Abstract
Globally, biological invasions can have strong impacts on biodiversity as well as ecosystem functioning. While less conspicuous than introduced aboveground organisms, introduced belowground organisms may have similarly strong effects. Here, we synthesize for the first time the impacts of introduced earthworms on plant diversity and community composition in North American forests. We conducted a meta‐analysis using a total of 645 observations to quantify mean effect sizes of associations between introduced earthworm communities and plant diversity, cover of plant functional groups, and cover of native and non‐native plants. We found that plant diversity significantly declined with increasing richness of introduced earthworm ecological groups. While plant species richness or evenness did not change with earthworm invasion, our results indicate clear changes in plant community composition: cover of graminoids and non‐native plant species significantly increased, and cover of native plant species (of all functional groups) tended to decrease, with increasing earthworm biomass. Overall, these findings support the hypothesis that introduced earthworms facilitate particular plant species adapted to the abiotic conditions of earthworm‐invaded forests. Further, our study provides evidence that introduced earthworms are associated with declines in plant diversity in North American forests. Changing plant functional composition in these forests may have long‐lasting effects on ecosystem functioning.
Keywords: introduced earthworms, plant communities, plant diversity, biological invasions, earthworm invasion, community composition, meta‐analysis
Introduction
Species invasions are among the strongest drivers of environmental change globally (Sala et al., 2000; Murphy & Romanuk, 2014) and can have large effects on biodiversity and ecosystem processes, functions and services (Wardle et al., 2004; Vilà et al., 2011; Walsh et al., 2016). Understanding the full extent of invasive species impacts is a fundamental challenge in ecology (Simberloff et al., 2013); species invasions can have cascading effects across trophic levels (Estes et al., 2011) and facilitate future invasions (Simberloff & Von Holle, 1999). While the ecological importance of linkages between above‐ and belowground communities and ecosystem processes is recognized (de Deyn & van der Putten, 2005), the impacts of introduced belowground organisms, such as earthworms, on aboveground communities are less understood than those of introduced aboveground organisms (Wardle et al., 2004; Cameron et al., 2016).
Introduced earthworms, which have been invading northern North American forests with little or no native earthworm fauna since the time of European settlement (Hendrix & Bohlen, 2002), have a profound influence on ecosystem functioning and services in North American forests. By re‐engineering soil physical and chemical characteristics (Bohlen et al., 2004; Hendrix et al., 2008; Eisenhauer, 2010), they accelerate nutrient cycling (Szlavecz et al., 2006; Sackett et al., 2013; Ewing et al., 2015) and water infiltration (Eisenhauer et al., 2012; Capowiez et al., 2014) and may increase CO2 emissions to the atmosphere (Lubbers et al., 2013). Significant impacts of introduced earthworms on ecosystem functioning could also be mediated through changes in biodiversity, given the wide range of above‐ and belowground organisms with which they interact (Edwards, 2004). Previous studies have documented effects of introduced earthworms on ecological communities in forests (Hale et al., 2006; Eisenhauer et al., 2007; Frelich et al., 2012; Fisichelli et al., 2013), but the generality or variability of such effects has yet to be systematically quantified. Here, we synthesize the impacts of invasive earthworms on the diversity and composition of understory plant communities in North American forests.
Previous studies have documented a range of effects of introduced earthworms on plant communities (e.g., Hale et al., 2006), including changes in both species diversity and composition. Introduced earthworms cause these changes in a number of ways: by predating and ingesting seeds and seedlings (Zaller & Saxler, 2007; Asshoff et al., 2010; Forey et al., 2011; Drouin et al., 2014; Clause et al., 2015; Cassin & Kotanen, 2016), altering seedbank composition (Eisenhauer et al., 2009; Nuzzo et al., 2015), modifying microhabitats where seeds germinate by removing leaf litter (Frelich et al., 2006), and accentuating drought events by accelerating drainage via constructing burrows (Larson et al., 2010; Eisenhauer et al., 2012). Furthermore, disturbances associated with introduced earthworms might confer a competitive advantage to graminoids (Hale et al., 2006; Holdsworth et al., 2007a; Nuzzo et al., 2009; Fisichelli et al., 2013; but see Dobson & Blossey, 2015), or facilitate invasion by other species (e.g., Heneghan et al., 2007; Nuzzo et al., 2009). Other common stressors in North American forests, for example, deer browsing or allelopathic invasive plants, also may interact with introduced earthworms, potentially intensifying compositional changes in plant communities (Fisichelli et al., 2013; Dávalos et al., 2015; Hale et al., 2016). The overall impact of ecosystem engineers on plant diversity is generally considered to be positive (Romero et al., 2015) but has yet to be systematically investigated for earthworms across North American forests.
Variation in the impacts of introduced earthworms on biodiversity could reflect differences in invasion history. Different assemblages of introduced earthworms have been strongly correlated with distinct stages of invasion (Hale et al., 2005; Holdsworth et al., 2007b; Loss et al., 2013). Recently invaded forests are usually dominated by epigeic earthworms, such as Dendrobaena octaedra, while forests with longer invasion histories also have endogeic and anecic species where environmental conditions, for example, soil pH, texture and moisture, permit their presence (Hale et al., 2005; Frelich et al., 2006; Holdsworth et al., 2007b; Loss et al., 2013). The combined impact of multiple earthworm ecological groups on forest understories can be dramatic, as these earthworm assemblages can completely remove surface leaf litter, reduce organic matter in upper soil horizons (Hale et al., 2005; Nuzzo et al., 2009; Resner et al., 2015), and cause significant declines in plant diversity (Hale et al., 2006; Holdsworth et al., 2007a). Therefore, accounting for earthworm invasion history is essential for reaching a general understanding of how diversity and composition of plant communities are affected by introduced earthworms.
Changes in biodiversity due to introduced earthworms could lead to significant alterations in the provisioning of vital ecosystem functions in North American forests, such as nutrient and water cycling, which has important implications for the development of future management and conservation strategies. In the present study, we assessed for the first time impacts of introduced earthworms on plant diversity and composition across North American forests using meta‐analytic techniques. We predict that the presence and abundance of introduced earthworms (i) decrease plant species diversity (Bohlen et al., 2004) and (ii) systematically favor graminoids (Frelich et al., 2012; Fisichelli et al., 2013) and non‐native plant species (Heneghan et al., 2007; Nuzzo et al., 2009). Further, we expect that the magnitude of all abovementioned effects will increase with the number of earthworm ecological groups, reflecting a greater variety of potential disturbance mechanisms likely to result in changes in plant communities (Hale et al., 2006).
