Abstract
Aim
Respiratory distress syndrome (RDS) is a major cause of mortality and morbidity in premature infants. By the time symptoms appear, it may already be too late to prevent a severe course, with bronchopulmonary dysplasia or mortality. We aimed to develop a rapid test of lung maturity for targeting surfactant supplementation.
Methods
Concentrations of the most surface‐active lung phospholipid dipalmitoylphosphatidylcholine and sphingomyelin in gastric aspirates from premature infants were measured by mass spectrometry and expressed as the lecithin/sphingomyelin ratio (L/S). The same aspirates were analysed with mid‐infrared spectroscopy. Subsequently, L/S was measured in gastric aspirates and oropharyngeal secretions from another group of premature infants using spectroscopy and the results were compared with RDS development. The 10‐minute analysis required 10 μL of aspirate.
Results
An L/S algorithm was developed based on 89 aspirates. Subsequently, gastric aspirates were sampled in 136 infants of 24–31 weeks of gestation and 61 (45%) developed RDS. The cut‐off value of L/S was 2.2, sensitivity was 92%, and specificity was 73%. In 59 cases, the oropharyngeal secretions had less valid L/S than gastric aspirate results.
Conclusion
Our rapid test for lung maturity, based on spectroscopy of gastric aspirate, predicted RDS with high sensitivity.
Keywords: Gastric aspirate, Lung surfactant, Mid‐infrared spectroscopy, Prematurity, Respiratory distress syndrome
Abbreviations
- CI
Confidence interval
- DPPC
Dipalmitoylphosphatidylcholine
- FiO2
Fraction of oxygen in inspired gas
- FTIR
Fourier transform infrared spectroscopy
- L/S
Lecithin/sphingomyelin ratio
- MS
Mass spectroscopy
- nCPAP
Nasal continuous positive airway pressure
- RDS
Respiratory distress syndrome
Key notes.
Respiratory distress syndrome (RDS) is a major cause of mortality and morbidity in premature infants, and when symptoms appear, it may already be too late to prevent a severe course
We developed a rapid mid‐infrared spectroscopy test to predict the development of RDS and accurately target treatment.
A low lecithin/sphingomyelin ratio in gastric aspirate was a sensitive predictor for RDS and may be useful for targeting early surfactant supplementation.
Introduction
Respiratory distress syndrome (RDS) remains a major cause of mortality and morbidity in premature infants despite the increased use of antenatal steroids and early nasal continuous positive airway pressure (nCPAP). The majority of premature infants with a gestational age of less than 30 weeks have immature lungs and approximately half still require treatment with surfactant when treated with nCPAP or mechanical ventilation 1, 2. In recent years, there have been several improvements in treatment, which have helped reduce the incidence and severity of RDS and bronchopulmonary dysplasia. Minimal use of oxygen in the delivery room 3 and in the neonatal period is an important development in care. Very early treatment with nCPAP, combined with minimal use of mechanical ventilation, especially during initial treatment, reduces ventilator‐induced lung damage and is now widely used 4, 5. Over the years, early rescue treatment with surfactant has been strongly associated with a more favourable outcome compared to late rescue treatment 6, 7, 8. Better outcomes are also seen with a combination of nCPAP and surfactant 7, 8. Finally, less invasive administration of surfactant via a thin catheter has emerged as an alternative method, especially in the most preterm infants 9. However, we need to treat selectively with surfactant, and very soon after birth, in order to substantially improve the prognosis of RDS and bronchopulmonary dysplasia. In many cases, this needs to happen before symptoms are present. In order to do so, we need a bedside test that can quickly identify which infants have surfactant deficiency to target treatment effectively 10. Prophylactic treatment has been widely used, but it has been shown to increase the combined mortality and incidence of bronchopulmonary dysplasia 11.
