Summary
Otlertuzumab (TRU‐016) is a humanized anti‐CD37 protein therapeutic that triggers direct caspase‐independent apoptosis of malignant B cells and induces antibody‐dependent cell‐mediated cytotoxicity. Patients with relapsed chronic lymphocytic leukaemia (CLL) received either otlertuzumab (20 mg/kg) weekly by IV infusion for two 28‐day cycles then every 14 days for four 28‐day cycles and IV bendamustine (70 mg/m2) on Days 1 and 2 of each cycle for up to six 28‐day cycles or bendamustine alone. Thirty‐two patients were treated with otlertuzumab and bendamustine and 33 with bendamustine alone. Overall response rate according to the International Workshop on Chronic Lymphocytic Leukaemia criteria was 69% in the otlertuzumab and bendamustine arm and 39% in the bendamustine alone arm (P = 0·025). Median progression‐free survival (PFS) was 15·9 months in the otlertuzumab and bendamustine arm and 10·2 months in the bendamustine alone arm (P = 0·0192). There was a higher incidence of pyrexia (34% vs. 12%) and neutropenia (59% vs. 39%) with the combination but this did not result in a higher incidence of severe (grade 3/4) infections (13% vs. 27%). This combination significantly increased the response rate and prolonged the PFS over single agent bendamustine in patients with relapsed or refractory CLL.
Keywords: otlertuzumab, CLL, bendamustine
Significant advances have occurred in the treatment of chronic lymphocytic leukaemia (CLL) but, in general, most patients eventually relapse after first line therapy (Hallek et al, 2010; Burger et al, 2012; Fischer et al, 2012; Goede et al, 2014). Bendamustine alone or in combination with rituximab has emerged as an effective treatment strategy for patients with CLL (Kath et al, 2001; Bremer, 2002; Bergmann et al, 2005; Fischer et al, 2011, 2012). In studies of bendamustine monotherapy, depending on the patient population, salvage therapy has resulted in an overall response rate (ORR) between 40–93% and a complete response (CR) rate between 7–30% (Kath et al, 2001; Aivado et al, 2002; Bremer, 2002; Bergmann et al, 2005; Lissitchkov et al, 2006). In a phase 1/2 trial conducted by the German CLL Study Group, single agent bendamustine in patients with relapsed/refractory CLL, produced an ORR of 56% and a CR rate of 12%; progression‐free survival (PFS) and overall survival (OS) were not reported (Bergmann et al, 2005). In another trial conducted by the German CLL Study Group, the combination of bendamustine and rituximab was evaluated in a single arm Phase 2 study of patients with relapsed/refractory CLL and produced an ORR of 59% with a CR rate of 9% and a PFS of 15 months with an OS of 34 months (Fischer et al, 2011).
CD37 is a heavily glycosylated cell surface protein that is expressed constitutively at high levels on human B cells and transformed mature human leukaemic B cells (Campo et al, 1991; Belov et al, 2001; Barrena et al, 2005). Reduced cell surface expression of CD20 on peripheral B‐CLL cells compared to B cells from healthy donors is a well‐known hallmark of B‐CLL (Ginaldi et al, 1998; D'Arena et al, 2000; Rawstron et al, 2001). While the expression of CD37 on CLL cells also appears lower than expression on normal B cells (Peters et al, 1994; Barrena et al, 2005; Rafiq et al, 2013), CD37 represents a solid alternative target for CLL with a surface antigen density similar to or higher than that of CD20 (Peters et al, 1994; Press et al, 1994). Anti‐CD37 antibodies currently in Phase 1 development for treatment of B cell malignancies include the monoclonal antibody BI836826 for CLL and the anti‐CD37 immunoconjugates 131I‐MB‐1, IMGN529 and 177Lu‐tetulomab (BetalutinTM) for non‐Hodgkin lymphoma (Robak & Robak, 2014).
Otlertuzumab (TRU‐016) is a CD37‐specific, single‐chain, homodimeric therapeutic protein built on the ADAPTIR (modular protein technology) platform, consisting of antibody‐derived, single‐chain variable fragments linked to immunoglobulin (Ig) constant domains (Byrd et al, 2009). Otlertuzumab binds to CD37 and, like monoclonal antibodies, employs the effector function of Fc‐dependent cytotoxicity (FcDCC), also known as antibody‐dependent cellular cytotoxicity (ADCC). Otlertuzumab does not induce complement activation. It does induce apoptosis directly via binding to the CD37 receptor, which results in upregulation of BIM (also termed BCL2L11), a pro‐apoptotic protein (Lapalombella et al, 2012). Because otlertuzumab delivers its signal via interaction with CD37 rather than CD20, this drug offers the possibility for therapeutic benefit when CD20 is shed, blocked or removed from the surface of the targeted B cells, a limitation that has been reported in CLL (Jilani et al, 2003; Kennedy et al, 2003; Gopal et al, 2008). In preclinical models, treatment with otlertuzumab resulted in increased anti‐tumour activity when combined with other therapeutic drugs used for B‐cell malignancies (Baum et al, 2009; Algate et al, 2010; Smolewski et al, 2014).
In vitro studies show additive activity of otlertuzumab with bendamustine (Algate et al, 2010). These findings were extended to in vivo xenograft models, where otlertuzumab plus bendamustine resulted in a greater inhibition of tumour growth as compared to that attained with each individual drug (Algate et al, 2010).
The first‐in‐human Phase 1 trial of otlertuzumab demonstrated single‐agent clinical activity and appeared to be well tolerated in an advanced CLL patient population (Byrd et al, 2014). Thus, we hypothesized that the addition of otlertuzumab to bendamustine could further improve the response in CLL patients. The Phase 1b portion of this study demonstrated that otlertuzumab in combination with bendamustine was well tolerated and showed a positive response in subjects with relapsed CLL (Awan et al, 2012). The randomized Phase 2 study reported here evaluates the safety, pharmacokinetics and efficacy of otlertuzumab and bendamustine compared to bendamustine alone.
Patients, materials, methods
This research was approved by the relevant institutional review boards or ethics committees and all human participants gave written informed consent. This trial was registered at www.clinicaltrials.gov as NCT01188681.
The objective of this study was to compare the efficacy and safety of otlertuzumab in combination with bendamustine to bendamustine alone in patients with relapsed CLL. In addition, this study investigated the pharmacokinetics and pharmacodynamics of otlertuzumab and the development of antibodies to otlertuzumab.
Eligibility criteria and study design
Previously treated patients ≥18 years of age with a diagnosis of CLL by the 2008 International Workshop on CLL Criteria (IWCLL) (Hallek et al, 2008) and with Rai stage I–II (intermediate risk) or III‐IV (high risk) CLL (Rai et al, 2000) who had refractory or relapsed disease after 1–3 prior treatments were included. Patients were required to have the following: an Eastern Cooperative Oncology Group (ECOG) performance status ≤2; creatinine clearance (CrCl) >40 ml/min; serum creatinine, total bilirubin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) ≤2·0× upper limit of normal (ULN); an absolute neutrophil count (ANC) ≥1·2 × 109/l; platelet count ≥75 × 109/l; and no anti‐cancer or investigational therapy within 30 days of treatment. Patients were not eligible if they had received treatment with rituximab or other B‐cell depleting agents within 30 days before first study drug dose or alemtuzumab within 12 weeks before first study drug dose; were refractory to fludarabine or other purine analogue therapy; discontinued prior bendamustine secondary to toxicities; or had major surgery within 30 days before first study drug dose.
