.2015.01.013. doi: 10.1016/j.mric.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Dong C, Zhou M, Liu D, Long X, Guo T, Kong X. Diagnostic accuracy of computed tomography for chronic thromboembolic pulmonary hypertension: A systematic review and meta-analysis. PLoS One. 2015;10:e0126985. doi: 10.1371/journal.pone.0126985. doi: 10.1371/journal.pone.0126985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Armini AM. Diagnostic advances and opportunities in chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2015;24:253–62. doi: 10.1183/16000617.00000915. doi: 10.1183/16000617.00000915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jenkins D. Pulmonary endarterectomy: The potentially curative treatment for patients with chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2015;24:263–71. doi: 10.1183/16000617.00000815. doi: 10.1183/16000617.00000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Machuca T, de Perrot M. When to refer a patient with chronic thromboembolic pulmonary hypertension for pulmonary endarterectomy. Can J Cardiol. 2015;31:509–14. doi: 10.1016/j.cjca.2015.01.042. doi: 10.1016/j.cjca.2015.01.042. [DOI] [PubMed] [Google Scholar]
- 13.Roig Figueroa V, Herrero Pérez A, de la Torre Ferrera N, Hernández García E, Aller Alvarez JL, Para Cabello J. Iloprost for chronic thromboembolic pulmonary hypertension. Arch Bronconeumol. 2004;40:326–8. doi: 10.1016/S1579-2129(06)60310-8. [PubMed] [Google Scholar]
- 14.Suntharalingam J, Treacy CM, Doughty NJ, Goldsmith K, Soon E, Toshner MR, et al. Long-term use of sildenafil in inoperable chronic thromboembolic pulmonary hypertension. Chest. 2008;134:229–36. doi: 10.1378/chest.07-2681. doi: 10.1378/chest.07-2681. [DOI] [PubMed] [Google Scholar]
- 15.Becattini C, Manina G, Busti C, Gennarini S, Agnelli G. Bosentan for chronic thromboembolic pulmonary hypertension: Findings from a systematic review and meta-analysis. Thromb Res. 2010;126:e51-6. doi: 10.1016/j.thromres.2010.01.007. doi: 10.1016/j.thromres.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Ghofrani HA, D’Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369:319–29. doi: 10.1056/NEJMoa1209657. doi: 10.1056/NEJMoa1209657. [DOI] [PubMed] [Google Scholar]
- 17.Hoeper MM. Pharmacological therapy for patients with chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2015;24:272–82. doi: 10.1183/16000617.00001015. doi: 10.1183/16000617.00001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Firth AL, Yao W, Ogawa A, Madani MM, Lin GY, Yuan JX. Multipotent mesenchymal progenitor cells are present in endarterectomized tissues from patients with chronic thromboembolic pulmonary hypertension. Am J Physiol Cell Physiol. 2010;298:C1217–25. doi: 10.1152/ajpcell.00416.2009. doi: 10.1152/ajpcell.00416.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: A report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: Developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc. and the Pulmonary Hypertension Association. Circulation. 2009;119:2250–94. doi: 10.1161/CIRCULATIONAHA.109.192230. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Sitbon O, Humbert M, Jaïs X, Ioos V, Hamid AM, Provencher S, et al. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation. 2005;111:3105–11. doi: 10.1161/CIRCULATIONAHA.104.488486. doi: 10.1161/CIRCULATIONAHA.104.488486. [DOI] [PubMed] [Google Scholar]
- 21.Tonelli AR, Alnuaimat H, Mubarak K. Pulmonary vasodilator testing and use of calcium channel blockers in pulmonary arterial hypertension. Respir Med. 2010;104:481–96. doi: 10.1016/j.rmed.2009.11.015. doi: 10.1016/j.rmed.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 22.Zhang HL, Liu ZH, Wang Y, Xiong CM, Ni XH, He JG, et al. Acute responses to inhalation of Iloprost in patients with pulmonary hypertension. Chin Med J. 2012;125:2826–31. doi: 10.3760/cma.j.issn.0366-6999.2012.16.005. [PubMed] [Google Scholar]
- 23.Reichenberger F, Mainwood A, Doughty N, Fineberg A, Morrell NW, Pepke-Zaba J. Effects of nebulised iloprost on pulmonary function and gas exchange in severe pulmonary hypertension. Respir Med. 2007;101:217–22. doi: 10.1016/j.rmed.2006.05.019. doi: 10.1016/j.rmed.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 24.Leuchte HH, Baezner CJ, Baumgartner RA, Mernitz P, Neurohr C, Behr J. Acute hemodynamic responses to supplemental oxygen and their prognostic implications in pulmonary hypertension. Respiration. 2013;85:400–7. doi: 10.1159/000340009. doi: 10.1159/000340009. [DOI] [PubMed] [Google Scholar]
- 25.Ulrich S, Fischler M, Speich R, Popov V, Maggiorini M. Chronic thromboembolic and pulmonary arterial hypertension share acute vasoreactivity properties. Chest. 2006;130:841–6. doi: 10.1378/chest.130.3.841. doi: 10.1378/chest.130.3.841. [DOI] [PubMed] [Google Scholar]
- 26.Krug S, Hammerschmidt S, Pankau H, Wirtz H, Seyfarth HJ. Acute improved hemodynamics following inhaled iloprost in chronic thromboembolic pulmonary hypertension. Respiration. 2008;76:154–9. doi: 10.1159/000107977. doi: 10.1159/000107977. [DOI] [PubMed] [Google Scholar]
- 27.Medarov BI, Judson MA. The role of calcium channel blockers for the treatment of pulmonary arterial hypertension: How much do we actually know and how could they be positioned today? Respir Med. 2015;109:557–64. doi: 10.1016/j.rmed.2015.01.004. doi: 10.1016/j.rmed.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Kurzyna M, Araszkiewicz A, Blaszczak P, Grabka M, Hawranek M, Kopec G, et al. Summary of recommendations for the haemodynamic and angiographic assessment of the pulmonary circulation. Joint statement of the Polish Cardiac Society's Working Group on Pulmonary Circulation and Association of Cardiovascular Interventions. Kardiol Pol. 2015;73:63–8. doi: 10.5603/KP.2015.0011. doi: 10.5603/KP.2015.0011. [DOI] [PubMed] [Google Scholar]
- 29.Montani D, Savale L, Natali D, Jaïs X, Herve P, Garcia G, et al. Long-term response to calcium-channel blockers in non-idiopathic pulmonary arterial hypertension. Eur Heart J. 2010;31:1898–907. doi: 10.1093/eurheartj/ehq170. doi: 10.1093/eurheartj/ehq170. [DOI] [PubMed] [Google Scholar]
- 30.Kramm T, Eberle B, Guth S, Mayer E. Inhaled iloprost to control residual pulmonary hypertension following pulmonary endarterectomy. Eur J Cardiothorac Surg. 2005;28:882–8. doi: 10.1016/j.ejcts.2005.09.007. doi: 10.1016/j.ejcts.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Kramm T, Eberle B, Krummenauer F, Guth S, Oelert H, Mayer E. Inhaled iloprost in patients with chronic thromboembolic pulmonary hypertension: Effects before and after pulmonary thromboendarterectomy. Ann Thorac Surg. 2003;76:711–8. doi: 10.1016/s0003-4975(03)00728-8. doi: 10.1016/s0003-4975(03)00728-8. [DOI] [PubMed] [Google Scholar]