Abstract
In Escherichia coli K-12 overexpressing CpxR, transcription of the ung gene for uracil-DNA glycosylase was repressed, ultimately leading to the induction of mutation. Gel shift, DNase I footprinting, and in vitro transcription assays all indicated negative regulation of ung transcription by phosphorylated CpxR. Based on the accumulated results, we conclude that ung gene expression is negatively regulated by the two-component system of CpxR/CpxA signal transduction.
The cpxRA two-component system of Escherichia coli, consisting of CpxA sensor kinase or phosphatase and CpxR cognate response regulator, regulates biofilm formation, motility, chemotaxis, host cell invasion, and virulence (5, 8, 13, 21). By using the generally accepted DNA sequence for binding CpxR (two tandem GTAAA motifs separated by a 5-bp linker, the CpxR box), DeWulf et al. (6) performed 15-bp weighted matrix analysis for CpxR recognition sites on the entire E. coli genome and identified new target genes (ung, ompC, psd, mviA, aroK, rpoE, secA, and aer) that might be regulated positively or negatively by CpxR/CpxA. The expression of cpxRA increases sharply at the onset of the stationary growth phase (5), suggesting the involvement of the Cpx system in stationary phase survival.
Uracil-DNA glycosylase (Ung), the most abundant type among all of the glycosylases in bacterial cells (16, 20), excises uracil residues from DNA, which arise as a result of either misincorporation of dUTP by DNA polymerase or deamination of DNA cytosine (9, 10, 11, 16, 27). The ung mutants show the specific mutator effect of a G · C-to-A · T transition as Duncan and Weiss reported previously (9). The ung gene expression remains constant up to the early stationary phase of E. coli but declines in the late stationary phase (26). However, the mechanisms underlying these phenomena are not understood well.
In order to clarify the possible involvement of the Cpx system in repression of ung expression, the effects of CpxR overexpression on ung expression are investigated in this study.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The E. coli strains used are listed in Table 1. They were grown at 37°C in a Luria-Bertani (LB) medium (pH 7.5) containing 1% Bacto Tryptone (Difco), 0.5% Bacto Yeast Extract (Difco), and 1% NaCl. The following antibiotics were added to the medium: ampicillin, 100 μg/ml; chloramphenicol, 25 μg/ml; tetracycline, 12.5 μg/ml; and kanamycin, 25 μg/ml.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Genotype or relevant characteristicsa | Source or reference |
|---|---|---|
| E. coli strains | ||
| BW25113 | lacIqrrnBT14 ΔlacZWJ16hsdR514 ΔaraBADAH33 ΔrhaBADLD78 | 4 |
| BW27559 | BW25113 Δ(cpxR)623 | 23 |
| BL21 | F−ompT hsdSB(rB− mB−) gal (λcI857 ind1 Sam7 nin5 lacUV5-T7 gene1) dcm (DE3) | Novagen |
| BD2314 | ung-152::Tn10 lacY1 gal-6 trpC45 his-68 purC50 tyrA2 rpsL125 malA1 xylA7 mtl-2 thi-1 [fluA2 tsx-70 supE44(AS)] | 9 |
| BWung | BW25113 ung-152::Tn10 | BW25113 × P1(BD2314) → Tcr |
| CC102b | P90C ara Δ(lac proB)XIII/F′ lacIZ proB+ | 2 |
| CC102ung | CC102 ung-152::Tn10 | CC102 × P1(BD2314) → Tcr |
| Plasmids | ||
| pCA24N | Cmr, expression vector | H. Moric |
| pCA24ΔGFP | ΔGFP, pCA24N | H. Morid |
| pET-21a (+) | Apr, expression vector | Novagen |
| pBAD18 | Apr, a vector containing the arabinose PBAD promoter | 12 |
| pRS551 | Apr, Kmr, lacZ operon fusion vector | R. W. Simons |
| p41-5ΔGFP | SfiI fragment (698 bp) containing cpxR was cloned into the corresponding sites of pCA24ΔGFP | H. Aiba |
| pKH50-3 | BamHI-NotI fragment (698 bp) containing cpxR was cloned into the corresponding sites of pET-21a(+) | K. Yamamoto |
| pRSung | pRS551, EcoRI-BamHI (538 bp) fragment containing ung promoter | This study |
| pBADnlpE | EcoRI-XbaI fragment (751 bp) containing nlpE was cloned into the corresponding site of pBAD18 | This study |
Ap, ampicillin; Km, kanamycin; Cm, chloramphenicol; Tc, tetracycline.