Materials and methods
Data selection
To create a database of the effects of earthworm invasion on understory plant communities in North American forests, we performed a search in July 2014 in the ISI Web of Science database using the keywords (‘earthworm*’) AND (‘exotic’ OR ‘invasive’). These search terms were selected in order to include a wide array of studies that addressed the effects of introduced earthworms on aboveground communities. From the initial list of 359 studies (see PRISMA diagram; Appendix S1), we examined each title and abstract to determine whether they met the inclusion criteria. Our inclusion criteria were that each study: (i) was performed in a North American forest ecosystem, (ii) reported density, biomass, or presence/absence of introduced earthworms that were identified either to species or earthworm ecological group and (iii) reported the cover or presence/absence of plant species (or plant functional groups) in the forest understory. Subsequently, we communicated with authors of the selected studies to obtain raw data for earthworm and plant communities. When raw data were not available, means or effect sizes were extracted directly from figures and tables. Through personal knowledge of the authors, we also obtained data from three Masters theses. In total, we identified 14 unique studies meeting our inclusion criteria (Appendix S2 and Table S1) that allowed us to examine associations of introduced earthworm community properties (density, biomass, and ecological group richness) with understory plant community properties (diversity, species richness, and evenness: 13 studies, 233 effect sizes) and with cover of plant growth forms and native or non‐native status (11 studies, 412 effect sizes). As most studies used hierarchical sampling designs, effect sizes were calculated at the site level for each combination of earthworm and plant community measures to capture within‐study variation.
Data description and preparation
Studies meeting the inclusion criteria were published between 2006 and 2015 and were predominantly located in the Upper Midwest and Mid‐Atlantic regions of the United States, from Indiana, the United States to Alberta, Canada (Table S1). Plant communities were typically assessed using plots (median area = 1.4 m2; range = 0.25–100 m2) in which identity and percent cover were recorded for each species. Most studies assessed plant communities once; for those that did so over multiple years, we calculated the mean percent cover of each plant species over the study period. Species richness (number of species), diversity (Shannon–Wiener diversity), and evenness (Evar; Smith & Wilson, 1996) of the understory plant community (usually all species less than 1 m tall) were calculated at the plot level. Plant growth habits and plant native status were extracted from the USDA plant database (USDA, 2014) for each species and categorized into three functional groups; woody, herbaceous, and graminoid (grasses and sedges), and either native or non‐native. Cover of each plant functional group or plant native status was calculated as the summed percent of total cover in a plot.
In general, earthworm communities were sampled in subplots (0.06–0.25 m2) nested within vegetation plots. Earthworms were extracted using a variety of techniques, for example, liquid mustard, cover boards, or formalin (Table S1), sorted to species or earthworm ecological group, counted, and/or weighed. For each plot, we calculated three measures of introduced earthworm communities: total biomass (g m−2), total density (number of individuals m−2), and richness of earthworm ecological groups (0–3). Introduced earthworm species were categorized into three ecological groups, anecic, epigeic, and endogeic (Table S2), which reflect differences in habitat and feeding preferences (Bouché, 1977; Bohlen et al., 2004). While widely used, this classification system may not be optimal as earthworm ecological groups cover a range of sizes and feeding behaviors (Lavelle, 1983; Brown, 1995). For example, Lumbricus rubellus and Aporrectodea longa are typically classified as epigeic and anecic, respectively, but their feeding behavior places them between different ecological groups (‘epi/endogeic’ and ‘anecic–endogeic’, respectively; Eisenhauer et al., 2008; Ferlian et al., 2014). As earthworm communities were sampled with different frequencies across studies (Table S1), we calculated the mean of each measure of earthworm communities per plot across sampling periods.
Effect sizes
To estimate the direction and strength of the relationships between introduced earthworm and plant communities, Spearman's rank correlation coefficients were calculated because most data were not distributed normally (Myers & Sirois, 2014). Spearman's correlation coefficients were transformed to Pearson's correlations and then converted to Fisher's z transformation of r for analysis to normalize the distribution of data (Koricheva et al., 2013). Sampling variance for Fisher's z transformation of r was calculated using unbiased estimates following Hedges (1989). Differences in data collection in particular studies prevented effect sizes from being calculated for all possible combinations of plant and earthworm community measures (Table S1).
Data analysis
We used multilevel, meta‐analytic regression models to estimate mean effect sizes of the relationships between introduced earthworm and plant communities in North American forests with the ‘metafor’ package (Viechtbauer, 2010). These models account for the nonindependence of measures taken from the same study (Nakagawa & Santos, 2012; Koricheva et al., 2013). For all models, we used a random intercept term where ‘site’ was nested within ‘study’. Observations from studies were weighted by the inverse of the sampling variance (Viechtbauer, 2010). Separate models using restricted maximum likelihood estimation were fitted for each measure of plant diversity and each plant functional group or native status. In all models, introduced earthworm community measures were included as a categorical moderator variable to test whether these measures had similar or contrasting effects. Model assumptions were checked by visually inspecting residuals for homogeneity and Pearson residuals for normality. To assess whether publication bias affected the results of our analyses, we visually inspected contour funnel plots (Koricheva & Gurevitch, 2014). We assessed the sensitivity of our analyses to the type of earthworm sampling technique, for example, formalin, liquid mustard, or cover boards. To do so, we added earthworm sampling technique as a fixed effect and its interaction with earthworm community measures to our original models. All analyses were performed using R 3.2.3 (R Core Team, 2015). Effect sizes and sampling variances used for all analyses are included as Supporting Information (for metadata, see Appendices S3 & S4).
Results
Plant diversity
We found that plant species diversity was significantly and negatively correlated with richness of earthworm ecological groups (Fig. 1; 95% confidence intervals did not overlap with zero), but was not significantly correlated with earthworm biomass or density. Plant species evenness and richness were not significantly correlated with any measure of introduced earthworm communities (Fig. 1). For each plant community variable, effect sizes were similar for earthworm biomass, density, and ecological group richness (Table 1).
Figure 1.
