Abstract
Aims
We investigate the extent of and factors associated with denial of previously reported cannabis and other illicit drug use, and assess the potential of hair testing for measuring substance use in general population samples.
Design
Birth cohort study.
Setting
United Kingdom, 1991–present.
Participants
A total of 3643 participants who provided hair and self‐report measures of cannabis and other illicit drug use in the Avon Longitudinal Study of Parents and Children (ALSPAC) at age 18 years.
Measurements
Denial of ever use of cannabis and other illicit drugs at age 18 following previously reported use. Positive hair drug tests for cannabis and other illicit drugs, and expected numbers of false positives and false negatives based on expected sensitivity and specificity.
Findings
Cannabis and other illicit drug use was reported by 1223 and 393 individuals, respectively, before age 18 years. Of these 176 (14.4%) and 99 (25.2%), respectively, denied use at age 18. Denial of cannabis use decreased with the reporting of other substances and antisocial behaviour. Cannabis and other illicit drug use at age 18 was reported by 547 (22.5%) and 203 (8.4%) individuals, respectively. Of these, 111 (20.3%) and 13 (6.4%) were hair‐positive for cannabis and other illicit drugs, respectively. Based on hair testing for cannabis use we expect 0 [95% confidence interval (CI) = 0–169] false positives and 394 (95% CI = 323–449) false negatives compared to observed 362 potential false positives and 436 potential false negatives based on self‐report. In hair‐positive individuals, reporting the use of other substances and antisocial behaviour decreased the odds of a negative self‐report.
Conclusions
Hair analysis provides an unreliable marker of substance use in general population samples. People who report more frequent substance use before age 18 are less likely to later deny previous substance use at age 18 than people who report occasional use.
Keywords: ALSPAC, cannabis, hair drug testing, illicit drugs, recanting, self‐report
Introduction
Self‐report is the most commonly used method of collecting substance use data. However, it is prone to misclassification and could introduce bias in either direction, challenging the credibility of substance use research 1. Researchers have identified the phenomenon of recanting, or the denial of previously asserted life‐time substance use 2, 3. It has been suggested the errors encountered are less likely to be the result of intentional distortions but rather the result of poor comprehension, forgetting or even carelessness, as well as age of onset of reporting 4, 5, 6. Recanting, if not handled carefully, is likely to have a considerable impact upon our understanding of drug use and our efforts to prevent it 2. Knowledge of the extent and causes of under‐reporting and recanting could be used to adjust estimates based on survey reports 7, 8.
While self‐report measures are used widely, there is no true ‘gold standard’ for measuring illicit drug use (whereby the phenomenon of recanting is just one demonstration of the problems that can arise from using self‐report measures). Finding an alternative measure of drug use that is less prone to bias would be advantageous to epidemiological studies. Hair analysis holds potential for application to such studies as a biological measure of drug use. While urine and blood samples can be used to provide a measure of recent drug use (2–3 days for a single use of cannabis and up to 24 days for chronic use 9, 10, 11), hair analysis has a potential detection window of several months. Furthermore, hair testing is less invasive, accepted more readily in community settings 12 and can be stored at room temperature without the need for immediate processing 13. Fendrich and colleagues reported two main uses of drug testing in epidemiological studies: generating accurate prevalence measures and ‘correcting’ prevalence measures generated by self‐reported information 14. Several studies have assessed the plausibility of using hair samples as a replacement for urine in drug testing 15, 16, 17, 18, 19, sampling individuals with higher levels of consumption (e.g. prisoners 14, heroin overdose cases 15 and at‐risk users 19). These results are unlikely to be applicable to many epidemiological studies examining a general population sample.
Using data from the Avon Longitudinal Study of Parents and Children (ALSPAC), we aimed to (1) investigate the extent of recanting both cannabis and other illicit drug use; (2) assess the agreement between hair testing and self‐reported cannabis and other illicit drug use using previously reported sensitivity and specificity values to determine expected disagreement (false negative and false positives); and (3) identify factors associated with both recanting and self‐report disagreement in positive hair test results.
Methods
Design
To conduct these analysis, we obtained data collected from both questionnaires and clinic sessions in a large UK‐based birth cohort which was representative of the general population. Data were extracted from ALSPAC, a longitudinal study situated in Bristol, UK 20. Full details of the ALSPAC cohort are provided in the Supporting information. In brief, 14 541 pregnant women with expected delivery dates between 1 April 1991 and 31 December 1992 were recruited. These women, along with their offspring and partners, have been followed‐up ever since using both postal questionnaires and clinic sessions. This study design provides a large general population sample with measures of drug use at several time‐points.
Study population
This analysis utilizes data from 5217 individuals who attended a clinic session at age 18 years (mean = 17.8 years; range = 16.3–20.0 years). A total of 3643 hair samples were collected from this clinic. Samples weighing less than 5 mg were excluded from analysis (n = 563). Nineteen samples did not generate useable results when testing for 11‐nor‐9‐carboxy‐delta‐9‐tetrahydrocannabinol (THC‐COOH), a metabolite of cannabis, resulting in 3061 individuals with available hair drug test data. Of these, 2429 (79.35%) completed the self‐report questions on cannabis use. Twelve samples did not generate usable results when testing for other illicit drugs and their metabolites, resulting in 3068 individuals with available hair drug test data. Of these, 2425 (79.0%) completed the self‐report questionnaires on other illicit drug use (Fig. 1). Other measures of drug use and covariates were taken from questionnaires completed at age 14 (mean = 14.3 years; range = 14.0–16.2 years) and 17 years (mean = 16.7 years; range = 16.4–18.1 years) and a clinic session at age 15 years (mean = 15.5 years, range = 14.3–17.7 years).
