Abstract
Aims
To test the hypothesis that delivery of integrated care augmented by a web‐based disease management programme and nurse coordinator would improve treatment target attainment and health‐related behaviour.
Methods
The web‐based Joint Asia Diabetes Evaluation (JADE) and Diabetes Monitoring Database (DIAMOND) portals contain identical built‐in protocols to integrate structured assessment, risk stratification, personalized reporting and decision support. The JADE portal contains an additional module to facilitate structured follow‐up visits. Between January 2009 and September 2010, 3586 Chinese patients with Type 2 diabetes from six sites in China were randomized to DIAMOND (n = 1728) or JADE, plus nurse‐coordinated follow‐up visits (n = 1858) with comprehensive assessments at baseline and 12 months. The primary outcome was proportion of patients achieving ≥ 2 treatment targets (HbA1c < 53 mmol/mol (7%), blood pressure < 130/80 mmHg and LDL cholesterol < 2.6 mmol/l).
Results
Of 3586 participants enrolled (mean age 57 years, 54% men, median disease duration 5 years), 2559 returned for repeat assessment after a median (interquartile range) follow‐up of 12.5 (4.6) months. The proportion of participants attaining ≥ 2 treatment targets increased in both groups (JADE 40.6 to 50.0%; DIAMOND 38.2 to 50.8%) and there were similar absolute reductions in HbA1c [DIAMOND −8 mmol/mol vs JADE −7 mmol/mol (−0.69 vs −0.62%)] and LDL cholesterol (DIAMOND −0.32 mmol/l vs JADE −0.28 mmol/l), with no between‐group difference. The JADE group was more likely to self‐monitor blood glucose (50.5 vs 44.2%; P = 0.005) and had fewer defaulters (25.6 vs 32.0%; P < 0.001).
Conclusions
Integrated care augmented by information technology improved cardiometabolic control, with additional nurse contacts reducing the default rate and enhancing self‐care. (Clinical trials registry no.: NCT01274364)
What's new?
The value of quality improvement programmes in the management of chronic conditions has been established in a number of prospective studies and meta‐analyses.
The effect of the Joint Asia Diabetes Evaluation (JADE) programme, an information technology‐augmented integrated care model, on diabetes‐related outcomes has been demonstrated in several studies within developed healthcare systems.
This study represents one of the few quality improvement initiatives undertaken in a developing country and is the first to answer the question of whether initiatives such as JADE are effective in enhancing quality of care in underfunded healthcare systems.
Given the increasing demand for healthcare resources in developing countries, quality improvement has the potential to improve chronic care without substantial additional costs.
What's new?
The value of quality improvement programmes in the management of chronic conditions has been established in a number of prospective studies and meta‐analyses.
The effect of the Joint Asia Diabetes Evaluation (JADE) programme, an information technology‐augmented integrated care model, on diabetes‐related outcomes has been demonstrated in several studies within developed healthcare systems.
This study represents one of the few quality improvement initiatives undertaken in a developing country and is the first to answer the question of whether initiatives such as JADE are effective in enhancing quality of care in underfunded healthcare systems.
Given the increasing demand for healthcare resources in developing countries, quality improvement has the potential to improve chronic care without substantial additional costs.
Introduction
Achieving and maintaining recommended treatment targets decreases the risk of diabetes‐related vascular complications, mortality and associated healthcare costs 1, 2, 3, 4. In the UK Prospective Diabetes Study, an 11‐mmol/mol (1%) reduction in mean HbA1c led to 21% fewer deaths, 14% fewer myocardial infarctions and a 37% decrease in microvascular complications 5. Despite collective accord, there are major treatment gaps attributable to suboptimum self‐care, poor adherence to treatment and clinical inertia, resulting in low rates of treatment target attainment in both developed and developing regions 6, 7.
These barriers are amplified by systemic factors, especially in developing countries. In China, for example, the majority of chronic care occurs in an in‐patient setting with most clinical assessments and treatment covered or reimbursed; however, in a primary or ambulatory care setting, many laboratory tests and chronic medications require co‐payments or are not reimbursed. Electronic medical systems exist but are largely fragmented, resulting in duplication or overlapping of consultations, investigations and medications. Despite an initiative to promote primary care by establishing community‐based clinics, many patients prefer going to hospitals for specialist care, with long waiting times and short contact intervals. Patients typically return every 1–2 months, mainly to collect medications, often without pre‐booking, assessment or education, and with high default rates 8, 9.
One area of focus is the lack of structure for documenting risk factors and complications, as well as the arbitrary nature of risk stratification and patient follow‐up. In the USA, the Institute of Medicine recommended the following strategies to improve chronic care: redesign care processes based on best practices, use information technology to manage clinical data with decision support, transfer knowledge and skills to team members to coordinate care and use performance and outcome measures for quality control 10. In a meta‐analysis of strategies for quality improvement, promotion of self‐care and team change were associated with a 4‐mmol/mol (0.37%) mean reduction in HbA1c, along with improvements in LDL cholesterol and blood pressure (BP) 11.
Since 1995, the Chinese University of Hong Kong (CUHK) Diabetes Care and Research Team has re‐designed workflows and trained nurses to assess patients and deliver protocol‐based care. By changing workflows and through task delegation, we were able to improve medication adherence, with attainment of multiple targets and reduced risks of cardiovascular‐renal complications 12, 13. Based on these prototypes, we designed the web‐based Joint Asia Diabetes Evaluation (JADE) portal, consisting of two modules. The comprehensive assessment (CA) module comprises templates for periodic assessment, risk stratification, personalized reporting and automated decision support. The follow‐up module includes templates for documentation of modifiable risk factors, hypoglycaemia and key events to track clinical progress and reinforce adherence 14. A separate portal with a CA module identical to that of JADE, was also created to help doctors establish a diabetes monitoring database (DIAMOND) as a first step towards a comprehensive quality improvement programme. In this demonstration project, we examined the effectiveness of delivering integrated care in China with or without nurse‐coordinated follow‐up visits using the JADE and DIAMOND portals, respectively, on cardiometabolic control and health behaviours, including default rates.
Patients and methods
The study assessed a 1‐year multicentre randomized non‐blinded quality improvement programme. Between January 2009 and September 2010, patients with Type 2 diabetes aged ≥ 18 years were recruited from six tertiary hospitals in China. Exclusion criteria included Type 1 diabetes, life‐threatening conditions, reduced life expectancy or inability to understand the scope of the study. The study was approved by the New Territories East Cluster Clinical Research Ethics Committee and local institutional ethics boards at each participating site. All participants provided written informed consent.
A total of 3586 eligible patients were randomized in a 1:1 ratio to DIAMOND (CA only) or JADE (CA plus nurse‐coordinated structured follow‐up). At each centre, randomization was performed using computer‐generated codes kept in sealed, opaque envelopes, numbered 1 to 600 prefixed by the study site. Personnel at the site not participating in the study opened the envelope and informed consenting participants of their group assignment.
