Summary
Background
Eosinophilic oesophagitis (EoE) is a chronic disease, driven by food allergens. Elemental diets are effective for the management of children with EoE, but studies on the effect of elemental diets in adults are scarce and poor palatability challenges dietary adherence.
Aim
To assess the effects of an elemental diet (Neocate, Nutricia, Utrecht, the Netherlands) on the inflammation, symptoms and endoscopic signs in adult EoE patients.
Methods
In this prospective study, 21 patients with active EoE, confirmed by biopsies showing ≥15 eosinophils per microscopic high power field (HPF) and symptoms of oesophageal dysfunction were included. Patients underwent endoscopy before and 4 weeks after diet. Histological disease activity (peak eosinophil count/HPF), and endoscopic signs were scored by physicians. Symptoms and adherence to the diet were evaluated by questionnaires. Serum total IgE levels and total eosinophil counts were determined and the expression of inflammatory cytokines was analysed by qPCR.
Results
In total, 17 (81%) of the patients completed the diet, of whom 12 (71%) showed complete histological response (≤15 eosinophils/HPF) and 4 (24%) showed partial histological response (≥50% reduction of baseline eosinophil count). Peak eosinophil counts decreased significantly after the diet from 40 to 9 per HPF (P ≤ 0.001). A marked improvement in endoscopic signs was observed. Symptoms decreased significantly in all subjects, and 15 patients (88%) became completely asymptomatic (P ≤ 0.001). In 14 patients (82%), blood eosinophil count and serum IgE decreased (P ≤ 0.05).
Conclusion
Elemental diet reduces eosinophilic inflammation and induces clinical remission in adult patients with eosinophilic oesophagitis.
Introduction
Eosinophilic oesophagitis (EoE) is a chronic immune‐mediated disease of the oesophagus, clinically characterised by dysphagia and food impaction.1 There has been a substantial increase in prevalence of EoE in the Western world, and EoE has become the main cause of dysphagia in children and young adults.2, 3, 4 Although the pathophysiology of EoE is complex and only partly understood, food allergens are thought to be the trigger of a T‐helper 2 (Th‐2) cell‐mediated immune response, whereby associated inflammatory cytokines can initiate or maintain the process of tissue remodelling of the oesophagus.5, 6 The theory of EoE being an allergy‐driven disease is further supported by the studies showing that EoE can be brought in remission by dietary management.7
EoE can be managed using pharmaceutical [(topical) corticosteroids)] and/or dietary therapy. Topical steroids are moderately effective in paediatric and adult EoE patients.8, 9 However, side effects preclude long‐term use, and since the underlying cause is not affected, the inflammation recurs once the (topical) steroid is withdrawn.8, 10, 11 Studies have shown that, without treatment, progressive narrowing of the oesophagus occurs.12 Dietary elimination of disease‐triggering allergens could provide a long‐term, drug‐free and sustainable solution, which is desirable since EoE is a chronic disease and most patients are children or young adults. The three main approaches in dietary management are (i) allergy test‐directed elimination, based on positive allergy test results, (ii) empiric elimination, based on most common allergic triggers (i.e. milk, wheat, egg, soy, peanuts/tree nuts, and fish/shellfish) and (iii) elimination of all table foods and substitution by an exclusively elemental (amino acid‐based) diet. A recent systematic review showed that empiric elimination diets yield moderate response rates (71%), whereas the effect of test‐directed elimination diets is questionable (45%).13 On the other hand, elemental diets are highly effective in children, showing rapid resolution of symptoms and histological remission in more than 95% of the patients.2, 13, 14 The effect of an elemental diet has only been investigated once in adult patients with EoE.15 In that study by Peterson et al., the elemental diet induced complete histological disease remission in 72% of the patients. However, symptoms did not improve and drop‐out rates were rather high (38%). Patient adherence to an elemental diet is challenging, not only in adults but also in children, due to unpalatability and monotony of the formula. Nevertheless, over the past decade the quality and variability in the formulas has improved. More research on the effect and feasibility of elemental diets in adult patients with EoE is thus required.
The aim of this study was to assess the efficacy of an exclusive elemental diet (Neocate Nutricia, Utrecht, the Netherlands) on the eosinophilic inflammation, symptoms and endoscopic signs in adult EoE patients. In addition, we evaluated patient adherence to a new improved formula with two available flavours.
Methods
Study subjects
In this prospective study, patients were included between December 2014 and September 2015. Subjects were recruited from the out‐patient clinic of the Academic Medical Centre in Amsterdam, a referral hospital for EoE in the Netherlands. Adults were eligible for enrolment if EoE was diagnosed according to current guidelines, defined as typical symptoms of EoE (dysphagia and/or food impaction), and presence of more than 15 eosinophils per high power field (HPF) during acid‐suppressant treatment.16 Patients were excluded if they had a severe comorbidity scored as ASA class III or higher, a history of other gastrointestinal diseases, had undergone surgery of the digestive tract (except from appendectomy), or were unable to stop anticoagulants or topical or systemic steroids in the two months preceding the study and during the study. The study protocol was approved by the local Medical Ethics Committee of the institution and registered in the Dutch trial registry (Trialregister.nl NTR4892).