Dosing and premedication
In the combination arm, otlertuzumab (20 mg/kg) was administered by intravenous (IV) infusion over 2–3 h over six 28‐day cycles. Dosing was weekly for the first 2 cycles, then on Day 1 and 15 of the next 4 cycles. In both arms, bendamustine (70 mg/m2) was dosed on Days 1 and 2 of every 28‐day cycle for 6 cycles. The dose of bendamustine was reduced when the ANC had not recovered to >1 × 109/l and platelet count had not recovered to >50 × 109/l by 28 days after dosing. After the first delay, bendamustine was reduced to 50 mg/m2, at the second occurrence bendamustine was reduced to 30 mg/m2, at the third occurrence, bendamustine was discontinued and at the fourth occurrence otlertuzumab was discontinued. Otlertuzumab was supplied by Emergent BioSolutions (Seattle, WA, USA). Bendamustine was purchased commercially. Patients were to receive full supportive care, including transfusions of blood and blood products, antibiotics, antiemetics, growth factors, as appropriate. Growth factors were not to be used prophylactically in the first cycle.
Safety assessments
Toxicity was assessed at each evaluation according to the National Cancer Institutes (NCI) Common Terminology Criteria for Adverse Events (CTCAE), version 4.03 (http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf).
Response assessments
Response was assessed monthly by the investigator on the basis of complete blood count (CBC); clinical assessment including measurement of lymph node size and spleen and liver size by physical examination; and evaluation of constitutional symptoms secondary to CLL. The primary endpoint, overall response to treatment, was assessed by the investigator 2 months after the end of treatment using the 2008 IWCLL criteria (Hallek et al, 2008) which included clinical assessment, CBC, computed tomography (CT) scan results, bone marrow and aspirate results, and duration of response. Response was also assessed per the 1996 NCI Working Group Criteria (Cheson et al, 1996). Progression during the follow‐up period was determined by clinical criteria and CBC. A CT scan was not required to confirm progression (Blum et al, 2007).
Pharmacokinetic analyses
Serum samples for pharmacokinetic (PK) analysis were analysed by a qualified and sensitive enzyme‐linked immunosorbent assay specifically developed for otlertuzumab using a monoclonal antibody specific for the CD37 binding domain of otlertuzumab. For terminal elimination half‐life calculations, a minimum of 3 time points with detectable levels of otlertuzumab were required to be included in final PK parameter estimates. Based on this requirement, 6 patients treated with otlertuzumab and bendamustine were excluded from mean parameter estimates. Actual times after otlertuzumab dose administration for individual subjects were used in all PK calculations; however, the proscribed times were used for graphing. Patients not receiving a full dose of otlertuzumab were excluded from PK parameter calculations, such as mean C max and total area under the concentration curve (AUC). Values for C max and time to reach C max (T max) were obtained by direct inspection of data. Area under the concentration‐time curve (AUCt) was determined by the log‐linear trapezoidal rule from time 0 to the last observed concentration (C t) at time t using GraphPad Prism® Version 6.01 (GraphPad Software, San Diego, CA, USA). Otlertuzumab PK parameters were estimated using validated WinNonlin Professional Version 6.3 software (Pharsight Corporation, Mountain View, CA, USA) with non‐compartmental methods when a patient had sufficient late time points available for PK analysis. Individual concentration‐time profiles were plotted and the terminal disposition rate constant (λz) was determined by the log‐linear regression of at least 3 points judged to be in the terminal phase. Descriptive statistics, such as means, standard deviations and precision [coefficient of variation (CV%)] were calculated for variables using Microsoft® Excel® 2010 (Microsoft Corporation, Redmond, WA, USA).
Statistical methods
An adaptive (minimization) randomization procedure was used to assign the patients at a 1:1 ratio to either otlertuzumab and bendamustine or bendamustine alone. The stratification factors in decreasing order of importance were:
High risk genomic features (del[17p13·1] or TP53 mutation): yes or no
Cumulative Illness Rating Scale >6 or ≤6
CrCl <60 ml/min or ≥60 ml/min.
Randomization was conducted using a centralized Interactive Response Technology on a competitive basis for patient enrolment across all study sites.
The primary endpoint was the investigator‐assessed ORR for otlertuzumab plus bendamustine and bendamustine alone. The primary efficacy analysis was to compare the combination of otlertuzumab and bendamustine to bendamustine alone with respect to the ORR [patients with partial response (PR) or CR]. Patients whose disease response could not be assessed were considered non‐responders. Assuming an ORR of 58% with bendamustine and an ORR of 80·7% with the combination of otlertuzumab and bendamustine (equivalent to a 39% improvement over 58%) it was assumed that a sample size of 60 evaluable patients would yield 80% power to show the combination has a significantly higher ORR than bendamustine alone at a one‐sided significance level of 20%. Secondary endpoints were CR rate, PFS and OS.
Data analyses were based on descriptive statistics. For continuous variables, these statistics included the following: mean, median, standard deviation, minimum and maximum. Final study analyses were conducted after the last patient stopped study treatment and response was assessed using intent‐to‐treat analysis. Time‐to‐event variables were described using Kaplan–Meier estimates, as well as mean and median time with 2‐sided 80% confidence intervals of the mean and median (Ellis et al, 2008).
Results
Patient characteristics and treatment
Sixty‐seven patients were enrolled and 65 were treated at 20 sites between December 2011 and April 2013. One patient randomized to the combination arm had bladder cancer and was not treated and one patient randomized to the control arm (i.e., treatment with bendamustine alone) withdrew consent before beginning treatment. Baseline characteristics are summarized in Table 1 and prior therapies are summarized in Table 2. Treatment arms were generally well balanced; however, patients in the combination arm were older, had more prior regimens, longer time since first diagnosis, and more bulky disease. More patients in the control arm were Rai Stage III or IV (36·4%) compared to the combination arm (28·1%). Two patients in the combination arm and 5 in the control arm had 17p13 deletions and 4 patients in the combination arm and 6 in the control arm had TP53 mutation. Five patients in the combination arm and 3 in the control arm were refractory to prior treatment, defined as relapse within 6 months after prior treatment. In all but one of these patients, a patient in the control arm, prior treatment included rituximab. One patient in each arm was refractory to bendamustine.
Table 1.
