Abstract
The evolution of the capsular biosynthetic (cps) locus of serogroup 6 Streptococcus pneumoniae was investigated by analyzing sequence variation within three serotype-specific cps genes from 102 serotype 6A and 6B isolates. Sequence variation within these cps genes was related to the genetic relatedness of the isolates, determined by multilocus sequence typing, and to the inferred patterns of recent evolutionary descent, explored using the eBURST algorithm. The serotype-specific cps genes had a low percent G+C, and there was a low level of sequence diversity in this region among serotype 6A and 6B isolates. There was also little sequence divergence between these serotypes, suggesting a single introduction of an ancestral cps sequence, followed by slight divergence to create serotypes 6A and 6B. A minority of serotype 6B isolates had cps sequences (class 2 sequences) that were ∼5% divergent from those of other serotype 6B isolates (class 1 sequences) and which may have arisen by a second, more recent introduction from a related but distinct source. Expression of a serotype 6A or 6B capsule correlated perfectly with a single nonsynonymous polymorphism within wciP, the rhamnosyl transferase gene. In addition to ample evidence of the horizontal transfer of the serotype 6A and 6B cps locus into unrelated lineages, there was evidence for relatively frequent changes from serotype 6A to 6B, and vice versa, among very closely related isolates and examples of recent recombinational events between class 1 and 2 cps serogroup 6 sequences.
The capsular biosynthetic loci (cps) of encapsulated bacteria typically have a modular construction, with central genes that specify the immunochemical differences between capsular serotypes flanked by genes common to all or several serotypes (2, 10). In pneumococci, the central cps genes have a low percent G+C content compared to the flanking cps genes and the rest of the genome and may have been acquired by horizontal gene transfer from distantly related species (2, 10). Although it appears that the serotype-specific regions of the cps locus have been introduced into pneumococci on multiple occasions from unknown sources, the number of times this may have happened, and the time scale over which such events occurred, are obscure. Thus, we do not know if some serotypes are very much younger than others and whether new pneumococcal serotypes arise at intervals of a few decades, a few centuries, or many millennia. It is also not known why there are so many (90) different capsular types in pneumococci (12) or whether the emergence of new serotypes is positively selected for by the host immune system and, if so, the intensity of this selection. Similarly, we know little about the evolutionary relationships among the capsular loci of the immunologically cross-reactive serotypes within a serogroup. Are the serological similarities of the serotypes within a single serogroup a reflection of their divergence from a recent common ancestral serotype, or does convergence occur, in which the introduction of very different cps sequences has resulted in immunochemically similar capsular polysaccharides? The answers to many of these questions should start to emerge now that the sequences of the complete cps loci of all 90 serotypes are becoming available (1, 7, 11, 13, 14, 17, 20-25, 31, 32; http://www.sanger.ac.uk/Projects/S_pneumoniae/CPS/).
The evolution of individual serotypes can be addressed by exploring the patterns of sequence diversity among isolates of a single serotype and relating these to the levels of genetic relatedness and patterns of evolutionary descent of the isolates. The evolution of the cps locus is likely to be complicated. Many of the cps genes are common to several serotypes, so a history of recombination among these homologs may confound attempts to discern ancestry. However, one or more genes within the serotype-specific region of the cps locus often have no significant nucleotide sequence similarity with the cps genes of other pneumococcal serotypes or serogroups or with any other genes in the sequence databases, although their gene products may have homology with enzymes involved in polysaccharide biosynthesis in other species (18). Unlike the flanking cps genes, in which sequence variation can arise by point mutation or by homologous recombinational replacements from the homologs in other serotypes, the accumulation of variation within a serotype-specific gene can occur initially only by point mutation and, once some variation has accumulated, by recombination between different isolates of the same serotype.
Serotype-specific cps genes that have no significant sequence similarity to genes in other pneumococcal serotypes or serogroups can therefore be used to study the evolution of a serotype or of the multiple serotypes within a multitypic serogroup. In this paper, we explore the origins of the two serotypes (6A and 6B) within pneumococcal serogroup 6 by analyzing patterns of sequence variation within the central cps region and relating the sequence variation within these genes to the genetic relatedness and inferred patterns of recent evolutionary descent of the isolates. The structures of the capsular polysaccharides of serotype 6A and 6B are identical, except for the linkage between the l-rhamnopyranosyl and d-ribitol residues in the tetrasaccharide repeat unit, which is 1→3 in serotype 6A but 1→4 in serotype 6B (15, 16, 27). In addition to showing surprisingly frequent interconversion between serotypes 6A and 6B among closely related isolates and the spread of serogroup 6 sequences to distantly related pneumococcal lineages, we demonstrate that the difference between serotypes 6A and 6B correlates with a single nonsynonymous substitution in the putative rhamnosyl transferase gene (wciP).
MATERIALS AND METHODS
Bacterial isolates.
A group of 56 serogroup 6 isolates were from the study by Robinson et al. (28), selected to cover the diversity of serotype 6A and 6B isolates among those clonal complexes in which isolates of both serotypes were observed and all available examples of serogroup 6 isolates that were identical, or very closely related, in genotype by multilocus sequence typing (MLST) but which differed in serotype (6A versus 6B). That study used an MLST scheme that characterized loci different from those in the standard pneumococcal MLST scheme, and the selected isolates from the study were therefore retyped using the standard MLST scheme of Enright and Spratt (8). The matrix of pairwise differences in the allelic profiles of all isolates of serotypes 6A and 6B within the public MLST database (http://spneumoniae.mlst.net), and of the additional isolates we selected and characterized by MLST from the study of Robinson et al. (28), was used to construct a dendrogram (data not shown). The implied genetic relatedness of these isolates was used to select additional isolates from our collection at Imperial College that further covered the diversity of sequence types (STs) of these two serotypes. In addition, we analyzed all isolates of serogroup 6 in the Imperial College collection that were identical by MLST or differed at only one MLST locus (single-locus variants [SLVs]) but whose serotypes varied. The final set of 102 serogroup 6 isolates chosen for further study included 43 isolates of serotype 6A and 59 isolates of serotype 6B.
Characterization of isolates.