G·C-A·T tester strain.
NotI digestion and self ligation of pCA24N.
Construction of plasmids.
The plasmids used are listed in Table 1. A DNA fragment (538 bp) containing the ung promoter region was prepared by PCR using E. coli BW25113 genome DNA as template and a pair of primers, ung-EcoRI-F (5′-TGGAACTTCACGGAATTCAATGTCA-3′) and ung-BamHI-R (5′-AAAATAGGGATCCTGCTTCTCTTCA-3′). After digestion with EcoRI and BamHI, the PCR-amplified fragment was inserted at the corresponding site of pRS551 to generate the plasmid pRSung. A DNA fragment (751 bp) containing the nlpE coding region was prepared by PCR using E. coli BW25113 genome DNA as a template along with a pair of primers, NLPEAF (5′-ATGCGCGGCAGAATTCGCAGCGGTCGGGAA-3′) and NLPEAR (5′-TGCGTTTGTTTCTAGATCAAGACGGGTTAC-3′). After digestion with EcoRI and BamHI, the PCR-amplified fragment was inserted at the corresponding site of pBAD18 to generate the plasmid pBADnlpE.
Preparation of the labeled probe for S1 nuclease, gel shift, and DNase I footprinting assays.
Probe A was generated by PCR amplification of the ung promoter region with the primers ung-S1F (5′-TGATGCCTCCCCGGCAAAAT-3′) and 32P-labeled ung-S1R (5′-TAGGGTTGCTGCTTCTCTTC-3′), and E. coli BW25113 genome DNA (100 ng) was used as the template for the Ex Taq DNA polymerase. The PCR product with 32P at its terminus was recovered from a polyacrylamide gel and then used for S1 nuclease, gel shift, and DNase I footprinting (noncoding strand) assays. The ung-S1R and labeled ung-S1F primers were used to prepare probe B for an S1 nuclease assay (transcription of yfiD). Probe C was generated by PCR amplification of the cpxR promoter region with the primers cpxRA-S1F (5′-GTTATCGCCTGAACCGACTT-3′) and 32P-labeled cpxRA-S1R (5′-GAAGCCTTCCATCTCGAGCA-3′), and E. coli BW25113 genome DNA (100 ng) was used as the template for the Ex Taq DNA polymerase. The PCR product with 32P at its terminus was recovered from a polyacrylamide gel and then used for S1 nuclease. The cpxRA-S1R and labeled cpxRA-S1F primers were used to prepare probe D for an S1 nuclease assay (transcription of cpxP). The labeled primers were prepared with 10 μCi of [γ-32P]ATP (5,000 Ci/mmol) by T4 polynucleotide kinase (Toyobo).
RNA isolation and S1 nuclease assay.
To prepare total RNA for the S1 nuclease assay, overnight cultures were diluted 100-fold in 100 ml of LB medium grown to an optical density at 600 nm (OD600) of 0.6 or 1.2 at 37°C. Subsequent purification steps were carried out as described previously (19). Ten thousand counts per minute of probe A (2 fmol) was incubated with 100 μg of total RNA in hybridization buffer (80% formamide, 0.4 M NaCl, 20 mM HEPES [pH 6.4]) at 75°C for 10 min, followed by further incubation at 37°C overnight; it was then digested with 50 U of S1 nuclease. The undigested DNA was precipitated by ethanol, dissolved in formamide dye solution (95% formamide, 0.05% bromophenol blue, 0.05% xylenecyanol), and analyzed by electrophoresis on a 6% polyacrylamide gel containing 8 M urea.
Purification of CpxR.
His-tagged CpxR for gel shift, DNase I footprinting, and in vitro transcription assays was purified from pKH50-3/BL21(DE3) as described previously (28).
Gel shift assay.
Ten thousand counts per minute of probe A (2 fmol) was incubated at 37°C for 10 min with CpxR in 12.5 ml of 50 mM Tris-HCl (pH 7.8), 50 mM NaCl, 3 mM Mg acetate, 0.1 mM EDTA, and 0.1 mM dithiothreitol. After addition of the DNA dye solution (40% glycerol, 0.025% bromophenol blue, 0.025% xylenecyanol), the mixture was directly subjected to a 6% polyacrylamide gel electrophoresis.
DNase I footprinting assay.