Baseline characteristics
| Otlertuzumab + bendamustine (N = 32) | Bendamustine (N = 33) | |
|---|---|---|
| Age (years) | ||
| Median | 65 | 60 |
| Range | 44–82 | 48–79 |
| ≥70 years | 10 (31·3%) | 9 (27·3%) |
| Sex | ||
| Male | 20 (63%) | 25 (75·8%) |
| Female | 12 (37·5%) | 8 (24·2%) |
| Race | ||
| White | 30 (93·8%) | 32 (97·0%) |
| Black | 2 (6·3%) | 1 (3·0%) |
| Rai stage | ||
| 0 | 2 (6·3%) | 1 (3·0%) |
| I | 8 (25·0%) | 5 (15·2%) |
| II | 13 (40·6%) | 15 (45·5%) |
| III | 2 (6·3%) | 2 (6·1%) |
| IV | 7 (21·9%) | 10 (30·3%) |
| β2‐microglobulin (mg/l) | ||
| Median | 3·2 | 3·4 |
| (Min, Max) | (1·6, 12·9) | (1·7, 7·6) |
| β2‐microglobulin (mg/l) group | ||
| n | 31 | 33 |
| 0–3·0 | 14 (45·2%) | 12 (36·4%) |
| 3·1–4·0 | 8 (25·8%) | 10 (30·3%) |
| 4·1–5·0 | 2 (6·5%) | 4 (12·1%) |
| 5·1–10·0 | 6 (19·0%) | 7 (21·2%) |
| >10·0 | 1 (3·2%) | 0 |
| Direct anti‐globulin test | ||
| n | 29 | 30 |
| Positive | 1 (3·4%) | 8 (26·7%) |
| Negative | 28 (96·6%) | 22 (73·3%) |
| Number of prior therapies | ||
| 1 | 12 (38%) | 20 (61%) |
| 2 | 14 (44%) | 9 (27%) |
| ≥3 | 6 (19%) | 4 (12%) |
| Median (range) sum of product diameters | 27·91 (5·3, 325·2) | 40·37 (1·9, 966·6) |
| 17p13 deletion | 2 (6·3%) | 5 (15·2%) |
| TP53 mutation | 4 (12·5%) | 6 (18·2%) |
| Refractory to rituximab | 5 (15·6%) | 3 (9·1%) |
All values are given as n (%) unless otherwise stated.
Min, minimum; Max, maximum; STD, standard deviation.
Table 2.
Previous therapies
| Characteristic | Otlertuzumab + bendamustine (N = 32) | Bendamustine (N = 33) |
|---|---|---|
| Cyclophosphamide | 28 | 26 |
| Fludarabine | 24 | 23 |
| Rituximab | 27 | 21 |
| FCR | 16 | 10 |
| Prednisone | 5 | 6 |
| Bendamustine | 3 | 2 |
| Chlorambucil | 11 | 4 |
| Cladribine | 6 | 3 |
| Lumiliximab | 5 | 2 |
| Vincristine | 3 | 3 |
| FCR‐L | 4 | 2 |
| FCR | 4 | 1 |
The numbers of patients who received each prior therapy are shown.
FCR, fludarabine, cyclophosphamide, rituximab; FCR‐L, fludarabine, cyclophosphamide, rituximab, lumiliximab; FCR, fludarabine, cyclophosphamide, rituximab.
The median number of cycles received was 6 for each cohort (range 1–6 in the combination arm and 2–6 in the control arm). Exposure to otlertuzumab and bendamustine is summarized in Table 3. Bendamustine exposure was similar between treatment arms. Median treatment duration for otlertuzumab was 156 days for the combination arm (range 2–193). Median treatment duration for bendamustine was 143 days in both arms. Seven patients (22%) in the combination arm and 12 patients (36%) in the control arm discontinued study treatment before completing all 6 cycles (Table 4). Three patients (9%) in the combination arm and 7 patients (21%) in the control arm discontinued study treatment due to adverse events (Table 5). In the combination arm, 3 patients (9%) discontinued treatment due to disease progression and one withdrew to have a stem cell transplant. In the control arm, 3 patients (9%) discontinued treatment due to disease progression, one patient withdrew for an unspecified reason, and one patient withdrew consent. One patient in the control arm died during treatment due to acute heart failure.
Table 3.
Exposure to otlertuzumab and bendamustine
| Otlertuzumab + bendamustine (N = 32) | Bendamustine (N = 33) | ||
|---|---|---|---|
| Otlertuzumab | Bendamustine | ||
| Number of total otlertuzumab infusions receiveda | |||
| Expected | 17 | 12 | 12 |
| Mean (SD) | 15·0 (3·57) | 10·5 (2·79) | 10·2 (2·79) |
| Median | 17·0 | 12·0 | 12·0 |
| Min, Max | 2, 18 | (2, 12) | (4, 12) |
| Average dose received per infusionb(mg) | |||
| Mean (SD) | 1438·6 (335·67) | 122·1 (24·39) | 130·1 (16·59) |
| Median | 1381·0 | 120·4 | 131·0 |
| Min, Max | 910, 2273 | 75, 174 | 91, 158 |
| Total treatment durationc (days) | |||
| Expected | 168 | 168 | 168 |
| Mean (SD) | 145·0 (42·61) | 129·4 (41·35) | 126·5 (39·58) |
| Median | 156·0 | 143·0 | 143·0 |
| Min, Max | 2, 193 | 2, 173 | 43, 170 |
Min, minimum; Max, maximum; SD, standard deviation.
Number of total otlertuzumab infusions received = Total number of otlertuzumab infusion across all treatment cycles.
Average otlertuzumab dose received per infusion = otlertuzumab dose received per infusion summed across all treatment cycles/number of total otlertuzumab infusions received.
Total treatment duration = End date of the last otlertuzumab infusion – beginning date of first otlertuzumab infusion.
Table 4.
Reasons for treatment discontinuation before completing treatment
| Otlertuzumab + bendamustine (N = 32) | Bendamustine (N = 33) | |
|---|---|---|
| Patients who did not complete 6 cycles | 7 (22%) | 12 (36%) |
| Adverse event | 3 (9%) | 7 (21%) |
| Disease progression | 3 (9%) | 3 (9%) |
| Stem cell transplant | 1 (3%) | 0 |
| Death | 0 | 1 (3%) |
| No reason given | 0 | 1 (3%) |
All values are given as n (%).
Table 5.
Adverse events resulting in early discontinuation of study drug
| Otlertuzumab + bendamustine (n = 3) |
| Thrombocytopenia, neutropenia |
| Thrombocytopenia |
| Neutropenia, diarrhoea |
| Bendamustine (n = 7) |
| Pure red cell aplasia |
| Thrombocytopenia |
| Thrombocytopenia |
| Haemolytic anaemia, hypogammaglobulinaemia |
| Immune haemolytic anaemia |
| Pyrexia |
| Bronchial infection |
Clinical responses
As shown in Table 6, ORR per IWCLL criteria was higher in the combination arm compared to the control arm (69% vs. 39%, P = 0·026). Response rate per the NCI Working Group criteria were 81% in the combination arm compared to 67% in the control arm. Per the IWCLL criteria, in the combination arm, 3 patients (9%) had a CR, 1 patient had CR with incomplete marrow recovery, and 19 (59%) had PR. In the control arm, 1 patient (3%) had a CR and 12 (36%) had PR. Overall response rate by baseline characteristic is illustrated in Fig 1 and summarized by 17p deletions and TP53 mutations in Table 7.
Table 6.