Chromosomal DNA from each isolate was prepared, and MLST was carried out using the seven pneumococcal housekeeping loci and primers described previously (8). For each locus, sequences were obtained on both DNA strands using an ABI3700 DNA sequencer (Applied Biosystems, Warrington, United Kingdom). Alleles at each locus were assigned by interrogating the pneumococcal MLST website (http://spneumoniae.mlst.net). The trace files of sequences that were different from those of all known alleles were carefully checked and were assigned new allele numbers. All of the isolates have been deposited in the MLST database. Serotyping was carried out using the Quellung reaction with sera purchased from the Statens Seruminstitut (Copenhagen, Denmark). Some isolates were cross-checked for serotype by inhibition enzyme-linked immunosorbent assay, as previously described (29). This test used two antibodies: Hyp6BM8 recognizes a cross-reactive epitope and binds with equal avidity to serotype 6A and 6B capsular polysaccharides, while Hyp6BM1 recognizes a serotype 6B-specific epitope and fails to bind to serotype 6A polysaccharide. The results were all concordant between the two serotyping systems.
The relatedness of isolates was examined by constructing a dendrogram from the matrix of pairwise differences in the allelic profiles of all isolates using the unweighted pair-group method with arithmetic averages (UPGMA). Nonoverlapping groups of related STs were identified using eBURST, with the default setting for the definition of groups (9; http://eburst.mlst.net/). With this definition, all STs assigned to the same eBURST group have alleles at ≥6 of the 7 MLST loci in common with at least one other member of the group, and the eBURST group is equated with a clonal complex in which all STs have probably descended from a single recent common ancestor or founding ST. The predicted founding ST of each eBURST group, and the predicted patterns of descent of all other STs in the group from the founder, were computed by eBURST and displayed as eBURST diagrams (9). STs that are directly linked to each other in eBURST diagrams differ at only a single locus (i.e., they are SLVs). For each ST in an eBURST group, the statistical support for being the founder of the group was assessed by the percent recovery of the ST as the founder in 1,000 bootstrap resamplings (9).
Analysis of the serogroup 6 cps locus.
The annotated sequences of the cps loci of two serotype 6A isolates deposited in GenBank (accession numbers AY078347 and AF246898) and of three serotype 6B isolates in GenBank (AF316640 [14], AF298581, and AF246897), and additional sequences of a serotype 6A and a 6B isolate from the Sanger Institute cps website (http://www.sanger.ac.uk/Projects/S_pneumoniae/CPS/), were aligned using the ClustalW algorithm within MEGA version 3.0 (19). Primers were designed from these aligned sequences that allowed internal fragments of three of the central cps genes of serotype 6A and 6B isolates to be amplified from each isolate by PCR and sequenced. The primers used both for PCR amplification and for sequencing were wciP-up, 5′-ATGGTGAGAGATATTTGTCAC-3′, and wciP-down, 5′-AGCATGATGGTATATAAGCC-3′, for the wciP gene; S6-wzy-F, 5′-CCTAAAGTGGAGGGAATTTCG-3′, and S6-wzy-R, 5′-CCTCCCATATAACGAGTGATG-3′, for the wzy gene; and S6-wzx-F, 5′-TTCGAATGGGAATTCAATGG-3′, and S6-wzx-R, 5′-GCGAGCCAAATCGGTAAGTA-3′, for the wzx gene. The PCRs were performed in a total volume of 50 μl, consisting of 2 μl of DNA lysate, 50 pmol of each primer, 200 μM deoxynucleoside triphosphates, and 2.5 U of Taq DNA polymerase in 1× PCR buffer (QIAGEN Ltd., Crawley, United Kingdom). The conditions used for amplification of the PCR products were a denaturation step at 95°C for 5 min and 30 cycles of 95°C for 1 min, 58°C for 30 s, and 72°C for 1 min, followed by a final extension at 72°C for 10 min in a PTC-200 Thermal Cycler (MJ Research Inc., Waltham, Mass.). Sequencing was carried out as for the MLST gene fragments.
For each cps gene fragment, the sequences from the different isolates were compared, and each different sequence was assigned as a distinct allele and given a different allele number. The allele numbers assigned for the three cps gene fragments (in the order wciP-wzy-wzx) defined the cps profile of the isolate. The sequences of the three cps fragments were concatenated, maintaining the +1 reading frame, and a tree was constructed from the concatenated sequences (1,614 bp), and from the individual gene fragments, by the neighbor-joining method using MEGA version 3.0 (19). Variable nucleotides and amino acids within the cps sequences and translated products were displayed using MEGA version 3.0.
One serotype 6B isolate (GenBank accession number AF246897) displayed a divergent sequence compared with the other serogroup 6 isolates, and in this isolate there was an ∼300-bp indel between the wciN and wciO genes. The presence or absence of the indel was established by the size of the intergenic fragment amplified using primers at the end of wciN (WCI-up, 5′-ATTTGGTGTACTTCCTCC-3′) and the start of wciO (WCI-down, 5′-CCATCCTTCGAGTATTGC-3′). The PCRs were performed as described above. A predicted 958-bp fragment was obtained for isolates without the indel, and a 1,267-bp fragment was obtained for those with the indel.
The structures of the cps loci of serotype 6A and 6B isolates, the similarities between the sequences, and the percent G+C content were visualized using the Artemis Comparison Tool developed by Kim Rutherford at the Sanger Institute (http://www.sanger.ac.uk/Software/ACT/).
RESULTS
Genetic relatedness of serotype 6A and 6B isolates.
Fig. 1 shows a UPGMA dendrogram based on the pairwise differences in the MLST allelic profiles of the 102 serotype 6A and 6B isolates selected for further study. There were 78 different STs. Many of the isolates were relatively distantly related to each other, but there were several clusters of isolates with closely related STs. Four STs included isolates of both serotypes 6A and 6B, and there were several further cases in which isolates of both serotypes were present in STs that were SLVs, differing at only a single MLST locus (Fig. 1).
FIG. 1.
Relatedness of serogroup 6 isolates of S. pneumoniae. A UPGMA tree was constructed from the matrix of pairwise differences in the allelic profiles of the 102 isolates. The serotype of each isolate is shown, followed by the isolate name, ST number, and cps profile. The presence of the indel between the wciN and wciO genes is also indicated. STs that include isolates of both serotypes 6A and 6B are indicated by asterisks.
The central cps region of serogroup 6 isolates.