Forty thousand counts per minute of probe A or B (8 fmol) was incubated at 37°C for 10 min with CpxR in 25 μl of 50 mM Tris-HCl (pH 7.8), 50 mM NaCl, 3 mM magnesium acetate, 5 mM CaCl2, 0.1 mM EDTA, 0.1 mM dithiothreitol, and 25 mg of bovine serum albumin/ml. After incubation for 10 min, DNA digestion was initiated by the addition of 5 ng of DNase I (Takara). After digestion for 30 s at 25°C, the reaction was terminated by the addition of 45 μl of DNase I stop solution (20 mM EDTA, 200 mM NaCl, 1% sodium dodecyl sulfate, 250 μg of yeast tRNA/ml). Digested products were precipitated by ethanol, dissolved in formamide dye solution, and analyzed by electrophoresis on a 6% polyacrylamide gel containing 8 M urea.
In vitro transcription assay.
A 538-bp EcoRI-BamHI fragment from pRSung, including the ung promoter region, or a 205-bp EcoRI fragment from pKB252 (1), including the lacUV5 promoter region, was used as the template DNA. Single-round transcription by the reconstituted holoenzymes was carried out as described previously (14). In brief, 0.1 pmol of template was incubated with 0, 1.25, 2.5, 5, and 10 pmol of CpxR for 10 min at 37°C in the presence or absence of 10 mM acetylphosphate in a total volume of 33 μl. Into this reaction mixture, 0.5 pmol of RNA polymerase was added and incubated for 20 min at 37°C to form an open complex. Then a substrate-heparin mixture containing [α-32P]UTP was added and further incubated for 10 min at 37°C. The transcripts were subjected to a 6% polyacrylamide gel containing 8 M urea.
Assay of Ung activity.
Cell extract for examining Ung activity was prepared as described previously (16). Here, 5 ml of an overnight culture of E. coli cells was inoculated into 200 ml of LB medium (pH 7.5) containing 1 mM of IPTG (isopropyl-β-d-thiogalactopyranoside) and grown at 37°C until reaching an OD600 of 1.2. After harvesting by centrifugation, the obtained pellet was washed with 50 ml of H2O. The cells were again harvested by centrifugation, and the pellet was resuspended in 3 ml of a lysis buffer (50 mM Tris-HCl, pH 7.6, 100 mM NaCl, 10% glycerol, 1 mM dithiothreitol). After the cells were sonicated, the cell debris was removed by centrifugation at 15,000 rpm for 20 min at 4°C, and the supernatant was used for assaying Ung activity. The DNA oligonucleotide containing uracil (oligo Lac-461Ruracil, 5′-AGCGCCATGGCCTGACUCATTCCCCAGCGA-3′) was labeled with 32P at the 5′ end by T4 polynucleotide kinase (Toyobo) and hybridized to the unlabeled oligomer, LAC-461F (5′-TCGCTGGGGAATGGGTCAGGCCATGGCGCT-3′), at a molar ratio of 1:10 to form a duplex with a single U · G mismatch. The duplex was separated with a 20% polyacrylamide gel electrophoresis and recovered. It was reacted with cell extract for 30 min at 37°C in a treatment buffer (20 mM Tris-HCl, 10 mM EDTA) and then treated with 0.1 M NaOH to cleave at the apyrimidine site for another 30 min at 37°C. After that, the products were separated in a 20% polyacrylamide containing 8% urea and autoradiographed.
Lac+ mutation assay.
To measure the mutation frequency from G · C to A · T, a spontaneous Lac+ mutation assay using E. coli CC102 (2) was performed as described previously (17). A single colony was inoculated into 5 ml of LB medium containing 1 mM IPTG and grown with aeration for 16 h. The overnight culture was diluted 106-fold with minimal A salt (18). Then, 0.1 ml of the diluted culture was added to 10 ml of minimal A medium containing up to 0.2% glucose, 1 mM MgSO4, 0.0005% thiamine hydrochloride, and tetracycline and/or chloramphenicol. After incubation for 16 h, the culture was harvested by centrifugation. The cell pellet was resuspended in 1 ml of minimal A salt, plated on a minimal A medium (0.2% lactose or 0.2% glucose), and incubated at 37°C. The mutation frequency was then calculated as the ratio of lac+ cells to 1 × 109 cells.
RESULTS AND DISCUSSION
Effect of overexpression of CpxR on Ung activity.