Each centre was given a grant to support an additional CUHK project team‐trained nurse to perform the CA, guided by the JADE/DIAMOND portals and supervised by a physician. Both doctors and nurses received training on the use of the portals to perform a structured patient evaluation, document care processes and communicate results of clinical and laboratory assessments to participants. For participants randomized to JADE, the additional nurse was trained to use the follow‐up module to manage follow‐up appointments and facilitate ongoing patient support between clinic visits, while the DIAMOND group received usual care. Details of the JADE programme have been published and are included in Appendix S1 15. The CA report consists of 5‐year probabilities for coronary heart disease, heart failure, stroke and end‐stage renal disease, estimated using validated risk equations 16, 17, 18, 19. Patients were classified into one of four risk categories based on risk scores derived from risk equations, presence or absence of cardiovascular‐renal disease, and an ensemble of metabolic risk factors. Each risk category corresponded to a recommended care level that determined frequency of structured follow‐up visits and intensity of care 14.
Automated reports provided to patients and physicians contained trend lines of attained versus recommended metabolic targets, 5‐year probabilities of major events, and risk categories. The physician report included triggered reminders on treatment intensification, while the patient report included practice tips to promote lifestyle changes, treatment adherence and self‐monitoring, in the local language. Both participants and referring doctors in the DIAMOND group received their respective reports followed by usual care. Participants assigned to JADE were recommended to receive 2–4 h of diabetes education and at least two additional contacts by the nurse coordinator (telephone or face‐to‐face visits). Facilitated by the follow‐up module, nurses were asked to reinforce treatment and lifestyle adherence, encourage self‐monitoring of blood glucose (SMBG) and remind them about structured follow‐up appointments that included documentation of laboratory measurements, body weight, blood pressure, hypoglycaemia, self‐care and other major events in the JADE portal. The nurse coordinator issued a follow‐up report, discussed cardiometabolic control and clarified concerns regarding therapy. Participants in the lower risk categories (l or 2, with few risk factors/complications) were recommended to have structured follow‐up visits every 4–6 months and those in higher risk categories (3 or 4, with multiple risk factors and/or complications) every 2–3 months. At the end of 12 months, all participants underwent repeat CA, and non‐returnees were contacted by telephone to ascertain health status.
The primary outcome was the proportion of participants attaining ≥ 2 treatment targets (HbA1c < 53 mmol/mol (7%), BP < 130/80 mmHg, LDL cholesterol < 2.6 mmol/l) after 12 months. Other outcomes included default rates, change in quality‐of‐life measures, frequency of hypoglycaemia, adherence to lifestyle modification/self‐care activities, and new onset of physician‐documented diabetes‐related endpoints. Events were recorded using standard forms, with predefined diagnosis accompanied by a narrative, albeit not adjudicated. Power calculation and statistical analyses applied are available in Appendix S2.
Results
Between January 2009 and September 2010, 3586 eligible participants, representing ~65% of all subjects considered [mean age 56.5 ± 11.6 years, 54.4% men, median (interquartile range) disease duration of 5 (1–10) years, mean HbA1c 62 ± 22 mmol/mol (7.85 ± 2.02%)] were recruited from patients already receiving treatment at the clinics. The primary reasons for declining were testing costs and recurring travel to study site. A total of 1728 participants were assigned to DIAMOND and 1858 to JADE, with both groups having similar characteristics at baseline (Table 1).
Table 1.
Baseline characteristics of all randomized Chinese patients with type 2 diabetes
| n | DIAMOND | JADE | P | ||
|---|---|---|---|---|---|
| Number randomized | 1728 | 1858 | |||
| Demographics | |||||
| Mean ± sd age, years | 1728 | 56.8 ± 11.7 | 1858 | 56.1 ± 11.6 | 0.096 |
| Gender: men, n (%) | 1728 | 941 (54.5) | 1858 | 1011 (54.4) | 0.98 |
| Median (IQR) disease duration, years | 1705 | 5.0 (1.0, 10.0) | 1809 | 5.0 (1.0, 10.0) | 0.725 |
| Education, n (%) | 1724 | 1849 | 0.001 | ||
| < 6 years | 168 (9.7) | 132 (7.1) | |||
| 6–11 years | 415 (24.0) | 424 (22.8) | |||
| > 11 years | 1140 (66.0) | 1291 (69.5) | |||
| Unemployed, n (%) | 1724 | 1103 (64.0) | 1850 | 1153 (62.3) | 0.306 |
| Tobacco use, n (%) | 1712 | 1827 | 0.998 | ||
| Never | 1123 (65.6) | 1202 (65.8) | |||
| Former | 206 (12.0) | 208 (11.4) | |||
| Current | 383 (22.4) | 417 (22.8) | |||
| Alcohol use, n (%) | 1718 | 1830 | 0.773 | ||
| Never | 1187 (69.1) | 1269 (69.3) | |||
