Summary
The phase 3 FIRST (Frontline Investigation of REVLIMID + Dexamethasone Versus Standard Thalidomide) trial demonstrated that lenalidomide plus low‐dose dexamethasone (Rd) until disease progression (Rd continuous) is an effective treatment option for transplant‐ineligible patients with newly diagnosed multiple myeloma (NDMM). Given genetic differences between Asian and Western populations, this subanalysis of the FIRST trial examined the safety and efficacy of Rd (given continuously or for 18 cycles [Rd18]) and MPT (melphalan, prednisone, thalidomide) in 114 Asian patients from Mainland China, South Korea and Taiwan. Efficacy and safety with Rd continuous in Asian patients were consistent with those in the overall study population. The overall response rates were 77·8% for Rd continuous, 57·5% for MPT and 65·8% for Rd18. The risk of progression or death was reduced by 39% with Rd continuous versus MPT and by 35% with Rd continuous versus Rd18. Rd continuous improved the 3‐year survival rate compared with MPT (70·2% vs. 56·4%) and Rd18 (58·1%). Common grade 3/4 adverse events in the Rd continuous and MPT arms were neutropenia (25·0% vs. 43·6%), infection (19·4% vs. 28·2%) and anaemia (19·4% vs. 15·4%), respectively. Thromboembolic event rates were low, and no second primary malignancies were observed. Rd continuous is safe and effective in transplant‐ineligible Asian patients with NDMM.
Keywords: lenalidomide, multiple myeloma, transplant‐ineligible, newly diagnosed, Asia
Multiple myeloma is the third most common haematological cancer and accounts for approximately 13% of all haematological cancers diagnosed worldwide (Palumbo & Anderson, 2011; Ferlay et al, 2013). The incidence of multiple myeloma in Asian countries is lower than that in Western countries, and this has been partly attributed to differences in genetics (Kang et al, 2008). However, several recent reports show that the incidence of multiple myeloma is increasing rapidly in several Asian countries (Huang et al, 2007; Lee et al, 2010). Environmental pollution and the Westernization of diets may be some factors contributing to this rise (Kim et al, 2014). Aging is also associated with an increased risk for multiple myeloma (Turesson et al, 2010) and, as in Western countries, life expectancy is increasing in several Asian countries (Huang et al, 2007; Kim et al, 2014). There is a need for effective and well‐tolerated treatment regimens for multiple myeloma to help address the challenge of the rising incidence of this disease in Asia. Genetic differences across races can alter the pharmacokinetics and pharmacodynamics of drugs, which could potentially contribute to differences in drug response. Therefore, race is an important consideration when evaluating the efficacy and safety of a drug regimen (Yasuda et al, 2008; Ramamoorthy et al, 2015).
The standard of treatment for young patients with newly diagnosed multiple myeloma (NDMM) remains stem cell transplantation (SCT) (Attal et al, 2015; Mateos et al, 2015). For patients with NDMM who are SCT‐ineligible, usually those who are older than 65–70 years, the introduction of immunomodulatory drugs and proteasome inhibitors has increased the number of efficacious therapy options (Palumbo et al, 2014; Mateos et al, 2015; Moreau et al, 2015). However, in Asia, approximately two‐thirds of patients with multiple myeloma do not receive immunomodulatory agents, such as lenalidomide, or the proteasome inhibitor bortezomib in first‐line treatment regimens, which may be due to resource and reimbursement limitations (Tan et al, 2013; Kim et al, 2014; Lu et al, 2014). Furthermore, although the median age of patients with multiple myeloma in Asia is lower than that of patients in the United States at 62 vs. 65 years, respectively, patients in Asia are less likely to undergo SCT (Kim et al, 2014; Gonsalves et al, 2015). This has been attributed to a lack of resources and/or infrastructure for SCT facilities (Kim et al, 2014).
Lenalidomide plus low‐dose dexamethasone (Rd) is a recommended treatment option for patients with NDMM who are SCT‐ineligible (Tan et al, 2013; Palumbo et al, 2014; Moreau et al, 2015; National Comprehensive Cancer Network, 2016). Data from this randomized, phase 3 FIRST (Frontline Investigation of REVLIMID + Dexamethasone Versus Standard Thalidomide) study demonstrated that Rd until disease progression (Rd continuous) is a safe and effective treatment option for this patient population (Benboubker et al, 2014). In this trial, Rd continuous reduced the risk of disease progression or death by 28% compared with MPT [hazard ratio (HR), 0·72 (95% CI, 0·61–0·85); P < 0·001] and by 30% compared with the limited 18 cycles of Rd [Rd18; HR, 0·70 (95% CI, 0·60–0·82); P < 0·001] (Benboubker et al, 2014). In addition, Rd continuous reduced the risk of death by 22% compared with MPT [HR, 0·78 (95% CI, 0·64–0·96); P = 0.02] (Benboubker et al, 2014). This subanalysis of the FIRST study examines the safety and efficacy of Rd continuous for the treatment of specifically those SCT‐ineligible patients with NDMM treated in Asia (Mainland China, South Korea, and Taiwan).