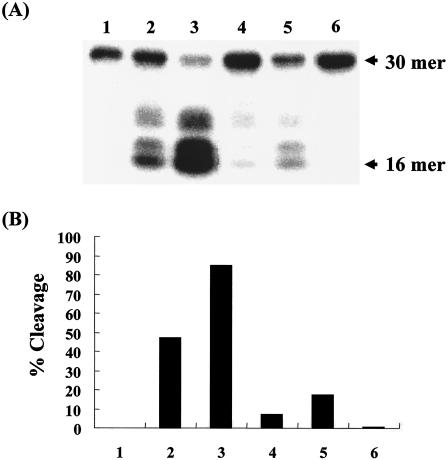
To determine whether the Cpx system is involved in the regulation of ung expression, we examined the Ung activity in crude cell extracts from E. coli BW25113(p41-5ΔGFP) overexpressing CpxR. When E. coli BW25113(p41-5ΔGFP) was incubated in the presence of IPTG, it was confirmed that the cpxR transcript from the lac promoter on the plasmid was overexpressed (Fig. 1A, lane 4). To measure Ung activity, we used a model substrate consisting of a synthetic polynucleotide of 30 nucleotides in length containing a single uracil residue at position 17 from the 5′ end. When cell extracts from E. coli BW25113(pCA24ΔGFP) without CpxR expression were used, the labeled polynucleotide (30-mer) containing uracil was cleaved after treatment with 0.1 M NaOH to form a 16-mer oligonucleotide and two other bands as cleaved DNA products (Fig. 2A, lanes 2 and 3). These bands were not detected in the absence or presence of the cell extracts from BWung (Fig. 2A, lanes 1 and 6). When CpxR was induced by addition of IPTG in BW25113(p41-5ΔGFP) (Fig. 2A, lanes 4 and 5), Ung activity decreased to about fivefold lower than that of BW25113(pCA24ΔGFP) (Fig. 2A, lanes 2 and 3), implying that the Cpx system repressed ung expression.
FIG. 1.

Overexpression of cpxR. The arrow indicates cpxR transcript from the lac promoter on the plasmid p41-5ΔGFP. BW25113(pCA24ΔGFP) (lanes 1 and 2) and BW25113(p41-5ΔGFP) (lanes 3 and 4) were grown to mid-log phase (OD600, 0.6) in the absence (lanes 1 and 3) or presence (lanes 2 and 4) of 1 mM IPTG. The S1 nuclease assay for the cpxR transcript was performed as described in Materials and Methods.
FIG. 2.
Assay of uracil-DNA glycosylase (Ung) activity. (A) Ung activity was assayed as described in Materials and Methods. For this assay, cell extracts (lanes 2 and 4, 25 ng; lanes 3 and 5, 50 ng) from BW25113(pCA24ΔGFP) (lanes 2 and 3), BW25113(p41-5ΔGFP) (lanes 4 and 5), and BWung (lane 6, 50 ng) were used. The 30-mer oligonucleotide was cleaved by Ung and NaOH to form a 16-mer product. In lane 1, no cell extract was used. (B) Cleaved DNA products (from panel A) were quantified with BAS 1000 Mac (Fuji film). Percent cleavage was determined by dividing the intensity of the cleaved product (16-mer) by the total intensity, which was defined as the sum of the intensities of the intact substrate and the cleaved products.
As described previously (9), a G · C-to-A · T transition was stimulated in E. coli ung mutants (CC102ung) (Table 2). We investigated the possible effects of CpxR overexpression on the mutator activity of the G · C-to-A · T transition by using a spontaneous Lac+ mutation assay (17). The results in Table 2 indicate that the G · C-to-A · T transition frequency for strain CC102(p41-5ΔGFP), overexpressing CpxR, was stimulated to about sixfold more than that for CC102(pCA24ΔGFP).
TABLE 2.
G · C→A · T mutation frequency
| Strain | No. of Lac+ colonies per 109 cellsa (±SD) |
|---|---|
| CC102 | 1.7 (±0.021) |
| CC102ung | 5.9 (±0.253) |
| pCA24ΔGFP/CC102 | 1.8 (±0.085) |
| p41-5ΔGFP/CC102 | 11.2 (±1.060) |
Each value represents the average of the data from three independent experiments.
Negative regulation of ung gene expression by overexpression of CpxR.
