Abstract
Aberrant connectivity is believed to contribute to the pathophysiology of autism spectrum disorder (ASD). Recent neuroimaging studies have increasingly identified such impairments in patients with ASD, including alterations in sensory systems. However, the cellular substrates and molecular underpinnings of disrupted connectivity remain poorly understood. Utilizing eye‐specific segregation in the dorsal lateral geniculate nucleus (dLGN) as a model system, we investigated the formation and refinement of precise patterning of synaptic connections in the BTBR T + tf/J (BTBR) mouse model of ASD. We found that at the neonatal stage, the shape of the dLGN occupied by retinal afferents was altered in the BTBR group compared to C57BL/6J (B6) animals. Notably, the degree of overlap between the ipsi‐ and contralateral afferents was significantly greater in the BTBR mice. Moreover, these abnormalities continued into mature stage in the BTBR animals, suggesting persistent deficits rather than delayed maturation of axonal refinement. Together, these results indicate disrupted connectivity at the synaptic patterning level in the BTBR mice, suggesting that in general, altered neural circuitry may contribute to autistic behaviours seen in this animal model. In addition, these data are consistent with the notion that lower‐level, primary processing mechanisms contribute to altered visual perception in ASD. Autism Res 2017, 10: 212–223. © 2016 The Authors Autism Research published by Wiley Periodicals, Inc. on behalf of International Society for Autism Research.
Keywords: autism spectrum disorder, brain circuit, synaptic patterning, visual system, lateral geniculate nucleus, eye‐specific segregation, BTBR mouse
Introduction
Autism spectrum disorder (ASD) is an increasingly prevalent neurodevelopmental disorder, characterised by deficits in socio‐emotional functions and language development, as well as repetitive and/or restrictive behaviours [DiCicco‐Bloom et al., 2006; Geschwind, 2009; Lai, Lombardo, & Baron‐Cohen, 2014; Llaneza et al., 2010]. In addition, ASD manifests clinically in a broad and heterogeneous fashion, consistent with the notion that there are likely a plethora of possible etiological factors, including both genetic and environmental influences. Thus, ASD remains a clinical syndrome, and not a specific etiologically defined disorder. The prominent heterogeneity in autism has been a challenge for investigators to uncover its neurobiological mechanisms and identify interventions for affected individuals. Currently, only co‐morbid manifestations of the disorder can be alleviated, but not the core symptoms [DiCicco‐Bloom et al., 2006; Geschwind, 2009; Lai et al., 2014; Llaneza et al., 2010; Veenstra‐VanderWeele & Warren, 2015].
For normal brain function and behaviour, neural circuits must maintain a precise organisation, but become disrupted in various neurological diseases. ASD has been characterised as a distributed central nervous system disorder that affects multiple brain regions [DiCicco‐Bloom et al., 2006; Lai et al., 2014; Maximo, Cadena, & Kana, 2014]. Recent studies have pointed to improper development of neural circuits as a potentially unifying mechanism in autism. At molecular and cellular levels, synaptic dysfunction has been a main focus of earlier studies, mainly because multiple autism susceptibility genes are involved in the postsynaptic signalling of glutamatergic synapses [Betancur, Sakurai, & Buxbaum, 2009; Bourgeron, 2009; Buxbaum et al., 2012; Peca & Feng, 2012; Zoghbi & Bear, 2012]. In addition, changes in the size, shape and number of dendritic spines have been observed in both patients and animal models [Hutsler & Zhang, 2010; Phillips & Pozzo‐Miller, 2015; Tang et al., 2014; Zoghbi & Bear, 2012]. At the tissue level, disruption in functional connectivity has been consistently identified using neuroimaging techniques [Maximo et al., 2014]. The identified impairments include both focal deficits in particular brain regions and aberrant long‐range connections between different lobes. Although much has been learned, many aspects in the development of neural circuits in ASD remain unknown. Specifically, whether precise patterns of synaptic connections are formed and maintained in autistic brains is uncertain.
Primary sensory processing could be critical for higher‐order social and cognitive tasks. Although impairment in sensory function has been reported since the earliest descriptions of ASD, its importance has only more recently been appreciated. For example, hyper‐ or hyporeactivity to sensory input or unusual interests in sensory aspects of the environment are included as part of the core symptoms of ASD only in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM‐5) released in 2013. Regarding visual processing, studies have observed both superior and deficient performance during behavioural or perceptual tasks in individuals with ASD. Overall, there is consensus that autistic patients could perform better when local visual details are the focus of the task [Baum, Stevenson, & Wallace, 2015]. However, there is much debate about whether “higher‐level,” integrative mechanisms or “lower‐level,” primary processing contribute to the observed changes in visual function [Baum et al., 2015].
Eye‐specific segregation in the dorsal lateral geniculate nucleus (dLGN) is a model system to study how precise patterning of synaptic connections form and refine during development [Guido, 2008; Huberman, Feller, & Chapman, 2008; Shatz, 1996]. The LGN is a relay centre along the visual pathway located in the dorsal part of the thalamus. It receives input from the retina via the optic nerve and projects to the visual cortex. The adult dLGN is characterised by its stereotyped organisation into discrete eye‐specific domains, which are evident as non‐overlapping regions of afferents from the two eyes. For a given species, axons of retinal ganglion cells from the contralateral and ipsilateral eye project to distinct domains with stereotyped shape, position, and cross‐sectional area in the adult dLGN. During normal development, the retino‐geniculate afferents from the two eyes are initially overlapping before they gradually segregate into eye‐specific regions. In mice, eye‐specific segregation in dLGN occurs during the first few weeks after birth. This process can be conveniently studied by labelling retinal afferents from both eyes with an anterograde tracer [Guido, 2008; Huberman et al., 2008; Shatz, 1996].
The BTBR T + tf/J (BTBR) inbred strain has been increasingly used to explore the neurobiology of ASD and to identify potential interventions [Llaneza et al., 2010; McFarlane et al., 2008; Moy et al., 2007; Ruskin et al., 2013; Smith, Rho, Masino, & Mychasiuk, 2014]. This model was identified in an extensive effort to characterise ten inbred mouse strains possessing autism‐like behaviours [Moy et al., 2007], and was later shown to display behavioural phenotypes relevant to all three core diagnostic symptoms of autism [McFarlane et al., 2008]. Additional tests conducted in multiple independent laboratories further confirmed that BTBR animals display prominent deficits in multiple social interaction and communication assays, and exhibit repetitive and stereotyped behaviours. Thus, the BTBR strain has been considered a consistent and robust animal model of autism, reflecting the more common form of non‐syndromic ASD [Gaugler et al., 2014]. Although its phenotype is probably not due to a single genetic change, making the study of the molecular and cellular mechanisms potentially challenging, it nonetheless offers an opportunity to identify structural and functional changes underlying the robust behavioural phenotype. Notably, studies using magnetic resonance imaging have uncovered profound impairments in both structural and functional connectivity [Dodero et al., 2013; Ellegood, Babineau, Henkelman, Lerch, & Crawley, 2013; Ellegood et al., 2015; Fenlon et al., 2015; Nie et al., 2010; Sforazzini et al., 2016; Squillace et al., 2014].