Methods
Study design
The FIRST study [MM‐020/Intergroupe Francophone du Myélome (IFM) 07‐01] was a multinational, open‐label, randomized, phase 3 trial of Rd continuous versus Rd18 or 12 cycles of MPT in patients with NDMM who were SCT ineligible (Benboubker et al, 2014). The study was conducted at 246 treatment centres in 18 countries and included 3 countries in Asia: Mainland China, South Korea and Taiwan. Patients were randomly assigned 1:1:1 to receive 1 of 3 treatment arms using a validated interactive voice‐response system and stratified by age (≤75 years vs. >75 years), International Staging System disease stage (I or II vs. III) and country. All sites received approval from their institutional review boards or ethics committees. The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice.
Patients
Eligible patients had previously untreated, symptomatic multiple myeloma, were ≥65 years of age or <65 years of age and not candidates for SCT, and had an Eastern Cooperative Oncology Group performance score ≤2. Patients with any degree of renal impairment were included, except those requiring dialysis. Exclusion criteria were an absolute neutrophil count <1 × 109/l, an untransfused platelet count <50 × 109/l, a serum aspartate aminotransferase or alanine aminotransferase level >3 times the upper limit of normal, grade ≥2 peripheral neuropathy, or a history of malignancies other than multiple myeloma in the prior 3 years (excluding nonmelanoma skin cancer, carcinomas in situ of the cervix or breast, or incidental findings of prostate cancer). Written informed consent was provided by all patients.
Treatments
Patients in the Rd continuous and Rd18 groups received oral lenalidomide 25 mg/day on days 1–21 and oral dexamethasone 40 mg on days 1, 8, 15 and 22 in 28‐day cycles until disease progression or for 72 weeks (18 cycles), respectively. Patients in the MPT group received oral melphalan 0·25 mg/kg/day on days 1–4, oral prednisone 2 mg/kg per day on days 1–4, and thalidomide 200 mg/day in 42‐day cycles for 72 weeks (12 cycles). Starting dose adjustments for dexamethasone and thalidomide were based on age, melphalan was based on age, absolute neutrophil count, platelet count and the level of renal function, and those for lenalidomide based on the level of renal function. All patients were required by protocol to receive thromboprophylaxis.
Assessments
The primary endpoint was progression‐free survival (PFS). Key secondary endpoints included the overall response rate (ORR; defined as partial response or better), duration of response (DOR), overall survival (OS), time to second‐line anti‐myeloma therapy and safety. Progression and response were evaluated using the International Myeloma Working Group criteria (Durie et al, 2006) and reviewed by an independent response‐adjudication committee. Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0 (National Cancer Institute, 2006). Treatment‐emergent AEs (TEAEs) were defined as AEs occurring or worsening on or after the first dose of any study drug, and within 28 days after receiving the last dose.
Statistical analysis
Efficacy analyses for this subpopulation included all randomized Asian patients in Asia. Safety analyses included Asian patients in Asia who received ≥1 dose of any study drug. All analyses, except OS, were conducted using a data cut‐off of 24 May 2013, which was the date of the planned final PFS analysis. At the request of health regulatory authorities, an additional, previously unplanned, survival analysis was performed using an updated data cut‐off of 3 March 2014. The unstratified log‐rank test was used for comparisons of time‐to‐event endpoints. The Cox proportional‐hazards model was used to estimate HRs and 95% CIs. Response data were compared between treatment arms using the Fisher exact test. For this subanalysis of the FIRST trial, no statistical significance was defined. Additional statistical detail is provided in the primary publication of the FIRST trial (Benboubker et al, 2014).
Results
Of 1623 total randomized patients, 114 NDMM patients in the Asian subpopulation were randomly assigned to receive Rd continuous (n = 36), Rd18 (n = 38) or MPT (n = 40). Forty‐seven patients were enrolled in Mainland China, 56 patients in South Korea and 11 patients in Taiwan. Patient demographics and baseline characteristics were well balanced between the treatment arms, except for sex and severe renal insufficiency (creatinine clearance <30 ml/min; Table 1). Rd continuous and Rd18 arms had an imbalance of more men than women patients. Severe renal impairment at baseline was more frequent in the Rd18 and MPT arms compared with the Rd continuous arm. The median age of the Asian subpopulation was lower than the overall study population (68·0 vs. 73·0 years, respectively; Table 1). A slightly higher proportion of patients in Asia had advanced disease compared with the overall study population, as indicated by the rates of patients with International Staging System stage III disease (44·7% vs. 40·6%, respectively) and an Eastern Cooperative Oncology Group performance score of 2 (28·1% vs. 21·1), respectively; Table 1). In addition, a greater proportion of patients in Asia had severe renal impairment compared with the overall study population (18·4% vs. 9·1%, respectively; Table 1). In the intention‐to‐treat population, 762 patients were assessed for cytogenetics including 32 patients from Asia. Of the Asian patients with evaluable cytogenetics profiles, 21·9% (n = 7) had profiles associated with high‐risk, defined as t(4;14), del 17p or t(14;16). Six patients (18·8%) had t(4;14), 2 patients (6·3%) had del 17p, and no patients had t(14;16). As of the 24 May 2013 data cut‐off, one‐third of the patients (n = 12) in the Rd continuous arm were treated for >2 years, with median treatment durations of 18·4, 11·0 and 11·1 months, respectively, with Rd continuous, Rd18 and MPT. An updated survival analysis conducted with a data cut‐off of 3 March 2014 had a median follow‐up of 38·1 months.