To investigate the effect of CpxR overexpression on the transcription of the ung gene, we performed an S1 nuclease assay using RNAs prepared from CpxR-expressing BW25113(p41-5ΔGFP) as well as BW25113(pCA24ΔGFP). When CpxR was overexpressed by addition of IPTG (Fig. 1A, lane 4; Fig. 3A, lane 4; Fig. 3B, lane 8), the transcription of ung decreased by fivefold (Fig. 3A, lane 4) compared to that of BW25113(pCA24ΔGFP) (Fig. 3A, lane 2) while cpxP increased by 10-fold (Fig. 3B, lanes 6 and 8). In addition, we examined whether overproduction of the outer membrane lipoprotein NlpE can repress the ung expression (Fig. 3C, lane 4), since overproduction of NlpE activates the Cpx signal transduction pathway (3, 7, 25). When nlpE expression was induced by arabinose, the ung transcription was repressed by fivefold (Fig. 3C, lane 4) compared to that of BW25113(pBAD18) (Fig. 3C, lane 2), while the transcriptional level of cpxP increased by 10-fold (Fig. 3D, lanes 6 and 8). Neither IPTG nor arabinose repressed ung and cpxP transcriptions in BW25113 (data not shown); therefore, these results suggest that the ung expression was negatively regulated by the Cpx pathway.
FIG. 3.
Effects of overexpressed CpxR or NlpE on ung and cpxP transcription. BW25113(pCA24ΔGFP) (A and B, lanes 1, 2, 5, and 6) and BW25113(p41-5ΔGFP) (A and B, lanes 3, 4, 7, and 8) were grown to mid-log phase (OD600, 0.6) in the absence (A and B, lanes 1, 3, 5, and 7) or presence (A and B, lanes 2, 4, 6, and 8) of 1 mM IPTG. S1 nuclease assays for ung (A) and cpxP (B) transcripts were performed as described in Materials and Methods. BW25113(pBAD18) (C and D, lanes 1, 2, 5, and 6) and BW25113(pBADnlpE) (C and D, lanes 3, 4, 7, and 8) were grown to mid-log phase (OD600, 0.6) in the absence (C and D, lanes 1, 3, 5, and 7) or presence (C and D, lanes 2, 4, 6, and 8) of 0.2% arabinose. After that, S1 nuclease assays for ung (C) and cpxP (D) transcripts were performed. Lane AG represents the Maxam-Gilbert sequence ladder. The transcription start site is marked with an asterisk.
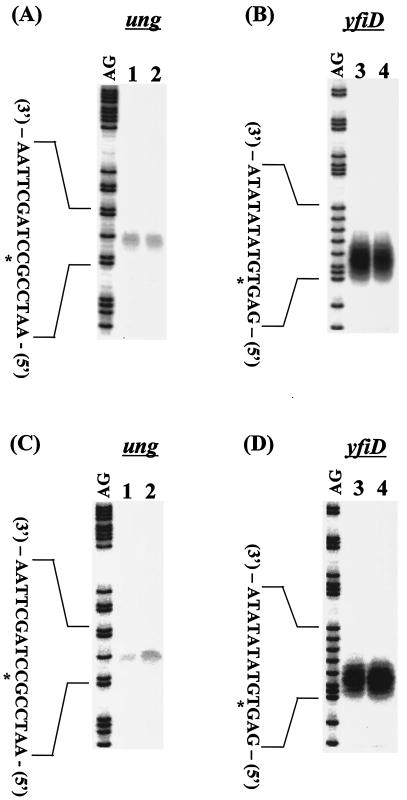
To confirm this result, we next examined the influence of cpxRA deletion on the expression of the ung gene. In wild-type BW25113 cells, the level of ung mRNA decreased in the stationary phase (Fig. 4C, lane 1), but the level of yfiD transcription did not change independently of the growth phase (Fig. 4B and D). The growth phase-coupled reduction in ung transcription was not observed in strain BW27559 (Fig. 4A and C, lane 2). These results indicate that CpxR negatively regulates ung transcription in the stationary phase and that this repression does not take place in the absence of the Cpx system.
FIG. 4.
ung and yfiD gene expression dependent on growth phase. E. coli BW25113 (A and C, lane 1; B and D, lane 3) and BW27559 (ΔcpxR) (A and C, lane 2; B and D, lane 4) were grown to mid-log phase (OD600 = 0.6) (A, B) or early stationary phase (OD600 = 1.2) (C, D). After that, S1 nuclease assays for ung (A, C) and yfiD (B, D) transcripts were performed. Lane AG represents the Maxam-Gilbert sequence ladder. The transcription start site is marked with an asterisk.