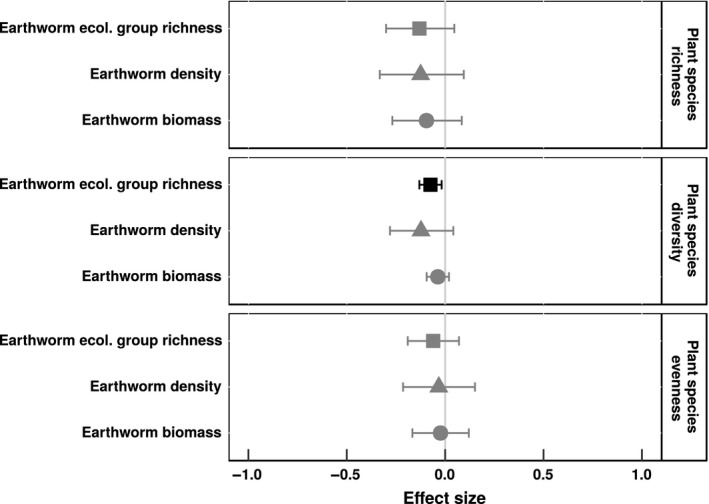
Mean effect sizes of relationships between introduced earthworm communities and plant species richness, diversity, and evenness of forest understory communities in North America. Whisker bars are 95% confidence intervals: Black whisker bars did not overlap with zero. Mean effect sizes are Pearson's correlation coefficients. Plant species diversity was calculated using Shannon–Wiener diversity, and plant species evenness was calculated using Evar (Smith & Wilson, 1996). Earthworm biomass is biomass of introduced earthworms (g m−2), earthworm density is number of introduced earthworms (individuals m−2), and earthworm ecological group richness is the number of introduced earthworm ecological groups.
Table 1.
Summary of meta‐analytic mixed‐effects models testing the relationships between introduced earthworm biomass, density, and ecological group richness and plant diversity, native and non‐native status, and functional group cover of forest understory communities in North America
| Response variable | Studies | Observations | AICc | Residual heterogeneity | L |
|---|---|---|---|---|---|
| Plant diversity | |||||
| Plant species richness | 13 | 83 | 56.1 | 209.2 | 0.4 |
| Plant species diversity | 10 | 75 | 43.0 | 112.8 | 0.4 |
| Plant species evenness | 10 | 75 | 28.2 | 98.0 | 0.5 |
| Plant functional groups | |||||
| Herbaceous cover | 11 | 102 | 35.5 | 188.8 | 3.4 |
| Graminoid cover | 9 | 88 | 37.1 | 203.9 | 1.2 |
| Woody cover | 11 | 102 | 458.6 | 816.6 | 3.4 |
| Plant native status | |||||
| Native plant cover | 10 | 61 | 67.1 | 262.0 | 7.9 |
| Non‐native plant cover | 9 | 59 | 60.5 | 221.4 | 8.7 |
Meta‐analytic mixed‐effects models evaluated the size effects representing the association between a measure of introduced earthworm community abundance or structure (density, biomass, richness of earthworm ecological groups) and plant diversity, plant native and non‐native status, or cover of plant functional groups. Plant species diversity is Shannon–Wiener diversity, plant species evenness is Smith and Wilson's evenness measure (‘Evar’; Smith & Wilson, 1996). Residual heterogeneity shows if the variability of the effect sizes not captured by the moderator variables is heterogeneous. The moderator variable in all models was a categorical factor representing measures of introduced earthworm communities. L is the likelihood ratio test statistic for model coefficients. Values of residual heterogeneity and L in black italics indicate statistical significance (α = 0.05).
Plant community composition
Graminoid cover was significantly positively correlated with introduced earthworm biomass, density, and ecological group richness (Fig. 2). In contrast, cover of neither herbaceous nor woody plants was significantly correlated with any measure of introduced earthworm communities. Non‐native plant cover exhibited a significant, positive correlation with introduced earthworm biomass (Fig. 3), but not with introduced earthworm density or richness of earthworm ecological groups. Native plant cover decreased, albeit nonsignificantly (mean effect size = −0.24; 95% confidence interval = −0.49–0.01), with increasing introduced earthworm biomass.
Figure 2.
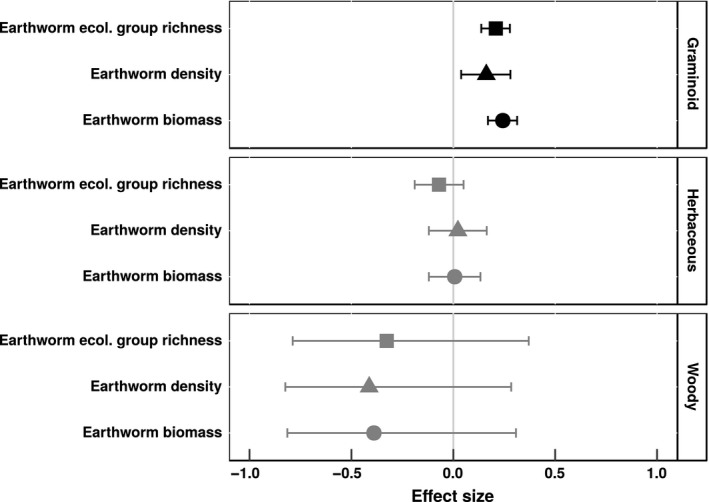
Mean effect sizes of relationships between introduced earthworm communities and cover of plant functional groups in forest understory communities in North America. Whisker bars are 95% confidence intervals: Black whisker bars did not overlap with zero. Effect sizes are Pearson's correlation coefficients. Earthworm biomass is biomass of introduced earthworms (g m−2), earthworm density is number of introduced earthworms (individuals m−2), and earthworm ecological group richness is the number of introduced earthworm ecological groups.
Figure 3.
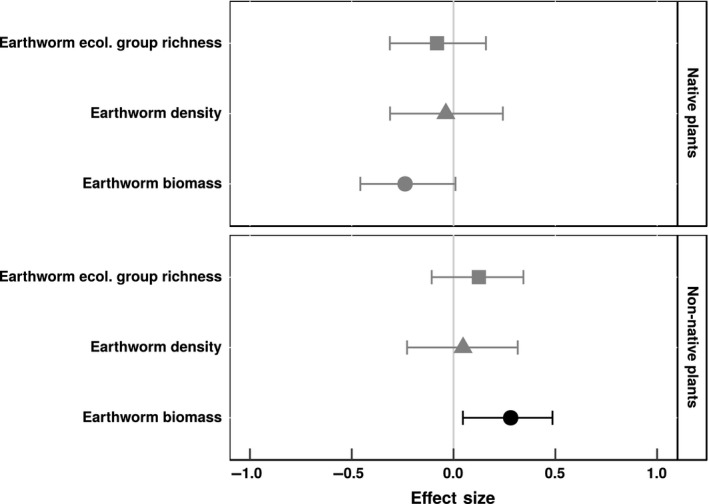
Mean effect sizes of relationships between introduced earthworm communities and cover of native and non‐native plants in forest understory communities in North America. Whisker bars are 95% confidence intervals: Black whisker bars did not overlap with zero. Effect sizes are Pearson's correlation coefficients. Earthworm biomass is biomass of introduced earthworms (g m−2), earthworm density is number of introduced earthworms (individuals m−2), and earthworm ecological group richness is the number of introduced earthworm ecological groups.