Biomarkers expressing lung maturity have been identified in amniotic fluid 12, gastric aspirate 13 and hypopharyngeal secretions 14. These fluids are partially produced in the foetal lungs as well as in the kidneys and the amniotic sac and therefore contain lung surfactant 15. The classic method to determine surfactant in amniotic fluid has been measuring lecithin/sphingomyelin ratio (L/S) with thin‐layer chromatography 12, 16. Sphingomyelin secretion remains fairly constant during pregnancy, whereas lecithin secretion increases in parallel with lung maturation. Consequently, L/S reflects lung maturity independently of dilution sampling effects 12. However, this well‐known diagnostic procedure to measure lung surfactant is slow and complex 16.
Gastric aspirate and oropharyngeal secretion are easier to obtain and measure than amniotic fluid 17, 18. Previously, we have measured the surfactant content in gastric aspirate with microbubble stability tests 19 and with lamellar body counts 20. We also conducted a randomised trial in very preterm infants with early surfactant guided by lamellar body counts in gastric aspirate versus traditional surfactant treatment 21. Use of lamellar body counts as a guide for early surfactant treatment led to a significant reduction in the need of oxygen at six hours of age and by day 28. Furthermore, the time on oxygen therapy was reduced and there was a trend towards a lower incidence of bronchopulmonary dysplasia. The earlier that the lamellar body counts were measured, the better the outcome. Dilution, however, was a problem with both the microbubble stability test and lamellar body counts. Furthermore, both these methods, and similar tests, are labour and laboratory intensive and require a minimum of two to three hours for analysis. This makes these methods less suitable for use as bedside tests.
The aim of this study was to predict the risk of RDS by measuring lung maturity. To achieve this, we developed a rapid test using spectroscopic analysis of L/S in small volumes of oropharyngeal secretion and from gastric aspirate obtained from gastric tubes placed as part of routine neonatal management. Subsequently, we validated this method in a number of premature infants by comparing the appearance of RDS with L/S.
Patients and methods
Study design
The study was a multicentre nonintervention diagnostic study with two phases.
In the method development phase, which was phase one of the study, gastric aspirate samples from a group of premature infants were analysed by mass spectroscopy (MS) to obtain reference values for the concentration of lecithin, which is also called phosphatidylcholine, and sphingomyelin. The same gastric aspirate samples were then measured by Fourier transform infrared spectroscopy (FTIR), and an algorithm was developed to quantify phosphatidylcholine and sphingomyelin concentrations by spectroscopy. These are detailed below and in Appendix S1. The concentration determined by MS of the lecithin species with the highest surface activity, dipalmitoylphosphatidylcholine (DPPC), was used to calculate the L/S. This phospholipid is only present at very low concentrations in blood or meconium.
During the clinical phase, which was phase two of the study, another population of premature infants had gastric aspirate and oropharyngeal samples obtained shortly after birth. These infants were monitored for the first five days of life and all clinically relevant data captured. L/S from this population was then determined by spectroscopy and analysed by the algorithm. The results were subsequently compared with the prevalence and severity of RDS.
The trial was approved by the research ethics committees of each centre. Informed oral and written parental consent was obtained either before birth or immediately following birth.
Pre‐analytical handling of samples
The gastric aspirates were frozen at −20°C shortly after they were drawn. Gastric aspirate may contain blood, cells, meconium, particular matters and mucus. Before the analyses in both phases of the study, the samples were thawed and mixed thoroughly followed by mild centrifugation of 300 g for three minutes. Cells and particulate matter precipitated and mucus gathered in the upper part of the sample. Sample aliquots for the analyses were taken from the clear midsection of the sample.
Oropharyngeal secretions were frozen at _20°C shortly after they were drawn. Before the analysis was carried out, the samples were thawed and 50 μL of distilled water was added to each oropharyngeal secretion sample due to the low volume available for analysis. The diluted oropharyngeal secretion samples were processed in the same way as the gastric aspirates.
Phase one: method development
To develop the L/S algorithm, 89 newborn infants from Denmark, UK and Sweden had gastric aspirate sampled from gastric tubes placed for routine clinical reasons shortly after birth. The infants had a median gestational age of 29.5, with a range of 24.2–33.7 weeks.