In the present study, we investigated circuit formation and refinement in the BTBR animals by examining retinal input and eye‐specific segregation in the dLGN. We compared the results to those from C57BL/6J (B6) mice, a strain with a normal social phenotype and low repetitive behaviours and which have been routinely used as controls for BTBR mice in ASD‐related studies [Ellegood and Crawley, 2015; Llaneza et al., 2010; Meyza et al., 2013]. In addition, B6 mice have been used to establish the developmental trajectory of eye‐specific segregation in mice [Jaubert‐Miazza et al., 2005; Stevens et al., 2007]. Here, we found altered retinal input fields and increased overlap between the ipsi‐ and contralateral afferents in the dLGN of BTBR animals, during development and at the adult stage.
Methods
Animals
Both neonatal (postnatal day 8, P8) and adolescent (postnatal day 30, P30) male B6 and BTBR mice were used. Importantly, retino‐geniculate segregation will have reached the mature pattern by the latter age. All surgical procedures were performed in accordance with the recommendations of the Canadian Council for Animal Care. The protocol was approved by the Health Sciences Animal Care Committee of the University of Calgary.
Anterograde Labelling
Established procedures were followed to label retino‐geniculate projections [Demas et al., 2006; Huberman, Stellwagen, & Chapman, 2002; Muir‐Robinson, Hwang, & Feller, 2002; Stellwagen & Shatz, 2002; Stevens et al., 2007]. Mice were anesthetised with ≤5% isofluorane vapours. Prior to natural eye opening, fused eyelids were separated to expose the temporal region of the eye. The sclera was pierced with a 30‐gauge needle and excess vitreous was drained. A Hamilton syringe, filled with a 0.2% solution of the B subunit of cholera toxin (CTB, Life Technologies) conjugated to either Alexa Fluor 555 (red) or Alexa Fluor 488 (green) dissolved in distilled water, was inserted into the hole made by the needle. A prescribed volume (2 μL for P8 and 5 μL for P30 animals) of solution was slowly injected into the eye. Each eye was injected with a different fluorescent conjugate so that the retinal input from both eyes could be visualised simultaneously in a single section of the dLGN. The following day, animals were deeply anesthetised and transcardially perfused with 4% paraformaldehyde in PBS.
Tissue Processing
Tissue was processed according to previously described methods [Cheng, Cai, & Belluscio, 2011]. Brains were removed and post‐fixed, embedded in gelatin, cryoprotected in 30% sucrose, sectioned in the coronal plane using a cryostat with the thickness of 90 μm, stained with 4′,6′‐diamidino‐2‐phenylindole (DAPI) and mounted onto slides.
Image Acquisition
Following established methods [Demas et al., 2006; Huberman et al., 2002; Muir‐Robinson et al., 2002; Stellwagen & Shatz, 2002; Stevens et al., 2007], images of 3–5 consecutive sections through the middle part of the dLGN were acquired with a Zeiss LSM 510 confocal microscope.
Image Analysis
Data analysis was performed following established methods [Demas et al., 2006; Huberman et al., 2002; Muir‐Robinson et al., 2002; Stellwagen & Shatz, 2002; Stevens et al., 2007], using a custom‐developed MATLAB program. An adaptive threshold paradigm similar to the ones used in previous reports [Demas et al., 2006; Huberman et al., 2002; Muir‐Robinson et al., 2002; Stellwagen & Shatz, 2002; Stevens et al., 2007] was used that specifies a level in the grey scale histogram where an abrupt increase in the intensity occurred (i.e., the distinction point between signal and residual background fluorescence). Specifically, pixel intensities were arranged in ascending order and the threshold was defined as the value at which the median intensity slope over N% (N = 1.5, 2, 2.5 or 3) of the total number of pixels in the image became positive. Thus, the program‐defined threshold took into account the entire image and was adaptive to the variations in the overall signal intensity among sections. Pixel intensity for each channel was then digitised so that every pixel greater than or equal to the defined threshold level was assigned a value of 1; otherwise, the pixel was given a value of 0. For terminal fields belonging to contralateral or ipsilateral axons, the total number of pixels in the defined area of LGN representing either green or red fluorescence was measured. Pixels that score 1 in both red and green channels were considered overlapping areas and were represented as yellow. A multi‐threshold protocol [Torborg, Hansen, & Feller, 2005] was further employed to compare the overlap across a range of signal to noise values, which has been used for direct statistical comparison of eye‐specific segregation between various strains of mice at different ages, including wild‐type and transgenic mice.
Statistical Analysis
Student's t‐test was performed to test statistical significance between BTBR and B6 data, assuming two‐tailed distribution and two‐sample unequal variance. Values represent the mean ± SEM, and significance was taken at P < 0.05.
Results
Retinal Input Field in the dLGN was Altered in BTBR Animals
To study retinal input in the dLGN, we labelled retinal afferents from both eyes with an anterograde tracer conjugated with different fluorophores (Fig. 1A). In the dLGN of control B6 animals at postnatal day 8 (P8), the ipsilateral domain (red signal) which receives input from the ipsilateral eye is surrounded by the contralateral domain (green signal), with stereotyped shape, position, and cross‐sectional area in the dorsal thalamus (Fig. 1B) [Guido, 2008; Huberman et al., 2008; Shatz, 1996]. The retinal input field in the BTBR animals of the same age was also organised into distinct ipsilateral and contralateral domains. However, it displayed an overall different structure (Fig. 1B), with a smaller cross‐sectional area (measured in Fig. 3A) and a more rounded shape. The relative position of dLGN in the dorsal thalamus was similar between B6 and BTBR animals (Fig. 1B). The changes in the general structure of the dLGN in the BTBR animals were striking, since studies examining the role of retinal activity, axon guidance molecules, and immune molecules have thus far not identified such overall deficits [Guido, 2008; Huberman et al., 2008; Shatz, 1996].
Figure 1.
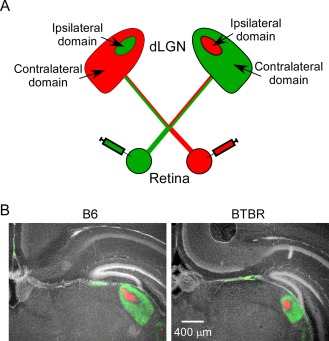
Retinal input to dLGN in the BTBR animals was smaller in cross‐sectional area and more rounded in shape, but with similar relative location, compared with that in the B6 animals. (A) Experimental design. CTB conjugated with green fluorophore was injected into one eye of the mouse, while CTB conjugated with red fluorophore injected into the other eye. CTB is taken up by retinal ganglion cells and then transported anterogradely by the optic nerve to the axon terminals in the dLGN. Domains with input from either contralateral or ipsilateral eye can be clearly recognised in dLGN, and given a certain species, they have characteristic shape, cross‐sectional area, and position. (B) Left panel: representative image of middle dLGN in the B6 animal. Green signal shows retina ganglion cell input from the contralateral eye, while red signal shows input from the ipsilateral eye. White signal shows DAPI nuclear staining. Right panel: compared with the B6 brain, retinal input to dLGN in the BTBR animals was smaller in cross‐sectional area and more rounded in shape. However, its location was relatively unchanged. Consistent with previous studies [Mercier, Kwon, & Douet, 2012], excessive separation of the two hippocampi was also observed in the BTBR brain. Scale bar: 400 μm.