Effect sizes for the relationships of native and non‐native plants with introduced earthworm communities differed significantly depending on the earthworm community measure (Table 1). Native plant cover and non‐native plant cover were more strongly related to introduced earthworm biomass than to either earthworm density or richness of earthworm ecological groups (Fig. 3).
Across‐study variation
For all models testing the association between plant diversity and community composition with introduced earthworm communities, the results showed a consistent and statistically significant amount of residual heterogeneity among studies (Table 1). In other words, our analysis detected that additional, unmeasured variables would be important for explaining the association between plant and earthworm communities. Visual inspection of contour funnel plots did not reveal publication bias toward over‐reporting relationships between plant diversity or community composition with non‐native earthworm abundance or community composition. Results from the sensitivity analyses indicated that different earthworm sampling techniques did not influence the association between plant diversity and community composition with introduced earthworm communities. For all models, the interaction of earthworm sampling technique and earthworm community measures was not statistically significant (P > 0.10).
Discussion
The extensive effects of introduced ecosystem engineers, such as earthworms, in North American forests could have transformative impacts on biodiversity and diversity‐dependent ecosystem functions (Ehrenfeld, 2010). Here, we present the first quantitative meta‐analysis showing that introduced earthworms are significantly associated with declines in plant species diversity and changes in plant community composition across multiple studies in North American forests.
Changes in plant diversity
Our analysis showed that earthworm invasion significantly influences plant species diversity. Forests with multiple earthworm ecological groups had significantly lower plant diversity than forests with fewer earthworm ecological groups. While particular earthworm species or ecological groups may have specific effects on seed fate and seedling growth and mortality (e.g., Asshoff et al., 2010; Eisenhauer et al., 2012; Fahey et al., 2013; Drouin et al., 2014; Groffman et al., 2015), our results highlight the combined impacts of multiple earthworm ecological groups on plant communities (Hale et al., 2005). Changes in plant species diversity likely occurred through a number of direct mechanisms, such as seed or seedling ingestion (Eisenhauer et al., 2010; Clause et al., 2015; Cassin & Kotanen, 2016), and indirect mechanisms, including altering water or nutrient availability, mycorrhizal associations, and soil structure (Hale et al., 2005; Holdsworth et al., 2007b; Dobson & Blossey, 2015; Resner et al., 2015; Paudel et al., 2016). While richness of earthworm ecological groups was positively and significantly correlated with earthworm density and biomass (Fig. S1 and Table S3), neither of the latter surrogates for earthworm activity exhibited significant associations with plant species diversity. In contrast to richness of earthworm ecological groups, variation in earthworm density or biomass may not fully capture the progression of earthworm invasion impacts (Loss et al., 2013) and, rather, may reflect changes in dominance of particular earthworm species, such as small‐bodied D. octaedra, large‐bodied L. terrestris (Hale et al., 2004) or entire ecological groups (Hale et al., 2005; Eisenhauer et al., 2007; Holdsworth et al., 2007b).
In line with recent global meta‐analyses on local‐scale changes in species richness (Vellend et al., 2013; Dornelas et al., 2014; but see Gonzalez et al., 2016), we found that plant species richness was robust to earthworm invasion. Despite no average change in plant species richness or evenness, there were clear changes in plant diversity and community composition. This result also could reflect scale‐dependent effects of earthworm invasions on plant diversity (Powell et al., 2013); similar to plant invasions, earthworm invasion may alter the slope and intercept of the species–area relationship by changing species abundance distributions.
Changes in plant community composition: native versus non‐native plant species
We provide clear evidence that non‐native plant cover is positively associated with biomass of introduced earthworms, thus supporting the ‘invasional meltdown’ hypothesis (Simberloff & Von Holle, 1999). Plant species that coevolved in the presence of earthworms, that is plant species of European and Asian origin, could have adaptations that confer tolerance to the presence of earthworms. Non‐native plant species may be adapted to similar soil conditions as those found in earthworm‐invaded forests, such as high soil pH and sparse litter cover (Nuzzo et al., 2009; Beauséjour et al., 2014; Whitfeld et al., 2014). The bioturbation of the forest floor associated with earthworm invasion also may favor non‐native plant species without obligate mycorrhizal associations (Lawrence et al., 2003; Paudel et al., 2016). Furthermore, introduced earthworms have been found to have positive, direct effects on non‐native plant species (Roth et al., 2015) by burying seeds in their burrows, which have high nutrient concentrations and may attenuate drought stress (Migge‐Kleian et al., 2006; Eisenhauer & Scheu, 2008). There is also growing evidence of a synergistic effect of introduced earthworms and deer herbivory on non‐native plant species (Dávalos et al., 2015). Particularly in newly invaded North American forests, introduced earthworms – in combination with deer herbivory – may be increasing disturbance frequency relative to historical norms (Frelich et al., 2012), which is an important factor in explaining increases in the abundance of non‐native species (Moles et al., 2012).
Our analysis showed that cover of native plant species did not change consistently in earthworm‐invaded forests. While the trend of decreasing native species cover with increasing introduced earthworm biomass supports the idea that earthworm invasion may lead to declines in native plant species diversity (Nuzzo et al., 2009), high across‐study variation reduced the overall strength of this signal. This result suggests that physical disturbance of the forest floor by introduced earthworms may increase the heterogeneity of regeneration sites, creating a greater diversity of favorable microhabitats for seed germination of both native and non‐native plant species (Asshoff et al., 2010; Nuzzo et al., 2015). In addition, we found that total plant cover increased significantly with earthworm biomass (Fig. S2 and Table S4), possibly indicating that earthworm invasion enhances germination of many plant species (Nuzzo et al., 2015). Results from previous studies have shown both positive and negative effects of introduced earthworms on seedling survival of native plant species. Dobson & Blossey (2015) found that twelve of fifteen native species were negatively affected by earthworm invasion, while other microcosm and field studies have reported both types of responses to the presence of introduced earthworms (Holdsworth et al., 2007a; Corio et al., 2009; Drouin et al., 2014). Native species’ responses to earthworms could be driven by tolerance to drought and frost upheaval (Dobson & Blossey, 2015), mycorrhizal associations (Lawrence et al., 2003; Paudel et al., 2016), or tolerance to root herbivory (Cameron et al., 2014).