Table 1.
Baseline characteristics of the Asian subpopulation and intention‐to‐treat population
| Characteristic | Asian subpopulation (n = 114) | ITT population (N = 1623) | ||||
|---|---|---|---|---|---|---|
| Rd continuous (n = 36) | Rd18 (n = 38) | MPT (n = 40) | Rd continuous (n = 535) | Rd18 (n = 541) | MPT (n = 547) | |
| Median age (range), years | 67·5 (44·0–83·0) | 67·0 (43·0–80·0) | 69·5 (51·0–86·0) | 73·0 (44·0–91·0) | 73·0 (40·0–89·0) | 73·0 (51·0–92·0) |
| ≥65 years, n (%) | 23 (63·9) | 26 (68·4) | 30 (75·0) | 504 (94·2) | 507 (93·7) | 520 (95·1) |
| Male, n (%) | 23 (63·9) | 23 (60·5) | 20 (50·0) | 294 (55·0) | 273 (50·5) | 287 (52·5) |
| ISS stage, n (%) | ||||||
| I/II | 21 (58·3) | 20 (52·6) | 22 (55·0) | 319 (59·6) | 322 (59·5) | 323 (59·0) |
| III | 15 (41·7) | 18 (47·4) | 18 (45·0) | 216 (40·4) | 219 (40·5) | 224 (41·0) |
| CrCl, n (%) | ||||||
| <30 ml/min | 3 (8·3) | 9 (23·7) | 9 (22·5) | 45 (8·4) | 47 (8·7) | 55 (10·1) |
| ≥30–50 ml/min | 3 (8·3) | 9 (23·7) | 5 (12·5) | 126 (23·6) | 120 (22·2) | 126 (23·0) |
| ≥50 ml/min | 30 (83·3) | 20 (52·6) | 26 (65·0) | 364 (68·0) | 374 (69·1) | 366 (66·9) |
| ECOG PS, n (%) | ||||||
| 0 | 4 (11·1) | 7 (18·4) | 5 (12·5) | 155 (29·0) | 163 (30·1) | 156 (28·5) |
| 1 | 22 (61·1) | 20 (52·6) | 24 (60·0) | 257 (48·0) | 263 (48·6) | 275 (50·3) |
| 2 | 10 (27·8) | 11 (28·9) | 11 (27·5) | 119 (22·2) | 113 (20·9) | 111 (20·3) |
| ≥3 | 0 | 0 | 0 | 2 (0·4) | 2 (0·4) | 2 (0·4) |
| Missing | 0 | 0 | 0 | 2 (0·4) | 0 | 3 (0·5) |
CrCl, creatinine clearance; ECOG PS, Eastern Cooperative Oncology Group performance score; ISS, International Staging System; ITT, intention‐to‐treat; MPT, melphalan, prednisone, thalidomide; Rd continuous, lenalidomide plus low‐dose dexamethasone until disease progression; Rd18, lenalidomide plus low‐dose dexamethasone for 18 cycles.
Efficacy
In this subpopulation, Rd continuous resulted in a PFS advantage compared with both MPT and Rd18 (Fig 1A). The median PFS was 21·1 months with Rd continuous versus 16·5 months with MPT and 17·4 months with Rd18. Rd continuous treatment reduced the risk of disease progression or death by 39% compared with MPT [HR, 0·61 (95% CI, 0·33–1·14)]. A similar reduction in the risk of progression or death was observed for Rd continuous compared with Rd18 [35%; HR, 0·65 (95% CI, 0·35–1·20)]. The 2‐year PFS rates were higher with Rd continuous versus MPT and Rd18 (48·0% vs. 24·9% and 35·6%, respectively).
Figure 1.