Figure 3.
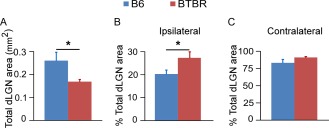
BTBR animals had less total cross‐sectional area of retinal field but greater percentage of ipsilateral input in the middle dLGN. (A) BTBR mice had significantly less total cross‐sectional area of retinal input compared with the B6 animals. (B) The percentage of ipsilateral domain to total dLGN area was significantly increased in the BTBR mice compared to the B6 controls. (C) There was no significant difference in the percentage of contralateral domain to total dLGN area between the two strains. Mean ± SEM (n = 5 animals for the B6 group, and n = 7 animals for the BTBR group, P < 0.05 by Student's t‐test).
BTBR Mice have Impaired Eye‐Specific Segregation during Neonatal Development
Next, we examined eye‐specific segregation in the dLGN of P8 animals by quantifying the degree of overlap between the contralateral and ipsilateral inputs. After removing background fluorescence, the pixels showing both contralateral (green) and ipsilateral (red) signal were considered regions of overlap (yellow) and counted (Fig. 2A). The degree of overlap was presented as the percentage of overlapping regions to the total dLGN area (Fig. 2B). Compared to B6 animals that have been routinely used as a reference strain in studying eye‐specific segregation [Jaubert‐Miazza et al., 2005; Stevens et al., 2007], the BTBR animals showed a significantly higher degree of overlap (P < 0.05) between the contralateral and ipsilateral input (Fig. 2B, n = 5 animals for the B6 group and n = 7 animals for the BTBR group). Importantly, this observation was independent of the threshold used (Fig. 2B), indicating that the difference between the two strains was robust across a range of signal‐to‐noise values.
Figure 2.
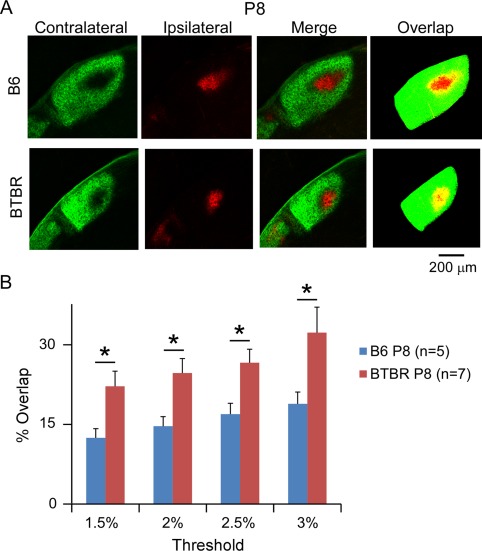
BTBR animals showed deficits in eye‐specific segregation during neonatal development. (A) Representative images of dLGN receiving both contralateral (green signal) and ipsilateral (red signal) input from the two eyes, and the overlap between the two (yellow pixels), which became apparent after the image was thresholded and digitised. BTBR mice at P8 had increased intermingling between the retinal ganglion cell axons from left and right eyes compared with B6 control. Scale bar: 200 μm. (B) Quantification of the percentage of dLGN receiving overlapping inputs in the BTBR vs. B6 animals at P8. BTBR mice exhibit significantly more overlap than the B6 mice, regardless of the threshold used. Data are represented as mean ± SEM (n = 5 animals for the B6 group, and n = 7 animals for the BTBR group, P < 0.05 by Student's t‐test).
BTBR Animals had Less Total dLGN Area but Greater Percentage of Ipsilateral Input in Cross Sections through the Middle dLGN
Further analysis showed that the total dLGN area receiving retinal input in cross sections through the middle part of the dLGN was significantly smaller (P < 0.05) in the BTBR vs. B6 animals (Fig. 3A), consistent with the overall finding mentioned above (Fig. 1B). Strikingly, the total dLGN area in the BTBR animals was only about 65% of that in the B6 animals (0.17 mm2 vs. 0.26 mm2), consistent with previously reported major reductions of grey matter in thalamus [Dodero et al., 2013]. Moreover, the percentage of the ipsilateral domain to the total dLGN area was significantly greater (P < 0.05) in the BTBR than B6 animals (Fig. 3B), while the percentage of the contralateral domain to the total dLGN area was similar between the two strains (Fig. 3C). These results indicate that a proportionally larger ipsilateral domain in the BTBR mice led to the observed increase of overlap between the contralateral and ipsilateral inputs.
Deficits in Eye‐Specific Segregation Continued into Mature Stage in the BTBR Mice
To investigate whether the increased overlap between the contralateral and ipsilateral input in the BTBR animals was due to delayed maturation of axonal refinement or persistent deficits, we further examined eye‐specific segregation in P30 animals. In mice, the refining process of eye‐specific segregation normally occurs during the first few weeks after birth, and by P30 it reaches the adult pattern [Guido, 2008; Huberman et al., 2008; Shatz, 1996]. The same method of quantifying the degree of segregation as used in the P8 animals was used here, and representative images of dLGN from both the B6 and BTBR animals were shown in Figure 4A. We found that the total cross‐sectional area of dLGN in P30 animals was greater than that in P8 animals for both strains, consistent with general brain growth during development. However, it was still significantly smaller in the BTBR compared with B6 animals at P30 (B6: 0.29 mm2, n = 6 animals; BTBR: 0.21 mm2, n = 6 animals, P < 0.01). In addition, the results showed that in both B6 and BTBR animals, the degree of overlap was reduced from P8 to P30 (Fig. 4B), supporting a refining process reported by previous studies [Jaubert‐Miazza et al., 2005; Stevens et al., 2007]. However, BTBR animals still showed a significantly higher degree of overlap between the contralateral and ipsilateral input across a range of thresholds used than the B6 animals (Fig. 4B, n = 6 animals for each group). Together, these data indicate that although refinement of eye‐specific segregation did occur in the BTBR animals, the process was impaired leading to persistent deficits in the retino‐geniculate circuit.
Figure 4.