Changes in plant community composition: graminoids
We predicted and found a positive relationship between graminoid cover and all measures of earthworm invasion. This finding suggests that earthworm invasion acts as a significant ecological filter that appears to drive strong changes in plant community composition. The long‐term effects of earthworm invasion on abiotic conditions in the forest understory, for example, rapid soil nutrient release and subsequent depletion, decreased soil water content, and increased surface runoff (Hale et al., 2005; Eisenhauer et al., 2012; Resner et al., 2015), may confer a competitive advantage to graminoids, particularly those with greater drought tolerance (Craine et al., 2013) and persistent bud banks (Bond, 2008; VanderWeide & Hartnett, 2015). Certain graminoids, such as those with greater tolerance of root herbivory (Cameron et al., 2014; Gilbert et al., 2014) or those without obligate mycorrhizal associations (e.g., Carex pensylvanica; Holdsworth et al., 2007a), also may respond positively to earthworm invasion. However, positive responses of graminoids to earthworms might be restricted to a subset of species within this functional group (Corio et al., 2009; Dobson & Blossey, 2015). Increasing graminoid cover in North American forests also may be attributable to positive, synergistic interactions with co‐occurring disturbances, such as deer browsing, fire history, forest management, and land‐use history (Powers & Nagel, 2008; Fisichelli et al., 2013). Given the prevalence of disturbances in northern North American forests that may influence biodiversity (Murphy & Romanuk, 2014), future studies should account for co‐occurring disturbances to add greater precision to estimates of the impacts of earthworm invasion on biodiversity of above‐ and belowground communities (Cameron et al., 2016).
Variation across studies
Across studies, impacts of earthworm invasion were significantly heterogeneous, likely due to variation in biophysical factors (Hale et al., 2005; Resner et al., 2015), invasion history (Hale et al., 2006), and co‐occurring disturbances (Fisichelli et al., 2013; Dávalos et al., 2015). Variation and error associated with measurement of earthworm and plant communities also may contribute to this heterogeneity. Estimates of earthworm density or biomass may be imprecise or inconsistent because of the scale of sampling, time of year, and year to year fluctuations in abundance (Callaham & Hendrix, 1997). In contrast, measures of plant communities likely reflect the history of earthworm invasion at a given site (Larson et al., 2010), which may also vary within studies (e.g., Hale et al., 2006; Holdsworth et al., 2007b). However, one‐time plant inventories along earthworm invasion fronts have a limited capacity to capture taxonomic and functional turnover of plant communities in response to earthworm invasion; repeated inventories, including those of uninvaded forests to establish pre‐invasion baselines, are vital for improving current estimates of biodiversity change (Eisenhauer et al., 2016; Gonzalez et al., 2016). Disentangling the impacts of earthworm invasion from biophysical and other co‐occurring disturbances on plant communities may be best addressed by combining field experiments (e.g., Dobson & Blossey, 2015) with quantitative trait and phylogenetic information (e.g., Cassin & Kotanen, 2016; Lemoine et al., 2016). Accounting for such factors in future studies, particularly in regions where plant communities have interacted with native earthworm fauna, would help to clarify the mechanisms through which earthworm invasion affects biodiversity.
Overall, our results show that earthworm invasion is associated with significant changes in the diversity of plant communities in North American forests. By changing the functional composition and facilitating the invasion of non‐native plant species, earthworm invasion may have long‐lasting impacts on ecosystem functioning and services in these forests. Furthermore, there is growing evidence that terrestrial, invertebrate invaders likely have strong impacts on other trophic levels and associated ecosystem functions (Wardle et al., 2011; Cameron et al., 2016), which may be accentuated with climate change (Eisenhauer et al., 2012). A more holistic approach to assessing the impacts of earthworm invasion, therefore, will be vital for developing management and conservation strategies that enhance the resilience of North American forests (Nimmo et al., 2015).
Supporting information
Data S1.
Appendix S1. PRISMA diagram
Appendix S2. References of studies included in meta‐analysis
Appendix S3. Metadata of ‘Cravenetal_Earthworms_PlantDiversity.csv’
Appendix S4. Metadata of ‘Cravenetal_EffectSizes_Earthworms_PlantFunctGroups.csv’
Table S1. Studies included in meta‐analysis and additional information about each study
Table S2. Introduced earthworm species and their corresponding ecological groups
Table S3. Summary of mixed‐effects model testing associations between introduced earthworm
biomass, density, and ecological group richness.
Table S4. Summary of mixed‐effects model testing the relationships between introduced earthworm biomass, density, and ecological group richness and total plant cover.
Fig. S1. Frequency of earthworm ecological group richness and correlations among measures of introduced earthworm abundance.
Fig. S2. Effect sizes of relationships between introduced earthworm communities and total plant cover.
Data S2. Cravenetal_Earthworms_PlantDiversity.csv
Data file containing effect sizes of relationships between introduced earthworm communities and plant species diversity, evennness, and richness of forest understory communities in North America.
Data S3. Cravenetal_Earthworms_PlantFunctionalGroups.csv
Data file containing effect sizes of relationships between introduced earthworm communities and cover of plant functional groups of forest understory communities in North America.
Acknowledgments
This project received support from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (grant agreement no 677232), the German Research Foundation (DFG FZT 118), and the German Academic Exchange Service (DAAD).
