Abstract
Introduction
Coffee is a widely consumed beverage containing antioxidant active compounds. During roasting the phytochemical composition of the coffee bean changes dramatically and highly polymeric substances are produced. Besides chlorogenic acids that are already present in green coffee beans, melanoidins show antioxidant capacity as well.
Objective
To employ post‐column derivatisation by coupling high performance size exclusion chromatography (HPSEC) to an antioxidant assay to investigate the effect of roasting on the properties of antioxidant active compounds in coffee brews.
Methodology
We have investigated the antioxidant capacity of Coffea arabica (Arabica) and C. canephora (Robusta) beans that were roasted over the full spectrum of roast conditions (four roasting speeds to three roast degrees) by comparing the results from HPSEC coupled on‐line to the ABTS assay with those from two batch assays, Folin Ciocalteu (FC) and oxygen radical absorbance capacity (ORAC) assay.
Results
The antioxidant capacity showed a general decrease towards slower and darker roasted coffee for all three assays, indicative of heat degradation of active compounds. Hence, low molecular weight (LMW) compounds such as chlorogenic acids (CGAs) decreased progressively already from relatively mild roasting conditions. In contrast, high molecular weight (HMW) compounds (e.g. melanoidins) increased from light to dark roast degrees with lowering magnitude towards slower roasting profiles.
Conclusion
By coupling HPSEC on‐line to the ABTS assay we were able to separately quantify the contribution of HMW and LMW compounds to the total antioxidant capacity, increasing our understanding of the roast process. © 2016 The Authors. Phytochemical Analysis Published by John Wiley & Sons Ltd.
Keywords: coffee, antioxidants, post‐column derivatisation, HPSEC, Folin–Ciocalteu, ABTS•+, ORAC
Short abstract
The composition of green coffee beans is complex, yet roasting adds to this complexity by producing highly polymeric melanoidins. Next to chlorogenic acids melanoidins also show antioxidant capacity, which was measured with batch assays as well as by on‐line coupling to size exclusion chromatography to investigate the roasting effect on the formation and/or degradation of high and low molecular weight compounds. Adding chromatography enabled us to separately quantify antioxidant activity of high and low molecular weight compounds to increase our understanding of the roasting process.
Introduction
Coffee is a highly valued beverage and is primarily produced commercially by roasting green coffee beans from two species, Coffea arabica (Arabica) and C. canephora (Robusta). The consumption of coffee beverages has been linked to several beneficial health effects, partly because of the high content of phenolic substances (Scalbert et al., 2005; Ludwig et al., 2014). However, many studies on the effects of polyphenols on health have focussed on analyses of their antioxidant properties irrespective of their origin, although there is now growing evidence that their beneficial modes of action are not identical (Ludwig et al., 2014; Vicente et al., 2014). Nevertheless, (poly)‐phenols, mostly of plant origin, have been commonly quantified collectively using one of the many antioxidant assays (Fraga et al., 2010; Lopez‐Alarcon and Denicola, 2013).
Green coffee beans contain large quantities of chlorogenic acids (CGAs), a family of water‐soluble phenols formed by esterification of (−)‐quinic acid with one or more cinnamic acids (Jaiswal et al., 2014). However, besides CGAs, coffee possesses substantial quantities of other molecules that contribute to the total antioxidant capacity, e.g. melanoidins and various volatile compounds (Yu et al., 2013). The polymeric melanoidins are not present in green coffee, but are formed during the roasting process by Maillard and caramelisation reactions, and have been associated with both antioxidant properties and health benefits [for review see (Moreira et al., 2012)]. Whilst melanoidins are formed, the roasting process also results in the loss of some of the CGAs that are either degraded or partly incorporated in the melanoidins maintaining their antioxidant active catechol group (Perrone et al., 2012). Furthermore, other factors associated with coffee preparation, e.g. brewing conditions, such as time, temperature and coffee to water ratio, also influence the compositions of coffee beverages (Voilley and Simatos, 1979).
Many articles have reported changes in coffee antioxidant contents as a function of roasting conditions (e.g. del Castillo et al., 2002; Cämmerer and Kroh, 2006), but the use of different methods/assays makes it difficult to find a consistent interpretation of the various results (Vignoli et al., 2014). In most cases batch assays are used to characterise various coffee brews, even though the values depend also on the choice of reference standard (Opitz et al., 2014).
Three separate assays were used in the present study: (i) coupling of the ABTS assay to high performance size exclusion chromatography (HPSEC), which involves monitoring the reduction of the 2,2‐azinobis‐(3‐ethylbenzothiazoline‐6‐sulphonate) stable radical (ABTS•+) by the sample after separation by size (Re et al., 1999), (ii) the Folin–Ciocalteu (FC) batch assay, which determines the total antioxidant capacity (TAC) (Singleton et al., 1999) by reduction at high pH of “molybdenum blue”, and (iii) the oxygen radical absorbance capacity (ORAC) batch assay, which assesses the ability of the sample to inhibit the reaction between peroxyl radicals and fluorescein (Ou et al., 2001). The principle of the FC and the ABTS assays is measurement of the reduction of an oxidising agent by an electron transfer reaction, which can be monitored photometrically via a change in colour upon reduction, whereas ORAC measures light emission from a fluorescent substrate, where the decay of a fluorescence probe is mediated by competition between a reactive species and antioxidant molecules (Prior et al., 2005). However, many more antioxidant assays have been published, and their advantages and disadvantages are discussed in detail by Apak et al. (2016). One main weakness that all in vitro assays have is their lack of direct biological relevance, since they do not measure bioavailability, in vivo stability, retention by tissues and reactivity in tissues (Huang et al., 2005); nor do they take into account possible activities of secondary products.
We have previously reported results which suggest that the time–temperature history (Smrke et al., 2013), O2 exposure during cooling/quenching (Goodman et al., 2011), and the coffee species (Opitz et al., 2014), all have significant impacts on antioxidant or free radical products of the beverage. However, the simple conduct of batch assays is inadequate for evaluating the contributions of various compounds to measured antioxidant values (Kusznierewicz et al., 2011; Celik et al., 2014). Especially the quantification of melanoidins has proven to be challenging and much effort was made to enhance the understanding of their formation as a function of the roast process (Echavarría et al., 2012). Hence, coupling an antioxidant assay to HPSEC should be helpful to quantify the formation of large molecular weight melanoidins and their contribution to the antioxidant capacity of coffee brews. And by separating them from low molecular weight (LMW) CGAs, the other large antioxidant contributor, relative contributions of high molecular weight (HMW) and LMW compounds can be assessed.