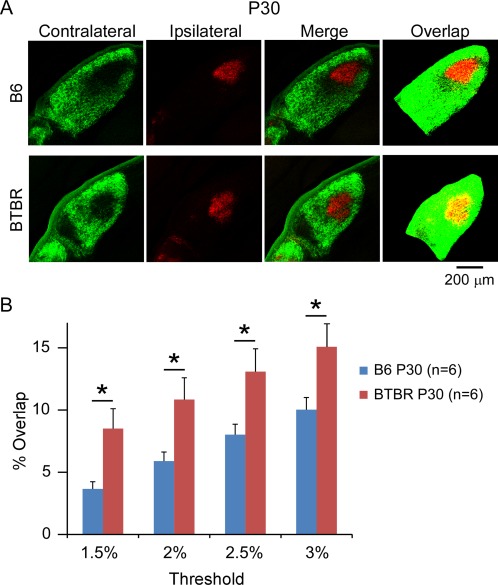
Deficits in eye‐specific segregation continued into mature stage in the BTBR animals. (A) Retinogeniculate projection patterns visualised after injecting CTB into left and right eyes of the B6 and BTBR mice. BTBR mice at P30 had more intermingling (yellow pixels in thresholded images, overlap) between retinal ganglion cell axons from left and right eyes compared with the B6 control. Scale bar: 200 μm. (B) Quantification of the percentage of dLGN receiving overlapping inputs in the BTBR vs. B6 animals at P30. BTBR mice exhibit significantly more overlap than B6 mice, independent of the threshold used. Data are represented as mean ± SEM (n = 6 animals for each group, P < 0.05 by Student's t‐test).
Aberrant Projections of Retino‐Geniculate Afferents were Observed in the BTBR Mice
Closer examination of retinal input in the dLGN revealed that in the B6 animals at P30, eye‐specific domains were well‐formed (Fig. 5A); the contralateral domain (Fig. 5B) contained little ipsilateral projections (Fig. 5B2) and vice‐versa (Fig. 5C, C2). However, in the BTBR animals at a similar age, aberrant retinal projections were observed in the dLGN. Substantial ipsilateral axons projected to the contralateral domain, even in regions far from the ipsilateral domain (Fig. 5E, E2). Similarly, many contralateral axons projected to the middle of the ipsilateral domain (Fig. 5F, F2). These data corroborate the observation of increased overlap between the contralateral and ipsilateral input in the BTBR animals and indicate that deficits in retino‐geniculate projection and segregation were not limited to the boundary of eye‐specific domains.
Figure 5.
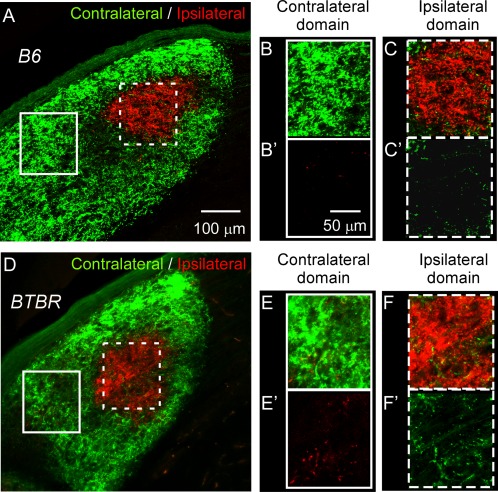
Aberrant projections in the dLGN of the BTBR animals. dLGN from P30 B6 (A–C2) or BTBR (D–F2) mice. Boxed regions in lower magnification images (A and D) correspond to higher magnification images on the right with boxes of the same style (B–C2 and E–F2, respectively). Boxes with solid white line show examples of the contralateral domain, while boxes with dotted white line show the ipsilateral domain. Only the ipsilateral input (red signal) is shown in (B2) and (E2); similarly, only the contralateral input (green signal) is shown in (C2) and (F2). There were increased ipsilateral projections within the contralateral domain in the BTBR mice (E and E2) compared to the B6 mice (B and B2). Similarly, there were increased contralateral projections within the ipsilateral domain in the BTBR mice (F and F2) compared to the B6 mice (C and C2). Scale bar in (A) and (D): 100 μm, in (B–C2) and (E–F2): 50 μm.
Discussion
Potential Mechanisms of Observed Deficits in Eye‐Specific Segregation in the BTBR Strain
Although impaired connectivity in the neural circuitry has been consistently found in both ASD patients and animal models from dendritic spines to functional levels, whether precise connections at the synaptic level are formed and maintained in autistic brains has remained unclear. Here, using eye‐specific segregation in the dLGN as a model system, we showed that specific circuit formation and refinement was disrupted in the BTBR mouse model of ASD, suggesting another possible cause of the impaired connectivity observed in the disorder.
A striking anatomical feature of the BTBR mice is agenesis of the corpus callosum [Meyza et al., 2013]. Interestingly, it has been hypothesised that dysgenesis of the corpus callosum constitutes a major risk factor for developing autism [Paul, Corsello, Kennedy, & Adolphs, 2014], and atypical development of the size and microstructure of the corpus callosum has been one of the most replicated neuroimaging findings in individuals with autism [Frazier & Hardan, 2009; Wolff et al., 2015]. Thus, it is likely that the dysgenesis of the corpus callosum in the BTBR mice may contribute to their autism‐like behaviour, and certainly plays an important role in the disrupted connectivity observed by neuroimaging methods in this model. Because the efferent projections from the dLGN travel through the corpus callosum to reach the visual cortex [Leyva‐Diaz & Lopez‐Bendito, 2013], it is possible that the impaired refinement we observed in the dLGN is secondary to the disrupted structure of the corpus callosum. Future work using the same analysis on other mouse models of ASD with a more normal structure of the corpus callosum would likely to provide more information on this issue.
The study of sensory systems has afforded great insights into the development and maintenance of the intricate neural circuits due to the existence of extraordinarily precise anatomical maps. These maps represent the particular peripheral input sources in the brain and have been found in the somatosensory cortex [Merzenich, Kaas, Sur, & Lin, 1978] including the barrel cortex [Woolsey & Van der Loos, 1970], the visual cortex [Guido, 2008; Hubel & Wiesel, 1977; Huberman et al., 2008; Raczkowski & Rosenquist, 1983; Shatz, 1996; Tusa, Palmer, & Rosenquist, 1978], the auditory cortex [Merzenich, Knight, & Roth, 1975], and the olfactory bulb [Axel, 1995; Cheng, Bai, Steuer, & Belluscio, 2013; Mombaerts, 2006]. Not only do they provide a way of organizing information flow through different stages in the brain, they also provide useful platforms to study circuit formation and refinement. In the visual system, eye‐specific segregation has been extensively investigated and landmark studies using individual axon labelling have clearly demonstrated elimination of both axon arbours and synapses that are formed inappropriately during the process [Campbell & Shatz, 1992; Sretavan & Shatz, 1984, 1987].