Mass spectroscopy
MS, which was the reference method, was carried out on the 89 gastric aspirate samples. 125 μL of aspirate was mixed with 10 μL of antioxidant (butylated hydroxytoluene, 50 g/L). Internal standards in chloroform to a final concentration of 1.0 nmol dimyristoylphosphatidylcholine per sample were then added. Once this was complete, samples were lipid‐extracted using dichloromethane and methanol 22 and the lipid‐containing lower organic phase removed and dried under warmed nitrogen gas. Once they were dried, the samples were capped with an airtight seal and stored at _20°C until analysis. Immediately prior to MS, the samples were reconstituted in 300 μL solvent comprising methanol, dichloromethane, water and concentrated ammonia (66:30:3:1, v:v).
MS analysis of the individual molecular species of lecithin, including DPPC and sphingomyelin, was performed by direct infusion on a Xevo TQ MS (Waters, Manchester, UK) fitted with an electrospray ionisation source with sample delivery at eight μL/min. Full positive and full negative scans were taken, followed by diagnostic tandem MS/MS precursor ion spectra for phosphatidylcholine (P184) and sphingomyelin (P168).
FTIR spectroscopy
Spectra were recorded in the mid‐infrared region (3200–900 cm−1) by a Tensor 27 FTIR spectrometer (Bruker, Massachusetts, USA). A nitrogen‐cooled mercury cadmium telluride detector and an attenuated total reflection cell, Harric BioATRcellII, with a silicon crystal were used. The temperature of the attenuated total reflection cell was 30°C. Spectra were recorded using OPUS software version 7.2 (Bruker, Massachusetts, USA). Gastric aspirate (20 μL) was transferred to a fresh vial containing 20 μL of internal standard (potassium thiocyanate 1 g/L). Then, 6 μL of this mixture was applied to the attenuated total reflection crystal. The sample was dried for two minutes under a gentle stream of air (40–45°C). Each sample was applied twice, and for each application, two spectra were recorded. The mean value of the four spectra was calculated, and the baseline was corrected and normalised with respect to the potassium thiocyanate peak at 2.000–2.110 cm−1. The spectroscopy after pre‐analytic handling of each sample was completed in less than 10 minutes.
Calibration of the FTIR methods
Calibration of the FTIR methods was carried out with the 89 gastric aspirate samples with reference concentrations of DPPC and total sphingomyelin measured by MS.
For each analyte, the spectra were processed using a stepwise selection of wave numbers giving the optimal least squares correlation between the measured mid‐infrared signal and the analyte concentration measured by the reference method. At each step of the analysis, a partial least square regression was evaluated by average cross‐validation. Wave numbers showing no or a small correlation between the mid‐infrared signal and measured concentration were excluded. The detection of the sample outliers was performed by leaving‐one‐out analysis, whereby calibration samples that deviated by more than 3.5 standard deviations from their measured concentration were excluded from the calibration set.
Algorithms for the quantification of DPPC and sphingomyelin were developed chemometrically to produce adequate correlation coefficients between the reference values and the associated computed values.
A more detailed overview of the stepwise calibration procedure is given in Appendix S1 in the online supplement section and has previously been published 23, 24.
Study phase two: L/S analyses
Infants with a gestational age from 24 to 31 completed weeks were eligible. Oropharyngeal secretion samples (30–100 μL) were secured from between the root of the tongue and the cheek with a 1‐mL syringe within a few minutes after birth. Gastric aspirate samples (0.3–2.5 mL) were drawn within half an hour after birth using a feeding tube attached to a 10‐mL syringe or a suction catheter (Ch 6) connected to a tracheal suction set. The feeding tube or suction catheters were placed while establishing nCPAP for respiratory stabilisation or intubation. Immediate treatment with nCPAP was the primary method of respiratory stabilisation at birth, and intubation was only carried out for resuscitation. Gastric aspirate and oropharyngeal secretion, as detailed in the section about pre‐analytical handling above, were stored at _20°C within one hour of birth for later analyses by FTIR spectroscopy.
