Abstract
BACKGROUND
Minimally processed ready‐to‐eat products are considered a high‐risk food because of the possibility of contamination with pathogenic bacteria, including Listeria monocytogenes from the animal reservoir, and the minimal processing they undergo. In this study, a sakacin‐A anti‐Listeria active package was developed and tested on thin‐cut veal meat slices (carpaccio).
RESULTS
Enriched food‐grade sakacin‐A was obtained from a cell‐free supernatant of a Lactobacillus sakei culture and applied (0.63 mg cm−2) onto the surface of polyethylene‐coated paper sheets to obtain an active antimicrobial package. The coating retained antimicrobial features, indicating that the process did not affect sakacin‐A functionality, as evidenced in tests carried out in vitro. Thin‐cut veal meat slices inoculated with Listeria innocua (a surrogate of pathogenic L. monocytogenes) were laid on active paper sheets. After 48 h incubation at 4 °C, the Listeria population was found to be 1.5 log units lower with respect to controls (3.05 vs 4.46 log colony‐forming units (CFU) g−1).
CONCLUSION
This study demonstrates the possibility of using an antimicrobial coating containing sakacin‐A to inhibit or decrease the Listeria population in ready‐to‐eat products, thus lowering the risk of food‐related diseases. © 2016 The Authors. Journal of The Science of Food and Agriculture published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: sakacin‐A, bacteriocin, active packaging, Listeria, carpaccio
INTRODUCTION
Human listeriosis is a relatively rare but serious zoonotic disease, with high morbidity, hospitalization and mortality in vulnerable populations. Among all zoonotic diseases under EU surveillance, listeriosis caused the most severe human disease, with 93.6% of cases hospitalized and 134 fatal cases (fatality rate 12.7% in 2010–2011).1 The relevance of this contamination is underlined by the updated compliance guideline recently released by the Food Safety and Inspection Service of the US Department of Agriculture, which ‘provides specific recommendations that official establishments producing post‐lethality exposed ready‐to‐eat meat and poultry products may follow to meet the requirements of 9 CFR part 430, the Listeria Rule’.2
Listeria spp. have the ability to grow in cool damp environments where other pathogens may not grow, and are capable of surviving freezing temperatures. For these reasons, ready‐to‐eat products that are consumed without cooking are of particular concern for Listeria monocytogenes contamination.3
Significant support in the fight against pathogenic microorganisms may derive from food packaging, which not only acts as a barrier against moisture, water vapor, gases and solutes but may also serve as a carrier of active substances such as antimicrobials in active packaging.4 Active packaging is defined as a mode of packaging in which package, product and environment interact to prolong shelf‐life or to enhance safety and/or quality of the food product.5
Recent research toward the development of new and eco‐friendly preservation methodologies is stimulated by the increasing demand for natural, disposable, biodegradable and/or recyclable food packaging materials.4 In this context, paper is a simple but promising low‐density material. In view of its properties (cost, acceptance, sustainability), paper may be regarded as an excellent material for controlled release of antimicrobials in active packaging, also taking into account that papermakers are capable of providing products with a broad range of mechanical, physical and chemical properties. Coatings such as waxes or polymeric materials can also be used to improve the otherwise poor barrier properties of paper.6
As regards natural antimicrobials, bacteriocins represent an attractive solution owing to their selective mode of action against foodborne and/or spoilage bacteria.7, 8 Sakacin‐A, produced by Lactobacillus sakei, belongs to the class IIa bacteriocins. Sakacin‐A is a Listeria‐targeting peptide of 41 amino acid residues with one disulfide bridge and a calculated molar mass of 4308 Da.9 In previous works, we investigated the production profile of sakacin‐A in a low‐cost medium formulation and the molecular mechanism of the antilisterial activity.10, 11 Sakacin‐A is able to kill Listeria cells by making their membranes permeable, and possesses also a slow hydrolytic action toward Listeria cell walls, suggesting that it can break specific bonds in the peptoglycan structure.12
The present study was aimed at obtaining an active packaging material by applying a sakacin‐A coating on the surface of polyethylene (PE)‐coated paper sheets, used for primary food packaging of thin‐cut veal meat. Beef carpaccio was taken as an example of a ready‐to eat‐food product, as it is considered a high‐risk food because of the possibility of contamination with pathogenic bacteria, including Listeria coming from the animal reservoir, and the minimal processing it undergoes.13
In the present research, an enriched sakacin‐A preparation obtained from a cell‐free supernatant of an L. sakei culture was coated onto the PE side of the aforementioned paper. The efficacy of the resulting ‘active package’ was assessed by performing an in vitro antimicrobial test against Listeria innocua, a surrogate strain commonly used in the laboratory to give a margin of safety to researchers and prevent unnecessary exposure to pathogens.14 Finally, a short‐term preliminary storage trial was carried out on actual carpaccio slices.