To further confirm the role of CpxR in the transcription of the ung gene, we performed in vitro transcription using reconstituted RNA polymerase holoenzyme Eσ70 and truncated DNA fragments (538 bp) containing the promoter region of ung in the presence or absence of CpxR. Addition of CpxR caused repression of ung transcription, while it did not affect the expression from the lacUV5 promoter (Fig. 5A). Such a repression was stimulated in the presence of 10 mM acetylphosphate (Fig. 5B, lanes 5 to 8).
FIG. 5.
In vitro transcription assay. Single-round transcription in vitro of 0.1 pmol ung DNA template (−452 to +86 of promoter region) (A, lanes 5 to 8; B, lanes 5 to 8) or lacUV5 DNA template (A, lanes 1 to 4; B, lanes 1 to 4) was performed in the presence (B) or absence (A) of 10 mM acetylphosphate. The amounts of CpxR were as follows: lanes 1 and 5, 0 pmol; lanes 2 and 6, 1.25 pmol; lanes 3 and 7, 2.5 pmol; lanes 4 and 8, 5 pmol. Electrophoresis was performed with a 6% polyacrylamide sequencing gel. Bold arrows indicate the ung and lacUV5 transcripts. (C) ung (circles) and lacUV5 (square) transcripts in the absence (open symbols) and presence (closed symbols) of CpxR were quantified with BAS 1000 Mac (Fuji film). The relative value in shown as a ratio between each transcript and that in the absence of CpxR.
Identification of the CpxR binding site.
To define the mechanism underlying the repression of ung transcription by CpxR, we tested whether CpxR directly interacts with the ung promoter region. For this purpose, we carried out gel shift assays using the 32P-labeled DNA fragment containing the ung promoter region. In the absence of acetylphosphate, a marked shift was observed only at higher concentrations of CpxR (Fig. 6A, lanes 4 to 6). In contrast, a significant gel shift was observed even at the lowest concentration of CpxR when 25 mM acetylphosphate was added (Fig. 6A, lanes 8 to 12). CpxR is a member of the OmpR family of winged helix-turn-helix transcription factors. We investigated the binding ability of another OmpR family protein, PhoP, and that of the bovine serum albumin to the ung promoter. No interaction of protein-ung promoter DNA was detected (Fig. 6B). These results indicate that the affinity of CpxR to the CpxR box of the ung promoter region is increased by phosphorylation.
FIG. 6.
Gel shift assay. (A) Probe A was incubated at 37°C for 10 min with CpxR (lanes 1 to 6) or CpxR phosphorylated by acetylphosphate at 37°C (lanes 7 to 12). The amounts of CpxR were as follows: lanes 1 and 7, 0 pmol; lanes 2 and 8, 1.25 pmol; lanes 3 and 9, 2.5 pmol; lanes 4 and 10, 5 pmol; lanes 5 and 11, 7.5 pmol; lanes 6 and 12, 10 pmol. (B) Probe A was incubated at 37°C for 10 min with PhoP (lanes 1 to 5) or BSA (lanes 6 to 10) at 37°C. The amounts of PhoP or BSA were as follows: lanes 1 and 6, 0 pmol; lanes 2 and 7, 1.25 pmol; lanes 3 and 8, 2.5 pmol; lanes 4 and 9, 5 pmol; lanes 5 and 10, 10 pmol).
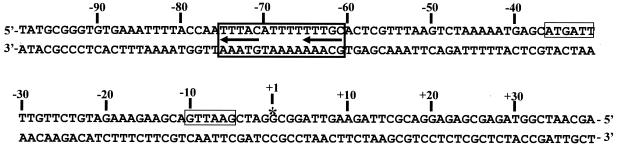
Next, we tried to identify the specific CpxR binding site on the ung promoter region by using a DNase I footprinting assay. CpxR was found to bind to the CpxR box containing the direct repeat between −61 and −86 upstream from the transcriptional start position (Fig. 7). The CpxR box in the ung promoter is located 61 bp upstream of the transcription start site (Fig. 8) and corresponds with the site predicted by DeWulf et al. (6). It is well known that the C-terminal domain of the α subunit of RNA polymerase (α-CTD) interacts with an AT-rich sequence (UP element) located upstream of the −35 hexamer (24). The CpxR box in the ung promoter also contains an AT-rich sequence corresponding to the UP element. CpxR binding to the ung promoter may repress its transcription by interfering with the ability of the α-CTD to bind to the UP element. In Salmonella enterica, the transcriptional factor PmrA binding to the 59 bp upstream site in the pmrD promoter represses pmrD transcription by interfering with the ability of the α-CTD to interact with a putative UP element that overlaps the AT-rich PmrA binding site (15). CpxR seems to repress the ung expression in a similar manner as shown for the pmrD promoter (15). Taken together, these results indicate that the ung gene transcription is directly controlled by phosphorylated CpxR.