Figure 2 shows the structures and percent G+C contents of the cps loci of serotype 6A and two serotype 6B isolates. The central region of the cps locus has a low-average percent G+C content (28.5%) compared to that of the flanking genes and to that typical of the pneumococcal genome (39.7%). Within the central region of low percent G+C are the wciN, wciO, wciP, wzy, and wzx genes. The wciO, wciP, wzy, and wzx sequences of the serotype 6A and 6B isolates showed >90% nucleotide sequence identity, and the first three genes had <65% identity to the sequences of any of the genes in the cps loci of those serogroups available at GenBank or at the S. pneumoniae cps website at the Sanger Institute (http://www.sanger.ac.uk/Projects/S_pneumoniae/CPS/) and to all other sequences at GenBank. The wzx gene of the serogroup 6 isolates also showed <65% identity to the cps regions of other pneumococcal serogroups and to other sequences in GenBank, except the sequences of serotype 19A and 19F pneumococci, where the similarity was ∼82%. In contrast, the upstream wzg, wzh, wzd, wze, wchA, and wciN genes and the downstream rhamnose biosynthetic genes (rmlA-rmlD) of the serotype 6A and 6B cps loci had high levels of sequence similarity with genes within the cps loci of pneumococci of several other serogroups.
FIG. 2.
cps regions of serotype 6A and 6B S. pneumoniae. The similarities between the cps loci of a serotype 6A isolate and a serotype 6B isolate with a class 1 cps sequence (both from the Sanger Institute website [http://www.sanger.ac.uk/Projects/S_pneumoniae/CPS/]) and a serotype 6B isolate with a class 2 cps sequence from GenBank (AF24897) were displayed using the Artemis Comparison Tool (http://www.sanger.ac.uk/Software/ACT/). The average, maximum, and minimum percent G+C contents along the cps locus and the locations of the cps genes are shown. The ∼300-bp indel between the wciN and wciO genes is indicated by a striped box. Blocks of red color indicate sequence homology between pairs of sequences.
Sequences of internal fragments of three genes within the serotype-specific region of the cps locus (wciP, wzy, and wzx) were obtained from the 102 serotype 6A and 6B isolates, and the alleles at each cps locus and the cps allelic profile were assigned for each isolate, as described in Materials and Methods.
Sequences of the wciP gene.
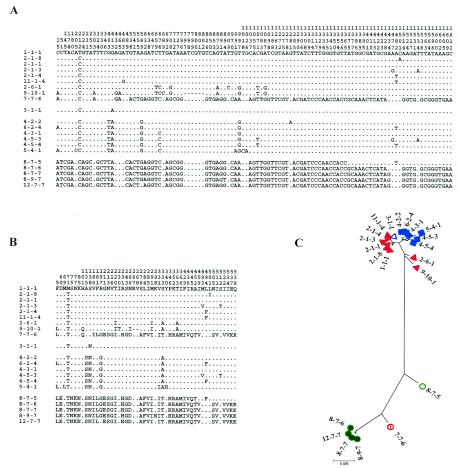
The wciP product is homologous to rhamnosyl transferases in other species, and since the serogroup 6 capsule contains rhamnose, it has been assigned this function. A 645-bp internal region of the gene was sequenced, and 11 distinct alleles were distinguished among the 102 isolates (Fig. 3). The alleles in serotype 6A isolates and in the majority of serogroup 6B isolates were relatively similar. However, two divergent alleles (wciP8 and wciP12), which differed from each other at only a single nonsynonymous site, were found in 14 serotype 6B isolates (Fig. 1). These divergent alleles differed at ∼3% of sites from the alleles in the majority of serotype 6B isolates. A single serotype 6A isolate (ACH-C2) had an allele that was a perfect mosaic (wciP7), in which the front 537 bp were identical to an allele found in two serotype 6A isolates (wciP9) and the rest of the fragment was identical to a divergent allele (wciP8) from serotype 6B isolates. The wciP1, -2, -7, -9, and -11 alleles were found exclusively among serotype 6A isolates, whereas wciP3, -4, -5, and -6 and the more divergent wciP8 and -12 were restricted to serotype 6B isolates. In no case was the same wciP allele found to be present in isolates of both serotypes 6A and 6B.
FIG. 3.
Allelic variation in the cps genes. (A) Neighbor-joining trees showing the relatedness among the alleles at wciP, wzy, and wzx. The scales represent a genetic distance of 0.5%. (B) The polymorphic nucleotide sites are shown for all alleles of the three cps genes. The nucleotides are numbered in vertical format. The nucleotide at each variable site is shown for the first allele, and only the nucleotides that differ from those in this sequence are shown. The dashes show the deletion in wzy-10. Alleles marked by an asterisk were not found in this study and are from published sequences of the cps genes deposited at GenBank (wciP10 in AF298581, wzy-8 and wzx-9 in AY078347, and wzx-10 in AF316640). (C) The polymorphic amino acids in the translated nucleotide sequences are shown. Dashes show the deletion in Wzy-10.
Sequences of the wzy gene.
The wzy gene product shows homology to polysaccharide polymerases. A 492-bp internal fragment of wzy was sequenced from the 102 isolates. There were nine alleles, two of which (wzy-1 and wzy-7) were found in both serotype 6A and 6B isolates (Fig. 3). Two alleles were more divergent (wzy-7 and wzy-9), and with one exception, these were found only in serotype 6B isolates. The exception was wzy-7, which was also present in the serotype 6A isolate ACH-C2. One allele (wzy-10) had a 6-bp deletion.
Sequences of the wzx gene.
The wzx gene product has been assigned by homology as a polysaccharide transporter or flippase. A 477-bp internal fragment of wzx was sequenced from the 102 isolates. There were eight alleles, four of which were present in both serotype 6A and 6B isolates (Fig. 3). Two of the alleles (wzx-6 and -7) were divergent, and a further allele was a perfect mosaic (wzx-5), the front 228 bp being identical to the divergent alleles wzx-6 and -7, whereas the rest of the fragment was identical to wzx-4. The divergent alleles were all present in serotype 6B isolates, except the serotype 6A isolate ACH-C2, which possessed wzx-6 (Fig. 1).
Sequences of the central region of the cps locus of serotype 6A and 6B isolates.
The sequences of the wciP, wzy, and wzx fragments were joined end to end (concatenated). Twenty-one different sequences (cps profiles) were present among the 102 serotype 6A and 6B isolates. The most prevalent cps profiles were 2-1-1 among the serotype 6A isolates (23 out of 43) and 4-2-2 and 8-7-7 among the serotype 6B isolates (31 and 9 out of 59). The polymorphic sites within the concatenated sequences (1,614 bp), and a neighbor-joining tree illustrating the relatedness among these sequences, are shown in Fig. 4.