Overall response rate per International Workshop on Chronic Lymphocytic Leukaemia (IWCLL) criteria
| Response by IWCLL criteria (Hallek et al, 2008) | Otlertuzumab + bendamustine (N = 32) | Bendamustine only (N = 33) | Rate ratio (95% CIb) | P‐valuec | ||
|---|---|---|---|---|---|---|
| n | % (95% CI) | n | % (95% CIa) | |||
| Overall response | 22 | 68·8 (50·0–83·9) | 13 | 39·4 (22·9–57·9) | 1·75 (1·08–2·83) | 0·026 |
| Complete response | 3 | 9·4 (2·0–25·0) | 1 | 3·0 (0·1–15·8) | ||
| Partial response | 19 | 59·4 (40·6–76·3) | 12 | 36·4 (20·4–54·9) | ||
| Stable disease | 5 | 15·6 (5·3–32·8) | 10 | 30·3 (15·6–48·7) | ||
| Progressive disease | 5 | 15·6 (5·3–32·8) | 10 | 30·3 (15·6–48·7) | ||
Overall response was assessed by the investigator 2 months after the end of treatment using the 2008 IWCLL criteria.
Exact 95% confidence interval.
Confidence interval for rate ratio based on normal approximation without continuity correction.
P‐value from Fisher's exact test for 2 × 2 tables.
Figure 1.
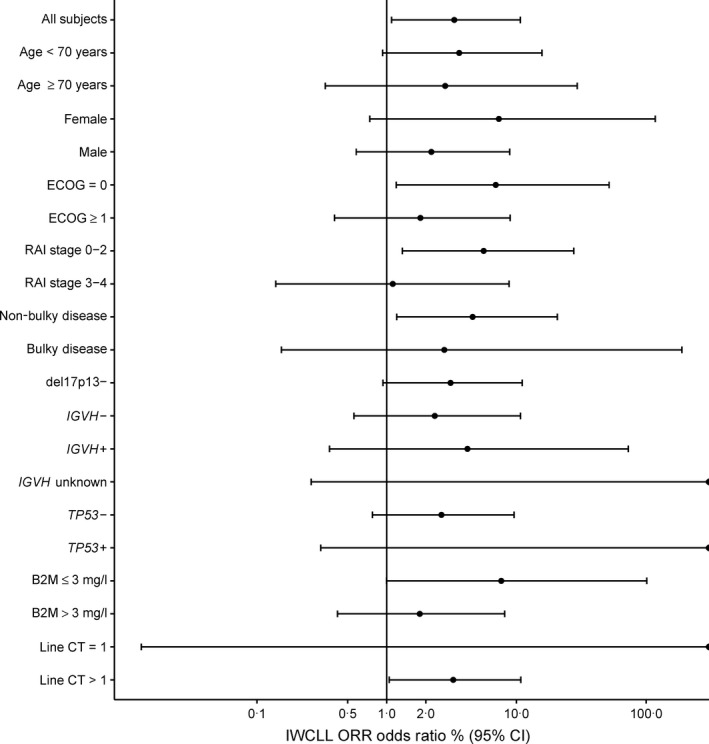
Odds ratio and 95% confidence intervals for overall response rate (per IWCLL criteria; Hallek et al, 2008) by characteristic. In this graph, ORR is shown by baseline characteristic. Points to the right of the vertical line indicate higher ORR in the combination arm compared to the control arm. The odds ratio is indicated by a black circle, and the 95% CI is indicated by a horizontal line. B2M, β2‐microglobulin; CI, confidence interval; Line CT, prior line(s) of chemotherapy; ECOG, Eastern Cooperative Oncology Group performance score; IWCLL, International Workshop on Chronic Lymphocytic Leukaemia; ORR, overall response rate.
Table 7.
Response rate by 17p13·1 deletion and TP53 mutations
| Otlertuzumab + bendamustine (N = 32) | Bendamustine (N = 33) | |||
|---|---|---|---|---|
| Patients with deletion/mutations | Response (IWCLL) | Patients with deletion/mutations | Response (IWCLL) | |
| 17p13·1 deletion only | 0 | NA | 2 | 0 |
| TP53 mutations only | 2 | 2 | 3 | 0 |
| Both mutations | 2 | 0 | 3 | 0 |
IWCLL, International Workshop on Chronic Lymphocytic Leukaemia.
Median PFS was longer in the combination arm compared to the control arm (15·9 months vs. 10·2 months, P = 0·0192, Fig 2A). Median OS was not reached in either arm (Fig 2B). After 2 years of follow‐up, no deaths had occurred in the combination arm and 3 deaths had occurred in the control arm.
Figure 2.
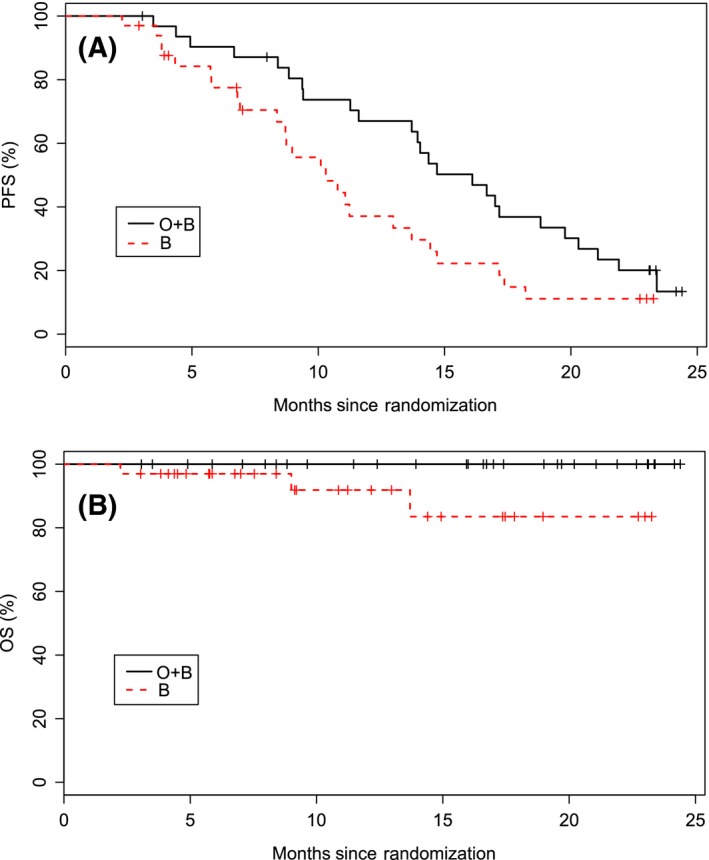
Kaplan–Meier curves are shown for progression free survival (A) and overall survival (B). Median progression free survival was significantly longer (P = 0·0192) in the combination arm (15·9 months) compared to the control arm (10·2 months). Median overall survival was not reached in either arm. The numbers below the graphs are the number of subjects at risk at the corresponding time points. B, bendamustine; HR, hazard ratio; O, otlertuzumab; OS, overall survival; PFS, progression‐free survival. [Colour figure can be viewed at wileyonlinelibrary.com]
Toxicities
Adverse events are summarized in Table 8. The overall incidence of adverse events was generally similar between treatment arms with 91% of patients in the combination arm and 100% in the control arm reporting an adverse event. Severe neutropenia occurred more frequently in the combination arm compared to the control arm (56% vs. 39%), as did severe thrombocytopenia (19% vs. 15%). There were fewer grade 3/4 infections in the combination arm compared to the control arm (13% vs. 27%). No patients in the combination arm and two patients in the control arm had febrile neutropenia. Serious adverse events were less frequent in the combination arm compared to the control arm (31% vs. 45%).