In the present paper, we describe the influence of the full spectrum of roasting conditions on the antioxidant properties of coffee beverages prepared from Arabica and Robusta beans taking into account two main roast parameters, speed of roasting and darkness of the roast, whilst comparing the results from two batch assays and one assay coupled on‐line to HPSEC. In comparison to a previous publication that was dedicated to method development (Smrke et al., 2013), we focussed on applying the HPSEC coupling to explore the full spectrum of possible roasting conditions for coffee. Adding the additional dimension of chromatographic separation according to size improved our understanding how very different roasting conditions affect the total antioxidant capacities of the coffee brews.
Experimental
Chemicals and reagents
5‐Caffeoylquinic acid (5‐CQA) (> 99%) was purchased from Acros Organic (Geel, Belgium), 5‐feruoyl quinic acid (5‐FQA) and 3,5‐di‐caffeoyl quinic acid (3,5‐Di‐CQA) from Chengdu Biopurify Phytochemicals Ltd (Chengdu, Sichuan, China), and gallic acid (GA), ABTS•+, fluorescein, 2,2′‐azobis(2‐methylpropionamidine) dihydrochloride (AAPH), FC reagent, salts and solvents from Sigma‐Aldrich (Buchs SG, Switzerland).
The FC reagent for the assay was prepared by 10× dilution of the commercial 2 M (with respect to acid) FC reagent. ABTS•+ solutions for the on‐line analysis were synthesised by oxidation with potassium persulphate (Re et al., 1999); equal volumes of 7 mM ABTS and 2.45 mM potassium persulphate solutions were mixed and the solution stored before use for at least 15 h at 4 °C. Fluorescein for the ORAC assay was prepared in a 0.37 μg/mL concentration and mixed with cooled AAPH solution. Standard solutions were prepared by dissolving the standards in water followed by appropriate dilution of the stock solution with water.
Coffee roasting
Two species of coffee [Coffea arabica (Arabica) from Costa Rica and C. canephora (Robusta) from Vietnam] were used in this study, and all samples were roasted in a 20 kg batch roaster (Roastmaster™20, Bühler AG, Uzwil, Switzerland). The roasting process was varied in order to produce a variety of roasted coffees covering the whole roasting range. Eight kilograms of green coffee beans were roasted in four profiles: fast (5–6 min), fast‐medium (8–9 min), medium‐slow (11–12 min) and slow (15–18 min), each to light, medium and dark roast degrees (see Tables 1 and 2). In order to obtain these samples, a large number of trial roasts were performed to establish highly reproducible roasting conditions (see Fig. 1 for the Arabica roasting trials; Robusta data not shown). Subsequently, five batches were roasted and the antioxidant analyses were performed on the three most similar ground samples as determined with a colour measuring instrument Colortest II (NeuhausNeotec GmbH, Reinbek, Germany). In addition, mass loss and the humidity content were analysed by gravimetric analysis.
Table 1.
Summary of data for Arabica coffee samples for different roast profiles and roast degrees expressed as the mean value ± standard deviation, (N = 3)
| Roast profile | Roast degree | Roast time (min:sec) | Final roast temperature (°C) | Colour of roasted and ground coffee (Colorette 3) | Mass loss (%) | Humidity (%) |
|---|---|---|---|---|---|---|
| Fast | light | 05:27 ± 0:02 | 200 ± 0 | 105.4 ± 0.9 a | 14.7 ± 0.0 | 2.60 ± 0.1 |
| medium | 05:49 ± 0:04 | 208 ± 0.6 | 77.9 ± 1.1 b | 16.4 ± 0.3 | 2.17 ± 0.03 | |
| dark | 06:22 ± 0:09 | 217 ± 0 | 53.6 ± 0.2 c | 18.8 ± 0.2 | 1.84 ± 0.04 | |
| Fast‐medium | light | 08:08 ± 0:13 | 204 ± 0 | 105.8 ± 0.2 a | 14.5 ± 0.3 | 2.01 ± 0.05 |
| medium | 08:44 ± 0:06 | 213 ± 0.6 | 78.1 ± 0.2 b | 16.6 ± 0.1 | 1.69 ± 0.14 | |
| dark | 09:10 ± 0:09 | 222 ± 1.0 | 53.4 ± 1.0 c | 18.9 ± 0.2 | 1.42 ± 0.06 | |
| Medium‐slow | light | 11:03 ± 0:18 | 203 ± 0.6 | 106.1 ± 0.4 a | 14.0 ± 0.4 | 1.78 ± 0.05 |
| medium | 11:27 ± 0:24 | 215 ± 0.6 | 77.7 ± 1.0 b | 15.9 ± 0.5 | 1.43 ± 0.04 | |
| dark | 11:43 ± 0:09 | 227 ± 1.7 | 53.8 ± 1.0 c | 17.6 ± 0.5 | 1.26 ± 0.05 | |
| Slow | light | 15:12 ± 0:19 | 201 ± 0 | 105.8 ± 0.4 a | 14.9 ± 0.1 | 1.74 ± 0.06 |
| medium | 16:41 ± 0:06 | 213 ± 1.0 | 78.6 ± 0.2 b | 16.8 ± 0.0 | 1.32 ± 0.03 | |
| dark | 17:53 ± 0:06 | 223 ± 0.6 | 53.8 ± 0.8 c | 19.2 ± 0.1 | 1.19 ± 0.08 |
Note: Statistical differences in roast colour were compared using analysis of variance (ANOVA) followed by a t‐test with Holm adjustment, different letters denote significant differences.
Table 2.
Summary of data for Robusta coffee samples for different roast profiles and roast degrees expressed as the mean value ± standard deviation (N = 3)
| Roast profile | Roast degree | Roast time (min:sec) | Final roast temperature (°C) | Colour of roasted and ground coffee (Colorette 3) | Mass loss (%) | Humidity (%) |
|---|---|---|---|---|---|---|
| Fast | Light | 05:11 ± 0:02 | 199 ± 0.6 | 105.4 ± 1.0 a | 13.0 ± 0.08 | 1.50 ± 0.01 |
| Medium | 05:14 ± 0:06 | 208 ± 0.6 | 78.9 ± 0.8 b | 15.0 ± 0.37 | 1.28 ± 0.02 | |
| Dark | 05:32 ± 0:07 | 217 ± 0.6 | 53.9 ± 0.7 c | 16.8 ± 0.09 | 1.31 ± 0.06 | |
| Fast‐medium | Light | 07:54 ± 0:02 | 212 ± 0.0 | 105.6 ± 1.0 a | 12.9 ± 0.00 | 1.41 ± 0.12 |
| Medium | 08:23 ± 0:11 | 220 ± 0.6 | 78.2 ± 0.2 b | 14.5 ± 0.00 | 1.24 ± 0.11 | |
| Dark | 08:26 ± 0:05 | 227 ± 0.6 | 54.9 ± 0.2 c | 16.2 ± 0.00 | 1.21 ± 0.04 | |
| Medium‐slow | Light | 10:55 ± 0:26 | 213 ± 0.0 | 104.7 ± 1.2 a | 14.4 ± 0.00 | 1.25 ± 0.00 |
| Medium | 11:32 ± 0:16 | 222 ± 0.6 | 77.7 ± 0.7 b | 15.5 ± 0.00 | 1.03 ± 0.03 | |
| Dark | 12:03 ± 0:17 | 226 ± 3.5 | 54.2 ± 0.5 c | 17.7 ± 0.01 | 1.07 ± 0.03 | |
| Slow | Light | 14:58 ± 0:40 | 213 ± 0.0 | 105.4 ± 0.6 a | 14.7 ± 0.00 | 1.11 ± 0.02 |
| Medium | 17:00 ± 0:10 | 222 ± 0.0 | 78.3 ± 0.2 b | 16.8 ± 0.00 | 0.99 ± 0.01 | |
| Dark | 17:54 ± 0:13 | 230 ± 0.0 | 54.0 ± 0.6 c | 18.5 ± 0.00 | 0.95 ± 0.05 |
Note: Statistical differences in roast colour were compared using analysis of variance (ANOVA) followed by a t‐test with Holm adjustment, different letters denote significant differences.