| Physical activity ≥ 3 times/week, n (%) | 1717 | 867 (50.5) | 1849 | 991 (53.6) | 0.064 |
| SMBG ≥ weekly, n (%) | 1576 | 677 (43.0) | 1713 | 780 (45.5) | 0.137 |
| Adherence to balanced diet, n (%) | 1719 | 1091 (63.5) | 1852 | 1205 (65.1) | 0.156 |
| Median (IQR) follow‐up, months | 1728 | 12.5 (5.28) | 1858 | 12.5 (3.98) | 0.449 |
| Complications and comorbidities, n (%) | |||||
| Retinopathy | 1681 | 228 (13.6) | 1808 | 241 (13.3) | 0.84 |
| Sensory neuropathy | 1722 | 143 (8.3) | 1852 | 113 (6.1) | 0.011 |
| Chronic kidney disease, n (%) | 1646 | 37 (2.2) | 1789 | 37 (2.1) | 0.717 |
| All heart events including heart failure, n (%) | 1726 | 164 (9.5) | 1845 | 155 (8.4) | 0.221 |
| Stroke, n (%) | 1720 | 54 (3.1) | 1850 | 42 (2.3) | 0.109 |
| Peripheral vascular disease, n (%) | 1556 | 164 (10.5) | 1688 | 122 (7.3) | 0.001 |
| Risk categories, n (%) | 0.483 | ||||
| Low: 1/2 | 1727 | 220 (12.7) | 1855 | 251 (13.5) | |
| High: 3/4 | 1727 | 1507 (87.3) | 1855 | 1604 (86.5) | |
| Treatments, n (%) | |||||
| Lifestyle modification only | 1728 | 385 (22.3) | 1858 | 388 (20.9) | 0.309 |
| On oral antidiabetic drug | 1728 | 1214 (70.3) | 1858 | 1321 (71.1) | 0.579 |
| Insulin | 1728 | 452 (26.2) | 1858 | 512 (27.6) | 0.345 |
| Any BP drugs | 1728 | 390 (22.6) | 1858 | 420 (22.6) | 0.98 |
| Angiotensin‐converting enzyme inhibitors | 1728 | 33 (1.9) | 1858 | 34 (1.8) | 0.86 |
| Angiotensin II receptor type 1 receptor blocker | 1728 | 207 (12.0) | 1858 | 220 (11.8) | 0.898 |
| Statins | 1728 | 544 (31.5) | 1858 | 597 (32.1) | 0.676 |
| Risk factor control | |||||
| Mean ± sd body weight, kg | 1715 | 69.52 ± 12.67 | 1845 | 69.57 ± 12.62 | 0.911 |
| Mean ± sd waist circumference, cm | |||||
| Women | 682 | 87.15 ± 9.97 | 713 | 85.96 ± 10.19 | 0.028 |
| Men | 800 | 91.46 ± 9.68 | 815 | 91.81 ± 9.48 | 0.47 |
| Mean ± sd BMI (kg/m2) | 1715 | 25.32 ± 3.62 | 1845 | 25.18 ± 3.58 | 0.246 |
| Mean ± sd diastolic BP (mmHg) | 1694 | 78.4 ± 10.4 | 1824 | 78.8 ± 11.0 | 0.243 |
| Mean ± sd systolic BP (mmHg) | 1694 | 125.8 ± 15.8 | 1824 | 125.0 ± 15.7 | 0.131 |
| Mean ± sd total cholesterol, mmol/l | 1653 | 4.95 ± 1.31 | 1785 | 4.91 ± 1.16 | 0.47 |
| Mean ± sd HDL cholesterol, mmol/l | |||||
| Women | 751 | 1.26 ± 0.31 | 799 | 1.29 ± 0.34 | 0.06 |
| Men | 891 | 1.1 ± 0.28 | 960 | 1.12 ± 0.27 | 0.108 |
| Mean ± sd LDL cholesterol, mmol/l | 1644 | 2.94 ± 0.89 | 1768 | 2.92 ± 0.88 | 0.446 |
| Mean ± sd haemoglobin, g/dl | 1446 | 14.49 ± 7.44 | 2545 | 14.31 ± 5.77 | 0.443 |
| Mean ± sd HbA1c, % (mmol/mol) | 1648 | 7.91 (53) ± 2.08 (15) | 1788 | 7.78 (59) ± 1.95 (15) | 0.057 |
| Median (IQR) triglyceride, mmol/l | 1654 | 1.54 (1.14) | 1788 | 1.48 (1.22) | 0.005 |
| Median (IQR) urine albumin to creatinine ratio, mg/mmol | 1506 | 1.20 (2.75) | 1606 | 1.25 (2.85) | 0.059 |
| Mean ± sd estimated GFR, ml/min/1.73 m2 | 1646 | 122.2 ± 38.74 | 1789 | 122.63 ± 41.88 | 0.754 |
| Obesity, n (%) | 1539 | 1081 (70.2) | 1607 | 1114 (69.3) | 0.575 |
| Dyslipidaemia, n (%) | 1669 | 1551 (92.9) | 1810 | 1668 (92.2) | 0.385 |
| Hypertension, n (%) | 1707 | 1275 (74.7) | 1835 | 1392 (75.9) | 0.421 |
| Macroalbuminuria, n (%) | 1506 | 75 (5.0) | 1606 | 89 (5.5) | 0.64 |
| Microalbuminuria, n (%) | 1506 | 330 (21.9) | 1606 | 385 (24.0) | 0.322 |
| Frequency of hypoglycaemic episodes ≥ once/month, n (%) | 1713 | 178 (10.4) | 1847 | 185 (10.0) | 0.712 |
| Attainment of treatment targets, n (%) | |||||
| HbA1c < 7.0% (53 mmol/mol) | 1648 | 692 (42.0) | 1788 | 811 (45.4) | 0.047 |
| BP < 130/80 (mmHg) | 1694 | 662 (39.1) | 1824 | 710 (38.9) | 0.926 |
| LDL cholesterol < 2.6 (mmol/l) | 1644 | 600 (36.5) | 1768 | 644 (36.4) | 0.966 |
| At least one target | 1595 | 1211 (75.9) | 1716 | 1300 (75.8) | 0.911 |
| At least two targets | 1595 | 555 (34.8) | 1716 | 624 (36.4) | 0.347 |
| All three targets | 1595 | 105 (6.6) | 1716 | 136 (7.9) | 0.137 |
| Quality of life | |||||
| Mean ± sd EQ‐VAS | 1715 | 83.01 ± 12.10 | 1830 | 82.83 ± 12.35 | 0.721 |
| Mean ± sd EQ‐5D index | 1708 | 0.91 ± 0.13 | 1817 | 0.91 ± 0.14 | 0.995 |
BP, blood pressure; DIAMOND, DIAbetes MONitoring Database; IQR, interquartile range; JADE, Joint Asia Diabetes Evaluation; SMBG, self‐monitoring of blood glucose; VAS, visual analogue scale.
Between March 2011 and December 2013, after a median (interquartile range) follow‐up of 12.5 (4.6) months, 2559 out of 3586 randomized participants (71.4%) returned for the second CA (CA2), with documentation of clinical and biochemical data and event rates. A total of 1027 participants (28.6%) did not return for CA2, but 636 of those were contacted by telephone for ascertainment of health status, while 371 (10.3%) were lost to follow‐up with no vitality ascertainment. In all, 24 participants had a cardiovascular event and 12 died. A final total of 2559 participants were included in the per‐protocol analysis (Fig. 1).
Figure 1.
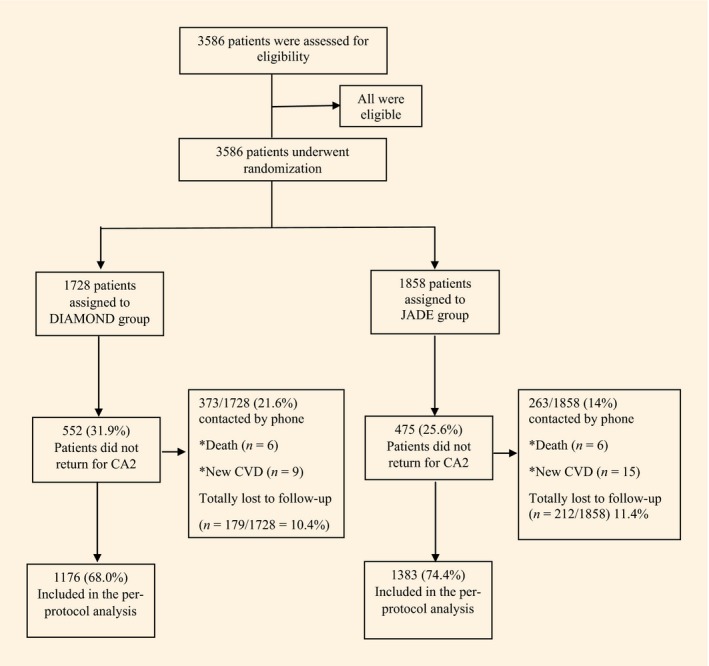
Randomization and disposition of patients included in the intend to treat and per protocol analyses.