Developmental axon pruning is thought to occur through one of the following cellular mechanisms: branch degeneration, branch retraction, or axosome shedding [Low & Cheng, 2005], although the exact mechanism responsible for elimination of incorrect retinal axon branches during eye‐specific segregation is not yet clearly understood. In contrast, many studies have shed light on the mechanisms of synaptic pruning during this developmental process. It has been established that spontaneous and coordinated spiking of retinal ganglion cells (retinal waves) mediates the elimination of synapses and segregation of retinal afferents from the two eyes [Guido, 2008; Huberman et al., 2008; Shatz, 1996]. Intriguingly, more recent studies have shown that immune signalling is involved in the synaptic pruning during eye‐specific segregation at the dLGN [Elmer & McAllister, 2012; Shatz, 2009; Wu, Dissing‐Olesen, MacVicar, & Stevens, 2015]. As an exaggerated inflammatory profile has been observed in the BTBR animals [Heo, Zhang, Gao, Miller, & Lawrence, 2011; Onore et al., 2013], it would be of interest to test whether altered immune signalling in the BTBR animals underlies the impairment reported here.
Contribution of Low‐Level Sensory Processing Mechanism to Altered Perception and Behaviour in ASD
Disturbance in sensory function is prevalent in ASD, reported in up to 87% of the patients [Baum et al., 2015]. Interestingly, it is often significantly correlated with the severity of other core domains of ASD such as social communication [Foss‐Feig, Heacock, & Cascio, 2012; Kern et al., 2007]. Recently, a concept has been advanced that “lower‐level” sensory processing may be an integral part of the “higher‐order” dysfunction observed in individuals with ASD [Baum et al., 2015]. This hypothesis is naturally plausible because sensory input is the basis of higher‐order social and cognitive functions. However, large‐scale integrative mechanisms such as those related to attention are equally possible to play an important role in the symptoms observed in ASD [Baum et al., 2015; Buschman & Kastner, 2015]. Thus, teasing apart the contributions of “lower” or primary vs. “higher” or integrative mechanisms would help us to better understand the neurobiological underpinnings of ASD and develop potential therapies.
In the visual system, it has been repeatedly observed that individuals with ASD perform better than typically developing children in tasks requiring information processing on local or detailed information [Baum et al., 2015]. However, there has been much debate about “primary” or “integrative” distinction discussed above. A recent study measured contrast sensitivity across a range of spatial frequencies in a large and defined group of autistic and normally developing participants, and found an increase in sensitivity at higher frequencies in the ASD group [Keita, Guy, Berthiaume, Mottron, & Bertone, 2014]. These data support the notion that early‐stage processing contributes to detail‐oriented perception in ASD, which may be further involved in higher‐level social or cognitive functions.
Studies in animal models of ASD have also shown structural alterations in early‐stage sensory areas. In addition to reduced grey matter volume of lateral thalamus mentioned above, a neuroimaging study also found statistically significant reductions in basal cerebral blood volume in brain regions including the thalamus and somatosensory cortex of BTBR mice, indicating decrease of resting‐state brain activity in these areas [Dodero et al., 2013]. A recent study using brain imaging together with histological analysis found that both the primary visual and somatosensory cortical areas were shifted medially in BTBR mice compared to B6 controls from P7 to P22, and cortical thickness of primary visual cortex was increased in the BTBR mice [Fenlon et al., 2015]. Another study used resting‐state BOLD functional magnetic resonance imaging to map the connectivity among brain regions, and found that the connectional profile of primary visual cortex had aberrant involvement of large ipsilateral and contralateral cortical areas such as the somatosensory, insular and orbitofrontal cortices, and showed an overall larger rostro‐caudal extension in the BTBR compared with B6 mice [Sforazzini et al., 2016]. In a mouse model of tuberous sclerosis complex, which has high co‐occurrence of ASD, defects in ipsilateral retino‐geniculate projections were observed, with normal contralateral projections and segregation between the two [Nie et al., 2010]. In the present study, we observed impairment in eye‐specific segregation at dLGN. Although its importance for visual function in mice remains unclear, our findings are consistent with the notion that early‐stage processing could contribute to altered visual perception in ASD.
Impaired Connectivity as a Unifying Mechanism of Autism
The core symptom domains that define ASD behaviourally, together with the vast heterogeneity of the clinical symptoms, suggest that these deficits likely involve widely distributed neural systems and networks. Accordingly, it has been proposed that ASD may represent a disorder of disrupted connectivity [Belger, Carpenter, Yucel, Cleary, & Donkers, 2011; DiCicco‐Bloom et al., 2006; Lai et al., 2014; Maximo et al., 2014; Stigler, McDonald, Anand, Saykin, & McDougle, 2011]. Functional neuroimaging studies have shown widespread deficits in neural networks associated with specific domains of information processing, but they have also indicated that not all brain systems are equally affected. Both over‐connectivity and under‐connectivity have been reported in ASD patients, and it has been hypothesised that increased short‐distance or local connection and decreased long‐distance connection may be cardinal features of the disease and underlie the behavioural impairments [Courchesne & Pierce, 2005]. Overall, several mechanisms have been proposed for the disrupted connectivity in ASD, including synaptic malfunction [Betancur et al., 2009; Bourgeron, 2009; Buxbaum et al., 2012; Hutsler & Zhang, 2010; Peca & Feng, 2012; Phillips & Pozzo‐Miller, 2015; Tang et al., 2014; Zoghbi & Bear, 2012], abnormal enlargement of the brain volume during early development [Courchesne et al., 2007; DiCicco‐Bloom et al., 2006], as well as abnormal white matter growth and reduced white matter integrity [DiCicco‐Bloom et al., 2006; Maximo et al., 2014; Travers et al., 2012].
The establishment and maintenance of precise wiring pattern is also important to ensure proper connectivity and function. However, it has been difficult to trace connections over large distances in the human brain and it remains unclear whether this aspect is affected in ASD. Recent advances in high‐definition fibre tracking [Fernandez‐Miranda et al., 2012; Verstynen, Jarbo, Pathak, & Schneider, 2011] may soon help advance our knowledge in this area. Alternatively, wiring pattern can be readily examined in animal models of ASD. Here, we report that precise connections between retinal afferents and relay neuron in the dLGN are disrupted in the BTBR mouse model of ASD, suggesting that impaired circuit formation and refinement may be a feature of this disorder. In the future, extending the analysis to other brain regions would shed light on whether the observed deficit in the wiring pattern is specific to dLGN or applies to other visual centres as well [Morin & Studholme, 2014]. For example, retinotopic map in the superior colliculus also develops through a process of refinement [Huberman et al., 2008]. It would be interesting to test whether it is preserved in the BTBR animals; if not, whether the impairment is also a lack of refinement. Similarly, retinotopic mapping is also present in the patterned projection from the LGN to visual cortex [Huberman et al., 2008], and it would be interesting to investigate its integrity as well. In addition, applying similar approaches to other sensory systems mentioned above would further shed light on the potential relation between precise wiring and animal behaviours in ASD. Finally, investigating eye‐specific segregation in other animal models of autism will provide information about specific synaptic patterning and connectivity in a more general context, and doing so in models with known genetic relation to autism may also greatly help to uncover the underlying mechanism of the impairment.