Figure 1.
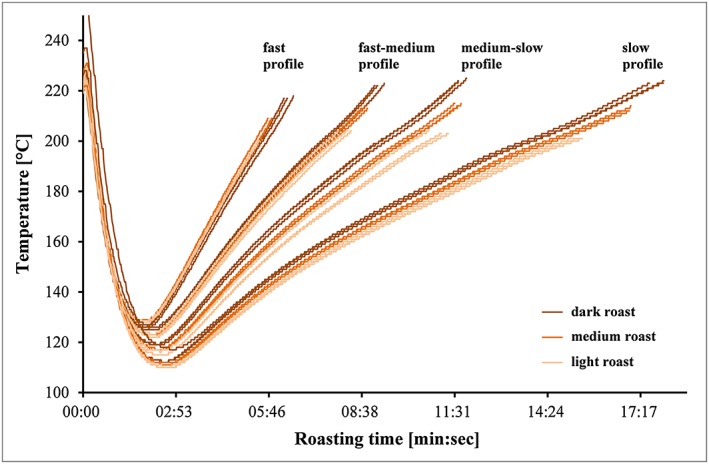
Time–temperature profiles of the different roast trials (N = 3 for each roast degree and N = 9 for each roast profile) that were conducted in this roasting campaign with Coffea arabica coffee. Coffee beans were roasted in four different roast profiles from fast, fast‐medium, medium‐slow to slow, as it is visible with increasing roasting time. Furthermore within each profile coffee beans were roasted to three roasting degrees (light, medium and dark) as it is visible with the increasing approximate temperature of the product. [Colour figure can be viewed at wileyonlinelibrary.com]
Coffee brew preparation
After storing for 12 days in a refrigerator at 4 °C, the coffee samples were ground with an espresso coffee grinder (Ditting KED 640, Bachenbülach, Switzerland) at a coarseness setting of seven. Samples were brewed in triplicate for the antioxidant assays using a 200 mL French press (Bodum, Triengen, Switzerland) in which 12 g of ground coffee powder was infused with approximately 200 mL [mean value 190.5 g ± 1 standard deviation (SD)] of hot water at 92 °C and stirred briefly. The coffee brew was then extracted for 4 min, before it was poured into 250 mL flasks (Schott, Germany) to let cool to room temperature. The extract was also diluted to adjust concentrations to the specific dynamic range of the antioxidant assays.
Antioxidant measurements
HPSEC coupled to on‐line ABTS antioxidant assay
Coffee brews were diluted with water (1:2.5), filtered (0.45 mm PET filters) and separated on a HPLC system (Agilent Series 1200, Santa Clara, CA, USA) using Supermultipore PW‐N HPSEC columns (TSKgel, Tosoh Bioscience, Stuttgart, Germany); the effluent was then coupled to the ABTS assay (scheme in Fig. 2) as described and validated by (Smrke et al., 2013). For size exclusion chromatography (SEC), all measurements were performed on a HPLC system (Agilent Series 1200, Santa Clara, CA, USA) with an eluent of 0.1 M ammonium phosphate buffer adjusted to pH 7 pumped isocratically at 0.4 mL/min, a sample injection volume of 5 μL, and a column oven temperature of 30 °C. The eluent was monitored at 210, 280, 325 and 405 nm using a diode array detector (DAD). An Ismatec ISM8273 peristaltic pump (IDEX Health and Science SA, Glattbrugg, Switzerland) was used for delivering the ABTS•+ reagent with a flow rate of 0.12 mL/min to the effluent via a T‐element after the DAD. The effluent was then passes through a reaction coil at 60 °C in an additional HPLC column compartment, which produces a 30 s delay to adjust and control the reaction conditions. The formation of the reduced ABTS product was monitored with a MWD (multiple wavelength detector, Agilent Series 1100) at 734 nm. Initially, the ABTS•+ concentration was manually set to get an initial baseline absorbance of 0.80 AU (absorbance unit) in order to ensure comparable starting conditions for all experiments. In each method the results are expressed relative to GA (in mg/L), which was used as a standard reference material.
Figure 2.
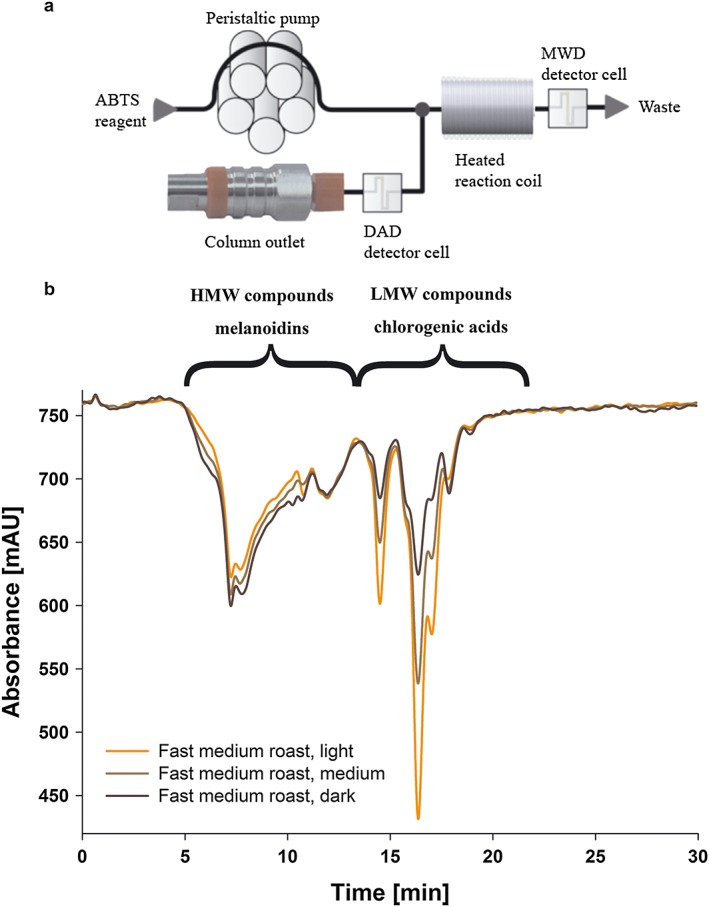
Measurement of decolourisation of ABTS•+ using HPSEC to analyse fast medium roasted Arabica coffee in three roast degrees (light, medium, dark). (a) Flow scheme of the post‐column derivatisation ABTS assay coupled to HPSEC, (b) chromatograms to illustrate the contribution of high molecular weight compounds, melanoidins (4–13 min) and low molecular weight compounds, chlorogenic acids (13–20 min) to the total antioxidant capacity (full integral) of the three roast degrees. [Colour figure can be viewed at wileyonlinelibrary.com]
Batch assays with flow injection analysis
For comparison, two validated assays, FC and ORAC, were employed. The FC assay used a flow injection analysis (FIA) method, while the ORAC assay used a stop flow sequential injection analysis (SIA) method (for details see Opitz et al., 2014). In both cases, the coffee extracts were diluted to adjust the concentration to the specific dynamic range of the assay (1:50 for FC and 1:400 for ORAC), filtered using 0.45 mm PET filters and analysed with a FIAlab 3200 (FiALab Instruments Inc., Bellevue, WA, USA) instrument. In each method the results are expressed as GA equivalents (mg GAE/L) relative to GA, which was used as a standard reference material.