References
- Asshoff R, Scheu S, Eisenhauer N (2010) Different earthworm ecological groups interactively impact seedling establishment. European Journal of Soil Biology, 46, 330–334. [Google Scholar]
- Beauséjour R, Handa IT, Lechowicz MJ, Gilbert B, Vellend M (2014) Historical anthropogenic disturbances influence patterns of non‐native earthworm and plant invasions in a temperate primary forest. Biological Invasions, 17, 1267–1281. [Google Scholar]
- Bohlen PJ, Scheu S, Hale CM, McLean MA, Migge S, Groffman PM, Parkinson D (2004) Non‐native invasive earthworms as agents of change in northern temperate forests. Frontiers in Ecology and the Environment, 2, 427–435. [Google Scholar]
- Bond WJ (2008) What limits trees in c₄ grasslands and savannas? Annual Review of Ecology, Evolution, and Systematics, 39, 641–659. [Google Scholar]
- Bouché M (1977) Strategies lombriciennes. Ecological Bulletins, 25, 122–132. [Google Scholar]
- Brown GG (1995) How do earthworms affect microfloral and faunal community diversity? In: The Significance and Regulation of Soil Biodiversity, vol. 63 (ed./eds Collins HP, Robertson GP, Klug MJ.), pp. 247–269. Springer, Dordrecht, The Netherlands. [Google Scholar]
- Callaham MA, Hendrix PF (1997) Relative abundance and seasonal activity of earthworms (Lumbricidae and Megascolecidae) as determined by hand‐sorting and formalin extraction in forest soils on the southern Appalachian Piedmont. Soil Biology and Biochemistry, 29, 317–321. [Google Scholar]
- Cameron EK, Cahill JF Jr, Bayne EM (2014) Root foraging influences plant growth responses to earthworm foraging. PLoS ONE, 9, e108873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron EK, Vilà M, Cabeza M (2016) Global meta‐analysis of the impacts of terrestrial invertebrate invaders on species, communities and ecosystems. Global Ecology and Biogeography, 25, 596–606. [Google Scholar]
- Capowiez Y, Sammartino S, Michel E (2014) Burrow systems of endogeic earthworms: Effects of earthworm abundance and consequences for soil water infiltration. Pedobiologia, 57, 303–309. [Google Scholar]
- Cassin CM, Kotanen PM (2016) Invasive earthworms as seed predators of temperate forest plants. Biological Invasions, 18, 1567–1580. [Google Scholar]
- Clause J, Forey E, Lortie CJ, Lambert AM, Barot S (2015) Non‐native earthworms promote plant invasion by ingesting seeds and modifying soil properties. Acta Oecologica, 64, 10–20. [Google Scholar]
- Core Team R (2015) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Corio K, Wolf A, Draney M, Fewless G (2009) Exotic earthworms of great lakes forests: a search for indicator plant species in maple forests. Forest Ecology and Management, 258, 1059–1066. [Google Scholar]
- Craine JM, Ocheltree TW, Nippert JB, Towne EG, Skibbe AM, Kembel SW, Fargione JE (2013) Global diversity of drought tolerance and grassland climate‐change resilience. Nature Clim. Change, 3, 63–67. [Google Scholar]
- Dávalos A, Nuzzo V, Blossey B (2015) Single and interactive effects of deer and earthworms on non‐native plants. Forest Ecology and Management, 351, 28–35. [Google Scholar]
- de Deyn GB, van der Putten WH (2005) Linking aboveground and belowground diversity. Trends in Ecology & Evolution, 20, 625–633. [DOI] [PubMed] [Google Scholar]
- Dobson A, Blossey B (2015) Earthworm invasion, white‐tailed deer and seedling establishment in deciduous forests of north‐eastern North America. Journal of Ecology, 103, 153–164. [Google Scholar]
- Dornelas M, Gotelli NJ, McGill B, Shimadzu H, Moyes F, Sievers C, Magurran AE (2014) Assemblage time series reveal biodiversity change but not systematic loss. Science, 344, 296–299. [DOI] [PubMed] [Google Scholar]
- Drouin M, Bradley R, Lapointe L, Whalen J (2014) Non‐native anecic earthworms (Lumbricus terrestris L.) reduce seed germination and seedling survival of temperate and boreal trees species. Applied Soil Ecology, 75, 145–149. [Google Scholar]
- Edwards CA (2004) Earthworm Ecology. CRC Press, Boca Raton, Florida. 441 pp. [Google Scholar]
- Ehrenfeld JG (2010) Ecosystem consequences of biological invasions. Annual Review of Ecology, Evolution, and Systematics, 41, 59–80. [Google Scholar]
- Eisenhauer N (2010) The action of an animal ecosystem engineer: identification of the main mechanisms of earthworm impacts on soil microarthropods. Pedobiologia, 53, 343–352. [Google Scholar]
- Eisenhauer N, Scheu S (2008) Invasibility of experimental grassland communities: the role of earthworms, plant functional group identity and seed size. Oikos, 117, 1026–1036. [Google Scholar]
- Eisenhauer N, Partsch S, Parkinson D, Scheu S (2007) Invasion of a deciduous forest by earthworms: changes in soil chemistry, microflora, microarthropods and vegetation. Soil Biology and Biochemistry, 39, 1099–1110. [Google Scholar]
- Eisenhauer N, Marhan S, Scheu S (2008) Assessment of anecic behavior in selected earthworm species: Effects on wheat seed burial, seedling establishment, wheat growth and litter incorporation. Applied Soil Ecology, 38, 79–82. [Google Scholar]
- Eisenhauer N, Schuy M, Butenschoen O, Scheu S (2009) Direct and indirect effects of endogeic earthworms on plant seeds. Pedobiologia, 52, 151–162. [Google Scholar]
- Eisenhauer N, Butenschoen O, Radsick S, Scheu S (2010) Earthworms as seedling predators: Importance of seeds and seedlings for earthworm nutrition. Soil Biology and Biochemistry, 42, 1245–1252. [Google Scholar]
- Eisenhauer N, Fisichelli NA, Frelich LE, Reich PB (2012) Interactive effects of global warming and “global worming” on the initial establishment of native and exotic herbaceous plant species. Oikos, 121, 1121–1133. [Google Scholar]
- Eisenhauer N, Barnes AD, Cesarz S et al (2016) Biodiversity–ecosystem function experiments reveal the mechanisms underlying the consequences of biodiversity change in real world ecosystems. Journal of Vegetation Science, doi: 10.1111/jvs.12435. [Google Scholar]
- Estes JA, Terborgh J, Brashares JS et al (2011) Trophic Downgrading of Planet Earth. Science, 333, 301–306. [DOI] [PubMed] [Google Scholar]
- Ewing H, Tuininga A, Groffman P et al (2015) Earthworms reduce biotic 15‐Nitrogen retention in northern hardwood forests. Ecosystems, 18, 328–342. [Google Scholar]
- Fahey TJ, Yavitt JB, Sherman RE, Maerz JC, Groffman PM, Fisk MC, Bohlen PJ (2013) Earthworm effects on the incorporation of litter C and N into soil organic matter in a sugar maple forest. Ecological Applications, 23, 1185–1201. [DOI] [PubMed] [Google Scholar]