Table 8.
Treatment‐emergent adverse events by system organ class and preferred term in 10% subjects in either arm
| Otlertuzumab + bendamustine (N = 32) | Bendamustine (N = 33) | |||
|---|---|---|---|---|
| All events (%) | Grade 3/4 (%) | All events (%) | Grade 3/4 (%) | |
| Any event | 91 | 66 | 100 | 70 |
| Infection | 59 | 13 | 61 | 27 |
| Neutropenia | 59 | 56 | 39 | 39 |
| Thrombocytopenia | 34 | 19 | 27 | 15 |
| Pyrexia | 34 | 3 | 12 | 0 |
| Anaemia | 31 | 13 | 33 | 15 |
| Nausea | 19 | 0 | 30 | 0 |
| Diarrhoea | 16 | 3 | 21 | 0 |
| Fatigue | 16 | 0 | 15 | 3 |
| Pruritus | 16 | 0 | 3 | 0 |
| Cough | 13 | 0 | 24 | 0 |
| Vomiting | 13 | 0 | 15 | 3 |
| Hyperuricemia | 13 | 0 | 9 | 3 |
| Chills | 13 | 0 | 6 | 0 |
| Headache | 6 | 0 | 15 | 0 |
| Constipation | 6 | 0 | 24 | 0 |
| Upper abdominal pain | 6 | 0 | 12 | 0 |
| Dizziness | 3 | 0 | 12 | 0 |
Pharmacokinetics and pharmacodynamics
Mean otlertuzumab concentration over time is shown in Fig 3. Mean PK parameter estimates are shown in Table 9. The half‐life of otlertuzumab ranged from 5 to 13 days, with a mean of 10 days. Average C max for patients dosed with 20 mg/kg otlertuzumab was approximately 1058 μg/ml. Average clearance and volume of distribution for otlertuzumab were 2·17 ml/day/kg and 28·8 ml/kg, respectively. The treatment schedule used for CLL patients maintained concentrations of otlertuzumab, with trough levels generally above 100 μg/ml. PK parameters were generally similar for males and females.
Figure 3.
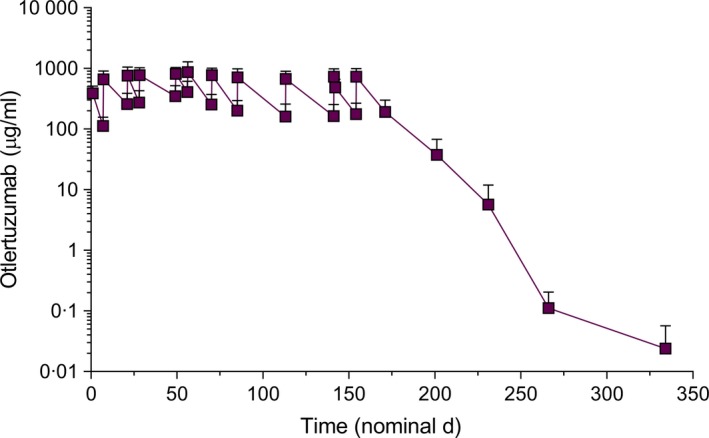
Mean otlertuzumab cncentrations over time. The mean half‐life is 10·0 days. [Colour figure can be viewed at wileyonlinelibrary.com]
Table 9.
Pharmacokinetic parameters
| HL lambda z (day) | C max (μg/ml) | AUC (day*μg/ml) | V (ml/kg) | CL (ml/day/kg) | |
|---|---|---|---|---|---|
| Mean | 10·0 | 1058·3 | 80791 | 28·8 | 2·17 |
| SD | 2·0 | 413·8 | 27809 | 11·5 | 1·25 |
| CV% | 20·5 | 39·1 | 34·4 | 39·9 | 57·6 |
CV%, coefficient of variation; SD, standard deviation; HL lambda z, apparent terminal elimination half‐life; C max, maximum observed concentration; AUC, area under the curve from the time of dosing, to the last measurable concentration; V, volume of distribution based on the terminal phase; CL, serum clearance.
The combination of otlertuzumab and bendamustine was more effective than bendamustine alone at reducing the malignant clone (Wilcoxon exact test, P < 0·0001; Fig 4). In the combination arm, the number of CD19+CD5+ cells was reduced, from a median of 28·464 × 109/l to 0·0039 × 109/l, at the end of treatment compared to a reduction, from 28·867 × 109/l to 0·453 × 109/l, in the control arm.
Figure 4.
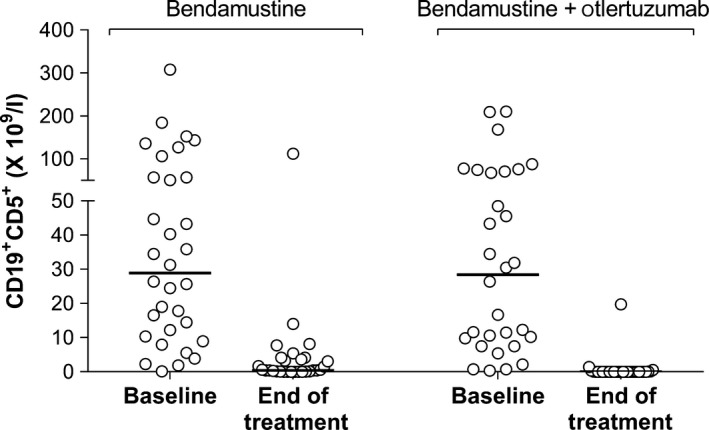
CD19+CD5+ counts at baseline and end of treatment.
Discussion
This multicentre Phase 2 study in patients with relapsed/refractory CLL demonstrates that otlertuzumab in combination with bendamustine results in a significantly higher response rate as measured by standard response criteria and PFS without additional toxicity that may be expected from a combination therapy. Although the numbers are small and imbalanced between treatment arms (Table 7), it is notable that the combination had some activity in patients with TP53 mutations (2/4 patients) although no patients with 17p deletions (0/2) had a response. Stratification took into account several factors so overall the randomization was balanced, although some individual components were not be balanced due to the small sample size of 65.
Otlertuzumab in combination with bendamustine was well tolerated; 78% of patients were able to receive all 6 cycles of therapy and the rate of discontinuation due to adverse events was actually lower than in the control arm (9% vs. 21%). This difference is driven by a higher occurrence of CLL‐related disease events in the control arm, which is consistent with the fact that the combination was more effective at reducing the malignant clone (Fig 4). The overall incidence of adverse events and severe and serious adverse events were generally similar between the treatments. Although there was a greater incidence of severe neutropenia with the combination, there was not a greater incidence of severe or serious adverse events, including infections, in the otlertuzumab plus bendamustine arm compared to the bendamustine arm. The addition of bendamustine did not have a major impact on the half‐life of otlertuzumab. In this combination study, the mean half‐life was 10 days; in a single‐agent study the mean half‐life was 8–9 days (Byrd et al, 2014).