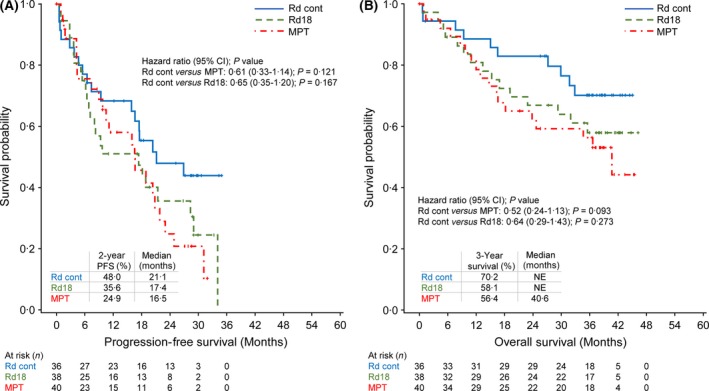
Progression‐free survival and overall survival in the Asian subpopulation (A and B, respectively). NE, not estimable; MPT, melphalan, prednisone, thalidomide; Rd cont, lenalidomide plus low‐dose dexamethasone until disease progression; Rd18, lenalidomide plus low‐dose dexamethasone for 18 cycles.
At updated OS analysis, the risk of death with Rd continuous was reduced by 48% versus MPT [HR, 0·52 (95% CI, 0·24–1·13)] and by 36% versus Rd18 [HR, 0·64 (95% CI, 0·29–1·43); Fig 1B]. In addition, Rd continuous improved the rate of 3‐year survival versus MPT and Rd18 (70·2% vs. 56·4% and 58·1%).
Rd continuous led to a higher ORR than MPT and Rd18 (77·8%, 57·5% and 65·8%, respectively; Table 2). Rd continuous more than doubled the rate of ≥ very good partial responses compared with MPT (52·8% and 22·5%, respectively). Rd continuous also prolonged the DOR compared with MPT and Rd18 (Table 2). The median time to second‐line anti‐myeloma therapy was 24·1 months with Rd continuous, 23·3 months with MPT and 21·0 months with Rd18 (Table 3).
Table 2.
Response rates and duration of response (International Myeloma Working Group criteria; Durie et al, 2006) in the Asian subpopulation
| Rd continuous (n = 36) | Rd18 (n = 38) | MPT (n = 40) | |
|---|---|---|---|
| ORR (≥partial response), n (%) | 28 (77·8) | 25 (65·8) | 23 (57·5) |
| Complete response | 5 (13·9) | 5 (13·2) | 6 (15·0) |
| Very good partial response | 14 (38·9) | 11 (28·9) | 3 (7·5) |
| Partial response | 9 (25·0) | 9 (23·7) | 14 (35·0) |
| Stable disease, n (%) | 3 (8·3) | 11 (28·9) | 10 (25·0) |
| Progressive disease, n (%) | 2 (5·6) | 1 (2·6) | 1 (2·5) |
| Not evaluable, n (%) | 3 (8·3) | 1 (2·6) | 6 (15·0) |
| Median DOR (95% CI), monthsa | NE (16·6‐NE) | 17·2 (5·5–32·2) | 13·8 (7·6–22·8) |
| DOR HR (95% CI) | |||
| Rd continuous versus MPT | 0·43 (0·19–0·95) | ||
| Rd continuous versus Rd18 | 0·53 (0·24–1·15) | ||
| Rd18 versus MPT | 0·83 (0·39–1·78) | ||
95% CI, 95% confidence interval; DOR, duration of response; HR, hazard ratio; MPT, melphalan, prednisone, thalidomide; NE, not estimable; ORR, overall response rate; Rd continuous, lenalidomide plus low‐dose dexamethasone until disease progression; Rd18, lenalidomide plus low‐dose dexamethasone for 18 cycles.
For patients achieving ≥partial response.
Table 3.
Time to second‐line antimyeloma treatment in the Asian subpopulation
| Rd continuous (n = 36) | Rd18 (n = 38) | MPT (n = 40) | |
|---|---|---|---|
| Median time to second‐line AMT (95% CI), months | 24·1 (18·6‐NE) | 21·0 (11·1‐NE) | 23·3 (11·9‐34·7) |
| HR (95% CI) | |||
| Rd continuous versus MPT | 0·83 (0·44–1·55) | ||
| Rd continuous versus Rd18 | 0·85 (0·46–1·61) | ||
| Rd18 versus MPT | 0·93 (0·50–1·73) | ||
95% CI, 95% confidence interval; AMT, anti‐myeloma therapy; HR, hazard ratio; MPT, melphalan, prednisone, thalidomide; NE, not estimable; Rd continuous, lenalidomide plus low‐dose dexamethasone until disease progression; Rd18, lenalidomide plus low‐dose dexamethasone for 18 cycles.