RDS was diagnosed when at least two of the four classical symptoms were present: the need for supplemental oxygen, tachypnoea, intercostal retractions and grunting, and a chest x‐ray consistent with RDS excluded other causes of respiratory distress. The severity of RDS was graded in four groups 19. It was graded as non‐RDS if the fraction of inspired oxygen (FiO2) was <0.40 in maximal nine hours after birth, with no need for supplemental oxygen later on. Mild RDS was when the FiO2 was 0.22–0.40 for more than nine hours after birth, but there was no need for surfactant or mechanical ventilation. Moderate RDS was the need for surfactant or FiO2 >0.40, but no mechanical ventilation. Severe RDS was the need for mechanical ventilation within the five‐day study. FiO2 was registered every hour for the first 24 hours and every second hour for the following 48 hours and twice on days 4 and 5.
The calculation of the L/S values by the DPPC and sphingomyelin algorithms was performed without any knowledge of the clinical outcomes.
Different factors in addition to the neonatal treatment of lung symptoms are known to influence the development of RDS and possibly also surfactant analyses. The factors considered in the following include the following: multiple births, birth by Caesarean section, gestational diabetes, asphyxia, antenatal steroid treatment and contamination with blood, meconium and mucus and viscous gastric aspirate.
Statistical analysis
We estimated that 135 infants with a gestational age of 24–31 weeks, with an estimated 55 with RDS 19, would be sufficient to evaluate the method.
We chose cut‐off values that maximised the sum of the sensitivity and specificity to improve the usability of L/S to diagnose RDS 25. The 95% confidence intervals (CI) for sensitivity and specificity were calculated. The L/S data were stratified into groups based on gestational ages of 24–25, 26–29 and 30–31 weeks.
Pearson's chi‐square test was used to examine the possible influence of Caesarean section on the accuracy of L/S as a predictor of RDS and the sensitivity of L/S versus gestational age. Two‐tailed p values of <0.05 were considered to indicate statistical significance.
Results
Infants were enrolled in the second phase of the study from March 2014 to January 2015 from eight Danish and three Swedish neonatal units. Gastric aspirate samples were obtained from 143 of the 144 enrolled infants. Seven cases were excluded – four due to prophylactic surfactant treatment before sampling, two due to severe infections and one due to congenital lung malformation – leaving 136 infants with gastric aspirates in the final cohort. Oropharyngeal secretions were available from 59 infants. For 58 infants, oropharyngeal secretions were available in addition to gastric aspirate, and for one infant, only oropharyngeal secretion was available. Of the included 136 infants, seven infants (5%) died before the end of the five‐day study period.
Table 1 shows the clinical characteristics of the infants who had gastric aspirates included in the analyses.
Table 1.
Demographic and clinical characteristics of the patients
| Characteristics | (N = 136) |
|---|---|
| GA, median (interquartile range), week | 29.8 (27.3–31.0) |
| Birth weight, median (interquartile range), g | 1260 (902–1506) |
| Apgar score at five minute, median (interquartile range), | 10 (8–10) |
| Antenatal corticosteroids, n (%) | 117 (86) |
| Caesarean section, n (%) | 85 (63) |
| Male gender, n (%) | 71 (52) |
In Figure 1, the L/S values from the gastric aspirates are compared with the development of RDS. RDS developed in 61 (45%) of the 136 infants and was classified as mild in 10 (16%), as moderate in 31 (51%) and as severe in 20 (33%). The optimal cut‐off value 25 of L/S was 2.2. The developed L/S method as a predictor of RDS from gastric aspirate had a sensitivity of 92% (95% CI: 82–97), a specificity of 73% (95% CI 62–83), a positive predictive value of 74% (95% CI: 66–85) and a negative predictive value of 92% (95% CI: 82–97). Gestational age was 24–25 weeks for 12 infants (9%), 26–29 weeks for 62 infants (46%) and 30–31 weeks for a further 62 infants (46%). The sensitivity was high in all three gestational age groups, and only one or two infants with RDS in each group had an L/S above the cut‐off 2.2. Gestational age and L/S with different degrees of RDS severity are shown in Table 2. Infants without RDS had L/S below 2.2 in 20 cases: eight of these (40%) needed extra oxygen and no infants diagnosed without RDS, with an L/S above 3.2. required extra oxygen.
Figure 1.