MATERIALS AND METHODS
Microorganisms and culture conditions
Lactobacillus sakei DSMZ 6333 was used as the sakacin‐A producer and L. innocua DSMZ 20649 as the indicator strain (Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Braunschweig, Germany). Lactobacillus sakei was maintained on MRS broth (Scharlau Chemie, Barcelona, Spain) and L. innocua on TSB (Tryptic Soy Broth; Difco, Darmstadt, Germany). Strains were stored as frozen stocks at −80 °C in appropriate liquid medium in the presence of 0.5 g mL−1 glycerol. Cultures were propagated twice before being used.
Sakacin‐A production by L. sakei was performed in a culture medium containing (g L−1) bacto peptone (10), meat peptone (8), yeast autolysate (4), glucose (10) and CaCO3 (3) in deionized water. All ingredients were purchased from Costantino (type FM, Fermentation Media, Torino, Italy). Trials were carried out in 2 L flasks, each containing 1.4 L of culture medium and inoculated with 70 mL of preculture. Cultures were incubated in stationary conditions at 30 °C for 20 h, corresponding to maximum bacteriocin concentration.12 Bacteriocin production was expressed as arbitrary units (AU mL−1), measured by agar diffusion assays using L. innocua as the indicator organism.10
Enriched sakacin‐A
A food‐grade freeze‐dried preparation of sakacin‐A was obtained from the culture broth of L. sakei after centrifugation at 10 000 × g for 35 min at 4 °C. The supernatant (culture filtrate, CF) was diafiltered in a 200 mL Amicon® cell (Millipore, Billerica, MA, USA) fitted with an Ultracel® PL‐3 membrane with molecular weight cut‐off (MWCO) 3000 Da (Millipore), by concentration to 40 mL and dilution to the original volume with Milli‐Q water. The concentration/dilution step was repeated three times. The final concentrate (40 mL) was frozen at −20 °C for 4 h and freeze‐dried at 25 °C and 1.33 Pa for 48 h in an Alpha 2‐4 LD plus lyophilizer (Christ, Osterode am Harz, Germany) connected to a Savant VP 190 vacuum pump (Savant Instruments Inc., Farmingdale, NY, USA).
Preparation of sakacin‐A active paper sheets
Trials were carried out incorporating the enriched sakacin‐A in a gelatin‐based coating solution, whose composition is reported in Table 1. To prepare the coating, gelatin was first dissolved in water at 60 °C, then glycerol and enriched sakacin‐A were added while stirring at room temperature. Sheets (A4, 210 mm × 297 mm, 75 g m−2) of a commercial PE‐coated paper (Burgo Group, Altavilla Vicentina, Italy) were used for the application of the coating solution onto the PE side. An automatic film applicator (SH1137, Sheen Instruments, West Molesey, UK) equipped with a 50 µm wire bar coater was used at a controlled speed of 110 mm s−1. Coated sheets were oven dried at 105 °C for 2 min. Sheets coated with gelatin/glycerol solution without sakacin‐A were also prepared as negative controls.
Table 1.
Mass composition of coating solution
| Coating component | g | g g−1 DW |
|---|---|---|
| Gelatin from cold water fish skin | 8.15 | 0.44 |
| Enriched sakacin‐A | 8.15 | 0.44 |
| Glycerol | 2.23 | 0.12 |
| Water | 81.5 | — |
| Total | 100 | 1 |
| Total (dry weight, DW) | 18.5 | — |
Antimicrobial activity of sakacin‐A and sakacin‐A active paper
An aliquot (300 μL) of overnight Listeria culture in TSB was added to 30 mL of soft TSA (TSB with 8 g L−1 agar) in a Petri dish and left at room temperature until solidification. Wells (8 mm diameter) were made on the plate and aliquots (150 μL) of sakacin‐A (either CF or enriched sakacin‐A) were poured inside. Bacteriocin activity (AU) was quantified as the reciprocal of the highest dilution that exhibited a clear zone of inhibition.11
In the case of coated paper sheets (both active and negative control), antimicrobial activity was evidenced qualitatively using the plate overlay assay. Samples (1 cm2) were placed on the surface of Listeria TSA plates prepared as described above. Solid cultures were then incubated overnight at 37 °C, and antimicrobial activity was evaluated by the formation of a clear halo of Listeria growth inhibition around the sample.