In the JADE group, 191 participants were in risk category 1–2, with the remaining 1192 in risk category 3–4. The mean frequencies of nurse‐coordinated follow‐up visits in risk categories 1–4 were 1.2, 1.8, 1.9 and 2.4 times per year, respectively. Follow‐up frequency for patients in DIAMOND was not captured because the DIAMOND portal was not designed to capture follow‐up frequency.
Compared with baseline, the proportion of participants attaining ≥ 2 treatment targets (DIAMOND 38.2 to 50.8%, P < 0.01; JADE 40.6 to 50.0%, P < 0.01); HbA1c < 53 mmol/mol (7%) (DIAMOND 45.8 to 61.1%, P < 0.01, JADE 49.0 to 58.5%, P < 0.01) and LDL cholesterol < 2.6 mmol/l (DIAMOND 36.4 to 52.9%, P < 0.01; JADE 39.5 to 53.4%, P < 0.01) increased similarly, with no between‐group difference (Fig. 2). The absolute change in HbA1c [DIAMOND −8 mmol/mol, JADE −7 mmol/mol (DIAMOND −0.69%, JADE −0.62%); P = 0.473] and LDL cholesterol (DIAMOND −0.32 mmol/l, JADE −0.28 mmol/l; P = 0.286) was similar in each group (Table 2). The proportion of participants with BP < 130 mmHg fell in the DIAMOND group but remained unchanged in the JADE group (DIAMOND 40.6 to 34.6%, P < 0.01; JADE 39.2 to 37.4%, P = 0.239) with no between‐group difference (Fig. 2). More patients had a reduction in systolic BP of ≥ 10 mmHg (DIAMOND 18.4 vs JADE 22.2%; P = 0.052) and in diastolic BP of ≥ 5 mmHg (DIAMOND: 26.6% vs JADE: 33.5%, P = 0.018) in the JADE than the DIAMOND group (Table 2).
Figure 2.
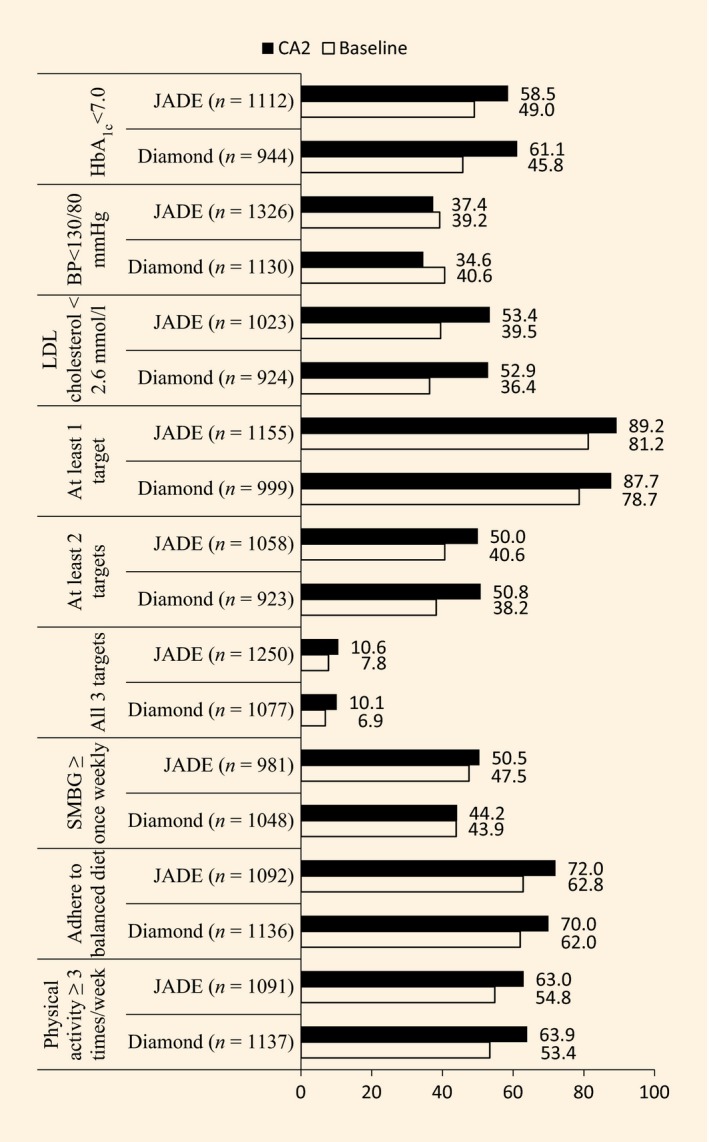
Proportions of patients in the Diabetes Monitoring Database (DIAMOND) and Joint Asia Diabetes Evaluation (JADE) groups attaining treatment targets at repeat assessment after 1 year of follow‐up. Between‐group comparisons adjusted for trial centre, age, gender, disease duration and baseline value. All P‐values for within‐group comparison (CA2 vs baseline) were P < 0.01 except that of JADE on blood pressure (BP) < 130/80 mmHg (P = 0.239). No significant difference for changes in target achievement between groups. Only patients with paired data for baseline and second comprehensive assessment (CA2) are included in analysis. SMBG, self‐monitoring of blood glucose.
Table 2.
Mean changes for HbA1c, blood pressure, lipids and quality of life measures as well as changes in medications and self‐care behaviour in the DIAMOND and JADE groups who underwent comprehensive assessments at baseline and after 12 months
| Month 12: baseline visit | Valid number | DIAMOND (95% CI) | Valid number | JADE (95% CI) | Crude P value | Adjusted P valuea |
|---|---|---|---|---|---|---|
| Metabolic control | ||||||
| Median (IQR) HbA1c, mmol/mol | 944 | −8 (−9, −7) | 1112 | −7 (−8, −6) | 0.372 | 0.473 |
| Median (IQR) HbA1c, % | 944 | −0.69 (−0.81, −0.57) | 1112 | −0.62 (−0.73, −0.50) | 0.372 | 0.473 |
| Median (IQR) SBP, mmHg | 1130 | 2.43 (1.49, 3.37) | 1326 | 1.64 (0.77, 2.50) | 0.221 | 0.091 |
| Median (IQR) DBP, mmHg | 1130 | 0.25 (−0.37, 0.86) | 1326 | −1.03 (−1.65, −0.41) | 0.004 | 0.057 |
| Median (IQR) LDL cholesterol, mmol/l | 924 | −0.32 (−0.38, −0.27) | 1023 | −0.28 (−0.34, −0.23) | 0.335 | 0.286 |
| Median (IQR) body weight, kg | 1148 | −0.13 (−0.48, 0.22) | 1353 | −0.02 (−0.29, 0.25) | 0.612 | 0.482 |
| HbA1c reduction ≥ 0.5%, n (%) | 944 | 400 (42.4) | 1112 | 420 (37.8) | 0.034 | 0.223 |
| Systolic BP reduction ≥ 10 mmHg, n (%) | 1130 | 208 (18.4) | 1326 | 294 (22.2) | 0.021 | 0.052 |
| Diastolic BP reduction ≥ 5 mmHg, n (%) | 1130 | 301 (26.6) | 1326 | 444 (33.5) | < 0.001 | 0.018 |
| LDL cholesterol reduction ≥ 30%, n (%) | 924 | 193 (20.9) | 1023 | 206 (20.1) | 0.682 | 0.511 |
| Body weight reduction ≥ 3%, n (%) | 1148 | 232 (20.2) | 1353 | 259 (19.1) | 0.503 | 0.344 |
| Smoking cessation, n (%) | 205 | 16 (7.8) | 199 | 21 (10.6) | 0.346 | 0.759 |
| Add on medication b, n (%) | ||||||
| Insulin | 50/885 (5.6) | 71/1027 (6.9) | 0.295 | 0.175 | ||
| Oral antidiabetic drug | 67/350 (19.1) | 96/383 (25.1) | 0.199 | 0.041 | ||
| BP‐lowering drugs | 63/894 (7.1) | 55/1072 (5.1) | 0.098 | 0.126 | ||
| Lipid‐regulating drugs | 106/793 (13.4) | 110/899 (12.2) | 0.337 | 0.586 | ||
| Quality of life | ||||||
| Median (IQR) EQ‐5D index | 1039 | 0.028 (0.018, 0.038) | 992 | 0.037 (0.027, 0.047) | 0.192 | 0.146 |
| Median (IQR) EQ‐VAS | 1001 | −0.80 (−1.52, −0.086) | 967 | 0.66 (−0.12, 1.44) | 0.005 | 0.478 |
BP, blood pressure; DIAMOND, DIAbetes MONitoring Database; IQR, interquartile range; JADE, Joint Asia Diabetes Evaluation.