CGA analysis
Green coffee brews were prepared in triplicate as described earlier, and analysed directly by HPLC‐MS (1200 series and 6130 series, Agilent Technologies, Santa Clara, CA, USA). CGAs were separated on a Luna (2) C18 column (3 mm × 150 mm, 3 μm; Phenomenex, Torrance, CA, USA) by running a gradient using eluent A (95% water and 5% acetonitrile, 0.1% FA) and eluent B (95% acetonitrile and 5% water, 0.1% FA), starting with 100% A, then to 98% A in 20 min, to 89% A in 15 min and to 35% A in 10 min, followed by a cleaning and regeneration cycle. Absorbance at 325 nm was used for quantification, and an external calibration curve was prepared using 5‐CQA, 5‐FQA and 3,5‐Di‐CQA as representatives of the three main CGA groups of caffeoyl‐quinic acids, feruoylquinic acids and di‐caffeoylquinic acids. CGA contents were also estimated by HPSEC for green and roasted coffee extracts by integration of area under the curve from 13 min to 20 min, taking the values recorded at 325 nm after calibrating with 5‐CQA.
Statistical analysis
Statistical data analyses were performed with R. One‐way analysis of variance (ANOVA) followed by a pairwise t‐test and Holm adjustment were used to compare the antioxidant values for all roast degrees among the roast profiles. For graphical visualisation of the data SigmaPlot 11.0 (Systat Software) was used.
Results and discussion
The results from the ABTS assay coupled on‐line to HPSEC and the two batch assays for brews prepared from beans roasted according to the various conditions are presented in Fig. 3 for Arabica and in Fig. 4 for Robusta samples. Although there was good reproducibility between replicate samples, there were appreciable differences associated with the coffee species, degree of roast and speed of roasting. Roasting was performed to get to the same roast colour (darkness of roast) and therefore same roast degree using different roast profiles. Therefore roast colours were not significantly different for the roast degrees light, medium or dark, independent if the coffee was roasted with a fast, fast‐medium, medium‐slow or slow roast profile (see Tables 1 and 2).
Figure 3.
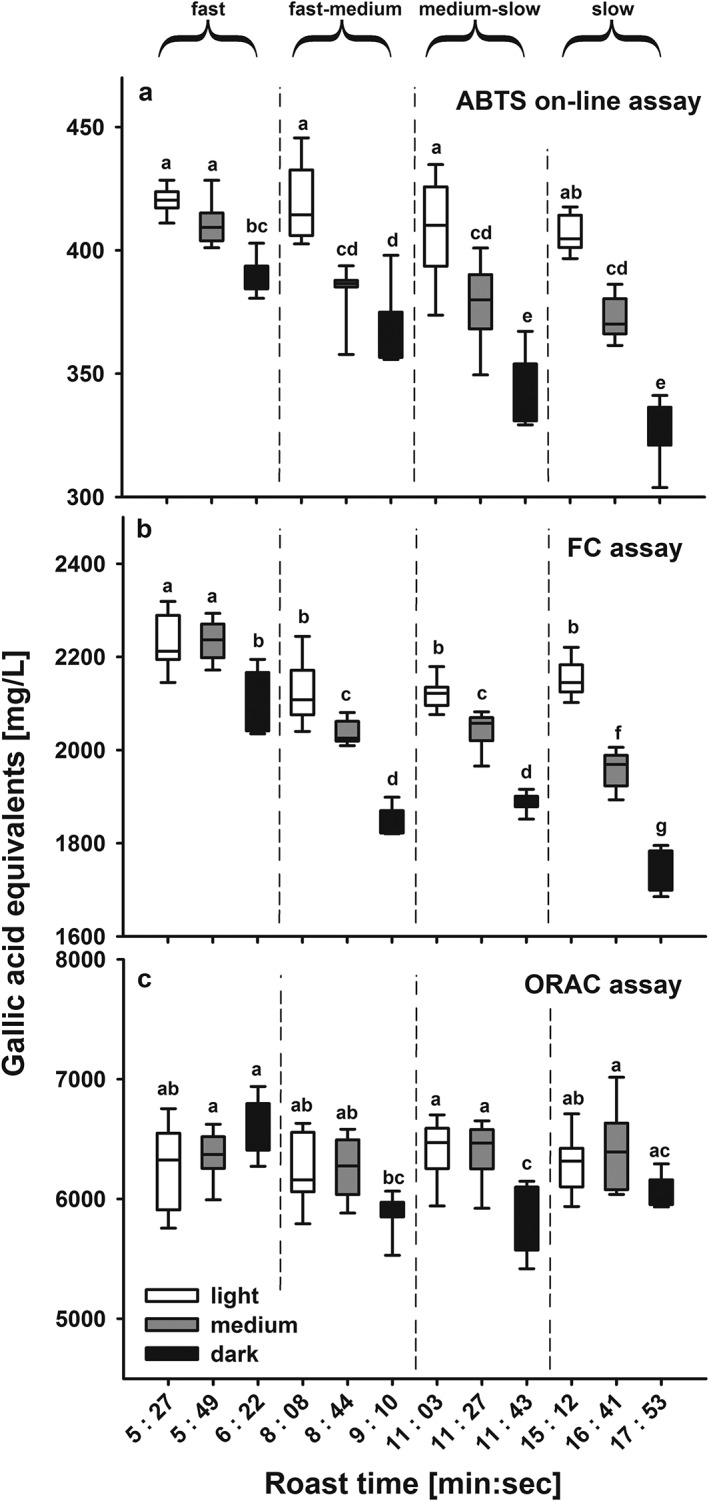
Measured antioxidant capacity as gallic acid equivalents (in mg/L) for Coffea arabica (Arabica) coffee beans as a function of roasting time and degree using (a) the ABTS assay coupled on‐line to HPSEC, (b) the FC batch assay, and (c) the ORAC batch assay. The roasting times varied according to the roasting profiles; which were fast: 5.27–6.21 min; fast‐medium: 8.08–9.10 min, medium‐slow: 11.03–11.43 min, and slow: 15.12–17.53 min. For each roast profile three roast degrees were obtained, the light roast denoted with a light brown colour, the medium roast with a medium brown colour and the dark roast with a dark brown colour. All roasts were roasted in triplicate and each roast brewed in triplicate (N = 9). Statistical differences were compared using ANOVA followed by a t‐test with Holm adjustment, different letters denote significant differences.