Utility of the BTBR Mouse Model of ASD
The BTBR inbred strain is considered a robust animal model of autism [Gaugler et al., 2014], mainly because it displays all the core behavioural features that define this condition—specifically, impaired social behaviours and communication, as well as stereotyped and restricted behaviours [Ellegood and Crawley, 2015; McFarlane et al., 2008; Meyza et al., 2013; Moy et al., 2007; Ruskin et al., 2013]. Its consistent behavioural phenotype likely entails an array of molecular and cellular mechanisms that have yet to be fully elucidated. The BTBR model has been increasingly used in ASD‐related studies during the relatively short time since its discovery. These studies have identified alterations in BTBR animals at many levels, including genetic and epigenetic, synaptic, neuroanatomic and functional connectivity, as well as immunological [Ellegood and Crawley, 2015; Llaneza et al., 2010; Meyza et al., 2013; Shpyleva et al., 2014]. While many of them share similarities with the features found in individuals with autism, the relation between these changes and the autism‐like behaviours largely remains to be established. More studies focused on establishing the causal relation between cellular and molecular pathways and the abnormal behaviours may prove fruitful. As mentioned above, the BTBR mouse is an inbred strain, and genetic characterisations thus far have not revealed alterations in high‐confidence risk genes related to ASD [Jones‐Davis et al., 2013; McFarlane et al., 2008]. Thus, the construct validity of the BTBR model has been questioned. A second difficulty with this model is that comparisons are necessarily made with other inbred strains (most commonly the B6 line). Because there are many genetic differences between these strains, it has been challenging to trace the divergence in physiology and behaviour back to molecular and cellular basis [Patterson, 2011]. Therefore, as discussed above, it would be advantageous to test whether observations could be generalised across different models of ASD. With its robust behavioural phenotype, the BTBR model could complement other animal models of autism, and may be more useful in modelling the form of non‐syndromic ASD.
In summary, we showed that eye‐specific segregation was impaired in the BTBR animals compared to B6 controls. Moreover, these abnormalities persisted into adulthood, and aberrant projections of retino‐geniculate afferents were observed in the BTBR mice. Thus, the formation and refinement of synaptic patterning, which normally maintains a precise organisation vital for brain function and behaviour, was disrupted in the BTBR mouse model. Taken together, these results suggest that disrupted connectivity in general may underlie the autistic behaviours observed in this model of ASD. The findings are also consistent with the hypothesis that early‐stage processing could contribute to altered visual perception in ASD.
Authors2 Contributions
NC designed the study and participated in the experiments, participated in the data analysis, and drafted the manuscript. MK participated in the experiments and helped to draft the manuscript. KM participated in the data analysis and helped to draft the manuscript. JMR helped to design the study and revised the manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors declare no competing interests.
Acknowledgments
The authors would like to thank Rose Tobias, Evelyn Ko and Elizabeth Hughes for general help regarding laboratory administration and maintenance. This work was funded by the Alberta Children's Hospital Research Institute at the University of Calgary.
References
- Axel, R. (1995). The molecular logic of smell. Scientific American, 273, 154–159. [DOI] [PubMed] [Google Scholar]
- Baum, S.H. , Stevenson, R.A. , & Wallace, M.T. (2015). Behavioral, perceptual, and neural alterations in sensory and multisensory function in autism spectrum disorder. Progress in Neurobiology, 134, 140–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belger, A. , Carpenter, K.L. , Yucel, G.H. , Cleary, K.M. , & Donkers, F.C. (2011). The neural circuitry of autism. Neurotoxicity Research, 20, 201–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancur, C. , Sakurai, T. , & Buxbaum, J.D. (2009). The emerging role of synaptic cell‐adhesion pathways in the pathogenesis of autism spectrum disorders. Trends in Neurosciences, 32, 402–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeron, T. (2009). A synaptic trek to autism. Current Opinion in Neurobiology, 19, 231–234. [DOI] [PubMed] [Google Scholar]
- Buschman, T.J. , & Kastner, S. (2015). From behavior to neural dynamics: An integrated theory of attention. Neuron, 88, 127–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum, J.D. , Betancur, C. , Bozdagi, O. , Dorr, N.P. , Elder, G.A. , & Hof, P.R. (2012). Optimizing the phenotyping of rodent ASD models: Enrichment analysis of mouse and human neurobiological phenotypes associated with high‐risk autism genes identifies morphological, electrophysiological, neurological, and behavioral features. Molecular Autism, 3, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, G. , & Shatz, C.J. (1992). Synapses formed by identified retinogeniculate axons during the segregation of eye input. Journal of Neuroscience, 12, 1847–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, N. , Bai, L. , Steuer, E. , & Belluscio, L. (2013). Olfactory functions scale with circuit restoration in a rapidly reversible Alzheimer's disease model. Journal of Neuroscience, 33, 12208–12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, N. , Cai, H. , & Belluscio, L. (2011). In vivo olfactory model of APP‐induced neurodegeneration reveals a reversible cell‐autonomous function. Journal of Neuroscience, 31, 13699–13704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne, E. , & Pierce, K. (2005). Why the frontal cortex in autism might be talking only to itself: Local over‐connectivity but long‐distance disconnection. Current Opinion in Neurobiology, 15, 225–230. [DOI] [PubMed] [Google Scholar]
- Courchesne, E. , Pierce, K. , Schumann, C.M. , Redcay, E. , Buckwalter, J.A. , Kennedy, D.P. , & Morgan, J. (2007). Mapping early brain development in autism. Neuron, 56, 399–413. [DOI] [PubMed] [Google Scholar]
- Demas, J. , Sagdullaev, B.T. , Green, E. , Jaubert‐Miazza, L. , McCall, M.A. , Gregg, R.G. , … Guido, W. (2006). Failure to maintain eye‐specific segregation in nob, a mutant with abnormally patterned retinal activity. Neuron, 50, 247–259. [DOI] [PubMed] [Google Scholar]