Antimicrobial activity of active paper on thin‐cut veal meat
Ready‐to‐eat thin‐cut veal meat (meant for consumption as raw food within 24 h, known in Italy as ‘carpaccio’) was purchased in a local store. Samples were aseptically cut into sections (5 cm × 10 cm, 7–8 g) and inoculated with an appropriate dilution of L. innocua overnight culture to obtain a microbial load of approximately 103 cells cm−2. After inoculation, samples were kept at room temperature for 30 min to allow for cell attachment. Inoculated slices were covered (top and bottom) with sections of sakacin‐A active paper sheets and transferred onto glass trays that were manually wrapped in PVC film under aerobic conditions (Fig. 1). Samples were then stored at 4 °C. A negative control set of trials was also performed employing paper sheets coated with gelatin in the absence of sakacin‐A. On days 0 and 2, individual meat slices were transferred aseptically into a Stomacher bag (VWR blender bag, Milano, Italy) containing 63–72 mL of sterile peptone water (10 g L−1 bacteriological peptone; Costantino) and blended in a Stomacher (Star Blender LB 400, VWR, Milano, Italy) at high speed for 3 min. Tenfold dilution series of the obtained suspension were made in the same sterile peptone water and used for L. innocua determination and total aerobic plate count. Selective L. innocua determination was performed employing the ALOA culture medium (Agar Listeria Ottaviani Agosti added with enrichment and selective supplements; Biolife, Milan, Italy). Plates were surface inoculated and incubated at 37 °C for 24–48 h. Total aerobic plate count was performed on Nutrient Agar (Scharlau Chemie) plates incubated at 30 °C for 48 h. Experiments were replicated twice. Counts were reported as log colony‐forming units (CFU) g−1 meat, and means and standard deviations were calculated.
Figure 1.
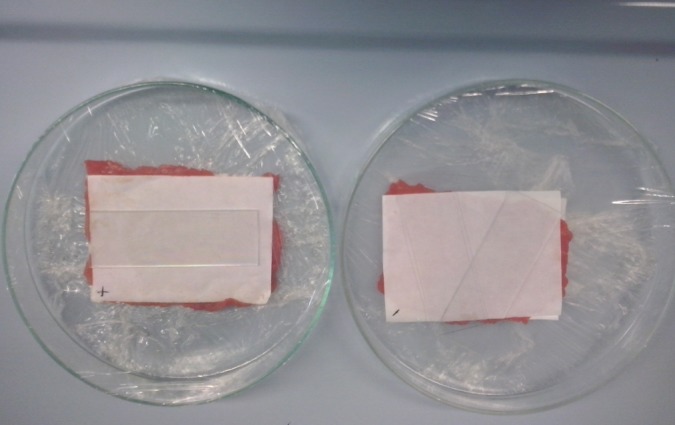
Thin‐cut meat slices intentionally inoculated with Listeria innocua and stored in contact with sakacin‐A active paper (left, +) and negative control (right, −).
Electrophoresis
Enriched sakacin‐A was characterized by Novex® NuPAGE® sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS‐PAGE) (Life Technologies, Carlsbad, CA, USA). A 1 mg portion of the freeze‐dried preparation was dissolved in 1 mL of denaturing buffer (0.125 mol L−1 Tris HCl, pH 6.8, 500 g L−1 glycerol, 17 g L−1 SDS, 0.1 g L−1 bromophenol blue, 10 mL L−1 2‐mercaptoethanol) diluted twofold with water. After treatment at 100 °C for 10 min, an appropriate volume of the solution was used for NuPAGE® SDS‐PAGE on NuPAGE® Novex® 4–12% Bis–Tris Protein Gel, run at a constant 200 V. Gels were stained with Simply Blue® Safe Stain. Molecular mass markers (Novex® Sharp Standard) covered the 3.5–260 kDa range.