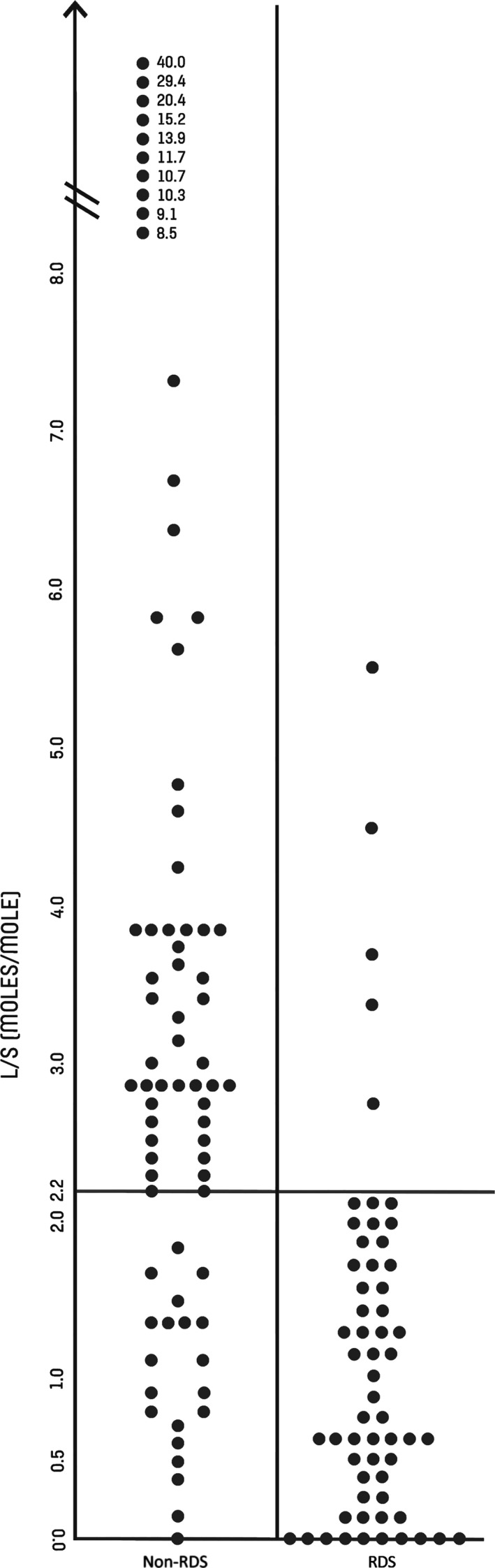
Lecithin/sphingomyelin ratio (L/S) in gastric aspirates determined by mid‐infrared spectroscopy in 61 infants with RDS and 75 infants with non‐RDS. The best cut‐off value is 2.2 moles/mole.
Table 2.
Gestational age and L/S ratio in different degrees of severity of RDS
| RDS severity | n | (N = 136) | |
|---|---|---|---|
| Gestational age (weeks) | L/S ratio (moles/mole) | ||
| Median (range) | Median (range) | ||
| Non‐RDS | 75 | 30.4 (25.0–31.9) | 3.0 (0–40.0) |
| Mild | 10 | 28.1 (25.0–31.9) | 1.3 (0.4–2.1) |
| Moderate | 31 | 30.0 (24.3–31.6) | 0.6 (0–5.3) |
| Severe | 20 | 26.2 (24.1–29.9) | 0.7 (0–4.5) |
| RDS (all degrees) | 61 | 28.7 (24.1–31.9) | 0.8 (0–5.3) |
One set of triplets and 19 pairs of twins were included in the study: the triplets and 12 of the 19 pairs of twins accounted for the 65% of the multiple births born by Caesarean section. In 12 pairs, the lowest L/S was found in the second twin, while in one pair, the L/S was the same in both twins. Twins and triplets were correctly diagnosed in 81% of cases compared with 82% of all infants in the cohort.