Figure 4.
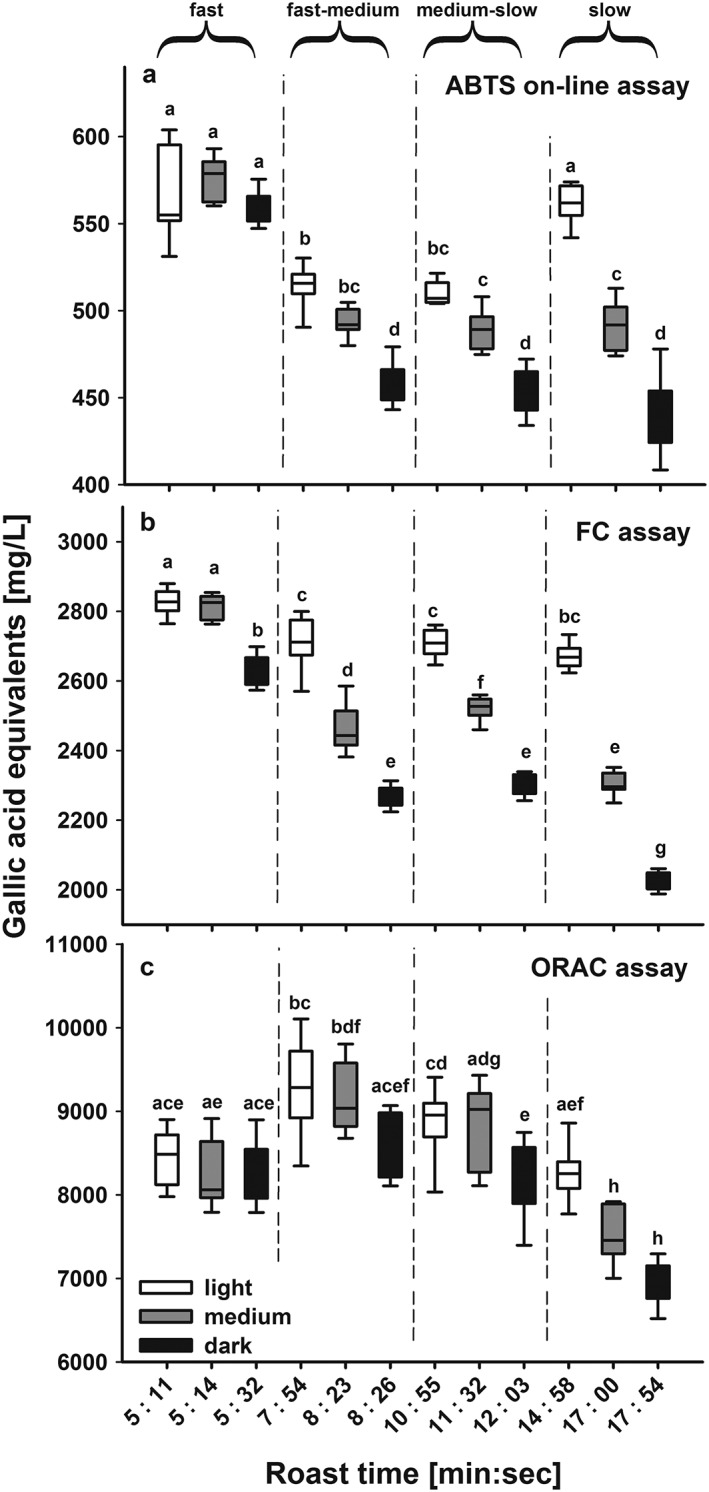
Measured antioxidant capacity as gallic acid equivalents (in mg/L) for Coffea canephora (Robusta) coffee beans as a function of roasting time and degree using (a) the ABTS assay coupled on‐line to HPSEC, (b) the FC batch assay, and (c) the ORAC batch assay. The roasting times varied according to the roasting profiles; which were fast: 5.11–5.31 min; fast‐medium: 7.53–8.25 min, medium‐slow: 10.55–11.37 min, and slow: 14.27–17.54 min. For each roast profile three roast degrees were obtained, the light roast denoted with a light brown colour, the medium roast with a medium brown colour and the dark roast with a dark brown colour. All roasts were roasted in triplicate and each roast brewed in triplicate (N = 9). Statistical differences were compared using ANOVA followed by a t‐test with Holm adjustment, different letters denote significant differences.
Coffee species
For each of the three assays, the results from the Robusta beans were significantly higher than those from the corresponding samples from Arabica beans. Comparing the antioxidant values between both coffee species over the wide range of different roasts, GAE (in mg/L) antioxidant values obtained with the ABTS on‐line assay were on average 1.32 (± 0.07 SD) times higher for Robusta than those for the corresponding Arabica coffee, 1.2 (± 0.04 SD) times for the FC assay and 1.5 (± 0.13 SD) times for the ORAC assay. The assays approximately reflected the 1.5 times higher content of CGAs in green beans of Robusta where the concentrations were 3.09 ± 0.07 g/L in Arabica and 4.72 ± 0.3 g/L in Robusta and are consistent with other reports (e.g. Perrone et al., 2008). Besides the three major caffeoylquinic acid‐isomers (5‐CQA, 3‐CQA, 4‐CQA), three feruoylquinic acids (3‐FQA, 4‐FQA and 5‐FQA) and four di‐caffeoylquinic acids (3,4‐Di‐CQA co‐eluting with 1,5‐Di‐CQA, 3,5‐Di‐CQA, 4,5‐Di‐CQA) were included in the quantification. Quantification of CGAs based on HPSEC‐DAD integral of green Arabica coffee resulted in values of 2.92 ± 0.15 g/L, which is comparable to the HPLC‐DAD concentrations. Although green Robusta coffee contained higher absolute antioxidant values, it also experienced a more pronounced loss of antioxidant capacity during roasting. The range of values between coffees roasted at fast or slow speeds was usually higher for Robusta coffee, e.g. 560 GAE for dark/fast to 430 for dark/slow roasted Robusta coffee compared to 390 GAE for dark/fast to 330 GAE for dark/slow roasted Arabica coffee. This higher relative degradation in Robusta beans can be explained by their higher CGA content, which is prone to degradation during the roasting process. Similarly, the ORAC values for dark/slow roasted Robusta coffee were only around ~75% of the level for light/fast‐medium roasted coffee, whereas dark/slow roasted Arabica coffee was still ~90% of that of the dark/fast coffee.