Safety
The rate of grade 3/4 TEAEs was lower with Rd continuous than with MPT (66·7% and 87·2%, respectively; Table 4). Despite a longer duration of treatment in the Rd continuous arm, the rate of grade 3/4 TEAEs was similar with Rd continuous and Rd18. Rd continuous was associated with a lower rate of grade 3/4 infections than MPT or Rd18. No patients in either Rd arm experienced grade 3/4 deep vein thrombosis, compared to 1 patient in the MPT arm (2·6%). Rates of grade 3/4 pulmonary embolism were low and similar in patients receiving Rd continuous, MPT and Rd18 (2·8%, 2·6% and 2·6%, respectively). No second primary malignancies were reported among any of the treatment arms by the cut‐off date of 24 May 2013.
Table 4.
Grade 3 or 4 treatment‐emergent adverse events (safety population) of interest in the Asian subpopulation
| Rd continuous (n = 36) | Rd18 (n = 38) | MPT (n = 39) | |
|---|---|---|---|
| Grade 3/4 TEAE, n (%) | 24 (66·7) | 24 (63·2) | 34 (87·2) |
| Haematological | |||
| Neutropenia | 9 (25·0) | 13 (34·2) | 17 (43·6) |
| Anaemia | 7 (19·4) | 2 (5·3) | 6 (15·4) |
| Thrombocytopenia | 5 (13·9) | 2 (5·3) | 2 (5·1) |
| Leucopenia | 2 (5·6) | 3 (7·9) | 3 (7·7) |
| Nonhaematological | |||
| Infection | 7 (19·4) | 12 (31·6) | 11 (28·2) |
| Pneumonia | 2 (5·6) | 9 (23·7) | 6 (15·4) |
| Hypokalaemia | 4 (11·1) | 2 (5·3) | 2 (5·1) |
| Hyperglycaemia | 4 (11·1) | 3 (7·9) | 1 (2·6) |
| Constipation | 3 (8·3) | 0 | 1 (2·6) |
| Peripheral sensory neuropathy | 1 (2·8) | 0 | 1 (2·6) |
| Cataract | 1 (2·8) | 1 (2·6) | 0 |
MPT, melphalan, prednisone, thalidomide; Rd continuous, lenalidomide plus low‐dose dexamethasone until disease progression; Rd18, lenalidomide plus low‐dose dexamethasone for 18 cycles; TEAE, treatment‐emergent adverse event.
Discussion
Rd continuous prolonged PFS and OS and improved ORR compared with MPT in the Asian subpopulation of the FIRST trial. PFS was prolonged by greater than 4 months with Rd continuous compared with MPT, and 2‐year PFS with Rd continuous was nearly doubled compared with MPT. An OS benefit was also seen with Rd continuous, with an increase observed in 3‐year survival compared with MPT. ORR was higher with Rd continuous versus MPT, largely due to a doubling of the rate of high‐quality responses ≥ very good partial responses. Rd continuous also prolonged DOR compared with MPT and Rd18, suggesting that continuous treatment can suppress tumour growth and prolong patient remissions.
Efficacy results in the Asian subpopulation are consistent with those in the overall FIRST trial population. Median PFS was similarly extended by 4·3 months with Rd continuous compared with MPT in the overall trial population (Benboubker et al, 2014). ORR was also similar for patients receiving Rd continuous in the Asian subpopulation (77·8%) and the overall trial population (75·1%) (Benboubker et al, 2014). Interestingly, a greater relative reduction in the risk of death (48%) was observed with Rd continuous versus MPT in the Asian subpopulation than in the overall study population (25%). It is important to note that the population of Asian patients was small, so these data should be interpreted with caution.
The safety profile of Rd continuous in the Asian subpopulation was generally consistent with that in the overall trial population (Benboubker et al, 2014). Unlike the general study population, patients in Asia receiving Rd continuous had a higher rate of grade 3/4 thrombocytopenia compared with those receiving MPT (13·9% vs. 5·1%, respectively). A low rate of grade 3/4 thromboembolic events was observed across all treatment arms in the Asian subpopulation (3·5% in the Asian subpopulation versus 6·3% in the overall study population), a finding that is consistent with other studies in patients from Asia with multiple myeloma (Shyu et al, 2009). No second primary malignancies have been observed as of data cut‐off in the Asian patient population.
The results presented here further support the improved patient outcomes with Rd, and as such, investigators have explored novel triplet regimens using the Rd backbone. In relapsed/refractory multiple myeloma, Rd has been the basis for new regimens in combination with the proteasome inhibitors carfilzomib (Stewart et al, 2015) and ixazomib (Moreau et al, 2016) and the monoclonal antibodies elotuzumab (Lonial et al, 2015) and daratumumab (Dimopoulos et al, 2016). In patients with NDMM, the addition of bortezomib to Rd (VRd) has improved clinical outcome in comparison with Rd alone (Durie et al, 2015). Results from ongoing studies with these novel treatment combinations in the frontline setting are eagerly awaited (ClinicalTrials.gov identifiers NCT01335399, NCT01668719 and NCT01850524).