FIG. 4.
Concatenated sequences of the cps genes of serogroup 6 isolates. (A) Polymorphic nucleotide sites within the concatenated wciP, wzy, and wzx genes are displayed as in Fig. 3. The cps profile corresponding to each sequence is shown. The first block of sequences are from serotype 6A isolates, and all of the sequences including and below the 3-1-1 profile are from serotype 6B isolates. The last block shows the divergent class 2 sequences. One serotype 6A sequence (cps profile 7-7-6) and one serotype 6B sequence (cps profile 8-7-5) are perfect mosaics between class 1 and class 2 sequences. (B) The polymorphic amino acids in the translated concatenated sequences are shown. The dashes in the cps profile 9-10-1 in panels A and B represent deleted nucleotides or amino acids. (C) Neighbor-joining tree constructed from the concatenated nucleotide sequences; taxa are labeled according to their cps profiles. ▴, serotype 6A isolates; ▪, serotype 6B isolates with class 1 sequences; •, serotype 6B isolates with class 2 sequences; ▵, serotype 6B isolates APH-10 and AAU-19 (cps profile 3-1-1); ○, serotype 6B isolates APO-445 and APO-446 (cps profile 8-7-5); ∅, serotype 6A isolate ACH-C2 (cps profile 7-7-6).
The majority of the sequences were very similar, and there was clustering of sequences according to the serotypes of the isolates. Within the main cluster of sequences (Fig. 4C) there was only one sequence (cps profile 3-1-1, present within two isolates, APH-10 and AAU-19) that clustered anomalously; these isolates were serotype 6B, but their cps region clustered within sequences restricted to serotype 6A isolates. Inspection of the sequence showed that this serotype 6B sequence differed at only one nucleotide site (a nonsynonymous substitution in wciP) from the most prevalent sequence among the serotype 6A isolates (cps profile 2-1-1).
Twelve serotype 6B isolates had very closely related sequences (cps profiles 8-7-7, 12-7-7, 8-7-6, and 8-9-7) that were >5% divergent from those of the other serotype 6B isolates (Fig. 4A and C). These 12 isolates all had divergent alleles at wciP, wzy, and wzx, indicating that their cps regions were likely to be divergent throughout the whole central cps region. Nine of these serotype 6B isolates had the same sequence (cps profile 8-7-7), and the other three each had a different sequence, but each differed from the prevalent class 2 sequence at only a single nucleotide site. We refer to these divergent sequences as class 2 sequences to distinguish them from the class 1 sequences in the majority of serotype 6B isolates and in all of the serotype 6A isolates we examined. One further sequence (cps profile 8-7-5), present in two serotype 6B isolates (APO-445 and APO-446), clustered on the tree between the class 1 and 2 sequences and was a perfect mosaic (Fig. 4C); the sequence throughout the wciP and wzy fragments, and the first half of wzx, was identical to that in the predominant class 2 sequence of serotype 6B isolates, whereas the rest of the wzx fragment was identical to a class 1 sequence in several serotype 6B isolates (Fig. 4A).
One serotype 6A isolate (ACH-C2) also had a sequence (cps profile 7-7-6) that clustered on the tree between the class 1 and class 2 sequences and was a mosaic (Fig. 4C). As noted earlier, the first part of the wciP fragment in ACH-C2 was identical to that in some of the serotype 6A isolates, whereas the sequence of the rest of this fragment, and of the entire wzx and wzy fragments, was identical to the class 2 sequences in a serotype 6B isolate (AIS-C31).
The average pairwise diversity among the eight distinct concatenated sequences from serotype 6A isolates (excluding the mosaic sequence) was 0.4%; the sequences differed on average at 6.2 nucleotide sites. The class 1 serotype 6B sequences were similarly uniform (0.3% average pairwise diversity; differences at an average of 4.5 sites), and those of class 2 serotype 6B isolates (excluding the mosaic sequence) were extremely uniform (0.1% diversity), with the three unique class 2 sequences each differing from the predominant class 2 sequence at only a single nucleotide site. The average divergence between the serotype 6A sequences and the class 1 serotype 6B sequences was only 0.6%, whereas the divergence between both the serotype 6A and the class 1 serotype 6B sequences and the class 2 serotype 6B sequences was 5.4%.
Distribution of the wciN-wciO intergenic indel.
The presence or absence of the indel between the wciN and wciO genes was examined in all serotype 6A and 6B isolates by PCR. The indel was restricted to those isolates with class 2 cps sequences (all of which were serotype 6B) and to the one serotype 6A isolate (ACH-C2) and two serotype 6B isolates (APO-445 and APO-446) that had mosaic cps sequences (Fig. 1). Only two of the serotype 6B isolates with class 2 cps sequences lacked this indel (ASA-20 and AIS-C31) (Fig. 1).
Horizontal spread of the serotype 6A and 6B cps genes into divergent pneumococcal lineages.
Figure 1 shows the considerable diversity of genotypes among the 102 serogroup 6 isolates. The majority of the serogroup 6 isolates that were distantly related to all other serogroup 6 isolates were divergent lineages, as they were also not closely related to isolates of other serogroups in the MLST database. A history of horizontal transfer was apparent from the presence of the same cps profiles in several distantly related genetic backgrounds. Although many cps profiles were found only in isolates of a single ST or were restricted to isolates that were closely related by MLST, 7 of 21 (33%) cps profiles were present in pneumococci that were distantly related and differed at ≥6 of the 7 MLST loci (Fig. 1). This was particularly true of the cps profiles 2-1-1, 4-2-2, and 8-7-7 that predominated, respectively, among serotype 6A and serotype 6B isolates with class 1 and 2 sequences. The lack of any sequence variation in the three cps gene fragments in divergent isolates that had sequence differences at all (or most) MLST loci is most readily explained by the recent horizontal spread of the serotype 6A and 6B cps locus through the pneumococcal population.