Figure 1.
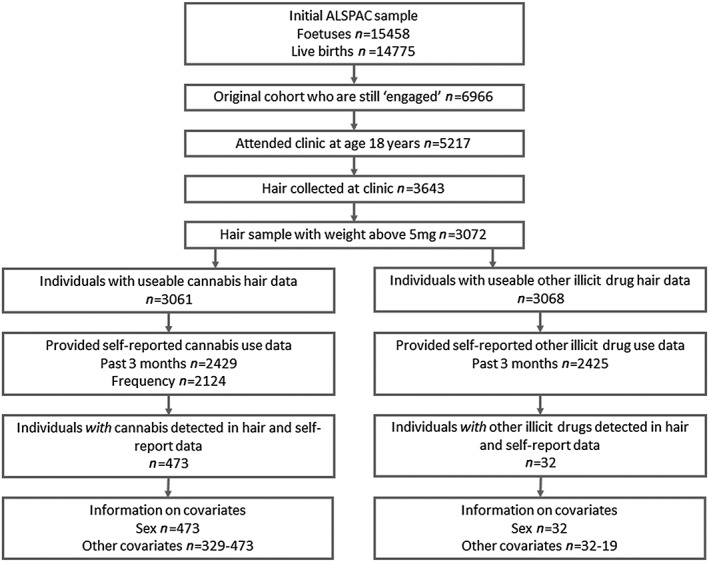
Sample derivation for analysis comparing hair drug testing and self‐report data at age 18 years
Measures
Current drug use (self‐reported)
Self‐reported measures at age 18 years included: cannabis use in the past 3 months (binary variable: yes/no); frequency of cannabis use (categorical variable: not in the past 3 months/monthly or less/two to four times per month/two to three times per week/four or more times per week); heavy cannabis use (four or more times per week) in the past 3 months (binary variable: yes/no); and other illicit drug use (cocaine, amphetamines, inhalants, sedatives, hallucinogens, opioids) in the past 3 months (binary variable: yes/no). All illicit drugs other than cannabis were combined.
Recanting measures
In addition to the drug use measures reported above, individuals were asked about ever use of substances at age 18 years. Previously reported ever use of cannabis and other illicit drugs was also available at ages 14, 15 and 17 years (binary variables: yes/no). Recanting was defined as denying ever use of cannabis or other illicit drug at age 18 years after reporting use at any of the earlier ages (binary variables: not recanted/recanted use). Individuals who had not reported use at earlier ages were excluded from analysis.
Hair drug measures
Measures relating to the detection of cannabis (measured using THC‐COOH) and other illicit drugs (opiates, amphetamines, cocaine, ecstasy and their metabolites) documented the results of the hair drug testing. Detection above cut‐off values ‘confirmed’ use within the past 3 months (binary variables: detected/not detected). Illicit drugs other than cannabis were grouped together. Details of the collection and extraction methods and cut‐off values for hair drug testing are provided in the Supporting information.
Covariates/predictors
Potential covariates/predictors were based on earlier analyses 3, 19. This included a measure of past reported frequency of cannabis or other illicit drug use at ages 14, 15 and 17 years (binary variables: used fewer than five times/used five times or more times). Other drug use measures included measures of licit drugs collected at 18 years [tobacco use in the past 30 days (binary variable: yes/no); blood cotinine levels representative of smoking within the past month using a cut‐off of 10 ng/ml (these were collected at the same visit as the hair sample) 21 (binary variable: yes/no); and current alcohol use (binary variable: non‐hazardous use/hazardous or harmful use)]. Details of the extraction methods for blood cotinine levels are provided in the Supporting information. Other measures included: sex; familial social class (binary variable: I, II and III non‐manual/III manual, IV and V 22); maternal education (binary variable: CSE, vocational or O‐level/A‐level or degree); GCSE English and Mathematics results (binary variables: A*–C grade/D–U grade); depressive symptoms using the Short Mood and Feelings Questionnaire (SMFQ) at 18 years [dichotomized to demonstrate high (score > 11) and low (score ≤ 11) levels of depressive symptoms 23, 24]; self‐reported antisocial behaviour in the past year at 18 years [binary variable: no behaviours/one or more behaviours, where behaviours are determined using core offences in the 2005 Offending, Crime and Justice Survey (mugging, shoplifting, break and enter, selling drugs, fire‐setting, selling and buying stolen goods 25)].