Study protocol
After signed informed consent, patients visited a dietician with expertise in EoE. Patients' eating habits, dietary eliminations and metabolic needs were analysed, using a complete 3‐day food diary. Although the amino acid‐based formula provides complete nutrition, considerable weight loss has been described as associated to its use.15 To guarantee sufficient intake, the minimal daily formula consumption was calculated based on a patients' body mass index and weekly physical activity. To diminish drop‐out rates, a 24‐h elemental diet test day was incorporated in the study protocol, to enable patients to take a weighted decision concerning study participation. During the diet, patients were carefully monitored by means of a weekly consult with their dietician or physician who evaluated body weight, side effects and patients' adherence to the diet. Adherence was monitored by means of weekly questionnaires in which patients reported protocol violations and daily formula consumption. Subsequently after the trial, patients specified their level of formula acceptance on a completely acceptable‐unacceptable Likert scale, in which 0 represented completely acceptable and 6 represented completely unacceptable. All participants underwent a baseline endoscopy and a follow‐up endoscopy after 4 weeks of elemental diet (Neocate Nutricia, Utrecht, the Netherlands). Before the endoscopies, patients underwent a venepuncture to obtain blood samples for evaluation of serum total immunoglobulin E (IgE) levels and total eosinophil counts.
Outcome parameters
The primary endpoint of this study was the proportion of patients in histological disease remission, defined as a peak eosinophil counts of less than 15 eosinophils/HPF (complete remission).17 Partial responders were patients with ≥15 eosinophils/HPF after diet, but with a decline of more than 50% of pre‐diet peak eosinophil count. Other secondary study endpoints were symptoms, quality of life (QoL), endoscopic features, serum IgE levels and serum total eosinophil counts and expression of genes encoding for inflammatory cytokines.
Symptoms of dysphagia were measured per questionnaire recording the frequency and severity of dysphagia on a 5‐point Likert‐type scale analogous to the Straumann Dysphagia Instrument (SDI). The total scores were composed of a sum of the frequency of symptoms, where 0 represents no symptoms in the past week and 5 represents daily symptoms, and the severity of symptoms where 0 represents no complains and 5 represents severe complains. Total scores ranged from 0 to 10. The Straumann Dysphagia Instrument is a questionnaire that has been used previously in placebo‐controlled clinical EoE trials.18, 19 To give an accurate estimation of dysphagia, the modified Straumann Dysphagia Instruments were obtained before and 1 week after completing the diet, when solid food were reintroduced, since it is difficult to measure dysphagia on a liquid diet. EoE is associated with a wide variety of symptoms, therefore, a validated Reflux Disease Questionnaire (RDQ) was obtained before and after diet.20 To evaluate the impact of the disease and the diet on the patients' quality of life (QoL) the validated Dutch Version 1.2 of the SF‐36 QoL questionnaire was completed before and after diet.21 This questionnaire measures health‐related QoL in eight domains: physical functioning, role of limitations due to physical problems, bodily pain, general health, vitality, role of limitations due to emotional problems, social functioning, and mental health. In addition to the pre and post‐diet measurements, QoL of the included subjects was also compared with QoL of a national reference cohort. Furthermore, during the first visit at the out‐patient clinic, past and current concomitant atopic diseases (i.e. allergic rhinitis, asthma, atopic dermatitis, food allergy), family history of atopic diseases, past treatments for EoE and patient characteristics were retrieved.
The elemental (amino acid‐based) diet
In this trial, a newly developed amino acid‐based formula was used (Neocate Nutricia, Utrecht, the Netherlands). This product is suitable and nutritionally adequate for adults when used as a short‐term sole source of nutrition. The formula was available in pineapple/orange or grape flavour and each package contained 237 kcal. Patients were only permitted to drink water or black/green tea without sugar during the diet. Since a full liquid diet diminishes chewing and salivary flow, and since saliva is essential for oral health, patients were allowed to use sugar‐free chewing gum, which is also beneficial to stimulate the oromotor function.22
Oesophagogastroduodenoscopy
The endoscopies were performed by a single gastroenterologist, according to a standardised protocol. Six biopsies were taken for histopathology and gene expression analysis. Endoscopic pictures of the oesophagus were recorded for evaluation of macroscopic signs, these pictures where blinded and incorporated in a slideshow (Microsoft PowerPoint 2010; Microsoft Inc., Redmond, WA, USA). Subsequently, the same gastroenterologist scored the depersonalised endoscopic images according to the Endoscopic Reference Score (EREFS).23 As previously formulated by Schoepfer et al., endoscopic features of EoE were sub‐classified as (i) inflammatory signs including whitish exudates, oedema, and linear furrows, and as (ii) fibrostenotic signs including rings, strictures and crêpe paper oesophagus.12
Histopathological analysis
Oesophageal biopsies, taken approximately 10–15 cm above the squamocolumnar junction were directly fixed in formalin and subsequently embedded in paraffin. After 24 h the biopsies were sectioned at 7 microns and stained with haematoxylin and eosin and tryptase. The biopsies were analysed by a gastrointestinal pathologist with special expertise in EoE, blinded to the patients' treatment status. First the area of most densely populated eosinophilia was determined in a low‐power view setting, subsequently a 400× magnification was used and the peak eosinophil count was determined per HPF (an area of 0.24 mm2). The peak mast cell counts were analysed likewise. In addition to the eosinophil count, we also evaluated (i) the presence of eosinophilic microabscesses (clusters of ≥4 eosinophils), (ii) basal hyperplasia, graded on a scale of 0 (absent hyperplasia) to 3 (severe hyperplasia in two upper third of total epithelial thickness) and (iii) spongiosis, which was scored analogous to basal hyperplasia.24 All analyses were performed using an Olympus BX41 microscope (Olympus Europe, Hamburg, Germany).