In a few cases, serogroup 6 isolates were similar in genotype to isolates of other serotypes. For example, STs 947 and 948 were SLVs of each other and were both serotype 6B and had the same cps profile (5-4-1), but they were not closely related to any other serogroup 6 isolates in the MLST database. However, using eBURST on the entire MLST database showed that they were within a large clonal complex whose predicted founder (9) was ST429, where almost all the other isolates were serogroup 23 (data not shown). These serotype 6B isolates therefore appear to have arisen from a serogroup 23 isolate by the replacement of its cps region with that from a serotype 6B isolate. Similarly, ST460 and ST529 (serotype 6A) were SLVs of each other and had the same cps profile (1-1-1) but were within a clonal complex whose predicted founder (ST97), and most SLVs, were serotype 10A, suggesting the introduction of serotype 6A cps sequences into a serotype 10A genetic background. In several other cases there also appeared to have been horizontal transfer of the serotype 6A or 6B cps genes into isolates of clonal complexes descended from founding genotypes expressing a different capsular serotype.
Variation in cps profiles within clonal complexes.
The relatedness of the serogroup 6 isolates inferred from the dendrogram showed several examples of isolates with very similar genotypes that differed in serotype, and there were four cases where serogroup 6 isolates of the same ST differed in serotype (Fig. 1). This suggested recent changes between serotypes 6A and 6B occurring within individual clones or clonal complexes. Two of the examples where isolates of the same ST differed in serotype could be attributed to recombination, as the cps profiles of the serotype 6A and 6B isolates within both ST1094 and ST315 were completely different, whereas those in the other two STs (ST361 and ST473) differed at only a single nonsynonymous site in wciP, and the differences were more likely to be due to point mutations (see below).
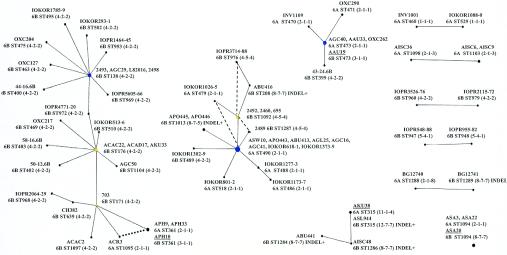
The eBURST algorithm was used to identify nonoverlapping groups of related STs (clonal complexes) and to predict the founding ST of each clonal complex and the patterns of evolutionary descent of all STs in the clonal complex from the predicted founding ST (9). The cps profiles and the sequences at the wciP, wzy, and wzx genes were then mapped onto the predicted patterns of descent of the isolates to explore the extents and mechanisms of serotype change among closely related isolates.
eBURST identified nine clonal complexes among the 78 STs that were resolved among the 102 serogroup 6 isolates (Fig. 5), and there were 29 singleton STs that differed in allelic profile from all other STs at two or more of the seven MLST loci. Five clonal complexes included only two STs, and in all but one case, the isolates of each ST (which are SLVs of each other) had identical serotypes and cps profiles. The other pair of STs (ST1288 and ST1289) included one serotype 6A isolate with the cps profile 2-1-8 and a serotype 6B isolate with a divergent class 2 cps profile (8-7-7). These isolates are therefore very closely related in overall genotype, differing by MLST at only a single locus, but their cps regions are very different, implying a change from serotype 6A to 6B (or vice versa) due to recombination at the cps locus.
FIG. 5.
eBURST groups among the serogroup 6 isolates. The eBURST algorithm (9) was applied to the 102 serogroup 6 isolates. STs that were not part of clonal complexes (singleton STs) are not shown, except in the case of ST1094, where the three isolates of this ST varied in serotype. The circles represent the individual STs, and the size of the circle indicates the abundance of the ST in the input data. STs linked by a line differ at a single MLST locus (i.e., they are SLVs). The isolate name is shown for each ST, followed by the serotype and cps profile. The presence of the indel between the wciO and wciN genes is also indicated. Where there are multiple isolates of an ST, the serotypes and cps profiles are the same unless otherwise indicated. Isolates of the same ST but different serotypes are underlined. ST1095 is an SLV of ST361, as well as of ST171, and taking account of the cps profiles of the isolates, the descent from ST361 to ST1095 (shown by the dotted line) is more parsimonious than the alternative descent from ST171 to ST1095 suggested by the eBURST algorithm. The dashed lines in the ST490 clonal complex identify STs that are double-locus variants, and taking account of the observed cps profiles, suggest possible alternative pathways of descent where the postulated intermediate SLVs are lacking.
One clonal complex included three STs, two of which were serotype 6B, and the single isolate of each of these STs shared the same class 2 cps profile (8-7-7). The third ST (ST315) was represented by one serotype 6B isolate with the cps profile 12-7-7 which differed at a single nonsynonymous substitution in wciP and by a serotype 6A isolate with a completely different cps profile (11-1-4). In the MLST database, there were an additional 18 isolates of ST315, all but one of which was serotype 6B, and 12 of the 13 SLVs of ST315 were also serotype 6B. The founder of this clonal complex was predicted to be ST315 (100% bootstrap support) and was almost certainly serotype 6B, and the serotype 6A isolate of this ST presumably arose by recombination at the cps locus.
There were four STs (seven isolates) within a small clonal complex whose predicted founder was ST473 (Fig. 5). Applying eBURST to the whole MLST database, combined with the additional isolates characterized here, confirmed that ST473 was the predicted founder (bootstrap support, 99%) and identified five further SLVs of ST473, all of which included only serogroup 6 isolates. Three of the four isolates of ST473, and those of two of its three SLVs, had the same cps profile (2-1-1) and were all serotype 6A (as were all isolates of the additional SLVs of ST473 in the MLST database). The ancestral state for this clonal complex was therefore almost certainly serotype 6A with the cps profile 2-1-1. However, the fourth isolate of ST473 (AAU-19) was serotype 6B, and its central cps region differed from that of the serotype 6A isolates by only a single nonsynonymous substitution in wciP, resulting in a change from the 2-1-1 to the 3-1-1 cps profile. The serotype of AAU-19 was rechecked and confirmed to be 6B. AAU-19 was from Australia, as was one of the serotype 6A isolates of ST473 with the ancestral cps profile 2-1-1. The isolate of the third SLV of ST473 (ST399) was also serotype 6B but had a completely different cps profile (4-2-2). Since the cps regions of this serotype 6B isolate differed from those of the other isolates in this clonal complex at multiple sites and at each of the three sequenced cps loci, it was considered to have arisen by a recombinational replacement at the cps locus, resulting in a change from serotype 6A to 6B.