The ORR for single agent bendamustine in relapsed CLL ranges from 40% to 93% with a CR rate of 7–30% (Kath et al, 2001; Aivado et al, 2002; Bremer, 2002; Bergmann et al, 2005; Lissitchkov et al, 2006; Niederle et al, 2013). These studies (Table 10) are confounded by the utilization of various doses of bendamustine, ranging from 50 to 100 mg/m2. The patient populations also varied significantly between the studies with a median of 1–5 prior treatments; one study (Kath et al, 2001) is confounded by the inclusion of some treatment‐naïve patients and another study (Lissitchkov et al, 2006) by the inclusion of fludarabine‐naïve patients. Response in these published studies with bendamustine was assessed using the 1996 NCI Working Group guidelines for CLL. The IWCLL criteria are more rigorous than the NCI Working Group criteria in that it requires CT scans and that the subject has been off study drug for at least 2 months for the assessment of a CR. This is reflected in the IWCLL response rate demonstrated in the control arm of our study of 39%, which is a lower response rate than reported from the historic literature. If one uses the NCI Working Group criteria, the overall response rate in the control arm is 67%. Additionally, the previous single agent studies with bendamustine were uncontrolled with small sample sizes (15–49). Hence, response rates for bendamustine from these single‐arm small trials is wide and cannot be generalized.
Table 10.
Bendamustine in patients with relapsed or refractory CLL
| Study | ORR | CR | PFS | N | Regimen | Prior regimens |
|---|---|---|---|---|---|---|
| Bergmann et al (2005) | 56% | 12% | NR | 16 | 100 mg/m2 days 1, 2 every 21–28 days | Median = 3, range 1–6 |
| Kath et al (2001) | 75% | 30% | NR | 20 | 50 or 60 mg/m2 days 1–5, every 4 weeks |
11 patients treatment‐naive For 9 previously treated patients: Median = 1, range 1–4 |
| Lissitchkov et al (2006) | 40% | 27% | NR | 15 | 100 mg/m2 days 1, 2 every 3 weeks |
4 patients received 4 prior treatments 11 patients ≤3 prior treatments All fludarabine‐naive |
| Aivado et al (2002) | 67% | 29% | TTF: 6 months | 21 | 100 mg/m2, days 1 and 2 every 4 weeks |
Median = 2 3 patients received up to 4 prior treatments |
| Bremer (2002) | 93% | 7% | NR (OS ~32 months) | 15 | 60 mg/m2 days 1–5 every 4 weeks | Median = 5, range 3–9 |
| Niederle et al (2013) | 76% | 27% | 20·1 months | 49 | 100 mg/m2, days 1and 2 every 4 weeks |
“Mostly chlorambucil based” Specific data not provided. |
CR, complete response; NR, not reported; ORR, overall response rate; OS, overall survival; PFS, progression‐free survival; TTF, time to treatment failure.
Bendamustine was used as the control for this study, because at the time the study was designed there was no randomized controlled data supporting the superiority of the combination of bendamustine and rituximab over bendamustine alone. Bendamustine has been used in combination with rituximab, a B‐cell depleting agent for the treatment of CLL. In a combination trial with rituximab in front‐line CLL (n = 117), the ORR was 88% with a CR rate of 23% and a PFS of 34 months (Fischer et al, 2012). The same combination was used in relapsed CLL and the ORR was 59% with a CR rate of 9% and a PFS of 15 months (Fischer et al, 2011). In the single agent study of bendamustine in relapsed CLL the ORR was 56% with a CR rate of 12% (Bergmann et al, 2005). These studies both used the older 1996 NCI Working Group criteria for response and none used a control group. These single arm studies suggested that the ORR with bendamustine and rituximab was similar to responses reported for bendamustine alone.
The strength of the current study is that it was a randomized, stratified and controlled study, and utilized the newer IWCLL 2008 response criteria (Hallek et al, 2008). Otlertuzumab adds to the efficacy of bendamustine without increasing toxicity. The favourable safety profile suggests that otlertuzumab may be a good partner for novel substances such as ibrutinib, venetoclax or idelalisib. The mechanism of action of otlertuzumab suggests synergy between otlertuzumab and idelalisib and this has been supported by pre‐clinical work (Lapalombella et al, 2012). Other preclinical studies show an additive effect of otlertuzumab with mTOR inhibitors (Algate et al, 2010), CD20 antibodies, PI3K inhibitors and ibrutinib (McMahan et al, 2014). Otlertuzumab is currently being studied in an ongoing clinical trial in combination with rituximab, obinutuzumab or ibrutinib and in combination with rituximab and idelalisib (NCT01644253). The place for otlertuzumab has yet to be defined in the rapidly evolving field of CLL and this will require additional studies in combination with the newer agents. The activity, safety profile and combinatorial activity of otlertuzumab with a wide range of drugs holds the promise that it may become a useful drug in the treatment armamentarium for CLL.
Authorship
TR and UJ, designed and performed research, wrote the manuscript, reviewed drafts of the paper and approved the final version. SCS and AJE helped planned the trial and wrote the manuscript, participated in data interpretation and summation, revised the manuscript and approved the final version. JMP, JCB, FA designed and performed research and reviewed drafts of the paper and approved the final version. AH, JK, JL, ELM, AM, HH, JAG, MK, MH performed research and reviewed drafts of the paper and approved the final version.
Conflict‐of‐interest disclosure
A.J.E. and S.C.S. have financial interests in otlertuzumab and are employees of Aptevo Therapeutics. The remaining authors declare no competing financial interests.
Acknowledgements
The authors wish to thank the patients who participated in this trial and their families who supported them. Additionally, we wish to thank the sub‐investigators and research staff at each site who made completion of this trial possible.
Presented in abstract form at the 55th annual meeting of the American Society of Hematology, New Orleans LA, December 7, 2013 and the 56th annual meeting of the American Society of Hematology, San Francisco CA, December 6, 2014.