qPCR
During the endoscopy, two additional oesophageal biopsies were taken and immediately immersed in RNA stabilisation reagent (RNAlater; Qiagen, Hilden, Germany). The first 24 h the biopsies were stowed at 4 °C, before storing them at −80 °C until analysis. RNA was extracted from homogenised biopsies according to the manufacturer's instructions of RNeasy Micro Kit (Qiagen). The Nanodrop Spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA) calculated the concentration of RNA and subsequently cDNA was synthesised by making use of a reverse transcriptase reaction that was performed according to the MBI Fermentas cDNA synthesis kit (Fermentas, Vilnius, Lithuania), using both the Oligo (dT) 18 and the D (N) 6 primers. Quantitative real‐time RTPCR was performed on the LightCycler 480 (Roche Diagnostic, Almere, The Netherlands) using SYBR Green PCR Master Mix (Roche Diagnostic) and primers from Invitrogen (Life Technologies Corporation, Carlsbad, CA). For quantitative real‐time PCR, samples were normalised for the mean of the two most stable housekeeping genes (Cyclophilin and Hypoxanthine‐Guanine Phosphoribosyl Transferase) as determined by analysis with geNorm method software. Transcript levels of interleukin‐5 (IL5), interleukin‐13 (IL13), eotaxin‐3 (CCL26), periostin (POSTN) and thymic stromal lymphopoietin (TSLP), were determined in duplicate.
Optional four‐food reintroduction schedule
Once remission was established after 4 weeks of elemental diet, patients were offered a stepwise food reintroduction schedule outside this study protocol, with the objective of identifying the disease‐triggering foods and establishing a normal diet. The four most common allergic triggers for EoE (i.e. milk, soy, wheat and egg) completed with patient‐specific allergenic foods, as determined by diet history, were sequentially reintroduced, starting with the food least likely to trigger EoE. First, soy was reintroduced, followed by egg, wheat and finally milk. Each food group was introduced during 1–2 weeks, as Peterson et al. showed that eosinophils increased within a week after introduction of the allergic food.15 After reintroduction of every food group, symptoms were evaluated using the modified Straumann Dysphagia Instrument. Endoscopies were optional during the food reintroduction phase. If symptoms did not recur and/or if peak eosinophil counts were less than 15/HPF within 2 weeks after reintroduction of a food group, this group was considered to be accepted. If symptoms recurred, reintroduction of the subsequent food group was postponed, and an endoscopy was recommended to determine histological disease activity. Patients could also decide to re‐eliminate the symptom‐provoking food group and evaluate whether symptoms dissolved again. This process was continued until all food groups were tested in the reintroduction phase.
Sample size calculation
A study by Peterson et al., that had a study design comparable to that of our study, demonstrated a significant effect of elemental diet in 18 adult EoE patients.15 They observed a decrease in the peak eosinophil count from 54 at baseline, with an estimated standard deviation of 32, to less than 10 eosinophils after diet (a difference of 44). The standard deviation of this difference was not reported. However, since the number of eosinophils after diet is bounded below 10, the standard deviation of the mean difference will be driven by the standard deviation at baseline. Since we expected a comparable histological response, we estimated our sample size based on a comparable delta. We defined a decrease in peak eosinophil count to less than 15 eosinophils/HPF as a meaningful and clinically significant reduction. Based on an expected mean difference of 39 (a decrease in eosinophil count from 54 to ≤15), a standard deviation of 40, an alpha of 0.05 and a power of 90%, we needed to evaluate at least 14 subjects.
Statistical analysis
Statistical analysis was performed using IBM spss Statistics version 22.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics were used to summarise findings. Categorical variables were described as percentages and continuous variables were expressed as median with interquartile ranges. Before and after diet, proportions and binary data were compared using McNemar's test. Ordinal data were compared using the Wilcoxon's signed rank test for variables that were not normally distributed. Data were compared between responders and partial/nonresponders using a Mann–Whitney U test or a chi‐squared test. We calculated correlations using Spearman correlation coefficient.
Results
Inclusion and patient characteristics
Thirty‐four EoE patients, who presented with clinically active disease and were otherwise also eligible for participation, were invited for a visit at the out‐patient clinic. After the test day, nine patients dropped out because they judged the elemental diet formulation to be unpalatable (n = 7), because of personal circumstances (n = 1) or because their use of an anticoagulant could not be interrupted (n = 1). Eventually, 25 patients were enrolled and underwent the first endoscopy, after which another four patients were excluded due to spontaneous disease remission at baseline (≤15 eosinophils/HPF). During the first two weeks of the diet, two patients dropped out because of unpalatability and the life style modifications that came along with the diet. One patient tolerated the diet but became very anxious about the second endoscopy and cancelled the procedure. In summary, of the 21 patients who started the diet, 18 patients underwent the second endoscopy. Finally, 17 patients were included for final analyses, since one patient was excluded due to poor adherence. An inclusion flow chart is shown in Figure 1. In our cohort, there was a male predominance, most patients were Caucasian, and most had a history of concurrent atopic diseases. Patient characteristics are listed in Table 1.
Figure 1.
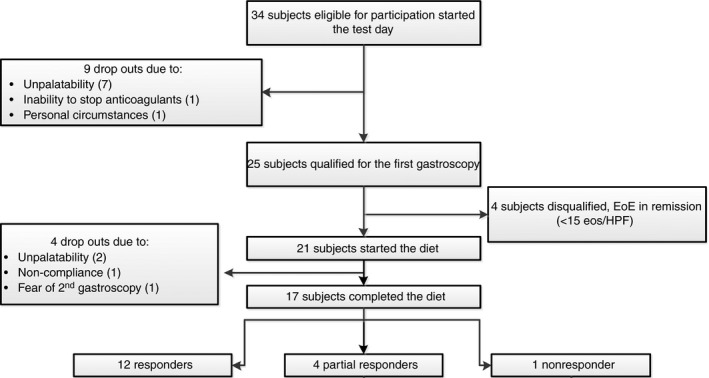
Flow chart showing the number of patients that were eligible for participations and patients who discontinued the trial. Of the 17 patients that completed the diet, 12 (71%) showed complete histological response (≤15 eosinophils per HPF) and 4 (24%) patients showed partial histological response (≥50% reduction of pre‐diet eosinophil counts), one patients was classified as a nonresponder.