Antioxidant assays
All three assays showed similar trends with coffee having lower antioxidant values when roasted to darker roasts, which is explainable by degradation of antioxidant active compounds (Vignoli et al., 2014).
HPSEC coupled to ABTS antioxidant assay
The flow scheme as well as chromatograms of light, medium and dark roast degrees of the fast‐medium roast profile are shown in Fig. 2 to illustrate the performance of HPSEC separation coupled to the ABTS assay. On‐line measurements resulted in an overall decrease of antioxidant capacity towards longer roasting times (Fig. 3(a) for Arabica and Fig. 4(a) for Robusta coffee) (Smrke et al., 2013; van der Werf et al., 2014). This decrease was significant regarding roast degree from light to dark roasts of both coffee species, but it was less prominent regarding roast profile, e.g. the light roasts of all four roasting speeds were not different for Arabica. Since Arabica coffee roasted to a light roast did not exceed a maximum temperature of 200 °C during roasting, which would indicate that only above that temperature degradation processes have an effect on ABTS•+ antioxidant activity (Fig. 1). For Robusta coffee, light roasts differed in their antioxidant capacity and light/fast as well as light/slow coffees had higher antioxidant capacity than the medium roasts, although the roast profiles (time–temperature profiles) were different in order to obtain similar roast degrees to the Arabica coffees (see Tables 1 and 2). For other roast degrees, roasting speed affected antioxidant capacity, e.g. for Arabica coffee from around 390 GAE for dark/fast roast to 330 GAE for dark/slow roast (Figs 3(a) and 4(a)).
Adding the additional dimension of HPSEC enabled separate quantification of HMW (essentially melanoidins) and LMW (mainly CGAs) fractions. Assessing the contribution of HMW and LMW fractions to the total antioxidant capacity separately, it became obvious that the HMW fraction always increased with increasing darkness of the roast, despite the overall decrease of antioxidant capacity (Fig. 5(a) for Arabica and Fig. 5(b) for Robusta). This increase with roasting degree is based on increased melanoidin formation, and partly also because of CGA incorporation into melanoidins (e.g. Perrone et al., 2012), which was evident for each roasting profile, but with lesser magnitude from fast to slow. The steepest increase of HMW‐based antioxidant capacity was thereby seen within the fast roasting profile, which indicates that in addition to roast duration, heat load per unit time also increases melanoidin production possibly due to polymer expansion with higher CGA incorporation together with less degradation of the catechol moiety (Echavarría et al., 2012). However, after an initial increase from light to medium roast degree for the slow Robusta profile, antioxidant capacity of the HMW decreased again slightly towards slow/dark roast (Fig. 5(b)), suggesting that the melanoidins are also subject to degradation by heat, and that (some of) their components lose antioxidant activity during roasting. This increase in HMW melanoidin content already at early roast stages could explain why the light Robusta roasts differed between the four profiles, whereas no increase was visible for Arabica coffee (Fig. 5).
Figure 5.
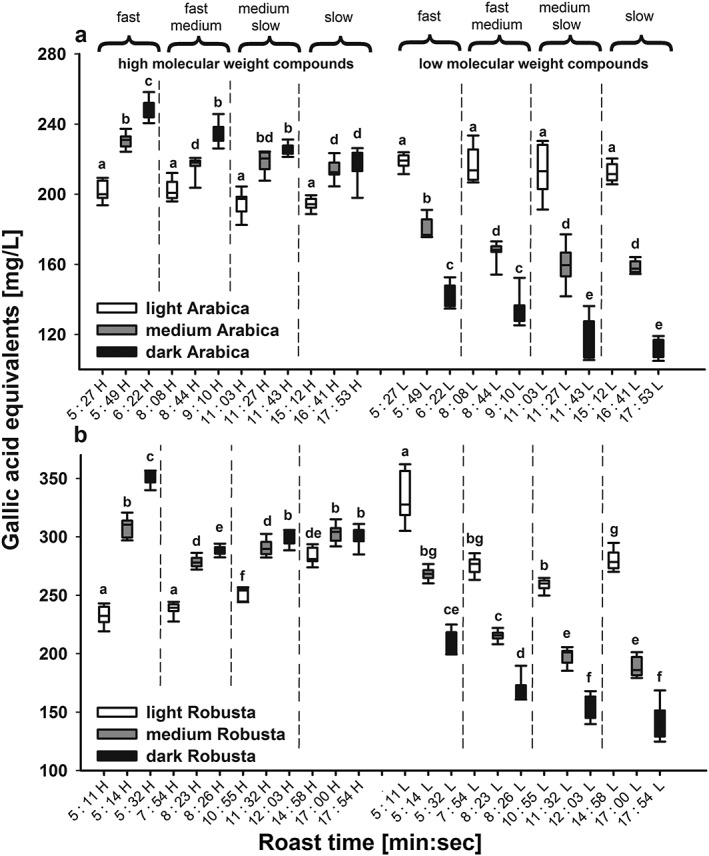
Measured antioxidant capacity as gallic acid equivalents (in mg/L) for (a) Coffea arabica and (b) C. canephora coffee beans as a function of roasting time and degree using the ABTS assay coupled on‐line to HPSEC. For comparison of high to low molecular weight compounds, high molecular weight compounds (H) are given on the left and low molecular weight compounds (L) on the right side of the figure. The roasting times varied according to the roasting profiles; which were fast: 5.27–6.21 min; fast‐medium: 8.08–9.10 min, medium‐slow: 11.03–11.43 min, and slow: 15.12–17.53 min. For each roast profile three roast degrees were obtained, the light roast denoted with a light brown colour, the medium roast with a medium brown colour and the dark roast with a dark brown colour. All roasts were roasted in triplicate and each roast brewed in triplicate (N = 9). Statistical differences were compared using ANOVA followed by a t‐test with Holm adjustment, different letters denote significant differences.