Statistical analyses
Individuals recanting previously reported cannabis and other illicit drug use were compared to individuals who did not recant use on a variety of predictors using logistic regression. Overall agreement between hair drug testing and self‐reported use of cannabis and other illicit drugs was assessed. Previously calculated sensitivity and specificity values of hair testing for THC‐COOH and other illicit drugs were used to calculate the expected number of false positives and negatives using the following equations:
where SRP is the number of individuals with a positive self‐report of drug use and SRN is the number of individuals with a negative self‐report of drug use. This allowed for examination of the reliability of hair drug testing to this sample, as one would expect to find that potential false positives and negatives fall within the expected boundaries. Expected false positives and negatives for THC‐COOH were calculated to compare: (a) any use in the past 3 months with no use; and (b) heavy use in the past 3 months with no use or light use. The sensitivity and specificity values used for THC‐COOH (calculated by Taylor and colleagues) were: 0.28 [95% confidence interval (CI) = 0.18–0.41] and 1.00 (95% CI = 0.91–1.00), respectively, for comparing any use in the past 3 months with no use; and 0.54 (95% CI = 0.33–0.73) and 0.95 (95% CI = 0.88–0.99), respectively, for comparing heavy use with no use and light use 26. The sensitivity and specificity values used for other illicit drugs (calculated by Ledgerwood and colleagues) were 0.93 and 0.69, respectively (no confidence intervals provided) 12. Finally, within hair‐positive individuals, we tested whether those with conflicting self‐report differed on a range of covariates using logistic regression. In individuals who were hair‐positive for other illicit drugs, analyses were conducted using all available data due to the small number of individuals who tested hair‐positive. All analyses were carried out using Stata version 13 27.
Results
Recanting
Cannabis recanting
Ever use of cannabis was reported by 1223 individuals at ages 14, 15 or 17 years. Of these, 176 (14.4%) did not report ever use at age 18 and therefore recanted use. Recanting cannabis use decreased with self‐reported use of cannabis five or more times in the past [odds ratio (OR) = 0.24, 95% CI = 0.11–0.50, P ≤ 0.001] and with the reporting of all other substances. Self‐reported antisocial behaviour at age 18 years also reduced the odds of recanting cannabis. None of the other predictors of recanting showed evidence of an association (Table 1).
Table 1.
Predictors of recanting use of cannabis at age 18 years using logistic regression (complete case analysis n = 546).
| Covariates | Recanted cannabis use | Total N (%) | OR (95% CI) | P | |
|---|---|---|---|---|---|
| No (n = 505) n (%) | Yes (n = 41) n (%) | ||||
| Sex | |||||
| Male (ref) | 196 (92.9) | 15 (7.1) | 211 (100) | 1.10 (0.57–2.13) | 0.778 |
| Female | 309 (92.2) | 26 (7.8) | 335 (100) | ||
| Past reported cannabis use frequency | |||||
| Fewer than 5 times (ref) | 230 (87.8) | 32 (12.2) | 262 (100) | 0.24 (0.11–0.50) | ≤ 0.001 |
| 5 or more times | 275 (96.8) | 9 (3.2) | 284 (100) | ||
| Alcohol consumption | |||||
| Non‐hazardous (ref) | 182 (88.8) | 23 (11.2) | 205 (100) | 0.44 (0.12–0.84) | 0.013 |
| Hazardous and harmful | 323 (94.7) | 18 (5.3) | 341 (100) | ||
| Tobacco (past 30 days) | |||||
| No (ref) | 222 (86.7) | 34 (13.3) | 256 (100) | 0.16 (0.07–0.37) | ≤ 0.001 |
| Yes | 283 (97.6) | 7 (2.4) | 290 (100) | ||
| Other illicit drugs (past 3 months) | |||||
| No (ref) | 392 (90.7) | 40 (9.3) | 432 (100) | 0.09 (0.01–0.64) | 0.016 |
| Yes | 113 (99.1) | 1 (0.9) | 114 (100) | ||
| Antisocial behaviour | |||||
| No (ref) | 350 (90.7) | 36 (9.3) | 386 (100) | 0.31 (0.12–0.81) | 0.017 |
| Yes | 155 (96.9) | 5 (3.1) | 160 (100) | ||
| Social class | |||||
| I, II and III non‐manual (ref) | 357 (93.5) | 25 (6.5) | 382 (100) | 1.54 (0.80–2.98) | 0.195 |
| III manual, IV and V | 148 (90.2) | 16 (9.8) | 164 (100) | ||
| Maternal education | |||||
| CSE, vocational, O‐level (ref) | 220 (90.9) | 22 (9.1) | 242 (100) | 0.70 (0.35–1.26) | 0.213 |
| A‐level, degree | 285 (93.8) | 19 (6.2) | 304 (100) | ||
| English GCSE | |||||
| D–U (ref) | 60 (90.9) | 6 (9.1) | 66 (100) | 0.79 (0.32–1.95) | 0.604 |
| A*–C | 445 (92.7) | 35 (7.3) | 480 (100) | ||
| Mathematics GCSE | |||||
| D–U (ref) | 84 (94.4) | 5 (5.6) | 89 (100) | 1.44 (0.55–3.77) | 0.462 |
| A*–C | 421 (92.1) | 36 (7.90 | 457 (100) | ||
| SMFQ at 18 years | |||||
| No (ref) | 372 (92.3) | 31 (7.7) | 403 (100) | 0.90 (0.43–1.89) | 0.785 |
| Yes | 133 (93.0) | 10 (7.0) | 143 (100) | ||
SMFQ (Short Moods and Feelings Questionnaire): no depressive symptoms (score ≤ 11) used as reference; GCSE = general certificate of secondary education; OR = odds ratio; CI = confidence interval.