- DiCicco‐Bloom, E. , Lord, C. , Zwaigenbaum, L. , Courchesne, E. , Dager, S.R. , Schmitz, C. , … Young, L.J. (2006). The developmental neurobiology of autism spectrum disorder. Journal of Neuroscience, 26, 6897–6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodero, L. , Damiano, M. , Galbusera, A. , Bifone, A. , Tsaftsaris, S.A. , Scattoni, M.L. , & Gozzi, A. (2013). Neuroimaging evidence of major morpho‐anatomical and functional abnormalities in the BTBR T+TF/J mouse model of autism. PLoS One, 8, e76655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellegood, J. , Anagnostou, E. , Babineau, B.A. , Crawley, J.N. , Lin, L. , Genestine, M. , … Lerch, J.P. (2015). Clustering autism: Using neuroanatomical differences in 26 mouse models to gain insight into the heterogeneity. Molecular Psychiatry, 20, 118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellegood, J. , Babineau, B.A. , Henkelman, R.M. , Lerch, J.P. , & Crawley, J.N. (2013). Neuroanatomical analysis of the BTBR mouse model of autism using magnetic resonance imaging and diffusion tensor imaging. Neuroimage, 70, 288–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellegood, J. , & Crawley, J.N. (2015). Behavioral and neuroanatomical phenotypes in mouse models of autism. Neurotherapeutics, 12, 521–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer, B.M. , & McAllister, A.K. (2012). Major histocompatibility complex class I proteins in brain development and plasticity. Trends in Neurosciences, 35, 660–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenlon, L.R. , Liu, S. , Gobius, I. , Kurniawan, N.D. , Murphy, S. , Moldrich, R.X. , & Richards, L.J. (2015). Formation of functional areas in the cerebral cortex is disrupted in a mouse model of autism spectrum disorder. Neural Development, 10, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez‐Miranda, J.C. , Pathak, S. , Engh, J. , Jarbo, K. , Verstynen, T. , Yeh, F.C. , … Friedlander, R. (2012). High‐definition fiber tractography of the human brain: Neuroanatomical validation and neurosurgical applications. Neurosurgery, 71, 430–453. [DOI] [PubMed] [Google Scholar]
- Foss‐Feig, J.H. , Heacock, J.L. , & Cascio, C.J. (2012). Tactile responsiveness patterns and their association with core features in autism spectrum disorders. Research in Autism Spectrum Disorders, 6, 337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier, T.W. , & Hardan, A.Y. (2009). A meta‐analysis of the corpus callosum in autism. Biological Psychiatry, 66, 935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaugler, T. , Klei, L. , Sanders, S.J. , Bodea, C.A. , Goldberg, A.P. , Lee, A.B. , … Buxbaum, J.D. (2014). Most genetic risk for autism resides with common variation. Nature Genetics, 46, 881–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind, D.H. (2009). Advances in autism. Annual Review of Medicine, 60, 367–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guido, W. (2008). Refinement of the retinogeniculate pathway. Journal of Physiology, 586, 4357–4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo, Y. , Zhang, Y. , Gao, D. , Miller, V.M. , & Lawrence, D.A. (2011). Aberrant immune responses in a mouse with behavioral disorders. PLoS One, 6, e20912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel, D.H. , & Wiesel, T.N. (1977). Ferrier lecture. Functional architecture of macaque monkey visual cortex. Proceedings of the Royal Society of London Series B, Biological Sciences, 198, 1–59. [DOI] [PubMed] [Google Scholar]
- Huberman, A.D. , Feller, M.B. , & Chapman, B. (2008). Mechanisms underlying development of visual maps and receptive fields. Annual Review of Neuroscience, 31, 479–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman, A.D. , Stellwagen, D. , & Chapman, B. (2002). Decoupling eye‐specific segregation from lamination in the lateral geniculate nucleus. Journal of Neuroscience, 22, 9419–9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutsler, J.J. , & Zhang, H. (2010). Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Research, 1309, 83–94. [DOI] [PubMed] [Google Scholar]
- Jaubert‐Miazza, L. , Green, E. , Lo, F.S. , Bui, K. , Mills, J. , & Guido, W. (2005). Structural and functional composition of the developing retinogeniculate pathway in the mouse. Visual Neuroscience, 22, 661–676. [DOI] [PubMed] [Google Scholar]
- Jones‐Davis, D.M. , Yang, M. , Rider, E. , Osbun, N.C. , da Gente, G.J. , Li, J. , … Sherr, E.H. (2013). Quantitative trait loci for interhemispheric commissure development and social behaviors in the BTBR T(+) tf/J mouse model of autism. PLoS One, 8, e61829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keita, L. , Guy, J. , Berthiaume, C. , Mottron, L. , & Bertone, A. (2014). An early origin for detailed perception in autism spectrum disorder: Biased sensitivity for high‐spatial frequency information. Scientific Reports, 4, 5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern, J.K. , Trivedi, M.H. , Grannemann, B.D. , Garver, C.R. , Johnson, D.G. , Andrews, A.A. , … Schroeder, J.L. (2007). Sensory correlations in autism. Autism, 11, 123–134. [DOI] [PubMed] [Google Scholar]
- Lai, M.C. , Lombardo, M.V. , & Baron‐Cohen, S. (2014). Autism. Lancet, 383, 896–910. [DOI] [PubMed] [Google Scholar]
- Leyva‐Diaz, E. , & Lopez‐Bendito, G. (2013). In and out from the cortex: Development of major forebrain connections. Neuroscience, 254, 26–44. [DOI] [PubMed] [Google Scholar]
- Llaneza, D.C. , DeLuke, S.V. , Batista, M. , Crawley, J.N. , Christodulu, K.V. , & Frye, C.A. (2010). Communication, interventions, and scientific advances in autism: A commentary. Physiology & Behavior, 100, 268–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low, L.K. , & Cheng, H.J. (2005). A little nip and tuck: Axon refinement during development and axonal injury. Current Opinion in Neurobiology, 15, 549–556. [DOI] [PubMed] [Google Scholar]
- Maximo, J.O. , Cadena, E.J. , & Kana, R.K. (2014). The implications of brain connectivity in the neuropsychology of autism. Neuropsychology Review, 24, 16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane, H.G. , Kusek, G.K. , Yang, M. , Phoenix, J.L. , Bolivar, V.J. , & Crawley, J.N. (2008). Autism‐like behavioral phenotypes in BTBR T+tf/J mice. Genes, Brain and Behavior, 7, 152–163. [DOI] [PubMed] [Google Scholar]
- Mercier, F. , Kwon, Y.C. , & Douet, V. (2012). Hippocampus/amygdala alterations, loss of heparan sulfates, fractones and ventricle wall reduction in adult BTBR T+ tf/J mice, animal model for autism. Neuroscience Letters, 506, 208–213. [DOI] [PubMed] [Google Scholar]
- Merzenich, M.M. , Kaas, J.H. , Sur, M. , & Lin, C.S. (1978). Double representation of the body surface within cytoarchitectonic areas 3b and 1 in “SI” in the owl monkey (Aotus trivirgatus). Journal of Comparative Neurology, 181, 41–73. [DOI] [PubMed] [Google Scholar]
- Merzenich, M.M. , Knight, P.L. , & Roth, G.L. (1975). Representation of cochlea within primary auditory cortex in the cat. Journal of Neurophysiology, 38, 231–249. [DOI] [PubMed] [Google Scholar]
- Meyza, K.Z. , Defensor, E.B. , Jensen, A.L. , Corley, M.J. , Pearson, B.L. , Pobbe, R.L. , … Blanchard, R.J. (2013). The BTBR T+ tf/J mouse model for autism spectrum disorders‐in search of biomarkers. Behavioural Brain Research, 251, 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts, P. (2006). Axonal wiring in the mouse olfactory system. Annual Review of Cell and Developmental Biology, 22, 713–737. [DOI] [PubMed] [Google Scholar]