The RDS predictions from L/S were significantly more precise with a p value of just below 0.05 in infants born by the vaginal route, which accounted for 37% of the deliveries, than by Caesarean section (Table 3a and b). None of the mothers of the included infants had gestational diabetes. No difference in specificity was found between the infants with Apgar scores of 10 and lower scores at five minutes. L/S predicted the development of RDS with the same precision in cases with and without antenatal corticosteroid treatment. Gastric aspirate was visually contaminated with blood in 32 (24%) of the cases. In 29 of these 32 cases, L/S correctly diagnosed the development of RDS. In addition, one case with RDS and two cases without RDS and blood contamination of the gastric aspirates were diagnosed incorrectly. Visual meconium contamination of the gastric aspirate was only seen in four cases, and all were diagnosed accurately without RDS. Mucus was found in different amounts in about half of the samples. Viscous gastric aspirate was not seen in any case.
Table 3.
L/S in gastric aspirates from infants born by Caesarean section vs. vaginal delivery
| (a) | |||||
|---|---|---|---|---|---|
| (N = 85) | Caesarean section | (N = 51) | Vaginal delivery | ||
| + RDS n | − RDS n | + RDS n | − RDS n | ||
| L/S < 2.2 | 33a | 15 | 23b | 5 | |
| L/S ≥ 2.2 | 5 | 32c | 0 | 23d | |
| (b) | ||
|---|---|---|
| (N = 136) | Prediction of RDS | |
|
Right n |
Wrong n |
|
| Caesarean section | 65 | 20 |
| Vaginal delivery | 46 | 5 |
Prediction was significantly better in the vaginal group, p < 0.05, relative risk 0.85 (CI 95%, 0.73–0.98).
Sensitivity 87% (CI 95%, 72–96).
Sensitivity 100% (CI 95%, 85–100).
Specificity 68% (CI 95%, 53–81).
Specificity 82% (CI 95%, 63–94).
The optimal cut‐off value 25 of L/S in oropharyngeal secretion was 3.7. The sensitivity, specificity and predictive values are shown in Table 4.
Table 4.
L/S in oropharyngeal secretions (N = 59)
Positive predictive value: 70% (CI 95%, 51–85).
Negative predictive value: 72% (CI 95%, 53–87).
Sensitivity 72% (CI 95%, 53–87).
Specificity 70% (CI 95%, 51–85).
Predicting RDS using the L/S ratio was significantly better than using the gestational age as a predictive indicator (Table 5a). When gestational age was used as a guide for predicting RDS, 43 (70%) of the 61 infants with RDS had a gestational age of less than 30 weeks, compared to 56 (92%) of the 61 infants with L/S below the cut‐off value when L/S was used to predict RDS. Both sensitivity and specificity were highest when predicted by L/S. Table 5b shows that the age from birth was median of six hours before the infants were treated with surfactant based on the clinical observations.
Table 5.
(a) Comparison of L/S and gestational age for prediction of RDS. (b) Characteristics of infants treated with surfactant
| (a) | ||
|---|---|---|
| N = 136 of which 61 developed RDS | ||
| L/S in gastric aspirate determined spectroscopically | Gestational age (week 24–31) | |
| Best cut‐off | 2.2 moles/molea | <30 week'sa |
| Sensitivity | 92%a | 70%a p < 0.01c |
| Specificity | 73%a | 60%a |
| Time to diagnosis | Less than one hour after birthb | At birth |
| (b) | ||
|---|---|---|
| (N = 51) | ||
| Main indication for surfactant treatment: FiO2 >0.40 | ||
| Time from birth to surfactant treatment: median six hours, range 0.1–48 h | ||
| Four infants were treated ≤one hour |
The best cut‐off value was that with the highest sum of sensitivity and specificity.
By the planned bedside test.
The p‐value was calculated by chi‐square test.
Discussion
For more than 50 years, clinicians have searched for a test to determine lung maturity in premature infants, and from the 1970s, several laboratory tests have been developed. Only a few are still in use, and they all have significant drawbacks, such as lack of specificity and sensitivity or a demand for skilled laboratory personnel and a long delay before the test results are available. Therefore, there is still an urgent unmet need for a quick and safe test to identify which newborn infants require surfactant. We have developed a fast method that does not require highly trained laboratory personnel and can be further developed to a point‐of‐care test. We chose to use gastric aspirates and oropharyngeal secretions, which are easy to obtain, and a process that does not require the use of complicated reagents.