Novex® NuPAGE® SDS‐PAGE was used also to quantify the amount of enriched sakacin‐A incorporated into the coating. A paper sample of 1 cm2 was cut, directly placed into 0.2 mL of denaturing buffer (diluted twofold with water) and heated at 100 °C for 10 min. The denaturation allows solubilization and extraction of all proteins present in the paper sample. After centrifugation at 10 000 × g for 2 min, an aliquot of the clarified suspension was used for NuPAGE® SDS‐PAGE. The intensity of individual bands was compared with the intensity of bands obtained with known amounts of the original enriched sakacin‐A preparation.15
Statistical analysis
Obtained data were submitted to two‐way analysis of variance. The Tukey–Kramer test was used for comparison of means, with significance assigned at P < 0.05.
RESULTS
Sakacin‐A production, enrichment and antimicrobial activity
Sakacin‐A production by L. sakei reached the maximum level at 20 h incubation, corresponding to the highest cell population (109 CFU mL−1), as reported by Trinetta et al. 11 The culture filtrate (CF) obtained after centrifugation showed an antimicrobial titer of 107 AU mL−1.
CF was ultrafiltered and diafiltered in order to concentrate the preparation and to remove low‐molecular‐weight compounds such as salts, small peptides, sugars, etc. that could interfere with the subsequent freeze‐drying process. The mass yield of freeze‐dried enriched sakacin‐A was approximately 3 g from 200 mL of CF.
The SDS‐PAGE analysis of enriched sakacin‐A is reported in Fig. 2, where a marked band at 3.5 kDa could be identified with sakacin‐A.12 No other noticeable proteins are evident. However, other peptides retained during the ultrafiltration step may be present, as suggested by the weak smear over the 3.5 kDa band.
Figure 2.

NuPAGE® SDS‐PAGE analysis of enriched sakacin‐A preparation: lane S, freeze‐dried enriched sakacin‐A preparation (20 µg); lane M, molecular mass markers.
The antimicrobial titer of enriched sakacin‐A was about 1.4 AU mg−1, as determined by agar well diffusion assay employing L. innocua as the indicator strain. Thus, on an activity basis, the overall yield of the enrichment process was 19.6%.
Antimicrobial activity of sakacin‐A active paper
In vitro measurements
Antimicrobial activity of the sakacin‐A solutions used for paper coating and of sakacin‐A active paper was demonstrated by a clear zone of growth inhibition on Listeria TSA plates (Fig. 3). No activity against the indicator strain was noticed in negative controls, demonstrating that neither the paper itself nor the gelatin coating had antimicrobial activity in the absence of sakacin‐A.
Figure 3.

Antimicrobial activity against Listeria innocua of (1) sakacin‐A/gelatin solution used for paper coating, (2) active paper (coated with sakacin‐A) and (3) negative control paper (coated with gelatin, in the absence of sakacin‐A).
Ready‐to‐eat carpaccio slices
Sakacin‐A‐coated paper sheets were evaluated for their antilisterial effectiveness on food samples. The Listeria population and total aerobic count (TAC) were determined in samples of ‘carpaccio’ (thin meat slices meant for consumption as raw meat within 24 h after purchasing) purposely contaminated with L. innocua (3.46 log CFU g−1) and then stored at 4 °C for 48 h (Table 2). Listeria innocua was used as a surrogate of foodborne pathogenic L. monocytogenes. A surrogate is a bacterium that has physiological characteristics nearly identical to a pathogenic bacterium of interest, and is used to give a margin of safety to researchers and prevent unnecessary exposure to pathogens.14
Table 2.
Listeria population and total aerobic count in samples of thin‐cut veal slices as such or inoculated with Listeria innocua and then stored for 48 h at 4 ± 1 °C in active paper sheets coated with sakacin‐A (active package) or paper sheets without sakacin‐A coating (negative control)
| Time (h) | Sample | Listeria population | Total aerobic count | ||
|---|---|---|---|---|---|
| Log CFU g−1 | Decimal reduction | Log CFU g−1 | Decimal reduction | ||
| 0 | As such | 0 | — | 4.79 ± 0.14a | — |
| 0 | + L. innocua | 3.46 ± 0.06a | — | 4.80 ± 0.07a | — |
| 48 | Negative control | 4.46 ± 0.09b | +1.00 | 5.14 ± 0.11b | +0.34 |
| 48 | Active package | 3.05 ± 0.17c | −0.41 | 4.51 ± 0.12c | −0.29 |
Means in the same column with different letters are significantly different (P < 0.05).