Adjusted for age, gender, disease duration, baseline value and trial centre.
New users/non users at baseline.
Both groups reported improved adherence to self‐care behaviours (P < 0.01 compared with baseline) with more patients in the JADE group performing SMBG at study end (50.5 vs 44.2%, P = 0.005; Fig. 1). In the DIAMOND group, 16 of 205 participants (7.8%) and 21 of 199 (10.6%) in the JADE group stopped smoking, with no between‐group difference (Table 2).
At study end, more participants in the JADE group initiated oral antidiabetic drug treatment (DIAMOND 19.1% vs JADE 25.1%; P = 0.041). New use of antihypertensive/lipid‐lowering drugs was significantly different from baseline but similar in both groups. The proportion of participants reporting hypoglycaemic episodes at least monthly fell similarly in both groups (DIAMOND 11.9 to 6.7%, P < 0.001; JADE 10.8 to 7.3%, P = 0.002). Scores on the EuroQol health status index, the EQ‐5D (visual analogue scale), tended to improve in the JADE group and declined in the DIAMOND group, with no between‐group difference.
Amongst returnees for CA2, the rate of incident diabetes‐related complications including, chronic kidney disease, sensory neuropathy, foot ulcer, loss of visual acuity or advanced eye disease, were similar between groups (Table 3).
Table 3.
New onset of diabetes‐related endpoints in JADE and DIAMOND study groups
| n | DIAMOND, n (%) | n | JADE, n (%) | |
|---|---|---|---|---|
| All patients with vitality status | 1549 | 1646 | ||
| Self‐reported new cardiovascular event (coronary heart disease or stroke) | 30 (1.9) | 42 (2.5) | ||
| Returnees | 1176 | 21 (1.8) | 1383 | 27 (2.0) |
| Non‐returnees | 373 | 9 (2.4) | 263 | 15 (5.7) |
| Death | 6 (0.3) | 6 (0.18) | ||
| Returnees for repeat assessment | 1176 | 1383 | ||
| New chronic kidney disease: 50% loss of estimated GFR | 900 | 16 (1.8) | 1026 | 12 (1.2) |
| New appearance of sensory neuropathy in patients without sensory neuropathy at baseline | 1072 | 49 (4.6) | 1048 | 52 (5.0) |
| Remission of sensory neuropathy in patients with sensory neuropathy at baseline | 96 | 70 (72.9) | 75 | 53 (70.7) |
| Worsening or new appearance of diabetic retinopathy | 992 | 28 (2.8) | 869 | 37 (4.3) |
| Improvement of diabetic retinopathy in patients with diabetic retinopathy at baseline | 59 | 59 (100) | 45 | 41 (91.1) |
| Improved visual acuity in at least one eye | 563 | 164 (29.1) | 657 | 228 (34.7) |
| Deteriorated visual acuity in at least one eye | 570 | 216 (37.9) | 658 | 239 (36.3) |
DIAMOND, DIAbetes MONitoring Database; JADE, Joint Asia Diabetes Evaluation.
Worsening /improvement of diabetic retinopathy is defined as advancement or stabilization in the grading by ophthalmologist (pre‐proliferative, proliferative, advanced).
n includes returnees and a subset of defaulters who could be reached for health status assessment at study end.
There were fewer defaulters in the JADE group than in the DIAMOND group (25.6 vs 32.0%; P < 0.001). At baseline, defaulters were younger (55.8 vs 56.7 years; P = 0.036), were more likely to have a positive smoking history (35.2 vs 33.9%; P = 0.002), were less well educated (> 11 years' education, 61.40 vs 70.70%; P = 0.017), and had worse cardiometabolic risk profile and higher rates of chronic kidney disease (3.20 vs 1.70%; P = 0.007) and macroalbuminuria (8.10 vs 4.20%; P < 0.001), despite similar disease duration. Although defaulters were more likely to be treated with insulin (30.70 vs 25.30%; P = 0.001) and lipid‐lowering drugs (38.20 vs 33.90%; P = 0.016), they were less likely to adhere to regular physical exercise (46.10 vs 54.50%; P < 0.001) and achieve HbA1c target (37.20 vs 46.40%; P < 0.001) or ≥ 2 treatment targets (30.60 vs 37.40%; P < 0.001; Table 4).
Table 4.