- Morin, L.P. , & Studholme, K.M. (2014). Retinofugal projections in the mouse. Journal of Comparative Neurology, 522, 3733–3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy, S.S. , Nadler, J.J. , Young, N.B. , Perez, A. , Holloway, L.P. , Barbaro, R.P. , … Crawley, J.N. (2007). Mouse behavioral tasks relevant to autism: Phenotypes of 10 inbred strains. Behavioural Brain Research, 176, 4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir‐Robinson, G. , Hwang, B.J. , & Feller, M.B. (2002). Retinogeniculate axons undergo eye‐specific segregation in the absence of eye‐specific layers. Journal of Neuroscience, 22, 5259–5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie, D. , Di Nardo, A. , Han, J.M. , Baharanyi, H. , Kramvis, I. , Huynh, T. , … Sahin, M. (2010). Tsc2‐Rheb signaling regulates EphA‐mediated axon guidance. Nature Neuroscience, 13, 163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onore, C.E. , Careaga, M. , Babineau, B.A. , Schwartzer, J.J. , Berman, R.F. , & Ashwood, P. (2013). Inflammatory macrophage phenotype in BTBR T+tf/J mice. Frontiers in Neuroscience, 7, 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson, P.H. (2011). Modeling autistic features in animals. Pediatric Research, 69, 34R–40R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul, L.K. , Corsello, C. , Kennedy, D.P. , & Adolphs, R. (2014). Agenesis of the corpus callosum and autism: A comprehensive comparison. Brain, 137, 1813–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peca, J. , & Feng, G. (2012). Cellular and synaptic network defects in autism. Current Opinion in Neurobiology, 22, 866–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, M. , & Pozzo‐Miller, L. (2015). Dendritic spine dysgenesis in autism related disorders. Neuroscience Letters, 601, 30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raczkowski, D. , & Rosenquist, A.C. (1983). Connections of the multiple visual cortical areas with the lateral posterior‐pulvinar complex and adjacent thalamic nuclei in the cat. Journal of Neuroscience, 3, 1912–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruskin, D.N. , Svedova, J. , Cote, J.L. , Sandau, U. , Rho, J.M. , Kawamura, M. Jr. , … Masino, S.A. (2013). Ketogenic diet improves core symptoms of autism in BTBR mice. PLoS One, 8, e65021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sforazzini, F. , Bertero, A. , Dodero, L. , David, G. , Galbusera, A. , Scattoni, M.L. , … Gozzi, A. (2016). Altered functional connectivity networks in acallosal and socially impaired BTBR mice. Brain Structure and Function, 221, 941–954. [DOI] [PubMed] [Google Scholar]
- Shatz, C.J. (1996). Emergence of order in visual system development. Proceedings of the National Academy of Sciences of the United States of America, 93, 602–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz, C.J. (2009). MHC class I: An unexpected role in neuronal plasticity. Neuron, 64, 40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpyleva, S. , Ivanovsky, S. , de Conti, A. , Melnyk, S. , Tryndyak, V. , Beland, F.A. , … Pogribny, I.P. (2014). Cerebellar oxidative DNA damage and altered DNA methylation in the BTBR T+tf/J mouse model of autism and similarities with human post mortem cerebellum. PLoS One, 9, e113712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, J.D. , Rho, J.M. , Masino, S.A. , & Mychasiuk, R. (2014). Inchworming: A novel motor stereotypy in the BTBR T+ Itpr3tf/J mouse model of autism. Journal of Visualized Experiments. 2014 july 5;(89). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squillace, M. , Dodero, L. , Federici, M. , Migliarini, S. , Errico, F. , Napolitano, F. , … Gozzi, A. (2014). Dysfunctional dopaminergic neurotransmission in asocial BTBR mice. Translational Psychiatry, 4, e427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sretavan, D. , & Shatz, C.J. (1984). Prenatal development of individual retinogeniculate axons during the period of segregation. Nature, 308, 845–848. [DOI] [PubMed] [Google Scholar]
- Sretavan, D.W. , & Shatz, C.J. (1987). Axon trajectories and pattern of terminal arborization during the prenatal development of the cat's retinogeniculate pathway. Journal of Comparative Neurology, 255, 386–400. [DOI] [PubMed] [Google Scholar]
- Stellwagen, D. , & Shatz, C.J. (2002). An instructive role for retinal waves in the development of retinogeniculate connectivity. Neuron, 33, 357–367. [DOI] [PubMed] [Google Scholar]
- Stevens, B. , Allen, N.J. , Vazquez, L.E. , Howell, G.R. , Christopherson, K.S. , Nouri, N. , … Barres, B.A. (2007). The classical complement cascade mediates CNS synapse elimination. Cell, 131, 1164–1178. [DOI] [PubMed] [Google Scholar]
- Stigler, K.A. , McDonald, B.C. , Anand, A. , Saykin, A.J. , & McDougle, C.J. (2011). Structural and functional magnetic resonance imaging of autism spectrum disorders. Brain Research, 1380, 146–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, G. , Gudsnuk, K. , Kuo, S.H. , Cotrina, M.L. , Rosoklija, G. , Sosunov, A. , … Sulzer, D. (2014). Loss of mTOR‐dependent macroautophagy causes autistic‐like synaptic pruning deficits. Neuron, 83, 1131–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torborg, C.L. , Hansen, K.A. , & Feller, M.B. (2005). High frequency, synchronized bursting drives eye‐specific segregation of retinogeniculate projections. Nature Neuroscience, 8, 72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers, B.G. , Adluru, N. , Ennis, C. , Tromp do, P.M. , Destiche, D. , Doran, S. , … Alexander, A.L. (2012). Diffusion tensor imaging in autism spectrum disorder: A review. Autism Research, 5, 289–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusa, R.J. , Palmer, L.A. , & Rosenquist, A.C. (1978). The retinotopic organization of area 17 (striate cortex) in the cat. Journal of Comparative Neurology, 177, 213–235. [DOI] [PubMed] [Google Scholar]
- Veenstra‐VanderWeele, J. , & Warren, Z. (2015). Intervention in the context of development: Pathways toward new treatments. Neuropsychopharmacology, 40, 225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstynen, T. , Jarbo, K. , Pathak, S. , & Schneider, W. (2011). In vivo mapping of microstructural somatotopies in the human corticospinal pathways. Journal of Neurophysiology, 105, 336–346. [DOI] [PubMed] [Google Scholar]
- Wolff, J.J. , Gerig, G. , Lewis, J.D. , Soda, T. , Styner, M.A. , Vachet, C. , … Network, I. (2015). Altered corpus callosum morphology associated with autism over the first 2 years of life. Brain, 138, 2046–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey, T.A. , & Van der Loos, H. (1970). The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Research, 17, 205–242. [DOI] [PubMed] [Google Scholar]
- Wu, Y. , Dissing‐Olesen, L. , MacVicar, B.A. , & Stevens, B. (2015). Microglia: Dynamic mediators of synapse development and plasticity. Trends in Immunology, 36, 605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoghbi, H.Y. , & Bear, M.F. (2012). Synaptic dysfunction in neurodevelopmental disorders associated with autism and intellectual disabilities. Cold Spring Harbor Perspectives in Biology, 4, [DOI] [PMC free article] [PubMed] [Google Scholar]