In summary, given a more restricted access to SCT and the increasing incidence of multiple myeloma in Asian countries, the availability of Rd continuous treatment has the potential to greatly benefit patients with NDMM in Asia (Kim et al, 2014). The efficacy results for Rd continuous from this subanalysis of the FIRST trial show that treatment outcomes in the Asian subpopulation and the overall study population are similar and support the use of Rd until disease progression as the standard of care for Asian patients with NDMM who are SCT‐ineligible.
Author contributions
J.H.L., S‐Y.H., A.E.H., J.H., G.C., H.S.E., W.M.C., W.Y. and T.F. have made substantial contributions to the conception and design of the work. J.H.L., K.K., J.Y.K., S‐Y.H., A.E.H., J.H., C.K.M., J.L., G.C., W.Y. and H.S.E have contributed to the acquisition, analysis, or interpretation of data for the work. J.H.L., S‐Y.H., A.E.H., J.J.L., J.H., G.C., H.S.E., W.Y. and T.F. have contributed to the drafting of the work. J.H.L., T.L., K.K., H.J.K., S.S.Y., J.O.L., S‐Y.H., A.E.H., J.H., J.L., C.H., G.C., H.S.E., W.M.C., W.Y. and T.F. have revised the manuscript critically for important intellectual content. All authors gave final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Acknowledgements
This work was supported with funding from the Intergroupe Francophone du Myélome and Celgene Corporation. The authors thank Kristina Hernandez, PhD, and Jennifer Leslie, PhD, for medical writing assistance, which was sponsored by Celgene Corporation.
References
- Attal, M. , Lauwers‐Cances, V. , Hulin, C. , Facon, T. , Caillot, D. , Escoffre, M. , Arnulf, B. , Macro, M. , Belhadj, K. , Garderet, L. , Roussel, M. , Mathiot, C. , Avet‐Loiseau, H. , Munshi, N.C. , Richardson, P.G. , Anderson, K.C. , Harousseau, J.L. & Moreau, P. (2015) Autologous transplantation for multiple myeloma in the era of new drugs: a phase III study of the Intergroupe Francophone du Myelome (IFM/DFCI 2009 trial). Blood (ASH Annual Meeting Abstracts), 126, 391. [Google Scholar]
- Benboubker, L. , Dimopoulos, M.A. , Dispenzieri, A. , Catalano, J. , Belch, A.R. , Cavo, M. , Pinto, A. , Weisel, K. , Ludwig, H. , Bahlis, N. , Banos, A. , Tiab, M. , Delforge, M. , Cavenagh, J. , Geraldes, C. , Lee, J.J. , Chen, C. , Oriol, A. , de la Rubia, J. , Qiu, L. , White, D.J. , Binder, D. , Anderson, K. , Fermand, J.P. , Moreau, P. , Attal, M. , Knight, R. , Chen, G. , Van Oostendorp, J. , Jacques, C. , Ervin‐Haynes, A. , Avet‐Loiseau, H. , Hulin, C. & Facon, T. ; FIRST Trial Team . (2014) Lenalidomide and dexamethasone in transplant‐ineligible patients with myeloma. The New England Journal of Medicine, 371, 906–917. [DOI] [PubMed] [Google Scholar]
- Dimopoulos, M.A. , Oriol, A. , Nahi, H. , San Miguel, J. , Bahlis, N.J. , Rabin, N. , Orlowski, R. , Komarnicki, M. , Suzuki, K. , Plesner, T. , Samoilova, O.S. , Yoon, S.S. , Yehuda, D.B. , Richardson, P.G. , Goldschmidt, H. , Reece, D. , Khokhar, N. , O'Rourke, L. , Chiu, C. , Qin, X. , Guckert, M. , Ahmadi, T. & Moreau, P. (2016) An open‐label, randomised phase 3 study of daratumumab, lenalidomide, and dexamethasone (DRd) versus lenalidomide and dexamethasone (Rd) in relapsed or refractory multiple myeloma (RRMM): POLLUX. Haematologica (EHA Abstracts), 101(s1), Abstract LB2238. [Google Scholar]
- Durie, B.G.M. , Harousseau, J. , Miguel, J.S. , Bladé, J. , Barlogie, B. , Anderson, K. , Gertz, M. , Dimopoulos, M. , Westin, J. , Sonneveld, P. , Ludwig, H. , Gahrton, G. , Beksac, M. , Crowley, J. , Belch, A. , Boccadaro, M. , Turesson, I. , Joshua, D. , Vesole, D. , Kyle, R. , Alexanian, R. , Tricot, G. , Attal, M. , Merlini, G. , Powles, R. , Richardson, P. , Shimizu, K. , Tosi, P. , Morgan, G. & Rajkumar, S.V. (2006) International uniform response criteria for multiple myeloma. Leukemia, 20, 1467–1473. [DOI] [PubMed] [Google Scholar]
- Durie, B.G.M. , Hoering, A. , Rajkumar, S.V. , Abidi, M.H. , Epstein, J. , Kahanic, S.P. , Thakuri, M.C. , Reu, F.J. , Reynolds, C.M. , Sexton, R. , Orlowski, R.Z. , Barlogie, B. & Dispenzieri, A. (2015) Bortezomib, lenalidomide and dexamethasone vs. lenalidomide and dexamethasone in patients (pts) with previously untreated multiple myeloma without an intent for immediate autologous stem cell transplant (ASCT): results of the randomized phase III trial SWOG S0777. Blood, 126, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay, J. , Soerjomataram, I. , Ervik, M. , Dikshit, R. , Eser, S. , Mathers, C. , Rebelo, M. , Parkin, D.M. , Forman, D. & Bray, F. (2013) GLOBOCAN 2012 v1.0, cancer incidence and mortality worldwide: IARC CancerBase no. 11. International Agency for Research on Cancer, lyon: Available from: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx. Accessed 31 October 2016. [Google Scholar]