FIG. 7.
DNase I footprinting assay. Probe A was incubated with various amounts of the purified CpxR (lane 1, 0 pmol; lane 2, 10 pmol; lane 3, 20 pmol; lane 4, 30 pmol; lane 5, 40 pmol; lane 6, 50 pmol; lane 7, 60 pmol; lane 8, 70 pmol; lane 9, 80 pmol) and subjected to DNase I footprinting assays. Lanes AG represent the Maxam-Gilbert sequence ladder. The black boxes and bold arrows indicate the CpxR binding region and the direct repeat, respectively.
FIG. 8.
ung promoter and CpxR box. The transcription start site is marked with an asterisk. The nucleotide number represents the distance from the transcription initiation site of the ung promoter. Putative recognition sequences for σ70 (−10 and −35) are boxed. The arrows indicate the direct repeat in the CpxR box shown previously (6).
In the present study, we demonstrated negative regulation of ung expression by overexpression of CpxR (see Fig. 2 and 3). Transcription of the ung gene at the stationary phase is about threefold higher in the cpxRA deletion strain than in the parental strain (Fig. 4C). DeWulf et al. (6) predicted positive control of the ung, ompC, psd, mviM, aroK, and secA genes by phosphorylated CpxR and negative control of rpoErseABC and aer. In this paper, we suggest that ung is expressed in the exponential growth phase but decreases in the stationary phase concomitantly with the increase in phosphorylated CpxR. This finding indicates that ung is a target of negative control by CpxR.
Furthermore, we also found an increase in the mutation level of E. coli overexpressing CpxR. The increased mutation might be attributable, at least in part, to the repression of ung expression by the increased CpxR. This is because it is not possible to repair DNA uracil residues, which arise as a result of either misincorporation of dUMP by DNA polymerase or deamination of DNA cytosine residues. This finding further implies that the CpxR/CpxA two-component system plays a role in controlling the mutation rate.
Acknowledgments
We thank R. W. Simons, J. H. Miller, B. K. Duncan, H. Mori, and Hirofumi Aiba for providing plasmids and E. coli strains.
This work was supported by the Program for Promoting Advancement of Academic Research at Private Universities and a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan.
REFERENCES
- 1.Backman, K., M. Ptashne, and W. Gilbert. 1976. Construction of plasmids carrying the cI gene of bacteriophage λ. Proc. Natl. Acad. Sci. USA 73:4174-4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cupples, C. G., and J. H. Miller. 1989. A set of lacZ mutations in Escherichia coli that allow rapid detection of each of the six base substitutions. Proc. Natl. Acad. Sci. USA 86:5345-5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danese, P. N., and T. J. Silhavy. 1997. The σE and the Cpx signal transduction systems control the synthesis of periplasmic protein-folding enzymes in Escherichia coli. Genes Dev. 11:1183-1193. [DOI] [PubMed] [Google Scholar]
- 4.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeWulf, P., O. Kwon, and E. C. C. Lin. 1999. The CpxRA signal transduction system of Escherichia coli: growth-related autoactivation and control of unanticipated target operons. J. Bacteriol. 181:6772-6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeWulf, P., A. M. McGuire, X. Liu, and E. C. C. Lin. 2002. Genome-wide profiling of promoter recognition by the two-component response regulator CpxR-P in Escherichia coli. J. Biol. Chem. 277:26652-26661. [DOI] [PubMed] [Google Scholar]
- 7.DiGiuseppe, P. A., and T. J. Silhavy. 2003. Signal detection and target gene induction by the CpxRA two-component system. J. Bacteriol. 185:2432-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorel, C., O. Vidal, C. Prigent-Combaret, I. Vallet, and P. Lejeune. 1999. Involvement of the Cpx signal transduction pathway of E. coli in biofilm formation. FEMS Microbiol. Lett. 178:169-175. [DOI] [PubMed] [Google Scholar]
- 9.Duncan, B. K., and B. Weiss. 1982. Specific mutator effects of ung (uracil-DNA glycosylase) mutations in Escherichia coli. J. Bacteriol. 151:750-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duncan, B. K. 1985. Isolation of insertion, deletion, and nonsense mutations of the uracil-DNA glycosylase (ung) gene of Escherichia coli K-12. J. Bacteriol. 164:689-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallinali, P., and J. Jiricny. 1996. A new class of uracil-DNA glycosylases related to human thymine-DNA glycosylase. Nature (London) 383:735-738. [DOI] [PubMed] [Google Scholar]