The ST490 clonal complex included 11 STs and 21 isolates (Fig. 5). ST490 was identified as the founding ST of the ST490 clonal complex (bootstrap support, 97%) by using eBURST on the 102 isolates combined with all other isolates in the MLST database. Using the combined data set, there were a further 10 STs compared to Fig. 5, and with a single exception, all isolates were serogroup 6. The eight isolates of ST490 among the 102 serogroup 6 isolates, as well as two of the SLVs of ST490, were serotype 6A and had the same cps profile (2-1-1). However, in this clonal complex there were five STs represented by serotype 6B isolates that had four different cps profiles that included both class 1 and class 2 sequences and a mosaic sequence. All four of these serotype 6B cps profiles differed at multiple nucleotide sites from each other and from the 2-1-1 profile found in all of the serotype 6A isolates within this clonal complex. Although it is difficult to provide a single evolutionary path from ST490 to all of its assumed descendants that is consistent with the changes in the cps profiles, it is apparent that changes from serotype 6A to serotype 6B (or from one serotype 6B cps profile to another) have occurred on at least four occasions within this one clonal complex.
The largest clonal complex identified by eBURST among the 102 isolates included 28 isolates and 21 STs; the great majority of them were serotype 6B and had the cps profile 4-2-2. By applying eBURST to the whole MLST database, ST176 was the predicted founder (bootstrap support, 92%) of this large clonal complex. Among the isolates of the clonal complex, variation in serotype and in cps profile was apparent within only one lineage descended from ST171, an SLV of ST176 (Fig. 5). ST361 was predicted to be descended from ST171, and two of the three isolates of this ST were serotype 6A (cps profile 2-1-1), and a descendant SLV of ST361 (ST1095) was also serotype 6A and had the same cps profile (Fig. 5). The other isolate of ST361 (APH-10) was serotype 6B, with the cps profile 3-1-1, which differed by only a single nonsynonymous substitution in wciP from the cps profile of the serotype 6A isolates of ST361. This substitution in the wciP gene of APH-10 (and in AAU-19 within the ST473 clonal complex, which has the same unusual cps profile) is believed to determine whether an isolate is serotype 6A or 6B (see Discussion). The most parsimonious explanation for the origin of APH-10 is that the ancestral cps profile of this clonal complex (4-2-2) changed by recombination to 2-1-1, resulting in a change from serotype 6B to 6A, and that the single substitution in wciP changed a serotype 6A isolate of ST361 back to serotype 6B, changing its cps profile to 3-1-1. Repeat serotyping of APH-10 confirmed that it was serotype 6B rather than 6A. The serotype 6B isolate APH-10, and serotype 6A isolates of this ST with the cps profile 2-1-1, were recovered in the Philippines.
DISCUSSION
The central cps region provides the most reliable information about the evolution of serogroups. This region in serotype 6A and 6B isolates has a lower percent G+C content than the flanking genes, which suggests that it has been introduced relatively recently, on an evolutionary timescale, from an unknown foreign source. The presence in pneumococci of many completely nonhomologous polysaccharide polymerases and glycosyl transferases in the serotype-specific region of the cps loci of the 90 different pneumococcal serotypes or serogroups also supports the view that these genes have been introduced on multiple occasions by horizontal gene transfer.
The sequence variation within the central cps region of all serotype 6A and 6B isolates was sufficiently low (<6%) to indicate that the sequences are all derived from a single recent common ancestral sequence, although this ancestral sequence may not necessarily have been present within a pneumococcus. The most likely scenario is that an ancestral serogroup 6 cps sequence was introduced into the pneumococcus from another species and that this ancestral sequence diverged, through random genetic drift or under selection for antigenic variation imposed by the host immune system. This resulted in two subfamilies of cps sequences that produced capsular polysaccharide structures that differed slightly in their immunochemistry and their rhamnose-ribitol linkages and which we now recognize as serotypes 6A and 6B. Although this scenario explains the high levels of sequence similarity within and between most of the serotype 6A and 6B isolates, it does not explain the origins of the more divergent class 2 cps sequences. The divergence between the class 1 and class 2 sequences is substantial (5.4%) and was greater than that found in typical housekeeping genes of the pneumococcus (8). Furthermore, the two classes of serotype 6B cps sequences are far more divergent from each other than class 1 serotype 6B sequences are from serotype 6A sequences.
Our favored scenario is that the class 2 sequence appeared by an independent introduction from a similar but unknown source. The class 2 cps sequences were more uniform than those of class 1; there were four class 2 cps profiles, and three of these differed from the predominant (presumably ancestral) sequence (the 8-7-7 cps profile) at only a single nucleotide site. The low level of sequence diversity among class 2 sequences, and the presence of perfect mosaics between class 1 and 2 sequences, is consistent with the recent appearance of the class 2 sequence in the pneumococcus and its recent recombination with class 1 sequences. Subsequently, the class 2 sequence has spread horizontally within the pneumococcal population and is now found in several serotype 6B isolates that appear from MLST to be only distantly related.
The introduction of genes encoding a structurally novel capsular polysaccharide from a distantly related species should be favored by natural selection, since it produces an antigenic variant of the pneumococcus against which there is no existing natural immunity. A more difficult question is whether immune selection drives the divergence of a single serotype into two related serotypes or favors the observed mutational or recombinational events that appear to have relatively frequently changed the serotype of isolates from 6A to 6B or vice versa. Selection for such events would require that the natural antibody response against the capsule of one of these serotypes is not fully protective against the subtly different capsule of the other serotype or that serotypes 6A and 6B differ in some other way that affects transmission to new hosts. There are no robust quantitative measures of the degree of cross protection from natural immunity between serotypes 6A and 6B, but studies of immunity induced by the conjugate vaccines, which include serotype 6B but not serotype 6A capsular polysaccharide, indicate a significantly less effective antibody response against serotype 6A isolates than against 6B isolates (30). Also, a study of hybridoma antibodies elicited by serotype 6B showed that while half of the antibodies bound with equal avidity to, and were able to opsonize, both 6A and 6B, the other half bound to serotype 6B alone and failed to opsonize serotype 6A isolates (29). Antibodies elicited by one serotype may provide reduced protection against acquisition or carriage of the other. Natural selection could therefore favor switches of serotype between 6A and 6B or the original divergence that appears to have split the ancestral serotype 6 into serotypes 6A and 6B.