Table 1.
Patients characteristics of the patients who completed the trial (n = 17)
| Gender, male | 12 (71%) |
| Age | 42 s.d. 12.5 years |
| Race, Caucasian | 13 (77%) |
| Body Mass Index | 24 (22–26) |
| Atopic diathesis | 12 (71%) |
| Food allergy | 8 (47%) |
| Asthma | 9 (53%) |
| Allergic rhinitis | 10 (59%) |
| Atopic dermatitis | 3 (18%) |
| Symptoms of acid regurgitation or heartburn | 10 (59%) |
| Prior topical steroid use | 10 (59%) |
| History of oesophageal stricture dilatation | 1 (6%) |
| Seasonal variation in dysphagia | 0 (0%) |
| Time since diagnosis | 3 (1.5–6.5) years |
| Diagnostic delaya | 5 (0–24) years |
| Weight loss after diet | 1.4 (0.9–2.4) kg |
Diagnostic delay is the time interval between the first symptoms and the diagnosis.
Histopathological response
Seventeen (81%) of the 21 patients who started the diet completed the trial, of whom 12 (71%) showed complete histological response (≤15 eosinophils per HPF) and another four (24%) patients showed partial histological response (≥50% reduction of pre‐diet eosinophil counts). In addition, all complete responders also had a reduction of more than 50% of pre‐diet eosinophil count. One patient was classified as nonresponder since there was still a remarkable oesophageal eosinophilia after diet, despite a reduction in peak eosinophil count of 33%. On a group level, peak eosinophil counts decreased significantly from 40 (29–80) to 9 (1.5–17.50) per HPF after diet, (P < 0.001). We found no significant effect of the diet on peak mast cell counts (Figure 2).
Figure 2.
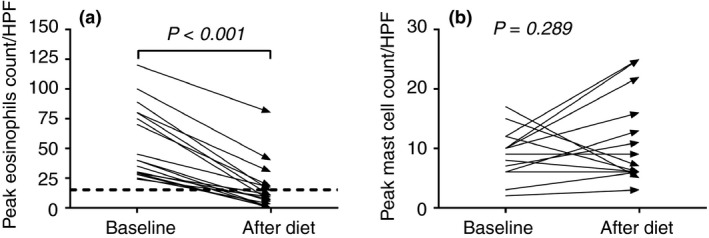
The left graph (a) represents the peak eosinophil counts per HPF of the included patients (n = 17) at baseline and after 4 weeks of elemental dietary treatment. There is a significant decline in peak eosinophil count after treatment (P < 0.001). The dotted line indicates the threshold of active EoE (>15 eos/HPF). The right graph (b) represents the peak mast cell count per HPF at baseline and after 4 weeks of elemental dietary treatment. We observed no significant difference in peak mast cell counts after treatment (P = 0.816).
The number of patients with moderate to severe spongiosis decreased significantly from 13 (76%) to five (30%) after the diet (P = 0.015). However, complete resolution of spongiosis after the diet was observed in a minority of patients (6%). An analogous decrease in basal layer hyperplasia was observed; 13 (76%) patients had moderate to severe basal hyperplasia before the diet, whereas after the diet, basal cell hyperplasia was mostly mild (65%) or absent (24%) (P = 0.006). Eosinophilic microabscesses were present in nine (53%) patients before the diet, and in two (12%) patients after diet (P = 0.008). Results are presented in Table 2.
Table 2.
Histopathological features before and after the diet
| Baseline, median (IQR) | After diet, median (IQR) | P‐value | |
|---|---|---|---|
| Peak eosinophil count | 40 (29–80) | 9 (1.5–17.50) | <0.001 |
| Peak mast cell count | 10 (6–12) | 7 (6–15.50) | 0.816 |
| Spongiosis | Present % | Present % | |
| None | 0 | 6 | 0.015 |
| Mild | 24 | 65 | |
| Moderate | 47 | 24 | |
| Severe | 29 | 5 | |
| Basal hyperplasia | |||
| None | 0 | 22 | 0.006 |
| Mild | 22 | 61 | |
| Moderate | 56 | 6 | |
| Severe | 22 | 11 | |
| Microabscesses | 53 | 12 | 0.008 |
IQR, interquartile range.
Baseline eosinophil counts were significantly higher in nonresponders and partial responders compared with the complete responders (P = 0.013). Apart from baseline eosinophil count, there were no significant differences between both groups regarding symptoms, patient characteristics or medical history.
Endoscopic features
At baseline, oedema was the most prominent endoscopic feature, observed in more than 90% of patients, followed by rings and furrows (both present in 82%). In all patients, at least one endoscopic feature was recorded at baseline. In 16 (94%) patients, endoscopic features improved after the diet. According to the EREFS, total endoscopic scores decreased from 7 (4–8) to 3 (2–6) after the diet (P < 0.001). The inflammatory scores decreased significantly from 4 (3–5) to 2 (1–3) (P < 0.001), whereas the fibrostenotic scores were not significantly reduced by the diet. The total endoscopic scores were positively correlated with the peak eosinophil counts (r = 0.61, P < 0.01). Results are presented in Table 3 and Figure 3.
Table 3.
Endoscopic features before and after the diet
Figure 3.