After 48 h, the Listeria population of carpaccio slices stored in contact with the active paper sheets was found to be lower than that used for the initial inoculum (from 3.46 to 3.05 log CFU g−1). On the contrary, in negative controls (carpaccio slices stored in contact with paper coated with gelatin in the absence of sakacin‐A), the Listeria population increased by about 1 log unit (from 3.46 to 4.46 log CFU g−1).
Meat slices stored in presence of sakacin‐A active paper sheets also evidenced a slight decrease in aerobic populations (∼0.3 log unit) after 48 h storage at refrigerated temperature. On the contrary, slices packed without sakacin‐A coating evidenced a 0.35 log unit increase with respect to the initial value. These experiments demonstrate the antimicrobial activity of the sakacin‐A active paper sheets and their effectiveness against L. innocua in storage trials.
Sakacin‐A coating and release
SDS‐PAGE was used to characterize the presence of enriched sakacin‐A on the active paper (Fig. 4, lanes 1 and 2) as well as its release from the coating in storage trials (Fig. 4, lanes 3 and 4). For this purpose, proteins present in the coating were completely extracted from the paper by solubilization under denaturing conditions (i.e. upon heating at 100 °C in SDS‐PAGE denaturing buffer).
Figure 4.
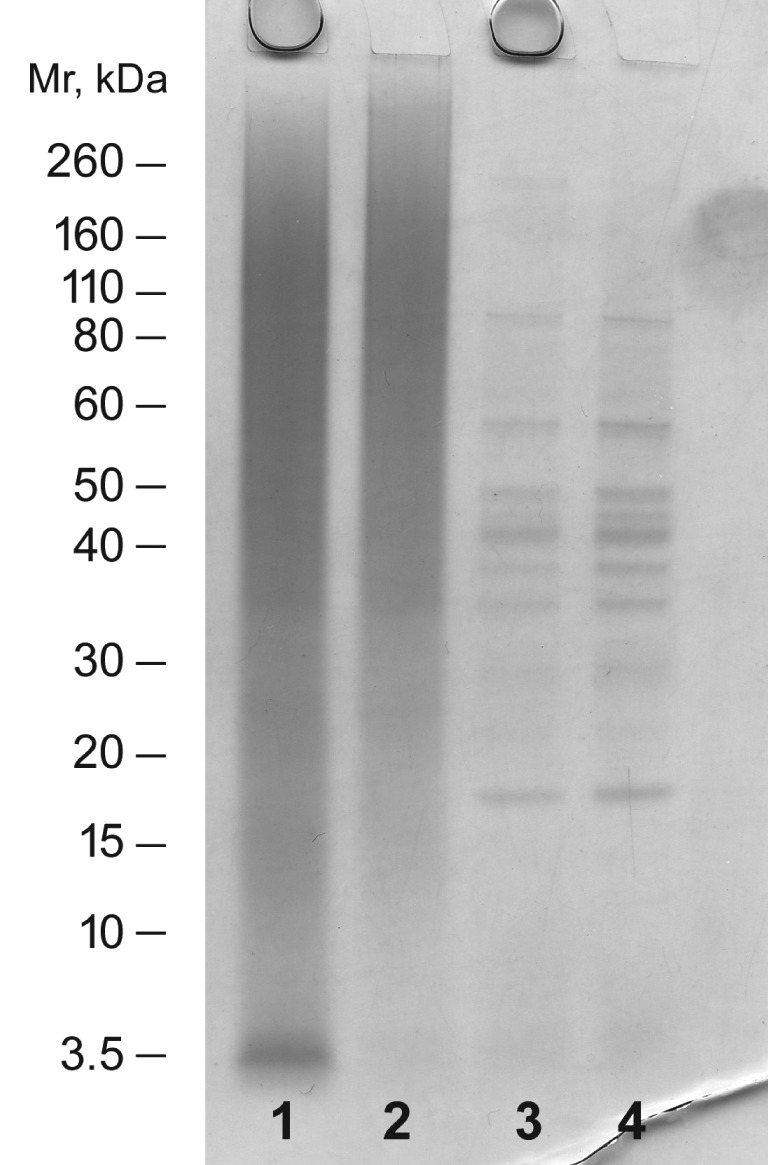
NuPAGE® SDS‐PAGE analysis of active paper and negative control: proteins extracted from active paper (with sakacin‐A) and negative control (without sakacin‐A) before (lanes 1 and 2 respectively) and 48 h after (lanes 3 and 4 respectively) being placed in contact with food.