Population characteristics at baseline for returnees versus defaulters
| Returnees | Defaulters | P | |
|---|---|---|---|
| Total number of participants, n (%) | 2559 (71.4) | 1027 (28.6) | – |
| Demographics | |||
| Mean ± sd age, years | 56.70 ± 11.56 | 55.80 ± 11.83 | 0.036 |
| Women, % | 54.4 | 54.5 | 0.942 |
| Education, % | < 0.001 | ||
| < 6 years | 6.7 | 12.7 | |
| 6–11 years | 11.6 | 11.8 | |
| > 11 years | 70.7 | 61.4 | |
| Unemployed, % | 64.2 | 60.5 | 0.041 |
| Smoking, % | 0.428 | ||
| Never | 66.1 | 64.8 | |
| Former | 11.6 | 11.8 | |
| Current | 22.3 | 23.4 | |
| Alcohol consumption, % | 0.002 | ||
| Never | 67.8 | 72.8 | |
| Former | 6.4 | 7.5 | |
| Occasional | 16.9 | 11.7 | |
| Regular | 8.9 | 8.0 | |
| Physical activity ≥ 3 times per week, % | 54.5 | 46.1 | < 0.001 |
| SMBG ≥ weekly, % | 45.1 | 42.4 | 0.163 |
| Mean ± sd disease duration, years | 6.43 ± 6.40 | 6.22 ± 6.11 | 0.369 |
| Complications and comorbidities, % | |||
| Chronic kidney disease | 1.7 | 3.2 | 0.007 |
| Coronary heart disease | 9.0 | 8.4 | 0.548 |
| Stroke | 3.0 | 1.9 | 0.052 |
| Peripheral vascular disease | 9.5 | 7.3 | 0.052 |
| Retinopathy | 13.9 | 12.5 | 0.282 |
| Sensory neuropathy | 7.0 | 7.6 | 0.489 |
| Risk categories | 0.101 | ||
| Low (1/2) | 13.8 | 11.7 | |
| High (3/4) | 86.2 | 88.3 | |
| Treatments, % | |||
| Lifestyle modification only | 21.2 | 22.3 | 0.487 |
| On oral antidiabetic drug | 71.4 | 69.0 | 0.163 |
| Insulin | 25.3 | 30.7 | 0.001 |
| On lipid drugs | 33.9 | 38.2 | 0.016 |
| Statins | 30.7 | 34.7 | 0.021 |
| On BP drugs | 23.2 | 21.0 | 0.154 |
| ACE inhibitors | 2.0 | 1.6 | 0.386 |
| AT1 receptor blockers | 12.3 | 11.0 | 0.279 |
| Risk factor control | |||
| Mean ± sd body weight, kg | 69. 68 ± 12.34 | 69.19 ± 13.36 | 0.308 |
| Mean ± sd BMI, kg/m2 | 25.68 ± 3.58 | 25.16 ± 3.64 | 0.361 |
| Mean ± sd waist circumference, cm | |||
| Women | 86.50 ± 10.11 | 86.68 ± 9.90 | 0.769 |
| Men | 91.44 ± 9.38 | 92.09 ± 10.04 | 0.214 |
| Mean ± sd diastolic BP, mmHg | 77.77 ± 9.6 | 77.76 ± 9.58 | 0.977 |
| Mean ± sd systolic BP, mmHg | 126.03 ± 15.07 | 126.72 ± 15.48 | 0.225 |
| Mean ± sd total cholesterol, mmol/l | 4.90 ± 1.18 | 4.99 ± 1.37 | 0.073 |
| Mean ± sd HDL cholesterol, mmol/l | |||
| Women | 1.29 ± 0.32 | 1.26 ± 0.33 | 0.118 |
| Men | 1.12 ± 0.28 | 1.08 ± 0.27 | 0.001 |
| Mean ± sd LDL cholesterol, mmol/l | 2.90 ± 0.86 | 3.00 ± 0.94 | 0.005 |
| Mean ± sd haemoglobin, g/dl | 14.44 ± 6.85 | 14.27 ± 6.05 | 0.524 |
| Mean ± sd HbA1c, % (mmol/mol) | 7.70 (61) ± 1.91 (14) | 8.20 (66) ± 2.24 (17) | < 0.001 |
| Median (IQR) triglyceride, mmol/l | 4.80 (4.15, 5.53) | 4.88 (4.19, 5.58) | 0.286 |
| Median (IQR) urine albumin to creatinine ratio, mg/mol | 1.19 (0.65, 3.25) | 1.35 (0.62, 4.23) | 0.059 |
| Mean ± sd estimated GFR, ml/min/1.73 m2 | 120.7 ± 39.6 | 126.7 ± 42.1 | < 0.001 |
| Obesity, % | 69.3 | 70.7 | 0.447 |
| Dyslipidaemia, % | 92.4 | 92.7 | 0.755 |
| Hypertension, % | 75.6 | 74.6 | 0.542 |
| Macroalbuminuria, % | 4.2 | 8.1 | < 0.001 |
| Microalbuminuria, % | 22.8 | 23.3 | 0.791 |
| Attainment of treatment targets, % | |||
| HbA1c < 7.0% (53 mmol/mol) | 46.4 | 37.2 | < 0.001 |
| BP < 130/80 mmHg | 50.0 | 47.1 | 0.154 |
| LDL cholesterol < 2.6 mmol/l | 37.1 | 34.8 | 0.206 |
| At least one target | 72.7 | 70.4 | 0.171 |
| At least two targets | 37.4 | 30.6 | < 0.001 |
| All three targets | 7.9 | 5.3 | 0.006 |
| Quality of life | |||
| Mean ± sd EQ‐VAS score | 83.38 ± 11.97 | 81.75 ± 12.80 | < 0.001 |
| Mean ± sd EQ‐5D index score | 0.91 ± 0.14 | 0.92 ± 0.14 | 0.156 |
BP, blood pressure; IQR, interquartile range; JADE, Joint Asia Diabetes Evaluation; SMBG, self‐monitoring of blood glucose.
Discussion
In this 12‐month randomized quality improvement programme in Chinese patients with Type 2 diabetes, we used a multi‐component web‐based portal to integrate care delivery focusing on workflow, task delegation and information technology. Irrespective of nurse support, both groups had improved cardiometabolic control, increased attainment of multiple treatment targets, enhanced self‐care and smoking cessation. The additional contacts by nurses during the follow‐up period did not further improve cardiometabolic control but reduced default rates and improved SMBG.
Given the multi‐component nature of the JADE/DIAMOND programme, it was challenging to identify the specific components that drove treatment effects, although these elements are known to individually and collectively improve diabetes care 11. In a 7‐year observational study consisting of 172 patients with Type 2 diabetes without history of cardiovascular‐renal complications, structured care provided by a diabetologist‐nurse team reduced cardiovascular‐renal disease and mortality by 50–70% compared with those attended by generalists in the medical clinic within the same institution 20. In another study evaluating peer empowerment in participants who also received structured care through the JADE programme, HbA1c was reduced by 0.3% (3 mmol/mol), with improvement in multiple targets attained and self‐care 21.
The addition of nurse‐coordinated follow‐up visits in the JADE group did not further enhance glycaemic control or target attainment. That said, JADE participants were more likely to have stable BP control and increased SMBG and were less likely to default, suggesting that ongoing support can be translated into beneficial actions. The nurse provided was envisaged to take on a multifunctional role to promote adherence to the care protocol and reinforce patient education. Given the translational nature of this study that examined integrated care in real‐world settings, we used data documented in the follow‐up module of the JADE portal to assess protocol adherence. We did not rigorously enforce and strictly record compliance to protocol‐recommended practice; thus, it was not possible to fully appraise intervention fidelity.
In China, delivery of chronic care is fragmented and infrastructure and capacity for team‐based care are still evolving. Nurses for instance, are often tasked with simple procedures such as teaching insulin injection and SMBG or performing blood glucose tests. Furthermore, patients are less willing to engage nurses, preferring to consult directly with doctors. As such, their abilities to educate and empower patients may be less advanced compared with fully trained diabetes nurses. This may partially explain the lack of difference between the two groups. Also, the nurse coordinators in the present study received basic training in diabetes care, but, unlike case managers, were not empowered with treatment authority. In a meta‐analysis, quality improvement initiatives that included case managers authorized to adjust medications without awaiting physician approval substantially improved patient care 22.