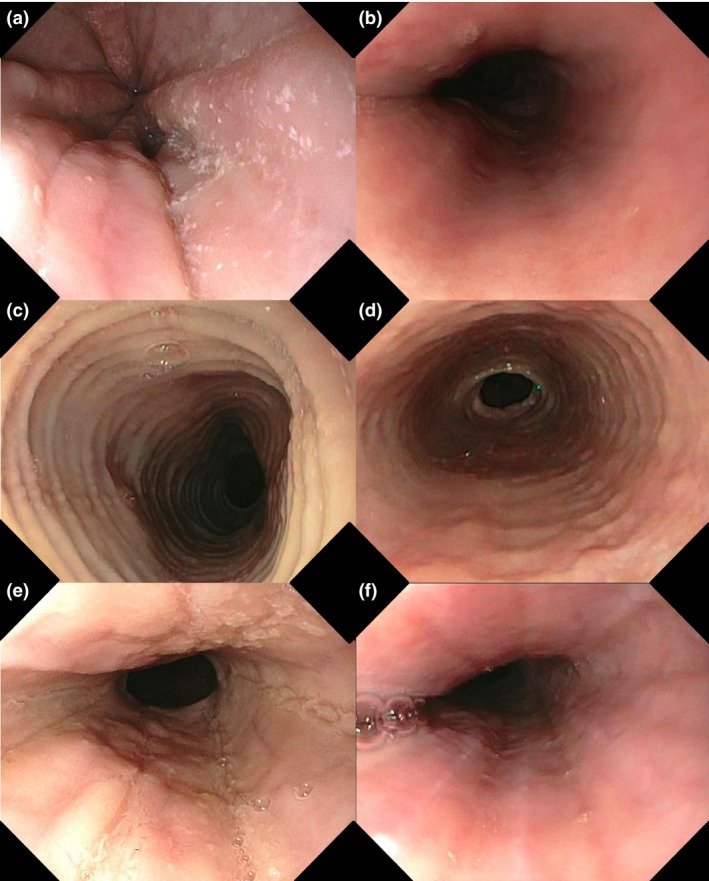
Endoscopic features of EoE and the effect of the elemental diet on these endoscopic features. Paired endoscopic images of three patients at baseline and after diet are presented, (a and b) patients 1, (c and d) patient 2, and (e and f) patients 3. The baseline images showing (a) white exudates, (c) concentric rings and (e) and longitudinal furrows. After successful elemental dietary treatment (<15 eos/HPF) the images are showing a reversibility of (b) white exudates, (d) concentric rings and (f) longitudinal furrows.
Symptoms and adherence
Initially, most patients presented with food impactions or severe dysphagia, and 13 (77%) patients had multiple dysphagia episodes per week. The modified Straumann Dysphagia Instrument decreased significantly from 6 (3–8) at baseline to 0 (0–0), measured 1 week after the diet, and 15 (88%) patients became completely asymptomatic. Apart from dysphagia and food impactions, a majority of patients suffered from chest pain and/or heartburn at baseline, and the Reflux Disease Questionnaire scores decreased from 9 (3.5–20) to 1 (0–8) after the diet. In two patients with coexisting active atopic dermatitis, the skin lesions dissolved completely during the diet, and in another patient with allergic rhinitis, there was a substantial improvement of rhinitis symptoms. The modified Straumann Dysphagia Instrument scores were positively correlated with the peak eosinophil counts (r = 0.55, P < 0.01). The patients' formula acceptance score was 1 (0–4), which represents mostly acceptable. The adherence questionnaires reported no protocol violations. Nevertheless, most patients had a lower formula consumption than was prescribed by the dietician. On weekly average patients consumed 3.6 packages too little. Reported weight loss was 1.4 (0.9–2.4) kilograms (kg).
With regard to the quality of life questionnaires, we observed that during the diet patients experienced a significant decline in social functioning [from 100 (75–100) at baseline to 75 (63–100) after diet, P = 0.01]. There was a substantial reduction in bodily pain after diet, which was scored conversely, the higher the score the lower the bodily pain, from 72 (44–96) at baseline to 84 (65–100) after diet, (P = 0.08), although it did not reach statistical significance. No differences were found in the other domains of the questionnaire. Comparison of the pre‐ and post‐diet quality of life scores of the EoE patients to the reference scores of the Dutch population showed that physical functioning and mental health scores after the diet were significantly higher than the reference values (physical functioning 92 ± 10.4 compared to 83 ± 23 and mental health 84 ± 13 compared to 77 ± 17, both P < 0.05).
Biomarkers and gene expression of inflammatory cytokines
The biomarkers and expression of cytokines related to EoE were investigated. Blood eosinophil count decreased significantly from 0.23 (0.14–0.55) to 0.14 (0.09–0.26) after the diet (P = 0.002). Serum total IgE decreased correspondingly, from 121 (41–353) to 108 (35–298) after the diet (P = 0.03). The diet significantly reduced the relative expression of the genes encoding the inflammatory cytokines IL‐5, IL‐13, POSTN and TSLP in oesophageal biopsy samples (Figure 4). The diet did not have an effect on the expression of CCL26 (P = 0.72). Peak eosinophil counts were significantly correlated with the expression of IL‐5 (r = 0.72, P < 0.01), IL‐13 (r = 0.65, P < 0.01), POSTN (r = 0.46, P < 0.01) and TSLP (r = 0.51, P < 0.01). There was no significant correlation between the peak eosinophils counts and the expression of CCL26 (r = 0.13, P = 0.51).
Figure 4.