- 12.Guzman, L.-M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hung, D. L., T. L. Raivio, C. H. Jones, T. J. Silhavy, and S. J. Hultgren. 2001. Cpx signaling pathway monitors biogenesis and affects assembly and expression of P pili. EMBO J. 20:1508-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kajitani, M., and A. Ishihama. 1983. Determination of the promoter strength in the mixed transcription system: promoters of lactose, tryptophan and ribosomal protein L10 operons from Escherichia coli. Nucleic Acids Res. 11:671-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato, A., T. Latifi, and E. A. Groisman. 2003. Closing the loop: the PmrA/PmrB two-component system negatively controls expression of its posttranscriptional activator PmrD. Proc. Natl. Acad. Sci. USA 100:4706-4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lutsenko, E., and A. S. Bhagwat. 1999. The role of the Escherichia coli Mug protein in the removal of uracil and 3,N4-ethenocytosine from DNA. J. Biol. Chem. 274:31034-31038. [DOI] [PubMed] [Google Scholar]
- 17.Mackay, W. J., S. Han, and L. D. Samson. 1994. DNA alkylation repair limits spontaneous base substitution mutation in Escherichia coli. J. Bacteriol. 176:3224-3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller, J. H. (ed.) 1992. A short course in bacterial genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 19.Minagawa, S., H. Ogasawara, A. Kato, K. Yamamoto, Y. Eguchi, T. Oshima, H. Mori, A. Ishihama, and R. Utsumi. 2003. Identification and molecular characterization of the Mg2+ stimulon of Escherichia coli. J. Bacteriol. 185:3696-3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mokkapati, S. K., A. R. Fernandez de Henestrosa, and A. S. Bhagwat. 2001. Escherichia coli DNA glycosylase Mug: a growth-regulated enzyme required for mutation avoidance in stationary-phase cells. Mol. Microbiol. 41:1101-1111. [DOI] [PubMed] [Google Scholar]
- 21.Otto, K., and T. J. Silhavy. 2002. Surface sensing and adhesion of Escherichia coli controlled by the Cpx-signaling pathway. Proc. Natl. Acad. Sci. USA 99:2287-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mori, H., K. Isono, T. Horiuchi, and T. Miki. 2000. Functional genomics of Escherichia coli in Japan. Res. Microbiol. 151:121-128. [DOI] [PubMed] [Google Scholar]
- 23.Oshima, T., H. Aiba, Y. Masuda, S. Kanaya, M. Sugiura, B. L. Wanner, H. Mori, and T. Mizuno. 2002. Transcriptome analysis of all two-component regulatory system mutants of Escherichia coli K-12. Mol. Microbiol. 46:281-291. [DOI] [PubMed] [Google Scholar]
- 24.Ross, W., K. K. Gosink, J. Salomon, K. Igarashi, C. Zou, A. Ishihama, K. Severinov, and R. L. Gourse. 1993. A third recognition element in bacterial promoter: DNA binding by the α subunit of RNA polymerase. Science 262:1407-1413. [DOI] [PubMed] [Google Scholar]
- 25.Snyder, W. B., L. J. B. Davis, P. N. Danese, C. L. Cosma, and T. J. Silhavy. 1995. Overproduction of NlpE, a new outer membrane lipoprotein, suppresses the toxicity of the Cpx signal transduction pathway. J. Bacteriol. 177:4216-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varshney, U., T. Hutcheon, and J. H. van de Sande. 1988. Sequence analysis, expression, and conservation of Escherichia coli uracil DNA glycosylase and its gene (ung). J. Biol. Chem. 263:7776-7784. [PubMed] [Google Scholar]
- 27.Wyszynski, M., S. Gabbara, and A. S. Bhagwat. 1994. Cytosine deaminations catalyzed by DNA cytosine methyltransferases are unlikely to be the major cause of mutational hot spots at sites of cytosine methylation in Escherichia coli. Proc. Natl. Acad. Sci. USA 91:1574-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamamoto, K., H. Ogasawara, N. Fujita, R. Utsumi, and A. Ishihama. 2002. Novel mode of transcription regulation of divergently overlapping promoters by PhoP, the regulator of two-component system sensing external magnesium availability. Mol. Microbiol. 45:423-438. [DOI] [PubMed] [Google Scholar]