Because cultural factors are an important component in education, we examined this result in light of education programmes implemented in Chinese populations. In a 1‐year prospective study in Hong Kong, a structured nurse education programme centred on cardiovascular disease risk every 3 months (mean total time 2.5 h) improved HbA1c, LDL cholesterol and BP (diastolic) compared with a control group 23. In Taiwan, the introduction of multidisciplinary care, combined with 2 h of diabetes education every 3 months for 1 year resulted in a 2‐mmol/mol (0.22%) reduction in HbA1c, with a nadir of 0.4% (4 mmol/mol) at 9 months, without changes in oral antidiabetic medications 24. In the JADE group, we also recommended nurses to deliver at least 2 h of education after the CA, but in a single session rather than multiple sittings. In a meta‐analysis, the benefit of patient self‐care education on HbA1c was most pronounced immediately after the intervention, with effects waning by 1–3 months 25. In the Taiwanese study, subjects received initial diabetes education followed by repeated reinforcement sessions every 3 months for 1 year. The reduction in HbA1c was evident at 3, 6 and 9 months but lost significance by 1 year, suggesting that physician or patient fatigue and loss of adherence may need to be addressed 24.
The fact that all participants benefitted from risk stratification with written feedback during the initial consultation might have contributed partially to a lack of separation and improvement in both groups. The proportion of participants with ≥ 2 treatment targets increased from 38.2 to 50.8% in the DIAMOND group and from 40.6 to 50.0% in the JADE group. Another reason for the lack of between‐group difference was patient‐structured follow‐up frequency in JADE. In the low‐risk category (care levels 1 and 2), the portal recommended 1–2 structured follow‐up visits per year and 4–5 visits for the high‐risk group (care levels 3 and 4). However, in the JADE group, the documented number of visits in the portal was 1.7 in participants at low risk and 2.0 in participants at high risk, the latter accounting for 86% of the JADE group. Given the fragmented nature of follow‐up medical visits in China, we anticipated the additional nurse‐coordinated visits to improve follow‐up frequency. The low number of these structured visits might have nullified the expected benefits, which highlights the challenges of implementing integrated care models in countries with traditional healthcare and financing systems. Moreover, patients' perspectives need to be considered, as additional visits for a silent disease like diabetes may not be welcomed because of the extra time, tests and costs involved. By study design, there was no documentation of interval measurements between baseline and repeat CA2 in the DIAMOND group. Thus, although a variance might have existed between the two groups, the failure to fully comply a structured follow‐up and education programme in the JADE group might have attenuated these differences by study end. Similar observations have been reported previously 24, 25, 26.
Despite the lack of between‐group differences in cardiometabolic control, the default rate, defined a priori, was lower in the JADE than in the DIAMOND group. The defaulters had higher HbA1c and LDL cholesterol, while concurrently were more likely to be prescribed insulin and lipid‐lowering drugs. Defaulters were younger, more likely to be in paid employment and were less likely to exhibit good self‐care behaviours. Patients less willing to participate in self‐care behaviours become increasingly dependent on polytherapy and with chronicity, drug regimens might become more complex, which could further exacerbate non‐adherence behaviours 27.
The present study has several limitations. Firstly, this was a real‐world application of integrated care augmented by information technology in China, where healthcare resources are limited. All six participating sites are leading centres, although many of the recommended tests in the CA were not reimbursed in an ambulatory setting. Unlike drug‐based clinical trials, none of the participants were compensated for their participation or diagnostic/care‐related expenditures. This might have led to selection of a more affluent population, although missing values for laboratory tests were found. As participating sites were selected from cosmopolitan cities, our findings cannot be extrapolated to rural populations. Secondly, while this was a randomized quality improvement programme, the treating doctors were not blinded to patient assignment and contamination was possible with participants in both the DIAMOND and JADE groups managed by the same physicians. Notwithstanding, the large sample size involving multiple centres, as well as the documentation of default rates/features known to be associated with higher mortality and treatment costs 28, 29, 30 are major strengths.
In the present study, we did not observe enhanced cardiometabolic control with the addition of a nurse coordinator to the web‐based CA module, although we did observe a reduction in default rates and improved SMBG. We also verified that incorporating a quality improvement programme using an innovative care platform, such as the JADE programme, is both feasible and effective in a low‐resource setting, albeit not without challenges. This prototype allowed the combining of logistics, task delegation and information technology to increase the efficiency and effectiveness of integrated care delivery with improvements in cardiometabolic control and self‐care, as well as reduced clinical inertia.
Funding sources
This study was supported by an unrestricted educational grant from Merck & Co., Inc. The sponsors had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data, preparation, review or approval of the manuscript, or decision to submit the manuscript for publication.
Competing interests
G.E.T., X.L., Y.Y.Z., R.O.P.Y., J.M.Y. and G.T.C.K. have no competing interests to declare. A.P.S.K., A.O.L., J.C.N.C. and R.C.W.M. have received honoraria for consultancy or giving lectures from Merck Sharp & Dohme. A.P.S.K., A.O.L., J.C.N.C., R.C.W.M., R.O., W.Y.S., W.Y.Y., W.H.L., X.H.G., L.N.J., W.P.J. and J.P.W. have received research grants through their institutions from Merck Sharp & Dohme. J.C.N.C. is the Executive Councillor and Chief Executive Officer of the Asia Diabetes Foundation, a charitable research organization, on a probono basis.
Supporting information
Appendix S1. Clinical assessment for subjects assigned to Diabetes Monitoring Database (DIAMOND) and Joint Asia Diabetes Evaluation (JADE) groups.
Appendix S2. Power calculation and statistical analysis.
Acknowledgements
The China JADE Study Group consists of: Yu Zhu, Beijing People's Hospital, Beijing, China; Xiaoping Xing, Fan Ping, Peking Union Hospital, Beijing, China; Junqing Zhang, Xiaowei Ma, First Hospital, Peking University Hospital, Beijing, China; Jing Hong, China‐Japan Friendship Hospital, Beijing, China; Xuhong Hou, Shanghai Sixth People's Hospital, Shanghai, China; Yanhua Zhu, Third Affiliated Hospital of Sun Yat‐Sen University, Guangzhou, China.