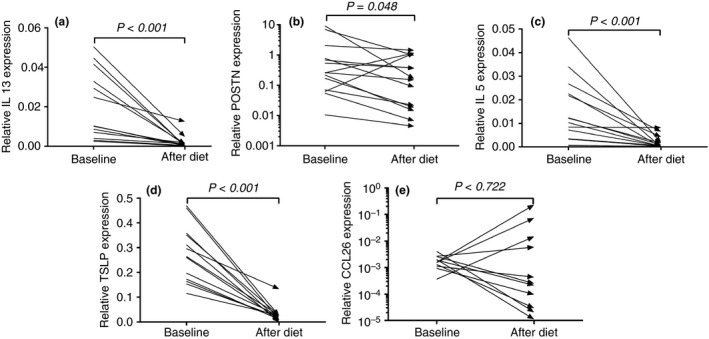
Logarithmic y‐axes were used to visualize the skewed data of POSSTN and CCL26. The relative expression of genes encoding inflammatory cytokines IL‐13, Periostin (POSTN), IL‐5, Thymic Stromal Lymphopoietin (TSLP) and eotaxin‐3 (CCL26) at baseline and after 4 weeks of elemental dietary treatment. Treatment significantly reduced the relative expression of: (a) IL‐13 (P < 0.001), (b) POSTN (P = 0.048), (c) IL‐5 (P < 0.001) and (d) TSLP (P < 0.001). The expression of (e) CCL26 was not significantly affected by elemental dietary treatment. Expression levels were given relative to housekeeping genes Cyclophilin and Hypoxanthine‐Guanine Phosphoribosyl Transferase.
Optional four‐food reintroduction schedule
After the elemental diet, eight complete responders and three partial responders (65% of the patients) continued with the optional food reintroduction process. Milk was most frequently identified as an offending allergen; in three patients histopathology confirmed disease recurrence after reintroduction of milk, and in two patients milk was suspected based on symptom recurrence (follow‐up endoscopy was not performed). In three patients, successively egg, nuts and/or seeds and wheat were identified as offending allergens, based on clinical‐pathological disease recurrence after reintroduction. In the remaining three patients, the stepwise reintroduction did not reveal the offending food allergens. Approximately six months after reintroduction of all food groups, six patients underwent another follow‐up endoscopy, which showed disease recurrence in all patients.25
Discussion
In this prospective study, we have shown that an elemental diet can induce histological remission in adult EoE patients. The elemental diet induced complete histological response in 72% and a partial response in 24% of the patients. These results underline the importance of food (allergens) as a driver of EoE in adults. The influence of food allergens in the pathophysiology of EoE was confirmed by disease recurrence after reintroduction of the offending foods. In addition, in response to the diet the gene expression of inflammatory cytokines declined significantly and this paralleled the decrease in oesophageal inflammation. These observations confirm the significant role of inflammatory cytokines and cytotoxic eosinophil secretory products in the pathophysiology of EoE. In our study, elemental dietary treatment did not only lead to histological response in the majority of patients but also to clinical response, and 15 patients became completely asymptomatic after the diet. The strength of our study lies in the prospective design, since most prior dietary studies are based on a retrospective analysis of data.
In this prospective study, adherence to the diet was monitored carefully by intensive counselling by physicians and a dietician and, as a result, the drop‐out rates after final inclusion were relatively low (14%). After the incorporated test day, patients were able to make a more weighted decision concerning study participation, which diminished the dropouts during the first treatment period and, thus, reduced the number of baseline endoscopies in patients who might have dropped out later. Another factor that might have increased adherence is the improved taste of the formula and the ability to vary between two flavours. The improved formula might also have encouraged adequate nutritional intake, since the reported weight loss in our study was considerable lower [1.4 (0.9–2.4) kg] when compared with previous literature (ranging from 3 to more than 7 kg).15 Nevertheless, elemental dietary treatment should be limited to short‐term use since a median weight loss of 1.4 kg within 4 weeks should not be underestimated. In addition, despite the decline in social functioning, probably explained by the social limitations associated with an exclusively liquid diet, patients' mental health status after diet was significantly higher compared to the Dutch reference population. The reduced drop‐out rates and positive patients' experience imply that elemental diet has become feasible in routine practice to induce a quick remission. However, one could argue that adherence to an elemental diet in clinical practice might be lower, since patients in this trial were monitored carefully by physicians and/or dieticians. Nevertheless, an elemental diet is, in combination with the subsequent food reintroduction process to identify the disease‐triggering allergen, a sustainable, drug‐free treatment for EoE.
The efficacy rate seen in this study, in terms of histological remission, is comparable to the study of Peterson et al., in which a complete histological response after elemental diet was reported in 72% of the adult EoE patients.15 Regardless of the comparable study design and histological response there are some differences; the group of Peterson considered peak eosinophil counts of ≤10 as complete response, whereas in our study, we used a threshold of ≤15 eosinophils/HPF. This could have overestimated our response rates since peak eosinophil counts of two patients in our cohort, who were classified as complete responders, ranged between 10 and 14. Nonetheless, according to an intention‐to‐treat analysis with inclusion of all patients that started the diet including the dropouts, our response rate would be 57% (12/21) compared to 45% (13/29) reported by Peterson et al. Moreover, subjects may be noncompliant or may drop out from the study due to a lack of clinical response.26 This explanation is less likely in our study population, since symptoms diminished in the majority of patients, regardless of histological disease status. This is in contrast to the previous study, which reported no improvement of dysphagia or chest pain. An interesting similarity is that in both studies symptoms and signs of concurrent atopic diseases (i.e. atopic dermatitis and allergic rhinitis) decreased during the diet. This suggests that food allergens may also play a role in nongastrointestinal allergic diseases.