More clinical factors may influence the development of RDS and the lung maturity test. For example, the second twin often has a lower L/S than the first‐born infant. This tendency is also seen in the actual study.
Blood contamination of the gastric aspirate does not appear to influence measurements of L/S. This could be due to phosphatidylcholine being measured as DPPC, which is only sparsely present in blood and meconium. Gastric aspirates were contaminated with meconium in four cases. This was too few to draw conclusions about the effect, although L/S accurately predicted RDS in all cases. As mentioned, we did not analyse the mucus in gastric aspirates. Detailed lipid analyses of mucus in fresh, frozen and thawed gastric aspirate are planned before the L/S analysis is introduced as a bedside test. Viscous gastric aspirate was not a problem in the actual study, and it is probable that the phenomenon is only seen at term.
Predicting RDS by L/S analyses of gastric aspirate was significantly poorer in infants born by Caesarean section compared to those born through the vaginal route. A possible explanation is that L/S in gastric aspirate after Caesarean section originates from a mix of amniotic fluid and newly produced lung fluid, both with different L/S, in contrast to L/S in gastric aspirate from infants born vaginally. We found it conceivable that swallowed amniotic fluid to some degree may be squeezed out of the stomach during natural birth as opposed to birth by Caesarean section. Accordingly, gastric aspirate obtained immediately after a vaginal birth may contain a higher proportion of newly swallowed fluid from the lungs.
The actual L/S test was found to have a lower specificity than sensitivity, and we found it likely that this might have been mainly explained by the fact that all the included infants were stabilised with nCPAP within a few minutes of birth. The efficacy of nCPAP may alleviate or mask symptoms of RDS, making the diagnosis of RDS challenging, but it would be unethical to withhold treatment with nCPAP. In our study, 40% of the infants with non‐RDS and L/S values below the cut‐off required supplemental oxygen during the first nine hours of life, while infants with non‐RDS and higher L/S values had no need for oxygen. This makes it probable that some infants with low L/S values, who were graded as non‐RDS, actually had subclinical surfactant deficiency. It has also been shown that nCPAP can increase L/S in repeated hypopharyngeal secretions 26. We previously developed two different methods for early diagnosis of RDS on gastric aspirate from infants similar to the population in the present study receiving early nCPAP 18, 19. These previously developed tests, like our new method, had specificity that was lower than sensitivity, supporting the suggestion that nCPAP may mask less pronounced surfactant deficiency.
The analysis of gestational age and L/S in the different classifications of RDS supports the conclusion that our method correctly predicted the pulmonary outcome in these groups. Gestational age and L/S were lower in all groups of RDS compared to non‐RDS. What is also remarkable is that there was no distinct correlation between gestational age and L/S, probably reflecting that gestational age only accounted for a smaller fraction of the total variation in the result of the lung maturity test 20, 27. This was also supported by the higher sensitivity and specificity of L/S compared to gestational age when used as a predictor of RDS. Surfactant treatment in the actual study, as judged by clinical observations, was given after a median of six hours and only four infants were treated an hour or less after birth. This indicates that fast determination of L/S by a bedside test is likely to allow earlier diagnosis of RDS and earlier rescue treatment with surfactant compared to rescue surfactant treatment based on routine clinical criteria.
L/S varied in amniotic fluid, depending on the locations it was sampled from, and it had the strongest correlation with the development of RDS when it was taken near to the infant's mouth 28. Consequently, we also analysed L/S in oropharyngeal secretion. The fluid was taken noninvasively and close to the oral cavity. The specificity of L/S in the oropharyngeal secretions was equal to the specificity in the gastric aspirates, but the sensitivity for the oropharyngeal secretions was lower than for the gastric aspirates. We expect that the sampling technique can be optimised and that fresh oropharyngeal secretions and gastric aspirates will be easier to process than frozen ones. Stichtenoth et al. 29 measured surface tension in upper airway aspirates and found acceptable correlations to lung maturity. However, the processing time was rather long and the dilution of the surfactant may have interfered with the surface tension measurements. Others have also successfully performed L/S analyses on hypopharyngeal secretions 14, 26, but by using methods that were slower and less accurate. The use of spectroscopy to measure L/S on amniotic fluid was initially explored by Liu et al. 30. This method held promise for rapid measurements of lung maturity in the laboratory, but the low accuracy of the reference method, which was carried out by thin‐layer chromatography, proved to be a fundamental problem 16. The increased foetal loss after amniocentesis, together with improvements in newborn respiratory care, obviates wide use of the method.