There was considerable evidence for recombination at the cps locus. In addition to evidence for the dissemination of the serotype 6A and 6B cps locus into distantly related lineages, resulting in changes of serogroup by recombination, a phenomenon well documented elsewhere (3-6, 26), there were a surprising number of examples of recombination between the cps regions of serotypes 6A and 6B leading to changes from one of the serotypes to the other. Many of the recombinational events that have spread the serotype 6A and 6B capsule between strains appear to be relatively recent; a third of the serogroup 6 cps profiles were found in very different genotypes, indicating that no sequence changes within the three cps genes had occurred since the horizontal-transfer events. Similarly, the mosaic cps sequences appear to have arisen recently, as no nucleotide differences have occurred in either the putative donor or recipient sequences since their formation. These results therefore provide supporting molecular evidence for changes between serotype 6A and 6B within clonal complexes that were proposed by Robinson et al. (28)
We analyzed only the central cps region and cannot address the typical size of the cps region that is replaced when isolates change by recombination from serotype 6A to 6B or when the serogroup 6 cps locus spreads horizontally into other lineages. Recombinational crossover points in pneumococci that have changed serotype have been identified previously, and changes of serotype by recombination appear to often include most or all of the cps locus (4, 5, 25). The majority of the recombinational replacements that introduced the class 2 sequences into other lineages must have been relatively large (at least 4.5 kb), since in all but two isolates the characteristic indel between wciN and wciO has been cotransferred along with wciP, wzy, and wzx.
Comparisons of the published complete sequences of the cps loci of serotype 6A and 6B isolates identify those nonsynonymous substitutions in the serotype-specific region of the locus that correlate with an isolate being serotype 6A or 6B. Within this region (wciN-wzx) of the serotype 6A and 6B isolates there were several nonsynonymous substitutions that correlated with serotype. Our examination of sequence variation in a much larger set of diverse serotype 6A and 6B isolates established that only one nonsynonymous substitution in wciP correlated perfectly with serotype; isolates with serine at residue 195 of WciP were serotype 6A, whereas those with asparagine were serotype 6B (Fig. 4B). The importance of wciP in determining serotype was also supported by examination of the class 2 cps sequences that, with one exception, were found in serotype 6B isolates and that also have asparagine at position 195. The one class 2 cps region present in a serotype 6A isolate (ACH-C2) was a mosaic that appears to have arisen by a recombinational replacement that introduced the front half of wciP (introducing the serine residue into WciP) from a serotype 6A isolate.
The most convincing evidence for the key role of residue 195 comes from the wciP3 allele, which was found in two serotype 6B isolates and was identical to the wciP2 allele of serotype 6A isolates except at one nucleotide site, which changes residue 195. The two isolates (AAU-19 and APH10) were distantly related and appear to have independently changed from serotype 6A to 6B by the same single-nucleotide change in wciP. In both cases, the unusual serotype 6B isolates were identical by MLST to multiple isolates of serotype 6A and (except for the single-nucleotide change) had the same wciP-wzy-wzx sequence. Based on these observations, we conclude that these isolates independently changed from serotype 6A to 6B by a point mutation within wciP rather than by recombination.
There is therefore strong evidence that residue 195 within WciP determines the serotype within serogroup 6, although experimental evidence is required to establish conclusively that mutagenesis of this single nucleotide in wciP changes the serotype. A key role for wciP in determining whether an isolate is serotype 6A or 6B is consistent with the functional assignment of its gene product (rhamnosyl transferase), since their capsular polysaccharides differ only in the nature of the chemical linkage of rhamnose to ribitol (15, 16, 27), which is catalyzed by a rhamnosyl transferase.
Acknowledgments
We thank Moon Nahm at the University of Alabama for providing monoclonal antibodies Hyp6BM1 and Hyp6BM8 and Pat Coan for serotyping and shipping of strains. We are grateful to Angela Brueggemann and Dai Griffith at the John Radcliffe Hospital, Oxford, United Kingdom, for confirming the serotypes of some of the isolates and Stephen Bentley and Julian Parkhill at the Sanger Institute, Cambridge, United Kingdom, for providing annotated sequences of the serotype 6A and 6B cps loci. We thank all those who contributed isolates to this study and to the MLST database and Jay Butler and Karen Rudolph for providing replacements for isolates lost in a freezer accident.
This work was supported by the Wellcome Trust. B.G.S. is a Principal Research Fellow of the Wellcome Trust.
REFERENCES
- 1.Arrecubieta, C., R. Lopez, and E. Garcia. 1994. Molecular characterization of cap3A, a gene from the operon required for the synthesis of the capsule of Streptococcus pneumoniae type 3: sequencing of mutations responsible for the unencapsulated phenotype and localization of the capsular cluster on the pneumococcal chromosome. J. Bacteriol. 176:6375-6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrett, B., L. Ebah, and I. S. Roberts. 2002. Genomic structure of capsular determinants. Curr. Top. Microbiol. Immunol. 264:137-155. [PubMed] [Google Scholar]
- 3.Coffey, T. J., C. G. Dowson, M. Daniels J. Zhou, C. Martin, B. G. Spratt, and J. M. Musser. 1991. Horizontal transfer of multiple penicillin-binding protein genes, and capsular biosynthetic genes, in natural populations of Streptococcus pneumoniae. Mol. Microbiol. 5:2255-2260. [DOI] [PubMed] [Google Scholar]
- 4.Coffey, T. J., M. C. Enright, M. Daniels, J. K. Morona, R. Morona, W. Hryniewicz, J. C. Paton, and B. G. Spratt. 1998. Recombinational exchanges at the capsular polysaccharide biosynthetic locus lead to frequent serotype changes among natural isolates of Streptococcus pneumoniae. Mol. Microbiol. 27:73-83. [DOI] [PubMed] [Google Scholar]
- 5.Coffey, T. J., M. Daniels, M. C. Enright, and B. G. Spratt. 1999. Serotype 14 variants of the Spanish penicillin-resistant serotype 9V clone of Streptococcus pneumoniae arose by large recombinational replacements of the cpsA-pbp1a region. Microbiology 145:2023-2031. [DOI] [PubMed] [Google Scholar]
- 6.Coffey, T. J., M. C. Enright, M. Daniels, P. Wilkinson, S. Berrón, A. Fenoll, and B. G. Spratt. 1998. Serotype 19A variants of the Spanish serotype 23F multiresistant clone of Streptococcus pneumoniae. Microb. Drug Resist. 4:51-55. [DOI] [PubMed] [Google Scholar]
- 7.Dillard, J. P., M. W. Vandersea, and J. Yother. 1995. Characterisation of the cassette containing genes for type 3 capsular polysaccharide biosynthesis in Streptococcus pneumoniae. J. Exp. Med. 181:973-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enright, M. C., and B. G. Spratt. 1998. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology 144:3049-3060. [DOI] [PubMed] [Google Scholar]
- 9.Feil, E. J., B. Li, D. M. Aanensen, W. P. Hanage, and B. G. Spratt. 2004. eBURST: Inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J. Bacteriol. 186:1518-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia, E., D. Llull, R. Munoz, M. Mollerach, and R. Lopez. 2000. Current trends in capsular polysaccharide biosynthesis of Streptococcus pneumoniae. Res. Microbiol. 151:429-435. [DOI] [PubMed] [Google Scholar]
- 11.Guidolin, A., J. K. Morona, R. Morona, D. Hansman, and J. C. Paton. 1994. Nucleotide sequence analysis of genes essential for capsular polysaccharide biosynthesis in Streptococcus pneumoniae type 19F. Infect. Immun. 62:5384-5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henrichsen, J. 1995. Six newly recognized types of Streptococcus pneumoniae. J. Clin. Microbiol. 33:2759-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iannelli, F., B. J. Pearce, and G. Pozzi. 1999. The type 2 capsule locus of Streptococcus pneumoniae. J. Bacteriol. 181:2652-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang, S. M., L. Wang, and P. R. Reeves. 2001. Molecular characterisation of Streptococcus pneumoniae type 4, 6B, 8, and 18C capsular polysaccharide gene clusters. Infect. Immun. 69:1244-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamerling, J. P. 2000. Pneumococcal polysaccharides: a chemical view, p. 81-114. In A. Tomasz (ed.), Streptococcus pneumoniae: molecular biology and mechanisms of disease. Mary Ann Liebert, Inc., Larchmont, N.Y.