In a recent systematic review, the overall efficacy of elemental diets (in adults and children) was estimated to be more than 90%. These efficacy rates are based on 13 studies, of which 12 were performed in children, and most of the studies were carried out retrospectively.13 The slightly lower response rate in our adult study may be explained by several factors. One factor of importance may be the impact of prolonged disease duration in our cohort; the average disease duration was 3 (1.5–6.5) years, with a diagnostic delay of 5 (0–24) years. Previous literature yielded that prolonged undiagnosed EoE leads to irreversible stricture formation.12 This could have occurred in our cohort, since inflammatory endoscopic features (oedema, linear furrows and white exudates) improved significantly after the diet, whereas fibrotic features (strictures and rings) did not. In addition, prolonged disease duration may have aggravated the oesophageal eosinophilia which could have affected response rates, since partial and nonresponders had a significantly higher baseline peak eosinophil count compared to complete responders. In a previous study of adult EoE patients treated with topical fluticasone, a similar trend towards lower treatment efficacy in EoE patients with more severe inflammation was observed.27
Our study has some limitations. First of all, since patients with more severe disease status were less likely to respond completely, one could argue that patients should have been treated longer, for example, six weeks as is done with elimination diets.28 Second, it might be argued that it is a short‐coming of our trial that no control group was included. However, adding a placebo arm in an elemental diet trial implies huge practical and ethical concerns. Furthermore, in a placebo‐controlled trial of fluticasone in adult EoE patients, none of the patients in the placebo arm showed histological response, suggesting that oesophageal eosinophilia is not affected by placebo.19 Another limitation is the use of a per protocol analysis, excluding patients who deviated from the protocol, which may have induced bias. Nevertheless, the results of per protocol analysis give a more realistic reflection of the effects of treatment when proposed in an optimal manner.29 In addition, we have mentioned the results of an intention‐to‐treat analysis in our discussion section. Last, symptoms were scored using a non‐validated questionnaire since at the time of the study design no validated Patients Reported Outcome (PRO) questionnaires were available.30
Once disease remission was established by the elemental diet, single food groups were reintroduced and repeated endoscopies were needed to identify the disease‐triggering food allergens. Ideally, the food reintroduction process would be guided by a less‐invasive marker, but unfortunately patient‐friendly, less‐invasive disease activity markers for EoE are still not known. Apart from the burden to the patients, repetitive endoscopies result in considerable cost to healthcare systems. In addition, the cost of the formula to individuals is a major drawback of elemental diet, especially in USA, where the costs are reimbursed in only 31% of the States.31 The perception of health insurance companies may change in the near future, since a recent cost‐utility analysis yielded that dietary treatment is more cost‐effective than topical steroids as a first‐line treatment for EoE.32 Fortunately, the Dutch healthcare system reimburses the costs, allowing patients to choose for elemental diet regardless of income.
In conclusion, this study strongly suggests that in adult EoE patients, elemental diet reduces eosinophilic inflammation and induces clinical remission in the majority of patients. Using a new amino acid‐based formula with increased palatability, which was systematically tested by the patients before entering the study, has led to improved adherence to the elemental diet. Elemental dietary management of EoE seems highly promising in selected and motivated EoE patients.
Authorship
Guarantor of the article: Marijn J. Warners.
Author contributions: Marijn J. Warners and Albert J. Bredenoord: data collection and analysis, writing the manuscript; Berber J. Vlieg ‐ Boerstra, Joanne Verheij, Bram D. van Rhijn and Wouter J. de Jonge: data collection and analysis, writing assistance; Marleen T. J. Van Ampting, Lucien F. Harthoorn and Andreas J. P. M. Smout: writing assistance.
All authors approved the final version of the manuscript.
Acknowledgements
Declaration of personal interests: Albert J. Bredenoord received research funding from Endostim and Danone and received speaker and/or consulting fees from MMS, Astellas, AstraZeneca, Almirall and Allergan; Andreas J. P. M. Smout received consultancy fees from AstraZeneca, Almirall and Allergan; Wouter J. de Jonge is supported by Mead Johnson Pediatric Nutrition Institute, GlaxoSmithKline, Galvani, Parthenon, Ernst and Young, and Schwabe GmbH; Marleen T. J. Van Ampting and Lucien F. Harthoorn are employees of Nutricia Research ‐ results of the current study do not affect salary. Marijn J. Warners, Berber J. Vlieg – Boerstra, Joanne Verheij and Bram D. van Rhijn have no personal interest.
Declaration of funding interests: This investigator‐initiated study was partly funded by a grant from Nutricia Research.
The Handling Editor for this article was Dr Colin Howden, and it was accepted for publication after full peer‐review.