Other illicit drug recanting
Ever use of other illicit drugs was reported by 393 individuals at ages 14, 15 and 17 years. Of these, 99 (25.2%) did not report ever use at age 18 and therefore recanted use. The odds of recanting other illicit drug use decreased with self‐reported use of other illicit drugs five or more times in the past (OR = 0.14, 95% CI = 0.03–0.66, P = 0.012) and with the reporting of other substances. There was evidence that individuals whose mother had a higher education were less likely to recant use of other illicit drugs. None of the other predictors of recanting showed evidence of an association (Supporting information, Table S1).
Comparison of self‐report and hair testing
Seventeen different drugs/metabolites from six drug classes were detected in at least one individual's hair (Table 2).
Table 2.
Descriptive data of all drugs detected using hair analysis.
| Drug class | Drug/metabolite | Positive cut off (ng/mg) | Total (N) | Detected (n) | Detected (%) | Range (ng/mg) |
|---|---|---|---|---|---|---|
| Cannabis | THC‐COOH | 0.004 | 3065 | 627 | 20.5 | 0.004–0.05 |
| Opiates | Dihydrocodeine | 0.2 | 919 | 4 | 0.4 | 0.32–31.27 |
| Codeine | 0.2 | 919 | 16 | 1.7 | 0.21–2.74 | |
| Cocaine | Cocaine | 0.2 | 904 | 2 | 0.2 | 0.71–55.16 |
| Norcocaine | 0.2 | 904 | 1 | 0.1 | 0.38 | |
| Benzoylecgonine | 0.2 | 904 | 1 | 0.1 | 0.37 | |
| Benzodiazepines | Midazolam | 0.2 | 1043 | 1 | 0.1 | 0.85 |
| Diazepam | 0.2 | 1043 | 1 | 0.1 | 0.86 | |
| Oxazepam | 0.2 | 1043 | 1 | 0.1 | 0.75 | |
| Alprazolam | 0.2 | 1043 | 2 | 0.2 | 0.38–0.38 | |
| Desmethyldiazepam | 0.2 | 1043 | 1 | 0.1 | 0.38 | |
| Methamphetamine | Methamphetamine | 0.2 | 884 | 6 | 0.7 | 0.22–1.22 |
| MDA | 0.2 | 884 | 2 | 0.2 | 0.21–0.29 | |
| MDEA | 0.2 | 884 | 2 | 0.2 | 0.45–0.46 | |
| MBDB | 0.2 | 884 | 1 | 0.1 | 0.26 | |
| MDMA | 0.2 | 884 | 14 | 1.6 | 0.24–13.89 | |
| Ketamine | Ketamine | 0.2 | 870 | 7 | 0.8 | 0.28–20.70 |
| Illicit drugs | All including cannabis | Various: see above | 3068 | 649 | 21.2 | NA |
| All excluding cannabis | 0.2 | 3068 | 32 | 1.3 | NA |
Only confirmation testing of each drug is shown, as there were no individuals who were detected in the screening phase without being detected in the confirmation stage (see hair testing methods in Supporting information). Drugs/metabolites detected in 0 individuals not shown. Opiates = morphine, A6MAM, heroin, Acetylcodeine; amphetamine; cocaine: aeme, cocaethylene; benzodiazepines: flurazepam, nitrazepam, lorazepam, temazepam, clonazepam. When excluding drugs that could be prescribed to an individual and therefore might not be used in an ‘illicit’ way, the number of individuals with other illicit drugs detected in their hair did not change, showing that these drugs were also being used alongside other illicit substances. THC‐COOH = 11‐nor‐9‐carboxy‐delta‐9‐tetrahydrocannabinol; MDA = 3,4‐methylenedioxyamphetamine; tenamfetamine; MDEA =3,4‐methylenedioxy‐N‐ethyl‐amphetamine; MBDB =1,3‐benzodioxolyl‐N‐methylbutanamine; MDMA = 3,4‐methylenedioxy‐methamphetamine. NA = not applicable.
Cannabis (THC‐COOH)
Information on cannabis use in the past 3 months was provided by 2429 individuals. Use of cannabis was reported by 547 (22.5%) individuals, 370 (67.6%) of whom reported using cannabis monthly or less, 80 (14.6%) used two to four times per month, 38 (6.9%) used two to three times per week and 55 (10.1%) used four or more times per week. Four (0.8%) did not report frequency of use. Of those reporting cannabis use in the past 3 months 111 (20.3%) had a positive hair test for cannabis, while 362 (19.2%) of those not reporting use in the past 3 months had a positive hair test (Table 3). There was minimal change to these proportions when excluding individuals with missing information on predictors used later in this analysis (complete case sample) (Supporting information, Table S2).
Table 3.