Protein extracted from the negative control (paper sheets without sakacin‐A) showed a broad distribution of proteins of medium–high molecular weight that can be assigned to the gelatin used for the preparation of the coating (Fig. 4, lane 2). No proteins are visible around 3.5 kDa, i.e. in the sakacin‐A region. As clearly shown in Fig. 4 (lane 1), a band corresponding to sakacin‐A is evident, in addition to the gelatin bands, in samples from the paper coated with sakacin‐A.
The intensity of this band was compared with the intensity of bands obtained with known amounts of the enriched sakacin‐A preparation,15 allowing us to estimate the concentration of sakacin‐A in coated paper sheets at 0.63 mg cm−2. This estimated concentration is in good agreement with the amount of enriched sakacin‐A used for the preparation of the coating and with the volume of the coating solution spread on the paper sheets.
As for sakacin‐A release in storage trials with food, active packaging containing non‐volatile antimicrobial agents is assumed to work through contact with wet food.15 The contact between food and packaging lead to the migration of the active compound from the packaging to the food surface and vice versa. The release of sakacin‐A was studied through SDS‐PAGE by extracting proteins from paper that had been in contact with meat slices.
In both samples (negative control and active paper, lanes 3 and 4 in Fig. 4), neither the sakacin‐A band nor the gelatin smear were visible. The absence of both sakacin‐A and gelatin in paper that had been in contact with food is indirect evidence that the antimicrobial agent must be transferred, together with gelatin, onto the food. In this contest, release of the coating agent and of its ‘bioactive’ content was triggered by the swelling of gelatin upon taking up water from the wet food. Otherwise, in the SDS‐PAGE traces of the above samples, new distinct bands are visible. These bands could be attributed to soluble meat proteins dissolved in the liquid phase and migrated from food to the paper.
DISCUSSION
The present research was aimed at developing a novel antimicrobial food packaging system employing a food‐grade preparation of sakacin‐A. The bacteriocin was produced in liquid cultures employing a medium formulated with low‐cost ingredients, suitable for large‐scale industrial processes.11
Purification is usually an essential step in the characterization of unknown bacteriocins, but it represents a time‐consuming and low‐yield step in such studies. Indeed, owing to the high number of steps in current protocols, protein yields are very often low.16 In practical terms, purification to homogeneity is redundant and sometimes not suitable for food‐grade application.17
In the present work, sakacin‐A was concentrated from a food‐grade medium by one‐step diafiltration, giving a freeze‐dried enriched sakacin‐A free from contaminant proteins and with an antimicrobial titer of 1.36 AU mg−1. Total activity yield was nearly 20% better than other methods of rapid purification (10% in Guyonnet et al. 16), although lower than vastly time‐consuming methods (83% in Holck et al. 9).
Incorporation of bacteriocins into food packaging materials to control the growth of spoilage and pathogenic organisms has been much researched in the last decades.4 Paper and paperboard for food packaging provide mechanical strength, are biodegradable and have good printability, but display poor barrier properties to liquids, gases and vapors. To overcome these drawbacks, coatings with polymeric matrices or waxes are used to improve paper barrier properties and grease resistance. In addition, coatings can also be added with antimicrobials, to obtain an active food package.18, 19
Two methods are commonly used to prepare antimicrobial paper‐based food package materials for meats and/or meat products. One is to incorporate them directly into polymers, i.e. heat‐press and casting.4 Ming et al. 20 incorporated nisin and pediocin in cellulose casings to reduce L. monocytogenes in meats and poultry. Pediocin‐coated bags completely inhibited the growth of inoculated L. monocytogenes during 12 weeks of storage at 4 °C. In a previous paper, we reported on the incorporation of lysozyme and lactoferrin as antimicrobials in a cellulose‐based food package. Those studies indicated that these proteins were simultaneously released in a biologically active form, and the synergism between the two antimicrobials was evident in tests against common food contaminants.15
Another method to incorporate bacteriocins is to coat or adsorb them onto polymer surfaces. La Storia et al. 21 investigated the interaction between three bacteriocins (nisin and bateriocins produced by Lactobacillus curvatus and Lactobacillus plantarum) and five different PE films used for food packaging. The results indicated that each film–bacteriocin pair showed different antimicrobial activity owing to the establishment of chemical and surface interactions. Guiga et al. 22 produced three‐layer films of ethylcellulose, hydroxypropylmethylcellulose and ethylcellulose (EC/HPMC/EC) including either Nisaplin® or nisin. These multilayer films showed significant antimicrobial activity against Kocuria rhizophila ATCC 9341 (formerly Micrococcus luteus).