However, the most dominant effect of roasting is the steep decline in LMW compounds with increasing darkness level for all roast profiles and for both coffee species (Fig. 5(a) and 5(b)). This decrease is caused by a decrease in CGA concentrations that are either destroyed or partly incorporated into melanoidins, e.g. in Arabica from 1.4 g/L (light roast/fast profile) to 0.36 g/L (dark roast/slow profile) based on the HPSEC‐DAD integral at 325 nm. The observation of the lowest concentration of CGA in dark/slow roasts also indicates that lower temperatures over a longer time span have a higher impact on CGA degradation than short roasting at higher temperatures. Furthermore, LMW compounds are already prone to degradation at very early stages of the roasting process and the highest antioxidant activities are observed at even lighter roast degrees (Opitz et al., 2014). In addition to incorporation of CGAs in melanoidins, many other new compounds with antioxidant capacity are generated during roasting, especially structural isomers of CGAs (van der Werf et al., 2014).
Batch assays with flow injection analysis
The FC assay is often considered to be most sensitive to phenolic compounds, and since the CGAs are the principal phenols in coffee beans, the results from the FC assay could be considered to approximate to the CGA contents of the coffee brews. For each roasting speed for both coffee species, the FC results showed a progressive decrease in antioxidant value with increasing darkness of the roast with the exception of the light and medium roasted samples prepared using the fast roast profile, where the values are similar at around 2300 GAE for Arabica coffee and 2800 GAE for Robusta coffee (Figs 3(b) and 4(b)). These results are consistent with previous reports (Moon et al., 2009; Smrke et al., 2013). A progressive increase in antioxidant capacity from green coffee beans to a very light roast degree, followed by a decrease towards darker roasts, was reported by (Opitz et al., 2014), which may be partly the consequence of higher extraction efficiencies and increased mass loss during the roasting process. This progressive decrease from light to dark roasted coffee became even more pronounced at slower roasting speed. This is most clearly seen when comparing equivalent roast degrees of the fast and slow roasting profiles (see Tables 1 and 2 for roast colours), whereas the differences between samples from the two intermediate roasting speeds were mostly not significant (Figs 3(b) and 4(b)). For the light roasts, differences between roast speeds were small, and only the fast roasted coffee had significantly higher antioxidant values than the other three light roasts, whereas a decrease in antioxidant activity was measured for the dark roasts. This effect of roast duration could be explained by increased degradation of antioxidant active compounds as a result of longer time for degrading antioxidant active substances.
The trends in the results obtained with the ORAC assay were small for coffee samples of both species with dark roasts tending to be lower than the respective light and medium roast degrees. For Arabica coffee, roasting had little effect on the antioxidant values, although the roast degree still had a higher impact than the roasting profile. In contrast, with Robusta coffee a more prominent decrease was measured with lower roasting speed (Figs 3(c) and 4(c)), and slow roasted coffee exhibited a clear decrease from light to dark roast in accordance with the results from the two other assays. However, this low variation in antioxidant values could be influenced by the longer reaction time of this assay, which also takes account of slow reacting antioxidants, and also its lower precision (Opitz et al., 2014). The advantage of the ORAC assay is its quantification by area‐under‐curve (AUC) measurement, which enables the simultaneous measurement of lag phase, initial rate, and total extent of inhibition of antioxidants (Apak et al., 2016). However, comparably longer reaction times could also allow recycling of oxidised phenolic compounds after polymerisation reactions; this would lead to an over‐estimation of the antioxidant capacity, whereas the presence of metal ions could lead to an under‐estimation of the ORAC values (Nkhili and Brat, 2011).
Relationships between the assays
Increasing roasting time and darkness of roast clearly correlate with lower antioxidant capacities of the coffee brews, as shown for on‐line ABTS as well as FC assay and partly for the ORAC assay. The effect was most clearly visible with the FC assay, which also had the highest precision and lowest variation among replicates (Opitz et al., 2014). The differences observed with the ORAC assay were mostly only of a minor extent, but darker roasts of Robusta coffee showed clearly lower antioxidant values.
More importantly, coupling an antioxidant assay to HPSEC revealed that the relative contents of HMW and LMW compounds are dependent on both the darkness of the roast and speed of roasting, as well as on the coffee bean genetics. By integrating HMW and LMW compounds separately, opposing trends for melanoidins and CGAs during roasting were revealed and CGAs were contributing more to the total antioxidant capacity for the light roasts, whereas melanoidins contributed more at medium and dark roasts. Thus whereas CGAs are transformed or degraded during roasting under mild conditions and reduced by half for the slow/dark roast, melanoidins show increasing antioxidant capacity with darkness of roast; this increase is likely also due to incorporation of CGAs in the melanoidin backbone, but there are probably also contributions from other unknown mechanisms.
While the incorporation of CGAs in melanoidins has been confirmed by many studies, inconsistent results have been published to what extent melanoidins are contributing to total antioxidant capacity (Perrone et al., 2012). Hence, coupling chromatography to antioxidant assays made it possible to reliably quantify only part of the antioxidant mixture, thereby lowering the competitive effect of complex matrices in batch assays. Thus far, mainly reversed phase chromatography methods have been employed to detect individual compounds with antioxidant activity (e.g. De Smet et al., 2015). However, detection and quantification of individual CGAs with HPSEC was possible only to a limited extent due to much lower peak capacities in SEC.
Armed with such knowledge it is possible to adjust the roasting conditions to optimise the overall antioxidant capacity of coffee beverages, and the relative proportions of the major types of antioxidant molecules. As a result of the formation of the highest amounts of melanoidins and lowest amounts of CGA degradation, fast roasting at high temperatures results in a coffee with high antioxidant capacity and low degradation of active compounds, whilst the actual roast degree can be selected to satisfy the preference of the consumer. The speed of roasting does have substantial effects on coffee bean composition and increasing the heat transfer within short roasting times would presumably lead to even higher antioxidant values for fast roasted coffee, although extremely high gas flow rates increases the prospect of product oxidation (Pascual et al., 2002).
Acknowledgements
The authors acknowledge Babette Klopprogge for preparing coffee brews and antioxidant measurements, Sergio Petrozzi for technical assistance. The authors also thank the Commission for Technology and Innovation (CTI) of Switzerland for their financial contribution to the CTI‐project “Modulating the Coffee Roasting Process to Optimize the Antioxidant Potential of Coffee” (Project no. 13897.1 PFIW‐IW).
Opitz, S. E. W. , Goodman, B. A. , Keller, M. , Smrke, S. , Wellinger, M. , Schenker, S. , and Yeretzian, C. (2017) Understanding the Effects of Roasting on Antioxidant Components of Coffee Brews by Coupling On‐line ABTS Assay to High Performance Size Exclusion Chromatography. Phytochem. Anal., 28: 106–114. doi: 10.1002/pca.2661.