Comparison of self‐report cannabis and other illicit drug measures with the detection of THC‐COOH (cannabis metabolite) or other illicit drugs (and metabolites) in hair.
| Self‐reported Drug use | Hair analysis | Total N (%) | Expected rates of detection using hair analysis | ||
|---|---|---|---|---|---|
| Not detected n (%) | Detected n (%) | Not detected n (95% CI) | Detected n (95% CI) | ||
| Cannabis use (past 3 months) | |||||
| No | 1520 (80.8) | 362 (19.2) | 1882 (100) | 1882 (1713–1882) 394 (323–449) | 0 (0–169) 153 (98–224) |
| Yes | 436 (79.7) | 111 (20.3) | 547 (100) | ||
| Total | 1956 (80.5) | 473 (19.5) | 2429 (100) | ||
| Heavy cannabis use (past 3 months) | |||||
| No | 1605 (80.8) | 381 (19.2) | 1986 (100) | 1887 (1748–1966) 62 (36–90) | 99 (20–238) 73 (45–99) |
| Yes | 104 (77.0) | 31 (23.0) | 135 (100) | ||
| Total | 1709 (80.6) | 412 (19.4) | 2121 (100) | ||
| Frequency of cannabis use | |||||
| Never/not in the past 3 months | 1276 (80.9) | 302 (19.1) | 1578 (100) | NA | NA |
| Monthly or less | 302 (81.6) | 68 (18.4) | 370 (100) | ||
| 2–4 times per month | 69 (86.3) | 11 (13.7) | 80 (100) | ||
| 2–3 times per week | 27 (71.1) | 11 (28.9) | 38 (100) | ||
| 4+ times per week | 35 (63.6) | 20 (36.4) | 55 (100) | ||
| Total | 1709 (100) | 412 (100) | 2121 (100) | ||
| Use of other illicit drugs (past 3 months) | |||||
| No | 2203 (99.1) | 19(0.9) | 2222 (100) | 2066 | 156 |
| Yes | 190 (93.6) | 13 (6.4) | 203 (100) | 63 | 140 |
| Total | 2393 (98.7) | 32 (1.3) | 2425 (100) | ||
Expected rates of detected calculated using previously reported sensitivity and specificity values for THC‐COOH and other illicit drugs in hair, 95% confidence intervals calculated where possible from previously reported values. Expected rates highlighted in bold type relate to the expected numbers of false positives and false negatives in the ALSPAC sample. THC‐COOH complete case sample n = 727; other illicit drugs using all available data. Heavy cannabis use defined as weekly cannabis use (i.e. two to three times per week or four or more times per week). THC‐COOH in hair compared to all self‐reported cannabis variables, all other illicit drugs and their compared to self‐report other illicit drug variable. CI = confidence interval; NA = not applicable.
The expected level of false positives (self‐report negative and hair‐positive) and false negatives (self‐report positive and hair‐negative) were 0 (95% CI = 0–169) and 394 (95% CI = 323–449), respectively, when comparing cannabis users with non‐users. There were 362 individuals with a positive hair test and negative self‐report (i.e. potential false positives) and 436 individuals with a negative hair test and positive self‐report (i.e. potential false negatives). Similar results were observed when comparing heavy users with light users and non‐users (Table 3). In individuals who were hair‐positive, those not reporting cannabis use were less likely to report the use of other drugs, including hazardous alcohol consumption, other illicit drugs and tobacco in the past 30 days, when compared with individuals who did report use (Table 4). They were also less likely to report antisocial behaviour at age 18 years. None of the other factors showed evidence of an association (Supporting information, Table S3).
Table 4.
Comparison of reporting licit and illicit substance use with self‐reported cannabis use in individuals who had cannabis metabolite THC‐COOH detected in their hair using logistic regression (complete case analysis n = 174).
| Use of other drugs | Self‐reported use (past 3 months) in those with cannabis detected in hair | Total N (%) | OR (95% CI) | P | |
|---|---|---|---|---|---|
| Yes (n = 29) n (%) | No (n = 145) n (%) | ||||
| Alcohol consumption | |||||
| Non‐hazardous | 4 (4.1) | 93 (95.9) | 97 (100) | 0.08 (0.03–0.26) | ≤ 0.001 |
| Hazardous and harmful | 25 (35.5) | 52 (67.5) | 77 (100) | ||
| Tobacco (past 30 days) | |||||
| No | 8 (6.0) | 126 (94.0) | 134 (100) | 0.04 (0.02–0.12) | ≤ 0.001 |
| Yes | 21 (52.5) | 19 (47.5) | 40 (100) | ||
| Serum cotinine (> 10 ng/ml) | |||||
| No | 18 (11.4) | 140 (88.6) | 158 (100) | 0.06 (0.02–0.20) | ≤ 0.001 |
| Yes | 29 (16.7) | 5 (31.30 | 16 (100) | ||
| Other illicit drugs (past 3 months) | |||||
| No | 18 (11.4) | 140 (88.6) | 158 (100) | 0.07 (0.02–0.23) | ≤ 0.001 |
| Yes | 11 (68.8) | 5 (31.2) | 16 (100) | ||
All measures self‐reported apart from serum cotinine levels. Serum cotinine cut‐off level of 10 ng/ml, which is representative of smoking within the past month (i.e. levels higher than that observed from passive smoking). OR = odds ratio; CI = confidence interval.
Other illicit drug use
Illicit drug use in the past 3 months was reported by 203 (8.4%) individuals, while 2222 (91.6%) individuals reported no use. A total of 32 of these individuals tested positive for illicit drugs and their metabolites in their hair. Of those reporting use in the past 3 months, 13 (6.4%) had a positive hair test for other illicit drugs, while 19 (0.9%) of those not reporting use had a positive hair test (Table 3). The expected levels of false positives and false negatives were 156 and 63, respectively. Nineteen individuals had a positive hair test and negative self‐report (i.e. potential false positive) and 190 individuals had a negative hair test and positive self‐report (i.e. potential false negative).