Trinetta et al. 23 produced sakacin‐A‐containing (1 mg cm−2) pullulan films by incorporation and tested them on turkey breast experimentally inoculated with L. monocytogenes. The results showed reductions of up to 3 log CFU g−1 after 3 weeks under refrigerated storage and demonstrated the efficacy of this bacteriocin against different epidemic clones of the pathogenic bacterium.
In our preliminary assessment employing simple packaging conditions, the application of a sakacin‐A/cellulose‐coated material on meat samples gave positive results at a concentration of sakacin‐A in the packaging material of 0.63 mg cm−2. In storage trials with veal ‘carpaccio’, all components of the active surface layer in the active paper appear to have migrated to the food after 48 h at 4 °C. Thus an anti‐Listeria active packaging solution, not to be regarded as a way to ‘clean’ a contaminated food product, can contribute significantly to reducing the L. monocytogenes population in food items and lowering the risk of food‐related diseases.
CONCLUSIONS
This work, which should be considered as a preliminary investigation into the potential applicability of sakacin‐A in an anti‐Listeria active package, provides an example in which the bacteriocin was coated onto the surface of a PE‐coated paper commonly used for primary food packaging. Coating was efficient (no peptide loss was observed) and the bacterocin was released in a biologically active form. Application of this material on actual meat samples also gave positive results. Future work will indicate whether the antimicrobial‐loaded paper sheets used here may find other practical uses (e.g. paper liners or wraps) and whether they will be effective to improve the safety and extend the shelf‐life of other meat products.
ACKNOWLEDGEMENTS
This work was supported by funds from the Fondazione Cariplo (2015‐0464 NANOSAK – Nanocellulose–sakacin A conjugates for food packaging purposes). The authors wish to acknowledge Professor Francesco Bonomi (DeFENS, University of Milan) for constructive comments and for text revision.
REFERENCES
- 1. EFSA (European Food Safety Authority) and ECDC (European Centre for Disease Prevention and Control) , The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food‐borne Outbreaks in 2011. EFSA J 11:3129–3379 (2013). [Google Scholar]
- 2. USDA‐FSIS , FSIS Compliance Guideline: Controlling Listeria monocytogenes in Post‐lethality Exposed Ready‐to‐eat Meat Products and Poultry Products. Available: http://www.fsis.usda.gov/wps/portal/fsis/topics/regulatory‐compliance/listeria [7 October 2015]. [Google Scholar]
- 3. Singh RP and Anderson BA, The major types of food spoilage: an overview, in Understanding and Measuring the Shelf‐life of Food, ed. by Steele R. Woodhead Publishing, Cambridge, pp. 3–23 (2004). [Google Scholar]
- 4. Suppakul P, Miltz J, Sonneveld K and Bigger SW, Active packaging technologies with an emphasis on antimicrobial packaging and its applications. J Food Sci 68:408–420 (2003). [Google Scholar]
- 5. López‐Rubio A, Almenar E, Hernandez‐Muñoz P, Lagarón JM, Catalá R and Gavara R, Overview of active polymer based packaging technologies for food applications. Food Rev Int 20:357–387 (2004). [Google Scholar]
- 6. Kirwan MJ, Paper and paperboard packaging, in Food and Beverage Packaging Technology, ed. by Coles R. and Kirwan MJ. Wiley‐Blackwell, Oxford, pp. 213–250 (2011). [Google Scholar]
- 7. Nguyen VT, Gidley MJ and Dykes GA, Potential of a nisin‐containing bacterial cellulose film to inhibit Listeria monocytogenes on processed meat. Food Microbiol 25:471–478 (2008). [DOI] [PubMed] [Google Scholar]