References
- Aivado, M. , Schulte, K. , Henze, L. , Burger, J. , Finke, J. & Haas, R. (2002) Bendamustine in the treatment of chronic lymphocytic leukemia: results and future perspectives. Seminars in Oncology, 29, 19–22. [DOI] [PubMed] [Google Scholar]
- Algate, P. , Wiens, J. , Nillson, C. , Sho, M. , Chao, D. , Starling, G.C. , Byrd, J.C. & Gordon, B. (2010) TRU‐016, an anti‐CD37 SMIPTM biologic, in combination with other therapeutic drugs in models of non‐hodgkin's lymphoma. Blood, 116, 3931. [Google Scholar]
- Awan, F. , Jaeger, U. , Rifkin, R. , Thirman, M. , Byrd, J. , Hallek, M. , Stromatt, S. & Pagel, J. (2012) Phase 1b study of TRU‐016, an anti‐CD37 SMIPTM protein, in combination with bendamustine vs bendamustine alone in relapsed chronic lymphocytic leukemia. Blood, 120, 1795. [Google Scholar]
- Barrena, S. , Almeida, J. , Yunta, M. , Lopez, A. , Fernandez‐Mosteirin, N. , Giralt, M. , Romero, M. , Perdiguer, L. , Delgado, M. , Orfao, A. & Lazo, P.A. (2005) Aberrant expression of tetraspanin molecules in B‐cell chronic lymphoproliferative disorders and its correlation with normal B‐cell maturation. Leukemia, 19, 1376–1383. [DOI] [PubMed] [Google Scholar]
- Baum, P.R. , Cerveny, C. & Gordon, B. (2009) Evaluation of the effect of TRU‐016, an anti‐CD37 directed SMIP in combination with other therapeutic drugs in models of non‐Hodgkin's lymphoma. Journal of Clinical Oncology, 27, 8571. [Google Scholar]
- Belov, L. , de la Vega, O. , dos Remedios, C.G. , Mulligan, S.P. & Christopherson, R.I. (2001) Immunophenotyping of leukemias using a cluster of differentiation antibody microarray. Cancer Research, 61, 4483–4489. [PubMed] [Google Scholar]
- Bergmann, M.A. , Goebeler, M.E. , Herold, M. , Emmerich, B. , Wilhelm, M. , Ruelfs, C. , Boening, L. & Hallek, M.J. ; German, CLL Study Group . (2005) Efficacy of bendamustine in patients with relapsed or refractory chronic lymphocytic leukemia: results of a phase I/II study of the German CLL Study Group. Haematologica, 90, 1357–1364. [PubMed] [Google Scholar]
- Blum, K.A. , Young, D. , Broering, S. , Lucas, M.S. , Fischer, B. , Lin, T.S. , Grever, M.R. & Byrd, J.C. (2007) Computed tomography scans do not improve the predictive power of 1996 national cancer institute sponsored working group chronic lymphocytic leukemia response criteria. Journal of Clinical Oncology, 25, 5624–5629. [DOI] [PubMed] [Google Scholar]
- Bremer, K. (2002) High rates of long‐lasting remissions after 5‐day bendamustine chemotherapy cycles in pre‐treated low‐grade non‐Hodgkin's‐lymphomas. Journal of Cancer Research and Clinical Oncology, 128, 603–609. [DOI] [PubMed] [Google Scholar]
- Burger, J.A. , Keating, M.J. , Wierda, W.G. , Hoellenriegel, J. , Ferrajoli, A. , Faderl, S. , Lerner, S. , Zacharian, G. , Huang, X. , James, D.F. , Buggy, J.J. , Kantarjian, H.M. & O'Brien, S.M. (2012) The Btk inhibitor ibrutinib (PCI‐32765) in combination with rituximab is well tolerated and displays profound activity in high‐risk chronic lymphocytic leukemia (CLL) patients. Blood (ASH Annual Meeting Abstracts), 120, 187. [Google Scholar]
- Byrd, J. , Lapalombella, R. , Ramanunni, A. , Andritsos, L. , Flynn, J. , Baum, P. , Thompson, P. & Muthusamy, N. (2009) Effect of CD37 small modular immuno‐pharmaceutical (SMIP) on direct apoptosis in chronic lymphocytic leukemia cells via transcriptional up‐regulation of the BH3 family member BIM. Journal of Clinical Oncology, 27, 15S. [Google Scholar]
- Byrd, J.C. , Pagel, J.M. , Awan, F.T. , Forero, A. , Flinn, I.W. , Deauna‐Limayo, D.P. , Spurgeon, S.E. , Andritsos, L.A. , Gopal, A.K. , Leonard, J.P. , Eisenfeld, A.J. , Bannink, J.E. , Stromatt, S.C. & Furman, R.R. (2014) A phase 1 study evaluating the safety and tolerability of otlertuzumab, an anti‐CD37 mono‐specific ADAPTIR therapeutic protein in chronic lymphocytic leukemia. Blood, 123, 1302–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo, E. , Cardesa, A. , Alos, L. , Palacin, A. , Cobarro, J. , Traserra, J. & Montserrat, E. (1991) Non‐Hodgkin's lymphomas of nasal cavity and paranasal sinuses. An immunohistochemical study. American Journal of Clinical Pathology, 96, 184–190. [DOI] [PubMed] [Google Scholar]
- Cheson, B.D. , Bennett, J.M. , Grever, M. , Kay, N. , Keating, M.J. , O'Brien, S. & Rai, K.R. (1996) National Cancer Institute‐sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood, 87, 4990–4997. [PubMed] [Google Scholar]
- D'Arena, G. , Musto, P. , Cascavilla, N. , Dell'Olio, M. , Di Renzo, N. & Carotenuto, M. (2000) Quantitative flow cytometry for the differential diagnosis of leukemic B‐cell chronic lymphoproliferative disorders. American Journal of Hematology, 64, 275–281. [DOI] [PubMed] [Google Scholar]
- Ellis, S. , Carroll, K.J. & Pemberton, K. (2008) Analysis of duration of response in oncology trials. Contemporary Clinical Trials, 29, 456–465. [DOI] [PubMed] [Google Scholar]
- Fischer, K. , Cramer, P. , Busch, R. , Stilgenbauer, S. , Bahlo, J. , Schweighofer, C.D. , Bottcher, S. , Staib, P. , Kiehl, M. , Eckart, M.J. , Kranz, G. , Goede, V. , Elter, T. , Buhler, A. , Winkler, D. , Kneba, M. , Dohner, H. , Eichhorst, B.F. , Hallek, M. & Wendtner, C.M. (2011) Bendamustine combined with rituximab in patients with relapsed and/or refractory chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. Journal of Clinical Oncology, 29, 3559–3566. [DOI] [PubMed] [Google Scholar]
- Fischer, K. , Cramer, P. , Busch, R. , Bottcher, S. , Bahlo, J. , Schubert, J. , Pfluger, K.H. , Schott, S. , Goede, V. , Isfort, S. , von Tresckow, J. , Fink, A.M. , Buhler, A. , Winkler, D. , Kreuzer, K.A. , Staib, P. , Ritgen, M. , Kneba, M. , Dohner, H. , Eichhorst, B.F. , Hallek, M. , Stilgenbauer, S. & Wendtner, C.M. (2012) Bendamustine in combination with rituximab for previously untreated patients with chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. Journal of Clinical Oncology, 30, 3209–3216. [DOI] [PubMed] [Google Scholar]
- Ginaldi, L. , de Martinis, M. , Matutes, E. , Farahat, N. , Morilla, R. & Catovsky, D. (1998) Levels of expression of CD19 and CD20 in chronic B cell leukaemias. Journal of Clinical Pathology, 51, 364–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goede, V. , Fischer, K. , Busch, R. , Engelke, A. , Eichhorst, B. , Wendtner, C.M. , Chagorova, T. , de la Serna, J. , Dilhuydy, M.S. , Illmer, T. , Opat, S. , Owen, C.J. , Samoylova, O. , Kreuzer, K.A. , Stilgenbauer, S. , Dohner, H. , Langerak, A.W. , Ritgen, M. , Kneba, M. , Asikanius, E. , Humphrey, K. , Wenger, M. & Hallek, M. (2014) Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. The New England Journal of Medicine, 370, 1101–1110. [DOI] [PubMed] [Google Scholar]