In individuals who were hair‐positive, those not reporting other illicit drug use were less likely to report the use of other drugs, including cannabis and tobacco, in the past 30 days compared to individuals who reported use (Supporting information, Table S4). These individuals were also more likely to be female and less likely to report antisocial behaviour at 18 years. None of the other factors showed evidence of an association (Supporting information, Table S5).
Discussion
Approximately ~14 and ~25% of individuals who had previously reported use of cannabis and other illicit drugs, respectively, recanted use at age 18 years. Individuals were less likely to recant use if they reported other drug use and antisocial behaviours. This demonstrates that drug use behaviours are subject to measurement error/reporting bias, along with other behaviours that might not be deemed ‘socially acceptable’. Individuals who reported heavier use in the past were less likely to recant their use, suggesting that those who ‘experiment’ once or twice with a drug are less likely to continue reporting use later in the life‐course compared to individuals who partake in a behaviour several times. This could be influenced by both reporting and recall biases.
When comparing self‐report with hair drug‐testing information, there were more potential false positives than expected for the detection of cannabis and more potential false negatives than expected for the detection of cannabis and other illicit drugs. However, there were far fewer false positives observed than expected (19 potential versus 190 expected) for the detection of other illicit drugs. The number of potential false positives and negatives observed differ from what is expected, considering the performance of hair testing for drug use. Although there is likely to be some disagreement between self‐report and biological measures, these findings suggest that previously reported sensitivity values are overestimated for application to a general population sample.
By examining the extent of recanting in a general population sample, we have shown that self‐reported information on drug use can introduce measurement bias (and that the bias is greater for ever use compared to frequent use of illicit drugs). However, we have also demonstrated that hair drug testing is unable to provide a reliable measure of past drug use.
Limitations of this research
This analysis has the advantage of a large sample size to examine the applicability of hair drug testing to general population samples. None the less, several limitations should be considered.
First, despite the large initial sample, the small numbers of individuals with cannabis and other illicit drugs detected in hair leaves low power for assessment of potential reasons behind any inconsistencies. This is particularly true when examining other illicit drugs, as an initial general population sample in the thousands can result in very few individuals with drugs detected in their hair. Secondly, different types and presentation forms of illicit drugs (besides combined use) show variations in time and usage pattern 28, 29, 30. Thus, combining the use of ‘other illicit drugs’ into one measure is a potential limitation. However, due to the small number of individuals both reporting the use of illicit drugs and with illicit drugs detected in their hair, it would not have been possible to examine these separately. Thirdly, while self‐reported information is unlikely to provide an accurate measure of actual exposure (e.g. strength of cannabis, how much an individual inhales, etc.), it is the most reliable measure of long‐term drug use and can be considered a ‘gold standard’ against hair drug‐testing measures. The low levels of drug use in this general population sample suggest that positive hair drug tests among people who do not self‐report cannabis use are more likely to be false positives than unreported heavy cannabis use. Here, we have demonstrated that individuals with a stronger drug history are less likely to recant self‐reported information, making it appear unlikely that individuals with heavy enough drug use to be detected are not reporting this. When examining a forensic or court sample, the opposite might apply. Finally, THC‐COOH alone was measured in hair as a marker of cannabis use. There are other metabolites of cannabis that can be detected in hair. While THC‐COOH in hair is less likely to be influenced by external contamination and might be considered the optimal metabolite, a previous analysis has shown that THC and cannabidiol show greater sensitivity and specificity 26. This analysis could have been enhanced by testing for additional cannabis metabolites.
Comparison with literature and implications
Percy and colleagues 3 have reported several characteristics associated with recanting. The results presented here are not consistent with those reported by Percy and colleagues on gender (Percy suggested that women are less likely to recant use). However, our results are consistent when assessing other substances and antisocial behaviour. The results in our study and that of Percy and colleagues illustrate measurement error/reporting bias 31. This has wider implications for epidemiological studies on correlates of drug use, in particular studies assessing cannabis use (as this is often the only illicit drug whose use is common enough in the general population for sample effects to be estimated). Furthermore, this analysis has shown that individuals who report heavier cannabis use in the past are less likely to recant their use, therefore a measure of ever use is more likely to be misclassified and biased than frequency of use.
Reported associations using self‐reported drug use could be attributed to measurement bias influenced by perceptions of social desirability. Social desirability suggests that individuals might under‐report in order to present themselves in a more favourable way or, conversely, over‐report to ‘show off’. This over‐ or under‐reporting would be dependent upon that individual's acceptability of the behaviour 32. This theory is supported by our results showing that the use of several drugs and recanting of cannabis and other illicit drugs are associated negatively. Previous literature suggests that under‐reporting varies by drug 33, with cannabis users being less likely to under‐report. Our results are in agreement with this, with a higher percentage of ever illicit drug users recanting use.
Two studies have assessed previously the use of hair analysis as a biological measure of drug use in a general population sample. Frendrich and colleagues 14 reported higher rates of cannabis use from self‐report than from hair testing. However, we have reported higher prevalence rates from self‐reported other illicit drug use. Ledgerwood and colleagues 12 reported that self‐report and hair testing generally met good, but not excellent, agreement. These previous studies had small samples sizes in their analysis (583 and 613, respectively) in comparison to the 2452 individuals used in this study. The results reported here, along with results published elsewhere, suggest that using hair as a biomarker of drug use for alcohol 34, cannabis and other illicit drugs provides a less reliable measure than self‐report data.