References
- 1. Liacouras CA, Furuta GT, Hirano I, et al Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol 2011; 128: 3–20. [DOI] [PubMed] [Google Scholar]
- 2. Henderson CJ, Abonia JP, King EC, et al Comparative dietary therapy effectiveness in remission of pediatric eosinophilic esophagitis. J Allergy Clin Immunol 2012; 129: 1570–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Rhijn BD, Verheij J, Smout AJ, et al Rapidly increasing incidence of eosinophilic esophagitis in a large cohort. Neurogastroenterol Motil 2013; 25: 47–52; e5. [DOI] [PubMed] [Google Scholar]
- 4. Sealock RJ, Rendon G, El‐Serag HB. Systematic review: the epidemiology of eosinophilic oesophagitis in adults. Aliment Pharmacol Ther 2010; 32: 712–9. [DOI] [PubMed] [Google Scholar]
- 5. Chehade M, Aceves SS. Food allergy and eosinophilic esophagitis. Curr Opin Allergy Clin Immunol 2010; 10: 231–7. [DOI] [PubMed] [Google Scholar]
- 6. Menard‐Katcher P, Marks KL, Liacouras CA, et al The natural history of eosinophilic oesophagitis in the transition from childhood to adulthood. Aliment Pharmacol Ther 2013; 37: 114–21. [DOI] [PubMed] [Google Scholar]
- 7. Kelly KJ, Lazenby AJ, Rowe PC, et al Eosinophilic esophagitis attributed to gastroesophageal reflux: Improvement with an amino acid‐based formula. Gastroenterology 1995; 109: 1503–12. [DOI] [PubMed] [Google Scholar]
- 8. Murali A, Gupta A, Attar B, et al Topical steroids in eosinophilic esophagitis: systematic review and meta‐analysis of placebo controlled randomized clinical trials. J Gastroenterol Hepatol 2016; 31: 1111–9. [DOI] [PubMed] [Google Scholar]
- 9. Sawas T, Dhalla S, Sayyar M, et al Systematic review with meta‐analysis: pharmacological interventions for eosinophilic oesophagitis. Aliment Pharmacol Ther 2015; 41: 797–806. [DOI] [PubMed] [Google Scholar]
- 10. Helou EF, Simonson J, Arora AS. 3‐yr‐follow‐up of topical corticosteroid treatment for eosinophilic esophagitis in adults. Am J Gastroenterol 2008; 103: 2194–9. [DOI] [PubMed] [Google Scholar]
- 11. Furuta GT, Liacouras CA, Collins MH, et al Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology 2007; 133: 1342–63. [DOI] [PubMed] [Google Scholar]
- 12. Schoepfer AM, Safroneeva E, Bussmann C, et al Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time‐dependent manner. Gastroenterology 2013; 145: 1230–6. e1‐2. [DOI] [PubMed] [Google Scholar]
- 13. Arias A, González‐Cervera J, Tenias JM, et al Efficacy of dietary interventions for inducing histologic remission in patients with eosinophilic esophagitis: a systematic review and meta‐analysis. Gastroenterology 2014; 146: 1639–48. [DOI] [PubMed] [Google Scholar]
- 14. Markowitz J. Elemental diet is an effective treatment for eosinophilic esophagitis in children and adolescents. Am J Gastroenterol 2003; 98: 777–82. [DOI] [PubMed] [Google Scholar]
- 15. Peterson KA, Byrne KR, Vinson LA, et al Elemental diet induces histologic response in adult eosinophilic esophagitis. Am J Gastroenterol 2013; 108: 759–66. [DOI] [PubMed] [Google Scholar]
- 16. Dellon ES, Gonsalves N, Hirano I, et al ACG clinical guideline: evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol 2013; 108: 679–92; quiz 693. [DOI] [PubMed] [Google Scholar]
- 17. Wolf WA, Cotton CC, Green DJ, et al Evaluation of histologic cutpoints for treatment response in eosinophilic esophagitis. J Gastroenterol Hepatol Res 2015; 4: 1780–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Straumann A, Conus S, Degen L, et al Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology 2010; 139: 1526–37; 1537, e1. [DOI] [PubMed] [Google Scholar]
- 19. Butz BK, Wen T, Gleich GJ, et al Efficacy, dose reduction, and resistance to high‐dose fluticasone in patients with eosinophilic esophagitis. Gastroenterology 2014; 147: 324–33; e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shaw MJ, Talley NJ, Beebe TJ, et al Initial validation of a diagnostic questionnaire for gastroesophageal reflux disease. Am J Gastroenterol 2001; 96: 52–7. [DOI] [PubMed] [Google Scholar]
- 21. Aaronson NK, Muller M, Cohen PD, et al Translation, validation, and norming of the dutch language version of the SF‐36 health survey in community and chronic disease populations. J Clin Epidemiol 1998; 51: 1055–68. [DOI] [PubMed] [Google Scholar]
- 22. Llena‐Puy C. The rôle of saliva in maintaining oral health and as an aid to diagnosis. Med Oral Patol Oral Cir Bucal 2006; 11: E449–55. [PubMed] [Google Scholar]
- 23. Hirano I, Moy N, Heckman MG, et al Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut 2013; 62: 489–95. [DOI] [PubMed] [Google Scholar]
- 24. Mueller S, Neureiter D, Aigner T, et al Comparison of histological parameters for the diagnosis of eosinophilic oesophagitis versus gastro‐oesophageal reflux disease on oesophageal biopsy material. Histopathology 2008; 53: 676–84. [DOI] [PubMed] [Google Scholar]
- 25. Lucendo AJ. Motor disturbances participate in the pathogenesis of eosinophilic oesophagitis, beyond the fibrous remodelling of the oesophagus [5]. Aliment Pharmacol Ther 2006; 24: 1264–7. [DOI] [PubMed] [Google Scholar]
- 26. Gupta SK. Intention‐to‐treat concept: a review. Perspect Clin Res 2011; 2: 109–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Rhijn BD, Verheij J, van den Bergh Weerman MA, et al Histological response to fluticasone propionate in patients with eosinophilic esophagitis is associated with improved functional esophageal mucosal integrity. Am J Gastroenterol 2015; 110: 1289–97. [DOI] [PubMed] [Google Scholar]
- 28. Lucendo AJ, Arias A, Gonzalez‐Cervera J, et al Efficacy of six‐food elimination diet in adult eosinophilic oesophagitis: a prospective study. Gut 2011; 60: 42. [Google Scholar]
- 29. Intention to treat analysis and per protocol analysis: complementary information. Prescrire Int 2012; 21: 304–6. [PubMed] [Google Scholar]
- 30. Safroneeva E, Straumann A, Coslovsky M, et al Symptoms have modest accuracy in detecting endoscopic and histologic remission in adults with eosinophilic esophagitis. Gastroenterology 2016; 150: 581–90; e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Food Allergy & Anaphylaxix Connection TEAM . Available at: http://www.foodallergyawareness.org/ (accessed 5 February 2016).
- 32. Cotton CC, Hiller S, Green DJ, et al Six‐food elimination diet or topical steroids for first‐line treatment of eosinophilic esophagitis: a cost‐utility analysis. Gastroenterology 2015; 148(Suppl. 1): s–2. [Google Scholar]