- Ferlian O, Cesarz S, Marhan S, Scheu S (2014) Carbon food resources of earthworms of different ecological groups as indicated by 13C compound‐specific stable isotope analysis. Soil Biology and Biochemistry, 77, 22–30. [Google Scholar]
- Fisichelli NA, Frelich LE, Reich PB, Eisenhauer N (2013) Linking direct and indirect pathways mediating earthworms, deer, and understory composition in Great Lakes forests. Biological Invasions, 15, 1057–1066. [Google Scholar]
- Forey E, Barot S, Decaëns T et al (2011) Importance of earthworm–seed interactions for the composition and structure of plant communities: A review. Acta Oecologica, 37, 594–603. [Google Scholar]
- Frelich LE, Hale CM, Scheu S, Holdsworth AR, Heneghan L, Bohlen PJ, Reich PB (2006) Earthworm invasion into previously earthworm‐free temperate and boreal forests. Biological Invasions, 8, 1235–1245. [Google Scholar]
- Frelich LE, Peterson RO, Dovčiak M, Reich PB, Vucetich JA, Eisenhauer N (2012) Trophic cascades, invasive species and body‐size hierarchies interactively modulate climate change responses of ecotonal temperate–boreal forest. Philosophical Transactions of the Royal Society B: Biological Sciences, 367, 2955–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert KJ, Fahey TJ, Maerz JC et al (2014) Exploring carbon flow through the root channel in a temperate forest soil food web. Soil Biology and Biochemistry, 76, 45–52. [Google Scholar]
- Gonzalez A, Cardinale BJ, Allington GRH et al (2016) Estimating local biodiversity change: a critique of papers claiming no net loss of local diversity. Ecology, 97, 1949–1960. [DOI] [PubMed] [Google Scholar]
- Groffman PM, Fahey TJ, Fisk MC, Yavitt JB, Sherman RE, Bohlen PJ, Maerz JC (2015) Earthworms increase soil microbial biomass carrying capacity and nitrogen retention in northern hardwood forests. Soil Biology and Biochemistry, 87, 51–58. [Google Scholar]
- Hale C, Reich PB, Frelich LE (2004) Allometric equations for estimation of ash‐free dry mass from length measurements for selected European earthworm species (Lumbricidae) in the western Great Lakes region. The American Midland Naturalist, 151, 179–185. [Google Scholar]
- Hale C, Frelich L, Reich P, Pastor J (2005) Effects of European earthworm invasion on soil characteristics in northern hardwood forests of Minnesota, USA. Ecosystems, 8, 911–927. [Google Scholar]
- Hale CM, Frelich LE, Reich PB (2006) Changes in hardwood forest understory plant communities in response to European earthworm invasions. Ecology, 87, 1637–1649. [DOI] [PubMed] [Google Scholar]
- Hale AN, Lapointe L, Kalisz S (2016) Invader disruption of belowground plant mutualisms reduces carbon acquisition and alters allocation patterns in a native forest herb. New Phytologist, 209, 542–549. [DOI] [PubMed] [Google Scholar]
- Hedges LV (1989) An unbiased correction for sampling error in validity generalization studies. Journal of Applied Psychology, 74, 469–477. [Google Scholar]
- Hendrix PF, Bohlen PJ (2002) Exotic earthworm invasions in North America: ecological and policy implications: expanding global commerce may be increasing the likelihood of exotic earthworm invasions, which could have negative implications for soil processes, other animal and plant species, and importation of certain pathogens. BioScience, 52, 801–811. [Google Scholar]
- Hendrix PF, Callaham M Jr, Drake JM, Huang C‐Y, James SW, Snyder BA, Zhang W (2008) Pandora's box contained bait: The global problem of introduced earthworms. Annual Review of Ecology, Evolution, and Systematics, 39, 593–613. [Google Scholar]
- Heneghan L, Steffen J, Fagen K (2007) Interactions of an introduced shrub and introduced earthworms in an Illinois urban woodland: impact on leaf litter decomposition. Pedobiologia, 50, 543–551. [Google Scholar]
- Holdsworth AR, Frelich LE, Reich PB (2007a) Effects of earthworm invasion on plant species richness in northern hardwood forests. Conservation Biology, 21, 997–1008. [DOI] [PubMed] [Google Scholar]
- Holdsworth AR, Frelich LE, Reich PB (2007b) Regional extent of an ecosystem engineer: Earthworm invasion in northern hardwood forests. Ecological Applications, 17, 1666–1677. [DOI] [PubMed] [Google Scholar]
- Koricheva J, Gurevitch J (2014) Uses and misuses of meta‐analysis in plant ecology. Journal of Ecology, 102, 828–844. [Google Scholar]
- Koricheva J, Gurevitch J, Mengeresen K (2013) Handbook of Meta‐Analysis in Ecology and Evolution. Princeton University Press, Oxford. [Google Scholar]
- Larson E, Kipfmueller K, Hale C, Frelich L, Reich P (2010) Tree rings detect earthworm invasions and their effects in northern Hardwood forests. Biological Invasions, 12, 1053–1066. [Google Scholar]
- Lavelle P (1983) The structure of earthworm communities In: Earthworm Ecology: From Darwin to Vermiculture (ed./eds Satchell JE.), pp. 449–466. Springer, Dordrecht, the Netherlands. [Google Scholar]
- Lawrence B, Fisk MC, Fahey TJ, Suárez ER (2003) Influence of nonnative earthworms on mycorrhizal colonization of sugar maple (Acer saccharum). New Phytologist, 157, 145–153. [DOI] [PubMed] [Google Scholar]
- Lemoine NP, Burkepile DE, Parker JD (2016) Quantifying differences between native and introduced species. Trends in Ecology & Evolution, 5, 372–381. [DOI] [PubMed] [Google Scholar]
- Loss SR, Hueffmeier RM, Hale CM, Host GE, Sjerven G, Frelich LE (2013) Earthworm invasions in northern hardwood forests: a rapid assessment method. Natural Areas Journal, 33, 21–30. [Google Scholar]
- Lubbers IM, van Groenigen KJ, Fonte SJ, Six J, Brussaard L, van Groenigen JW (2013) Greenhouse‐gas emissions from soils increased by earthworms. Nature Climate Change, 3, 187–194. [Google Scholar]
- Migge‐Kleian S, McLean MA, Maerz JC, Heneghan L (2006) The influence of invasive earthworms on indigenous fauna in ecosystems previously uninhabited by earthworms. Biological Invasions, 8, 1275–1285. [Google Scholar]
- Moles AT, Flores‐Moreno H, Bonser SP et al (2012) Invasions: the trail behind, the path ahead, and a test of a disturbing idea. Journal of Ecology, 100, 116–127. [Google Scholar]
- Murphy GE, Romanuk TN (2014) A meta‐analysis of declines in local species richness from human disturbances. Ecology and Evolution, 4, 91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers L, Sirois MJ (2014) Spearman Correlation Coefficients, Differences between In: Wiley StatsRef: Statistics Reference Online. John Wiley & Sons Ltd. [Google Scholar]