Conclusion
We have demonstrated the problem of recanting substance use in adolescents in the general population and shown that individuals recanting reporting are less likely to report the use of other substances, antisocial behaviour and depressive symptoms. However, despite the known problems with self‐reported data, our findings suggest that hair is an unreliable measure of substance use in a general population due to many factors, among them purity of drug consumed and differences in individuals’ metabolism. It is therefore not a viable tool in many epidemiological studies such as cohorts.
Declaration of interests
None.
Supporting information
Table S1 Predictors of recanting use of other illicit drugs at age 18 years using logistic regression
Table S2 Comparison of self‐report cannabis and other illicit drug measures with the detection of THC‐COOH (cannabis metabolite) or other illicit drugs (and their metabolites) in hair (complete case sample)
Table S3 Comparison of socio‐economic and behavioural measures and reporting cannabis use (past 3 months) in individuals who had THC‐COOH (cannabis metabolite) detected in their hair using logistic regression
Table S4 Comparison of reporting licit and illicit substance use with self‐reported other illicit drug use in individuals who had other illicit drugs (and metabolites) detected in their hair (results of logistic regression) using all available data
Table S5 Comparison of socio‐economic and behavioural measures and reporting other illicit drug use (past 3 months) in individuals who had other illicit drugs (and metabolites) detected in their hair (results from logistic regression) using all available data
Data 1. Supporting info item
Acknowledgements
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. The UK Medical Research Council and the Wellcome Trust (grant ref: 102205/2/13/2) and the University of Bristol provide core support for ALSPAC. This publication is the work of the authors and M.T. and M.H. will serve as guarantors for the contents of this paper. This research was funded specifically by a Wellcome Trust PhD Studentship awarded to M.T. (grant ref: 097088/Z/11/Z).
Taylor, M. , Sullivan, J. , Ring, S. M. , Macleod, J. , and Hickman, M. (2017) Assessment of rates of recanting and hair testing as a biological measure of drug use in a general population sample of young people. Addiction, 112: 477–485. doi: 10.1111/add.13645.
References
- 1. Johnson T., Fendrich M. Modeling sources of self‐report bias in a survey of drug use epidemiology. Ann Epidemiol 2005; 15: 381–389. [DOI] [PubMed] [Google Scholar]
- 2. Martino S. C., McCaffrey D. F., Klein D. J., Ellickson P. L. Recanting of life‐time inhalant use: how big a problem and what to make of it. Addiction 2009; 104: 1373–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Percy A., McAlister S., Higgins K., McCrystal P., Thornton M. Response consistency in young adolescents’ drug use self‐reports: a recanting rate analysis. Addiction 2005; 100: 189–196. [DOI] [PubMed] [Google Scholar]
- 4. Tourangeau R., Rips L. J., Rasinski K. The Psychology of Survey Response. Cambridge: Cambridge University Press; 2000. [Google Scholar]
- 5. Fendrich M., Mackesy‐Amiti M. E. Decreased drug reporting in a cross‐sectional student drug use survey. J Subst Abuse 2000; 11: 161–172. [DOI] [PubMed] [Google Scholar]
- 6. Shillington A. M., Roesch S. C., Reed M. B., Clapp J. D., Woodruff S. I. Typologies of recanting of lifetime cigarette, alcohol and marijuana use during a six‐year longitudinal panel study. Drug Alcohol Depend 2011; 118: 134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dariotis J. K., Pleck J. H., Sonenstein F. L., Astone N. M., Sifakis F. What are the consequences of relying upon self‐reports of sexually transmitted diseases? Lessons learned about recanting in a longitudinal study. J Adolesc Health 2009; 45: 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fendrich M. The undeniable problem of recanting. Addiction 2005; 100: 143–144. [DOI] [PubMed] [Google Scholar]
- 9. Musshoff F., Madea B. Analytical pitfalls in hair testing. Anal Bioanal Chem 2007; 388: 1475–1494. [DOI] [PubMed] [Google Scholar]
- 10. Dolan K., Rouen D., Kimber J. An overview of the use of urine, hair, sweat and saliva to detect drug use. Drug Alcohol Rev 2004; 23: 213–217. [DOI] [PubMed] [Google Scholar]
- 11. Lowe R. H., Abraham T. T., Darwin W. D., Herning R., Lud C. J., Huestis M. A. Extended urinary Δ9‐tetrahydrocannabinol excretion in chronic cannabis users precludes use as a biomarker of new drug exposure. Drug Alcohol Depend 2009; 105: 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ledgerwood D. M., Goldberger B. A., Risk N. K., Lewis C. E., Price R. K. Comparison between self‐report and hair analysis of illicit drug use in a community sample of middle‐aged men. Addict Behav 2008; 33: 1131–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pragst F., Balikova M. A. State of the art in hair analysis for detection of drug and alcohol abuse. Clinica Chimica Acta 2006; 370: 17–49. [DOI] [PubMed] [Google Scholar]
- 14. Frendrich M., Johnson T. P., Wislar J. S., Hubbell A., Spiehler V. The utility of drug testing in epidemiological research: results for a general population survey. Addiction 2004; 99: 197–208. [DOI] [PubMed] [Google Scholar]
- 15. Cirimele V., Kintz P., Mangin P. Testing human hair for cannabis. Forensic Sci Int 1995; 70: 175–182. [DOI] [PubMed] [Google Scholar]