- 8. Papagianni M, Ribosomally synthesized peptides with antimicrobial properties: biosynthesis, structure, function and applications. Biotechnol Adv 21:465–499 (2003). [DOI] [PubMed] [Google Scholar]
- 9. Holck A, Axelsson L, Birkeland SE, Aukrust T and Blom H, Purification and amino acid sequence of sakacin A, a bacteriocin produced from Lactobacillus sakei Lb706. J Gen Microbiol 138:2715–2720 (1992). [DOI] [PubMed] [Google Scholar]
- 10. Trinetta V, Rollini M, Limbo S and Manzoni M, Influence of temperature and sakacin A concentration on survival of Listeria innocua cultures. Ann Microbiol 58:633–639 (2008). [Google Scholar]
- 11. Trinetta V, Rollini M and Manzoni M, Development of a low cost culture medium for sakacin A production by L. sakei . Process Biochem 43:1275–1280 (2008). [Google Scholar]
- 12. Trinetta V, Morleo A, Sessa F, Iametti S, Bonomi F and Ferranti P, Purified sakacin A shows a dual mechanism of action against Listeria spp: proton motive force dissipation and cell wall breakdown. FEMS Microbiol Lett 334:143–149 (2012). [DOI] [PubMed] [Google Scholar]
- 13. Masana MO, Barrio YX, Palladino PM, Sancho AM and Vaudagna SR, High pressure treatments combined with sodium lactate to inactivate Escherichia coli O157:H7 and spoilage microbiota in cured beef carpaccio. Food Microbiol 46:610–617 (2015). [DOI] [PubMed] [Google Scholar]
- 14. Friedly BC, Crandall PG, Ricke S, O'Bryan CA, Martin EM and Boyd LM, Identification of Listeria innocua surrogates for Listeria monocytogenes in hamburger patties. J Food Sci 73:174–178 (2008). [DOI] [PubMed] [Google Scholar]
- 15. Barbiroli A, Bonomi F, Capretti G, Iametti S, Manzoni M, Piergiovanni L et al., Antimicrobial activity of lysozyme and lactoferrin incorporated in cellulose‐based food packaging. Food Control 26:387–392 (2012). [Google Scholar]
- 16. Guyonnet D, Fremaux C, Cenatiempo Y and Berjeaud JM, Method for rapid purification of class IIa bacteriocins and comparison of their activities. Appl Environ Microbiol 66:1744–1748 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coventry MJ, Gordon JB, Alexander M, Hickey MW and Wan J, A food‐grade process for isolation and partial purification of bacteriocins of lactic acid bacteria that uses diatomite calcium silicate. Appl Environ Microbiol 62:1764–1769 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Raheem D, Application of plastics and paper as food packaging materials – an overview. Emir J Food Agric 25:177–188 (2012). [Google Scholar]
- 19. Rodríguez A, Batlle R and Nerín C, The use of natural essential oils as antimicrobial solutions in paper packaging. Part II. Prog Org Coat 60:33–38 (2007). [Google Scholar]
- 20. Ming X, Weber GH, Ayres JW and Sandienew E, Bacteriocins applied to food packaging materials to inhibit Listeria monocytogenes on meats. J Food Sci 62:413–415 (1997). [Google Scholar]
- 21. La Storia A, Ercolini D, Marinello F and Mauriello G, Characterization of bacteriocin‐coated antimicrobial polyethylene films by atomic force microscopy. J Food Sci 73:48–54 (2008). [DOI] [PubMed] [Google Scholar]
- 22. Guiga W, Swesi Y, Galland S, Peyrol E, Degraeve P and Sebti I, Innovative multilayer antimicrobial films made with Nisaplin® or nisin and cellulosic ethers: physico‐chemical characterization, bioactivity and nisin desorption kinetics. Innovat Food Sci Emerg Technol 11:352–360 (2010). [Google Scholar]
- 23. Trinetta V, Floros JD and Cutter CN, Sakacin A‐containing pullulan film: an active packaging system to control epidemic clones of Listeria monocytogenes in ready‐to‐eat foods. J Food Saf 30:366–381 (2010). [Google Scholar]