- Nakagawa S, Santos ES (2012) Methodological issues and advances in biological meta‐analysis. Evolutionary Ecology, 26, 1253–1274. [Google Scholar]
- Nimmo DG, Nally RM, Cunningham SC, Haslem A, Bennett AF (2015) Vive la résistance: reviving resistance for 21st century conservation. Trends in Ecology & Evolution, 30, 516–523. [DOI] [PubMed] [Google Scholar]
- Nuzzo VA, Maerz JC, Blossey B (2009) Earthworm invasion as the driving force behind plant invasion and community change in northeastern North American forests. Conservation Biology, 23, 966–974. [DOI] [PubMed] [Google Scholar]
- Nuzzo V, Dávalos A, Blossey B (2015) Invasive earthworms shape forest seed bank composition. Diversity and Distributions, 21, 560–570. [Google Scholar]
- Paudel S, Longcore T, MacDonald B, McCormick MK, Szlavecz K, Wilson GWT, Loss SR (2016) Belowground interactions with aboveground consequences: Invasive earthworms and arbuscular mycorrhizal fungi. Ecology, 97, 605–614. [DOI] [PubMed] [Google Scholar]
- Powell KI, Chase JM, Knight TM (2013) Invasive plants have scale‐dependent effects on diversity by altering species‐area relationships. Science, 339, 316–318. [DOI] [PubMed] [Google Scholar]
- Powers MD, Nagel LM (2008) Disturbance dynamics influence Carex pensylvanica abundance in a northern hardwood forest 1. The Journal of the Torrey Botanical Society, 135, 317–327. [Google Scholar]
- Resner K, Yoo K, Sebestyen S, Aufdenkampe A, Hale C, Lyttle A, Blum A (2015) Invasive earthworms deplete key soil inorganic nutrients (Ca, Mg, K, and P) in a northern hardwood forest. Ecosystems, 18, 89–102. [Google Scholar]
- Romero GQ, Gonçalves‐Souza T, Vieira C, Koricheva J (2015) Ecosystem engineering effects on species diversity across ecosystems: a meta‐analysis. Biological Reviews, 90, 877–890. [DOI] [PubMed] [Google Scholar]
- Roth AM, Whitfeld TJS, Lodge AG, Eisenhauer N, Frelich LE, Reich PB (2015) Invasive earthworms interact with abiotic conditions to influence the invasion of common buckthorn (Rhamnus cathartica). Oecologia, 178, 219–230. [DOI] [PubMed] [Google Scholar]
- Sackett TE, Smith SM, Basiliko N (2013) Indirect and direct effects of exotic earthworms on soil nutrient and carbon pools in North American temperate forests. Soil Biology and Biochemistry, 57, 459–467. [Google Scholar]
- Sala OE, Chapin FS III, Armesto JJ et al (2000) Global biodiversity scenarios for the year 2100. Science, 287, 1770–1774. [DOI] [PubMed] [Google Scholar]
- Simberloff D, Von Holle B (1999) Positive interactions of nonindigenous species: invasional meltdown? Biological invasions, 1, 21–32. [Google Scholar]
- Simberloff D, Martin J‐L, Genovesi P et al (2013) Impacts of biological invasions: what's what and the way forward. Trends in Ecology & Evolution, 28, 58–66. [DOI] [PubMed] [Google Scholar]
- Smith B, Wilson JB (1996) A consumer's guide to evenness indices. Oikos, 76, 70–82. [Google Scholar]
- Szlavecz K, Placella SA, Pouyat RV, Groffman PM, Csuzdi C, Yesilonis I (2006) Invasive earthworm species and nitrogen cycling in remnant forest patches. Applied Soil Ecology, 32, 54–62. [Google Scholar]
- USDA (2014) The PLANTS Database. Available at: http://plants.usda.gov (accessed 1 March 2015).
- VanderWeide B, Hartnett D (2015) Belowground bud bank response to grazing under severe, short‐term drought. Oecologia, 3, 795–806. [DOI] [PubMed] [Google Scholar]
- Vellend M, Baeten L, Myers‐Smith IH et al (2013) Global meta‐analysis reveals no net change in local‐scale plant biodiversity over time. Proceedings of the National Academy of Sciences, 110, 19456–19459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viechtbauer W (2010) Conducting meta‐analyses in R with the metafor package. Journal of Statistical Software, 36, 1–48. [Google Scholar]
- Vilà M, Espinar JL, Hejda M et al (2011) Ecological impacts of invasive alien plants: a meta‐analysis of their effects on species, communities and ecosystems. Ecology Letters, 14, 702–708. [DOI] [PubMed] [Google Scholar]
- Walsh JR, Carpenter SR, Vander Zanden MJ (2016) Invasive species triggers a massive loss of ecosystem services through a trophic cascade. Proceedings of the National Academy of Sciences, 113, 4081–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle DA, Bardgett RD, Klironomos JN, Setälä H, van der Putten WH, Wall DH (2004) Ecological linkages between aboveground and belowground biota. Science, 304, 1629–1633. [DOI] [PubMed] [Google Scholar]
- Wardle DA, Bardgett RD, Callaway RM, Van der Putten WH (2011) Terrestrial ecosystem responses to species gains and losses. Science, 332, 1273–1277. [DOI] [PubMed] [Google Scholar]
- Whitfeld TJS, Roth AM, Lodge AG, Eisenhauer N, Frelich LE, Reich PB (2014) Resident plant diversity and introduced earthworms have contrasting effects on the success of invasive plants. Biological Invasions, 16, 2181–2193. [Google Scholar]
- Zaller JG, Saxler N (2007) Selective vertical seed transport by earthworms: Implications for the diversity of grassland ecosystems. European Journal of Soil Biology, 43 Supplement 1, S86–S91. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.
Appendix S1. PRISMA diagram
Appendix S2. References of studies included in meta‐analysis
Appendix S3. Metadata of ‘Cravenetal_Earthworms_PlantDiversity.csv’
Appendix S4. Metadata of ‘Cravenetal_EffectSizes_Earthworms_PlantFunctGroups.csv’
Table S1. Studies included in meta‐analysis and additional information about each study
Table S2. Introduced earthworm species and their corresponding ecological groups
Table S3. Summary of mixed‐effects model testing associations between introduced earthworm
biomass, density, and ecological group richness.
Table S4. Summary of mixed‐effects model testing the relationships between introduced earthworm biomass, density, and ecological group richness and total plant cover.
Fig. S1. Frequency of earthworm ecological group richness and correlations among measures of introduced earthworm abundance.
Fig. S2. Effect sizes of relationships between introduced earthworm communities and total plant cover.
Data S2. Cravenetal_Earthworms_PlantDiversity.csv
Data file containing effect sizes of relationships between introduced earthworm communities and plant species diversity, evennness, and richness of forest understory communities in North America.
Data S3. Cravenetal_Earthworms_PlantFunctionalGroups.csv
Data file containing effect sizes of relationships between introduced earthworm communities and cover of plant functional groups of forest understory communities in North America.