- 16. Uhl M., Sachs H. Cannabinoids in hair: strategy to prove marijuana/hashish consumption. Forensic Sci Int 2004; 145: 143–147. [DOI] [PubMed] [Google Scholar]
- 17. Huestis M. A., Gustafson R. A., Moolchan E. T., Barnes A., Bourland J. A., Sweeney S. A., et al. Cannabinoid concentrations in hair from documented cannabis users. Forensic Sci Int 2007; 169: 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Musshoff F., Driever F., Lachenmeier K., Lachenmeier D. W., Banger M., Madea B. Results of hair analyses for drugs of abuse and comparison with self‐reports and urine tests. Forensic Sci Int 2006; 156: 118–123. [DOI] [PubMed] [Google Scholar]
- 19. Gryczynski J., Schwartz R. P., Mitchell S. G., O'Grady K. E., Ondersma S. J. Hair drug testing results and self‐reported drug use among primary care patients with moderate‐risk illicit drug use. Drug Alcohol Depend 2014; 141: 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boyd A., Golding J., Macleod J., Lawlor D. A., Fraser A., Henderson J., et al. Cohort profile: the ‘Children of the 90s’—the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol 2013; 42: 111–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vartiainen E., Seppala T., Lillsunde P., Puska P. Validation of self reported smoking by serum cotinine measurement in a community‐based study. J Epidemiol Community Health 2002; 56: 167–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Galobardes B., Shaw M., Lawlor D. A., Lynch J. W. Indicators of socioeconomic position (part 2). J Epidemiol Community Health 2006; 60: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Joinson C., Heron J., Lewis G., Croudace T., Araya R. Timing of menarche and depressive symptoms in adolescent girls from a UK cohort. Br J Psychiatry 2011; 198: 17–23. [DOI] [PubMed] [Google Scholar]
- 24. mckenzie D. P., Toumbourou J. W., Forbes A. B., Mackinnon A. J., McMorris B. J., Catalano R. F., et al. Predicting future depression in adolescents using the short mood and feelings questionnaire: a two‐nation study. J Affect Disord 2011; 134: 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wilson D., Sharp C., Patterson A. Young People and Crime: Findings from the 2005 Offending, Crime and Justice Survey. Statistical Bulletin 17/06. London: Home Office; 2006.
- 26. Taylor M., Lees R., Henderson G., Lingford‐Hughes A., Macleod J., Sullivan J. et al Comparison of Cannabinoids in hair with self‐reported cannabis consumption in heavy‐, regular‐ and non‐users of cannabis. Drug Alcohol Rev 2016; DOI: 10.1111/dar.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. StataCorp . Stata Statistical Software: Release 13. College Station, TX: StataCorp; 2013.
- 28. Moss H. B., Goldstein R. B., Chen C. M., Yi H.‐Y. Patterns of use of other drugs among those with alcohol dependence: associations with drinking behavior and psychopathology. Addict Behav 2015; 50: 192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thurtle N., Dargan P. I., Hunter L. J., Lovett C., White J. A., Wood D. M. A comparison of recreational drug use amongst sexual health clinic users in London with existing prevalence data. Int J STD AIDS 2016; 27: 1309–1316. [DOI] [PubMed] [Google Scholar]
- 30. Woodcock E. A., Lundahl L. H., Stoltman J. J., Greenwald M. K. Progression to regular heroin use: examination of patterns, predictors, and consequences. Addict Behav 2015; 45: 287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Macleod J., Hickman M., Smith G. D. Reporting bias and self‐reported drug use. Addiction 2005; 100: 562–563. [DOI] [PubMed] [Google Scholar]
- 32. Edwards A. L. The Social Desirability Variable in Personality Assessment and Research. New York, NY: The Dryden Press; 1957. [Google Scholar]
- 33. Harrell A. V. The validity of self‐reported drug use data. The accuracy of responses on confidential self‐administered answer sheets In: L. H., A. H., editors. The Validity of Self‐Reported Drug Use: Improving the Accuracy of Survey Estimates. Washington, DC: US Department of Health and Human Services; 1997, pp. 37–58. [Google Scholar]
- 34. Lees R., Kingston R., Williams T., Henderson G., Lingford‐Hughes A., Hickman M. Comparison of ethyl glucuronide in hair with self‐reported alcohol consumption. Alcohol Alcohol 2012; 47: 267–272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Predictors of recanting use of other illicit drugs at age 18 years using logistic regression
Table S2 Comparison of self‐report cannabis and other illicit drug measures with the detection of THC‐COOH (cannabis metabolite) or other illicit drugs (and their metabolites) in hair (complete case sample)
Table S3 Comparison of socio‐economic and behavioural measures and reporting cannabis use (past 3 months) in individuals who had THC‐COOH (cannabis metabolite) detected in their hair using logistic regression
Table S4 Comparison of reporting licit and illicit substance use with self‐reported other illicit drug use in individuals who had other illicit drugs (and metabolites) detected in their hair (results of logistic regression) using all available data
Table S5 Comparison of socio‐economic and behavioural measures and reporting other illicit drug use (past 3 months) in individuals who had other illicit drugs (and metabolites) detected in their hair (results from logistic regression) using all available data
Data 1. Supporting info item