- Gonsalves, W.I. , Leung, N. , Rajkumar, S.V. , Dispenzieri, A. , Lacy, M.Q. , Hayman, S.R. , Buadi, F.K. , Dingli, D. , Kapoor, P. , Go, R.S. , Lin, Y. , Russell, S.J. , Lust, J.A. , Zeldenrust, S. , Kyle, R.A. , Gertz, M.A. & Kumar, S.K. (2015) Improvement in renal function and its impact on survival in patients with newly diagnosed multiple myeloma. Blood Cancer Journal, 5, e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, S.Y. , Yao, M. , Tang, J.L. , Lee, W.C. , Tsay, W. , Cheng, A.L. , Wang, C.H. , Chen, Y.C. , Shen, M.C. & Tien, H.F. (2007) Epidemiology of multiple myeloma in Taiwan: increasing incidence for the past 25 years and higher prevalence of extramedullary myeloma in patients younger than 55 years. Cancer, 110, 896–905. [DOI] [PubMed] [Google Scholar]
- Kang, S.H. , Kim, T.Y. , Kim, H.Y. , Yoon, J.H. , Cho, H.I. , Yoon, S.S. , Kang, D.H. , Suh, C.W. , Lee, J.H. & Lee, D.S. (2008) Protective role of CYP1A1*2A in the development of multiple myeloma. Acta Haematologica, 119, 60–64. [DOI] [PubMed] [Google Scholar]
- Kim, K. , Lee, J.H. , Kim, J.S. , Min, C.K. , Yoon, S.S. , Shimizu, K. , Chou, T. , Kosugi, H. , Suzuki, K. , Chen, W. , Hou, J. , Lu, J. , Huang, X.J. , Huang, S.Y. , Chng, W.J. , Tan, D. , Teoh, G. , Chim, C.S. , Nawarawong, W. , Siritanaratkul, N. & Durie, B.G. (2014) Clinical profiles of multiple myeloma in Asia‐an Asian Myeloma Network Study. American Journal of Hematology, 89, 751–756. [DOI] [PubMed] [Google Scholar]
- Lee, J.H. , Lee, D.S. , Lee, J.J. , Chang, Y.H. , Jin, J.Y. , Jo, D.Y. , Bang, S.M. , Kim, H.J. , Kim, J.S. , Kim, K. , Eom, H.S. , Min, C.K. , Yoon, S.S. , Kim, S.H. , Suh, C. & Cho, K.S. ; Korean Multiple Myeloma Working Party . (2010) Multiple myeloma in Korea: past, present, and future perspectives. Experience of the Korean Multiple Myeloma Working Party. International Journal of Hematology, 92, 52–57. [DOI] [PubMed] [Google Scholar]
- Lonial, S. , Dimopoulos, M. , Palumbo, A. , White, D. , Grosicki, S. , Spicka, I. , Walter‐Croneck, A. , Moreau, P. , Mateos, M.V. , Magen, H. , Belch, A. , Reece, D. , Beksac, M. , Spencer, A. , Oakervee, H. , Orlowski, R.Z. , Taniwaki, M. , Rollig, C. , Einsele, H. , Wu, K.L. , Singhal, A. , San‐Miguel, J. , Matsumoto, M. , Katz, J. , Bleickardt, E. , Poulart, V. , Anderson, K.C. & Richardson, P. ; ELOQUENT‐2 Investigators . (2015) Elotuzumab therapy for relapsed or refractory multiple myeloma. The New England Journal of Medicine, 373, 621–631. [DOI] [PubMed] [Google Scholar]
- Lu, J. , Lu, J. , Chen, W. , Huo, Y. , Huang, X. & Hou, J. ; Chinese Medical Doctor Association Hematology Branch . (2014) Clinical features and treatment outcome in newly diagnosed Chinese patients with multiple myeloma: results of a multicenter analysis. Blood Cancer Journal, 4, e239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos, M.V. , Ocio, E.M. , Paiva, B. , Rosinol, L. , Martinez‐Lopez, J. , Blade, J. , Lahuerta, J.J. , Garcia‐Sanz, R. & San Miguel, J.F. (2015) Treatment for patients with newly diagnosed multiple myeloma in 2015. Blood Reviews, 29, 387–403. [DOI] [PubMed] [Google Scholar]
- Moreau, P. , Attal, M. & Facon, T. (2015) Frontline therapy of multiple myeloma. Blood, 125, 3076–3084. [DOI] [PubMed] [Google Scholar]
- Moreau, P. , Masszi, T. , Grzasko, N. , Bahlis, N.J. , Hansson, M. , Pour, L. , Sandhu, I. , Ganly, P. , Baker, B.W. , Jackson, S.R. , Stoppa, A.M. , Simpson, D.R. , Gimsing, P. , Palumbo, A. , Garderet, L. , Cavo, M. , Kumar, S. , Touzeau, C. , Buadi, F.K. , Laubach, J.P. , Berg, D.T. , Lin, J. , Di Bacco, A. , Hui, A.M. , van de Velde, H. & Richardson, P.G. ; TOURMALINE‐MM1 Study Group . (2016) Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. The New England Journal of Medicine, 374, 1621–1634. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute . (2006) Common terminology criteria for adverse events (CTCAE) version 3.0. Available from: https://Ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed 31 October 2016.