Diabet. Med. 34: 440–450 (2017)
References
- 1. Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003; 348: 383–93. [DOI] [PubMed] [Google Scholar]
- 2. Gaede P, Lund‐Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008; 358: 580–591. [DOI] [PubMed] [Google Scholar]
- 3. Kong AP, Yang X, Ko GT, So WY, Chan WB, Ma RC et al Effects of treatment targets on subsequent cardiovascular events in Chinese patients with type 2 diabetes. Diabetes Care 2007; 30: 953–959. [DOI] [PubMed] [Google Scholar]
- 4. Gaede P, Valentine WJ, Palmer AJ, Tucker DM, Lammert M, Parving HH et al Cost‐effectiveness of intensified versus conventional multifactorial intervention in type 2 diabetes: results and projections from the Steno‐2 study. Diabetes Care 2008; 31: 1510–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA et al Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321: 405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999‐2010. N Engl J Med 2013; 368: 1613–1624. [DOI] [PubMed] [Google Scholar]
- 7. Cheng XB, Hsieh YT, Tu ST, Hsieh MC. Obesity and low target attainment rates in Chinese with type 2 diabetes. Eur J Intern Med 2012; 23: e101–105. [DOI] [PubMed] [Google Scholar]
- 8. Wang HH, Wang JJ, Wong SY, Wong MC, Mercer SW, Griffiths SM. The development of urban community health centres for strengthening primary care in China: A systematic literature review. Br Med Bull 2015; 116: 139–154. [DOI] [PubMed] [Google Scholar]
- 9. Chan JCN, Cockram CS. Organisation of diabetes care – Western Pacific Region (Hong Kong and China as examples) In: DeFronzo A, Feranninni E, Keen H, Zimmet P. eds. International textbook of diabetes mellitus. Oxford: John, Wiley & Son, 2004. [Google Scholar]
- 10. Institute of Medicine . Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press, 2001. [PubMed] [Google Scholar]
- 11. Tricco AC, Ivers NM, Grimshaw JM, Moher D, Turner L, Galipeau J et al Effectiveness of quality improvement strategies on the management of diabetes: a systematic review and meta‐analysis. Lancet 2012; 379: 2252–2261. [DOI] [PubMed] [Google Scholar]
- 12. Wu JY, Leung WY, Chang S, Lee B, Zee B, Tong PC et al Effectiveness of telephone counselling by a pharmacist in reducing mortality in patients receiving polypharmacy: randomised controlled trial. BMJ 2006; 333: 522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chan JCN, So WY, Yeung CY, Ko GTC, Lau IT, Tsang MW et al The SURE Study: Effects of Structured versus Usual care on Renal Endpoint in Type 2 diabetes: A randomized multi‐centre translational study. Diabetes Care 2009; 32: 977–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chan JCN, So WY, Ko G, Tong PCT, Yang XL, Ma RCW et al The Joint Asia Diabetes Evaluation (JADE) Program: A Web‐based Program to Translate Evidence to Clinical Practice in Type 2 Diabetes. Diabet Med 2009; 26: 693–699. [DOI] [PubMed] [Google Scholar]
- 15. Ko GT, So WY, Tong PC, Le Coguiec F, Kerr D, Lyubomirsky G et al From design to implementation–the Joint Asia Diabetes Evaluation (JADE) program: a descriptive report of an electronic web‐based diabetes management program. BMC Med Inform Decis Mak 2010; 10: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang X, So WY, Kong AP, Ho CS, Lam CW, Stevens RJ et al Development and validation of stroke risk equation for Hong Kong Chinese patients with type 2 diabetes: the Hong Kong Diabetes Registry. Diabetes Care 2007; 30: 65–70. [DOI] [PubMed] [Google Scholar]
- 17. Yang X, So WY, Kong AP, Ma RC, Ko GT, Ho CS et al Development and validation of a total coronary heart disease risk score in type 2 diabetes mellitus. Am J Cardiol 2008; 101: 596–601. [DOI] [PubMed] [Google Scholar]
- 18. Yang XL, So WY, Kong AP, Ho CS, Lam CW, Ng MH et al Modified end‐stage renal disease risk score for Chinese type 2 diabetic patients—the Hong Kong Diabetes Registry. Diabetologia 2007; 50: 1348–1350. [DOI] [PubMed] [Google Scholar]
- 19. Yang X, Ma RC, So WY, Kong AP, Ko GT, Ho CS et al Development and validation of a risk score for hospitalization for heart failure in patients with type 2 diabetes mellitus. Cardiovasc Diabetol 2008; 7: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. So WY, Tong PC, Ko GT, Leung WY, Chow CC, Yeung VT et al Effects of protocol‐driven care versus usual outpatient clinic care on survival rates in patients with type 2 diabetes. Am J Manag Care 2003; 9: 606–615. [PubMed] [Google Scholar]
- 21. Chan JC, Sui Y, Oldenburg B, Zhang Y, Chung HH, Goggins W et al Effects of telephone‐based peer support in patients with type 2 diabetes mellitus receiving integrated care: a randomized clinical trial. JAMA Intern Med 2014; 174: 972–981. [DOI] [PubMed] [Google Scholar]
- 22. Shojania KG, Ranji SR, McDonald KM, Grimshaw JM, Sundaram V, Rushakoff RJ et al Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta‐regression analysis. JAMA 2006; 296: 427–440. [DOI] [PubMed] [Google Scholar]
- 23. Ko GT, Li JK, Kan EC, Lo MK. Effects of a structured health education programme by a diabetic education nurse on cardiovascular risk factors in Chinese Type 2 diabetic patients: a 1‐year prospective randomized study. Diabet Med 2004; 21: 1274–1279. [DOI] [PubMed] [Google Scholar]
- 24. Tien KJ, Hung HC, Hsiao JY, Hsu SC, Hsin SC, Shin SJ et al Effectiveness of comprehensive diabetes care program in Taiwanese with type 2 diabetes. Diabetes Res Clin Pract 2008; 79: 276–283. [DOI] [PubMed] [Google Scholar]
- 25. Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self‐management education for adults with type 2 diabetes: a meta‐analysis of the effect on glycemic control. Diabetes Care 2002; 25: 1159–1171. [DOI] [PubMed] [Google Scholar]
- 26. Beverly EA, Fitzgerald SM, Brooks KM, Hultgren BA, Ganda OP, Munshi M et al Impact of reinforcement of diabetes self‐care on poorly controlled diabetes: a randomized controlled trial. Diabetes Educ 2013; 39: 504–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Garcıa‐Perez LE, Alvarez M, Dilla T, Gil‐Guillen V, Orozco‐Beltran D. Adherence to Therapies in Patients with Type 2 Diabetes. Diabetes Ther 2013; 4: 175–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Currie CJ, Peyrot M, Morgan CL, Poole CD, Jenkins‐Jones S, Rubin RR et al The impact of treatment noncompliance on mortality in people with type 2 diabetes. Diabetes Care 2012; 35: 1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Asche C, LaFleur J, Conner C. A Review of Diabetes Treatment Adherence and the Association with Clinical and Economic Outcomes. Clin Ther 2011; 33: 74–109. [DOI] [PubMed] [Google Scholar]
- 30. Karter AJ, Parker MM, Moffet HH, Ahmed AT, Ferrara A, Liu JY et al Missed appointments and poor glycemic control: an opportunity to identify high‐risk diabetic patients. Med Care 2004; 42: 110–115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Clinical assessment for subjects assigned to Diabetes Monitoring Database (DIAMOND) and Joint Asia Diabetes Evaluation (JADE) groups.
Appendix S2. Power calculation and statistical analysis.