References
- Apak R, Ozyurek M, Guclu K, Capanoglu E. 2016. Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and electron transfer (ET)‐based assays. J Agric Food Chem 64: 997–1027. [DOI] [PubMed] [Google Scholar]
- Cämmerer B, Kroh LW. 2006. Antioxidant activity of coffee brews. Eur Food Res Technol 223: 469–474. [Google Scholar]
- Celik SE, Ozyurek M, Guclu K, Capanoglu E, Apak R. 2014. Identification and anti‐oxidant capacity determination of phenolics and their glycosides in elderflower by on‐line HPLC‐CUPRAC method. Phytochem Anal 25: 147–154. [DOI] [PubMed] [Google Scholar]
- De Smet S, Miserez B, Alegre MR, Yapa MT, de Villiers A, Sandra P, Lynen F. 2015. Optimization of a high‐resolution radical scavenging assay coupled on‐line to reversed‐phase liquid chromatography for antioxidant detection in complex natural extracts. J Sep Sci 38: 724–731. [DOI] [PubMed] [Google Scholar]
- del Castillo MD, Ames JM, Gordon MH. 2002. Effect of roasting on the antioxidant activity of coffee brews. J Agric Food Chem 50: 3698–3703. [DOI] [PubMed] [Google Scholar]
- Echavarría AP, Pagán J, Ibarz A. 2012. Melanoidins formed by Maillard reaction in food and their biological activity. Food Eng Rev 4: 203–223. [Google Scholar]
- Fraga CG, Celep GS, Galleano M. 2010. Biochemical actions of plant phenolics compounds: thermodynamic and kinetic aspects In Fraga CG (ed.), Plant Phenolics and Human Health: Biochemistry, Nutrition, and Pharmacology. John Wiley & Sons: Hoboken, NJ; 91–106. [Google Scholar]
- Goodman BA, Pascual EC, Yeretzian C. 2011. Real time monitoring of free radical processes during the roasting of coffee beans using electron paramagnetic resonance spectroscopy. Food Chem 125: 248–254. [Google Scholar]
- Huang D, Ou B, Prior RL. 2005. The chemistry behind antioxidant capacity assays J Agric Food Chem 53: 1841–1856. [DOI] [PubMed] [Google Scholar]
- Jaiswal R, Matei MF, Subedi P, Kuhnert N. 2014. Does roasted coffee contain chlorogenic acid lactones or/and cinnamoylshikimate esters? Food Res Int 61: 214–227. [Google Scholar]
- Kusznierewicz B, Piasek A, Bartoszek A, Namiesnik J. 2011. The optimisation of analytical parameters for routine profiling of antioxidants in complex mixtures by HPLC coupled post‐column derivatisation. Phytochem Anal 22: 392–402. [DOI] [PubMed] [Google Scholar]
- Lopez‐Alarcon C, Denicola A. 2013. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular‐based assays. Anal Chim Acta 763: 1–10. [DOI] [PubMed] [Google Scholar]
- Ludwig IA, Clifford MN, Lean MEJ, Ashihara H, Crozier A. 2014. Coffee: Biochemistry and potential impact on health. Food Funct 5: 1695–1717. [DOI] [PubMed] [Google Scholar]
- Moon JK, Yoo HS, Shibamoto T. 2009. Role of roasting conditions in the level of chlorogenic acid content in coffee beans: Correlation with coffee acidity. J Agric Food Chem 57: 5365–5369. [DOI] [PubMed] [Google Scholar]
- Moreira AS, Nunes FM, Domingues MR, Coimbra MA. 2012. Coffee melanoidins: Structures, mechanisms of formation and potential health impacts. Food Funct 3: 903–915. [DOI] [PubMed] [Google Scholar]
- Nkhili E, Brat P. 2011. Reexamination of the ORAC assay: Effect of metal ions. Anal Bioanal Chem 400: 1451–1458. [DOI] [PubMed] [Google Scholar]
- Opitz S, Smrke S, Goodman B, Keller M, Schenker S, Yeretzian C. 2014. Antioxidant generation during coffee roasting: A comparison and interpretation from three complementary assays. Foods 3: 586–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou B, Hampsch‐Woodill M, Prior RL. 2001. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J Agric Food Chem 49: 4619–4626. [DOI] [PubMed] [Google Scholar]
- Pascual EC, Goodman BA, Yeretzian C. 2002. Characterization of free radicals in soluble coffee by electron paramagnetic resonance spectroscopy. J Agric Food Chem 50: 6114–6122. [DOI] [PubMed] [Google Scholar]
- Perrone D, Farah A, Donangelo CM. 2012. Influence of coffee roasting on the incorporation of phenolic compounds into melanoidins and their relationship with antioxidant activity of the brew. J Agric Food Chem 60: 4265–4275. [DOI] [PubMed] [Google Scholar]
- Perrone D, Farah A, Donangelo CM, de Paulis T, Martin PR. 2008. Comprehensive analysis of major and minor chlorogenic acids and lactones in economically relevant Brazilian coffee cultivars. Food Chem 106: 859–867. [Google Scholar]
- Prior RL, Wu XL, Schaich K. 2005. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem 53: 4290–4302. [DOI] [PubMed] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice‐Evans C. 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26: 1231–1237. [DOI] [PubMed] [Google Scholar]
- Scalbert A, Manach C, Morand C, Remesy C, Jimenez L. 2005. Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr 45: 287–306. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela‐Raventos RM. 1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent In Oxidants and antioxidant, PT A, Packer L. (ed.). Elsevier: San Diego, CA; 152–178. [Google Scholar]
- Smrke S, Opitz SEW, Vovk I, Yeretzian C. 2013. How does roasting affect the antioxidants of a coffee brew? Exploring the antioxidant capacity of coffee via on‐line antioxidant assays coupled with size exclusion chromatography. Food Funct 4: 1082–1092. [DOI] [PubMed] [Google Scholar]
- van der Werf R, Marcic C, Khalil A, Sigrist S, Marchioni E. 2014. ABTS radical scavenging capacity in green and roasted coffee extracts. LWT Food Sci Technol 58: 77–85. [Google Scholar]
- Vicente SJV, Ishimoto EY, Torres E. 2014. Coffee modulates transcription factor Nrf2 and highly increases the activity of antioxidant enzymes in rats. J Agric Food Chem 62: 116–122. [DOI] [PubMed] [Google Scholar]
- Vignoli JA, Viegas MC, Bassoli DG, Benassi MD. 2014. Roasting process affects differently the bioactive compounds and the antioxidant activity of arabica and robusta coffees. Food Res Int 61: 279–285. [Google Scholar]
- Voilley A, Simatos D. 1979. Modeling the solubilization process during coffee brewing. J Food Proc Engin 3: 185–198. [Google Scholar]
- Yu XY, Zhao MY, Liu F, Zeng ST, Hu J. 2013. Antioxidants in volatile Maillard reaction products: Identification and interaction. LWT Food Sci Technol 53: 22–28. [Google Scholar]