- National Comprehensive Cancer Network . (2016) Clinical practice guidelines in oncology: Multiple myeloma (version 1.2017). Available from: https://www.nccn.org/professionals/physician_gls/PDF/myeloma.pdf. Accessed 31 October 2016. [DOI] [PMC free article] [PubMed]
- Palumbo, A. & Anderson, K. (2011) Multiple myeloma. The New England Journal of Medicine, 364, 1046–1060. [DOI] [PubMed] [Google Scholar]
- Palumbo, A. , Rajkumar, S.V. , San Miguel, J.F. , Larocca, A. , Niesvizky, R. , Morgan, G. , Landgren, O. , Hajek, R. , Einsele, H. , Anderson, K.C. , Dimopoulos, M.A. , Richardson, P.G. , Cavo, M. , Spencer, A. , Stewart, A.K. , Shimizu, K. , Lonial, S. , Sonneveld, P. , Durie, B.G. , Moreau, P. & Orlowski, R.Z. (2014) International Myeloma Working Group consensus statement for the management, treatment, and supportive care of patients with myeloma not eligible for standard autologous stem‐cell transplantation. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 32, 587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy, A. , Pacanowski, M.A. , Bull, J. & Zhang, L. (2015) Racial/ethnic differences in drug disposition and response: review of recently approved drugs. Clinical Pharmacology and Therapeutics, 97, 263–273. [DOI] [PubMed] [Google Scholar]
- Shyu, V.B. , Wang, P. & Chu, P. (2009) Low incidence of venous thromboembolism in Asian myeloma patients treated with thalidomide plus dexamethasone. Asia‐Pacific Journal of Oncology and Hematology, 2, 41. [Google Scholar]
- Stewart, A.K. , Rajkumar, S.V. , Dimopoulos, M.A. , Masszi, T. , Spicka, I. , Oriol, A. , Hajek, R. , Rosinol, L. , Siegel, D.S. , Mihaylov, G.G. , Goranova‐Marinova, V. , Rajnics, P. , Suvorov, A. , Niesvizky, R. , Jakubowiak, A.J. , San‐Miguel, J.F. , Ludwig, H. , Wang, M. , Maisnar, V. , Minarik, J. , Bensinger, W.I. , Mateos, M.V. , Ben‐Yehuda, D. , Kukreti, V. , Zojwalla, N. , Tonda, M.E. , Yang, X. , Xing, B. , Moreau, P. & Palumbo, A. ; ASPIRE Investigators . (2015) Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. The New England Journal of Medicine, 372, 142–152. [DOI] [PubMed] [Google Scholar]
- Tan, D. , Chng, W.J. , Chou, T. , Nawarawong, W. , Hwang, S.Y. , Chim, C.S. , Chen, W. , Durie, B.G. & Lee, J.H. (2013) Management of multiple myeloma in Asia: resource‐stratified guidelines. The Lancet. Oncology, 14, e571–e581. [DOI] [PubMed] [Google Scholar]
- Turesson, I. , Velez, R. , Kristinsson, S.Y. & Landgren, O. (2010) Patterns of multiple myeloma during the past 5 decades: stable incidence rates for all age groups in the population but rapidly changing age distribution in the clinic. Mayo Clinic Proceedings, 85, 225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda, S.U. , Zhang, L. & Huang, S.M. (2008) The role of ethnicity in variability in response to drugs: focus on clinical pharmacology studies. Clinical Pharmacology and Therapeutics, 84, 417–423. [DOI] [PubMed] [Google Scholar]