- 16.Kenne, L., B. Lindberg, and J. K. Madden. 1979. Structural studies of the capsular antigen from Streptococcus pneumoniae type 26. Carbohydr. Res. 73:175-182. [DOI] [PubMed] [Google Scholar]
- 17.Kolkman, M. A., W. Wakarchuk, P. J. Nuijten, and B. A. van der Zeijst. 1997. Capsular polysaccharide synthesis in Streptococcus pneumoniae serotype 14: molecular analysis of the complete cps locus and identification of genes encoding glycosyltransferases required for the biosynthesis of the tetrasaccharide subunit. Mol. Microbiol. 26:197-208. [DOI] [PubMed] [Google Scholar]
- 18.Kolkman, M. A., B. A. van der Zeijst, and P. J. Nuijten. 1998. Diversity of capsular polysaccharide synthesis gene clusters in Streptococcus pneumoniae. J. Biochem. 123:937-945. [DOI] [PubMed] [Google Scholar]
- 19.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 5:150-163. [DOI] [PubMed] [Google Scholar]
- 20.Llull, D., R. Lopez, E. Garcia, and R. Munoz. 1998. Molecular structure of the gene cluster responsible for the synthesis of the polysaccharide capsule of Streptococcus pneumoniae type 33F. Biochim. Biophys. Acta 1443:217-224. [DOI] [PubMed] [Google Scholar]
- 21.Llull, D., R. Munoz, R. Lopez, and E. Garcia. 1999. A single gene (tts) located outside the cap locus directs the formation of Streptococcus pneumoniae type 37 capsular polysaccharide. Type 37 pneumococci are natural, genetically binary strains. J. Exp. Med. 190:241-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morona, J. K., R. Morona, and J. C. Paton. 1997. Characterization of the locus encoding the Streptococcus pneumoniae type 19F capsular polysaccharide biosynthetic pathway. Mol. Microbiol. 23:751-763. [DOI] [PubMed] [Google Scholar]
- 23.Morona, J. K., R. Morona, and J. C. Paton. 1997. Molecular and genetic characterization of the capsule biosynthesis locus of Streptococcus pneumoniae type 19B. J. Bacteriol. 179:4953-4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munoz, R., M. Mollerach, R. Lopez, and E. Garcia. 1999. Characterisation of the type 8 capsular gene cluster of Streptococcus pneumoniae. J. Bacteriol. 181:6214-6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramirez, M., and A. Tomasz. 1998. Molecular characterisation of the complete 23F capsular polysaccharide locus of Streptococcus pneumoniae. J. Bacteriol. 180:5273-5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramirez, M., and A. Tomasz. 1999. Acquisition of new capsular genes among clinical isolates of antibiotic-resistant Streptococcus pneumoniae. Microb. Drug Resist. 5:241-246. [DOI] [PubMed] [Google Scholar]
- 27.Rebers, P. A., and M. Heidelberger. 1961. The specific polysaccharide of type VI pneumococcus. II. The repeating unit. J. Am. Chem. Soc. 83:3056-3059. [Google Scholar]
- 28.Robinson, D. A., D. E. Briles, M. J. Crain, and S. K. Hollingshead. 2002. Evolution and virulence of serogroup 6 pneumococci on a global scale. J. Bacteriol. 184:6367-6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun, Y., Y. Hwang, and M. H. Nahm. 2001. Avidity, potency, and cross-reactivity of monoclonal antibodies to pneumococcal capsular polysaccharide serotype 6B. Infect. Immun. 69:336-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Väkeväinen, M., C. Eklund, and J. Eskola. 2001. Cross-reactivity of antibodies to type 6B and 6A polysaccharide of Streptococcus pneumoniae, evoked by pneumococcal conjugate vaccines. J. Infect. Dis. 184:789-793. [DOI] [PubMed] [Google Scholar]
- 31.van Selm, S., M. A. Kolkman, B. A. van der Zeijst, K. A. Zwaagstra, W. Gaastra, and J. P. van Putten. 2002. Organization and characterization of the capsule biosynthesis locus of Streptococcus pneumoniae serotype 9V. Microbiology 148:1747-1755. [DOI] [PubMed] [Google Scholar]
- 32.van Selm, S., L. M. van Cann, M. A. Kolkman, B. A. van der Zeijst, and J. P. van Putten. 2003. Genetic basis for the structural difference between Streptococcus pneumoniae serotype 15B and 15C capsular polysaccharides. Infect. Immun. 71:6192-6198. [DOI] [PMC free article] [PubMed] [Google Scholar]