- Gopal, A.K. , Press, O.W. , Wilbur, S.M. , Maloney, D.G. & Pagel, J.M. (2008) Rituximab blocks binding of radiolabeled anti‐CD20 antibodies (Ab) but not radiolabeled anti‐CD45 Ab. Blood, 112, 830–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallek, M. , Cheson, B.D. , Catovsky, D. , Caligaris‐Cappio, F. , Dighiero, G. , Dohner, H. , Hillmen, P. , Keating, M.J. , Montserrat, E. , Rai, K.R. & Kipps, T.J. ; International Workshop on Chronic Lymphocytic Leukemia . (2008) Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute‐Working Group 1996 guidelines. Blood, 111, 5446–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallek, M. , Fischer, K. , Fingerle‐Rowson, G. , Fink, A.M. , Busch, R. , Mayer, J. , Hensel, M. , Hopfinger, G. , Hess, G. , von Grunhagen, U. , Bergmann, M. , Catalano, J. , Zinzani, P.L. , Caligaris‐Cappio, F. , Seymour, J.F. , Berrebi, A. , Jager, U. , Cazin, B. , Trneny, M. , Westermann, A. , Wendtner, C.M. , Eichhorst, B.F. , Staib, P. , Buhler, A. , Winkler, D. , Zenz, T. , Bottcher, S. , Ritgen, M. , Mendila, M. , Kneba, M. , Dohner, H. & Stilgenbauer, S. ; International Group Of, I. & German Chronic Lymphocytic Leukaemia Study Group . (2010) Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open‐label, phase 3 trial. Lancet, 376, 1164–1174. [DOI] [PubMed] [Google Scholar]
- Jilani, I. , O'Brien, S. , Manshuri, T. , Thomas, D.A. , Thomazy, V.A. , Imam, M. , Naeem, S. , Verstovsek, S. , Kantarjian, H. , Giles, F. , Keating, M. & Albitar, M. (2003) Transient down‐modulation of CD20 by rituximab in patients with chronic lymphocytic leukemia. Blood, 102, 3514–3520. [DOI] [PubMed] [Google Scholar]
- Kath, R. , Blumenstengel, K. , Fricke, H.J. & Hoffken, K. (2001) Bendamustine monotherapy in advanced and refractory chronic lymphocytic leukemia. Journal of Cancer Research and Clinical Oncology, 127, 48–54. [DOI] [PubMed] [Google Scholar]
- Kennedy, A.D. , Solga, M.D. , Schuman, T.A. , Chi, A.W. , Lindorfer, M.A. , Sutherland, W.M. , Foley, P.L. & Taylor, R.P. (2003) An anti‐C3b(i) mAb enhances complement activation, C3b(i) deposition, and killing of CD20+ cells by rituximab. Blood, 101, 1071–1079. [DOI] [PubMed] [Google Scholar]
- Lapalombella, R. , Yeh, Y.Y. , Wang, L. , Ramanunni, A. , Rafiq, S. , Jha, S. , Staubli, J. , Lucas, D.M. , Mani, R. , Herman, S.E. , Johnson, A.J. , Lozanski, A. , Andritsos, L. , Jones, J. , Flynn, J.M. , Lannutti, B. , Thompson, P. , Algate, P. , Stromatt, S. , Jarjoura, D. , Mo, X. , Wang, D. , Chen, C.S. , Lozanski, G. , Heerema, N.A. , Tridandapani, S. , Freitas, M.A. , Muthusamy, N. & Byrd, J.C. (2012) Tetraspanin CD37 directly mediates transduction of survival and apoptotic signals. Cancer Cell, 21, 694–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissitchkov, T. , Arnaudov, G. , Peytchev, D. & Merkle, K. (2006) Phase‐I/II study to evaluate dose limiting toxicity, maximum tolerated dose, and tolerability of bendamustine HCl in pre‐treated patients with B‐chronic lymphocytic leukaemia (Binet stages B and C) requiring therapy. Journal of Cancer Research and Clinical Oncology, 132, 99–104. [DOI] [PubMed] [Google Scholar]
- McMahan, C.F.H. , Miller, R. , Gordon, B. , Gottschalk, R. , Hoyos, G. & Gross, J. (2014) Evaluation of otlertuzumab (TRU‐016), an anti‐CD37 ADAPTIRTM therapeutic in preclinical combination studies with kinase inhibitors and a next generation anti‐CD20 Mab in vitro and in animal models of non‐hodgkin's lymphoma. Blood, 124, 3333. [Google Scholar]
- Niederle, N. , Megdenberg, D. , Balleisen, L. , Heit, W. , Knauf, W. , Weiss, J. , Freier, W. , Hinke, A. , Ibach, S. & Eimermacher, H. (2013) Bendamustine compared to fludarabine as second‐line treatment in chronic lymphocytic leukemia. Annals of Hematology, 92, 653–660. [DOI] [PubMed] [Google Scholar]
- Peters, R.E. , Janossy, G. , Ivory, K. , Al‐Ismail, S. & Mercolino, T. (1994) Leukemia‐associated changes identified by quantitative flow cytometry. III. B‐cell gating in CD37/kappa/lambda clonality test. Leukemia, 8, 1864–1870. [PubMed] [Google Scholar]
- Press, O.W. , Howell‐Clark, J. , Anderson, S. & Bernstein, I. (1994) Retention of B‐cell‐specific monoclonal antibodies by human lymphoma cells. Blood, 83, 1390–1397. [PubMed] [Google Scholar]
- Rafiq, S. , Siadak, A. , Butchar, J.P. , Cheney, C. , Lozanski, G. , Jacob, N.K. , Lapalombella, R. , McGourty, J. , Moledor, M. , Lowe, R. , Setter, B. , Jones, J. , Flynn, J.M. , Andritsos, L. , Devine, S. , Mo, X. , Jarjoura, D. , Tridandapani, S. , Algate, P. , Byrd, J.C. & Muthusamy, N. (2013) Glycovariant anti‐CD37 monospecific protein therapeutic exhibits enhanced effector cell‐mediated cytotoxicity against chronic and acute B cell malignancies. MAbs, 5, 723–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai, K.R. , Peterson, B.L. , Appelbaum, F.R. , Kolitz, J. , Elias, L. , Shepherd, L. , Hines, J. , Threatte, G.A. , Larson, R.A. , Cheson, B.D. & Schiffer, C.A. (2000) Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. The New England Journal of Medicine, 343, 1750–1757. [DOI] [PubMed] [Google Scholar]
- Rawstron, A.C. , Kennedy, B. , Evans, P.A. , Davies, F.E. , Richards, S.J. , Haynes, A.P. , Russell, N.H. , Hale, G. , Morgan, G.J. , Jack, A.S. & Hillmen, P. (2001) Quantitation of minimal disease levels in chronic lymphocytic leukemia using a sensitive flow cytometric assay improves the prediction of outcome and can be used to optimize therapy. Blood, 98, 29–35. [DOI] [PubMed] [Google Scholar]
- Robak, T. & Robak, P. (2014) Anti‐CD37 antibodies for chronic lymphocytic leukemia. Expert Opinion on Biological Therapy, 14, 651–661. [DOI] [PubMed] [Google Scholar]
- Smolewski, P. , Robak, P. , Cebula‐Obrzut, B. , Misiewicz, M. , Medra, A. , Majchrzak, A. , Witkowska, M. , Stromatt, S. & Robak, T. (2014) Pro‐apoptotic effect of an anti‐CD37 scFv‐Fc fusion protein, in combination with the anti‐CD20 antibody, ofatumumab, on tumour cells from B‐cell malignancies. European Journal of Cancer, 50, 2677–2684. [DOI] [PubMed] [Google Scholar]


