To the Editor: Imiquimod 5% cream is currently approved for topical treatment of condyloma acuminatum. The possible side effects of vitiligo or vitiligo-like hypopigmentation associated with imiquimod treatment have rarely been reported in the open literature. We herein presented 6 cases of imiquimod-induced genital hypopigmentation.
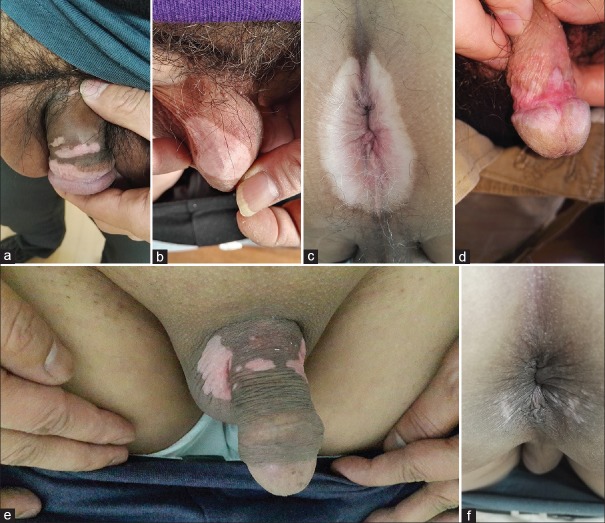
During the past 2 years, from January 2014 to December 2015, 6 male patients aged from 27 to 59 years were given imiquimod 5% cream for the treatment of condyloma acuminatum in STD Institute, Shanghai Dermatology Hospital. All patients followed the medicine instructions very carefully when applying the cream. After applying the cream for 1–2 weeks, all of them experienced local skin reactions ranging from mild erythema, edema to severe reactions such as excoriation, erosion, ulceration, along with burning, pain, itching in the treated area [Table 1]. When such adverse reactions happened, the patients discontinued the application for a short period until the skin recovered, and then they resumed. However, about 6–15 weeks later (12 weeks on average), noticeable color changes appeared on the application sites and even extended to the surrounding areas. Physical examinations showed that well-defined, localized and milky or slight white patches appeared on the treated areas [Figure 1a–1f]. All the patients denied the family medical history of vitiligo or similar disease and application of any other topical treatments. We suggested the patients stopping the cream immediately. Unfortunately, 6 months later, the hypopigmentation seemed to have no improvement.
Table 1.
Clinical characteristics of six patients
| Patients number | Age (years) | Lesion site | Time of using imiquimod before depigmentation (weeks) | Adverse reactions before depigmentation |
|---|---|---|---|---|
| 1 | 35 | Coronal sulcus, penile shaft, foreskin | 15 | Severe: Burning, pain, edema, erosions, exudate, ulceration |
| 2 | 45 | Scrotum | 13 | Mild: Itching, erythema |
| 3 | 59 | Perianal area | 14 | Severe: Pain, itching, edema, erosions, exudates, flaking |
| 4 | 27 | Glans and coronal sulcus | 12 | Mild: Itching, erythema |
| 5 | 38 | Shaft of penis, scrotum | 11 | Severe: Burning, pain edema, vesicles, erosions |
| 6 | 33 | Perianal area | 6 | Mild: Itching, erythema |
Figure 1.
(a) Vitiligo-like lesions on coronal sulcus, penile shaft, foreskin; (b) vitiligo-like hypopigmentation on scrotum; (c) vitiligo on perianal area; (d) hypopigmentation on glans and coronal sulcus; (e) vitiligo-like lesions on shaft of penis, scrotum; (f) hypopigmentation on perianal area.
Imiquimod is an immune response modifier which is widely used for the treatment of condyloma acuminatum. Most common adverse reaction is irritant contact dermatitis in application site, which range from mild to severe in intensity just as what we reported. In our cases, the causal relationship between topical use of imiquimod cream and localized vitiligo or vitiligo-like hypopigmentation was clear and exclusive. We noticed that hypopigmentation might occur by continuous application of the cream after the irritant reactions, and it might extend beyond the application site onto the surrounding skin. In addition, our cases showed that the average time of hypopigmentation occurrence was about 12 weeks after using imiquimod, which was very similar to Wang et al.'s and Li et al.'s reports.[1,2] It is difficult to explain the pathogenesis of such side effects. Mashiah and Brenner[3] considered that imiquimod-induced depigmentation was a cytotoxic T lymphocyte-mediated immune reaction, and Kim et al.[4] thought that imiquimod could induce local apoptosis of human melanocytes, but the exact mechanisms was ambiguous.
In conclusion, we reported these six cases to indicate that vitiligo or vitiligo-like hypopigmentation induced by imiquimod application is not a casual event, which rarely been reported in the open journals. Clinicians should be aware of this long-lasting or even irreversible potential side effect, which might be difficult for patients to accept.[5] For these reasons, such side effect should also be emphasized on the medicine package insert. In addition, more instructions and guidance should be given to the patients in advance who receive imiquimod therapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Xin Chen
References
- 1.Wang HW, Miao F, Shi L, Lü T, Huang Z, Wang XL. Imiquimod-induced localized vitiligo in wife and lichen planus in husband. Chin Med J. 2013;126:2593. doi: 10.3760/cma.j.issn.0366-6999.20123348. [PubMed] [Google Scholar]
- 2.Li W, Xin H, Ge L, Song H, Cao W. Induction of vitiligo after imiquimod treatment of condylomata acuminata. BMC Infect Dis. 2014;14:329. doi: 10.1186/1471-2334-14-329. doi: 10.1186/1471-2334-14-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mashiah J, Brenner S. Possible mechanisms in the induction of vitiligo-like hypopigmentation by topical imiquimod. Clin Exp Dermatol. 2008;33:74–6. doi: 10.1111/j.1365-2230.2007.02520.x. doi: 10.1111/j.1365-2230.2007.02520.x. [DOI] [PubMed] [Google Scholar]
- 4.Kim CH, Ahn JH, Kang SU, Hwang HS, Lee MH, Pyun JH, et al. Imiquimod induces apoptosis of human melanocytes. Arch Dermatol Res. 2010;302:301–6. doi: 10.1007/s00403-009-1012-0. doi: 10.1007/s00403-009-1012-0. [DOI] [PubMed] [Google Scholar]
- 5.Brown T, Zirvi M, Cotsarelis G, Gelfand JM. Vitiligo-like hypopigmentation associated with imiquimod treatment of genital warts. J Am Acad Dermatol. 2005;52:715–6. doi: 10.1016/j.jaad.2004.10.861. doi: 10.1016/j.jaad.2004.10.861. [DOI] [PubMed] [Google Scholar]