The spectroscopic part of our L/S method was very rapid, but at present, the method is still a laboratory test. To contribute to clinical decision‐making, results should be available within minutes after birth and a bedside version of the test is needed for our method to become useful in clinical settings. A new stand‐alone device is currently being developed with the aim that it will be used as a point‐of‐care test in neonatal wards, using custom‐made equipment for easy sampling and processing of gastric aspirate and potentially oropharyngeal secretions. The bedside test will be introduced in a randomised study, which will measure the benefit of this new method on clinical outcomes compared to standard treatment.
Conclusion
We have developed a rapid test to measure lung maturity on small volumes of gastric aspirate and oropharyngeal secretion in premature infants at birth. This test could help identify which infants will benefit from very early surfactant treatment, with the potential to significantly improve clinical outcomes resulting in less severe RDS, less need of mechanical ventilation and oxygen and less severe bronchopulmonary dysplasia.
Funding
This project received funding from the European Union's Horizon 2020 research and innovation programme.
Conflict of interest
Henrik Verder and Agnar Höskuldsson hold part of a patent for the spectroscopic analysis of biological samples. The authors have no other conflict of interest.
Supporting information
Appendix S1. Development of the algorithms for quantification of phosphatidylcholine (PC16:0/16:0) and sphingomyelin (SM) by FTIR.
Figure S1. Calibration result according to the developed algorithm for determination of the concentration of PC16:0/16:0.
Figure S2. Calibration result according to the developed algorithm for determination of the concentration of sphingomyelin.
Table S1. Comparison of L/S‐values measured on the same cases by mass spectrometry and by infrared spectroscopy.
Acknowledgements
We would like to thank Holbaek Hospital and Region Zealand, Denmark, for their vital co‐operation on this project and Paul Townsend and Kewi Zhang for technical assistance with the mass spectroscopy analyses. We also want to thank the nurses and doctors in the participating neonatal departments and the Department of Clinical Biochemistry, Holbaek Hospital, Vivi Kuhr Christensen for creating the database, Morgaine Matthews for helping with the manuscript and Ahmet Cizmeli for helping with the development of the algorithms.
References
- 1. Sandri F, Placka R, Ancora G, Simeoni U, Stranak Z, Martinelli S, et al. Prophylactic or early surfactant combined with nCPAP in very preterm infants. Pediatrics 2010; 125: e140. [DOI] [PubMed] [Google Scholar]
- 2. Bevilacqua G, Pamigiani S, Robertson B. Prophylaxis of respiratory distress syndrome by treatment with modified surfactant at birth: a multicentre prospective randomized trial. J Perinat Med 1996; 24: 609–20. [DOI] [PubMed] [Google Scholar]
- 3. Saugstad OD. Delivery room management of term and preterm newly born infants. Neonatology 2015; 107: 365–71. [DOI] [PubMed] [Google Scholar]
- 4. Verder H. Nasal CPAP has become an indispensable part of the primary treatment of newborns with respiratory distress syndrome. Acta Paediatr 2007; 96: 482–4. [DOI] [PubMed] [Google Scholar]
- 5. van Marter LJ, Dammann O, Alred EN, Leviton A, Pagano M, Moore M, et al. Chorioamnionitis, mechanical ventilation, and postnatal sepsis as modulators of chronic lung disease in preterm infants. J Pediatr 2002; 140: 171–6. [DOI] [PubMed] [Google Scholar]
- 6. Soll RF. Early versus delayed selective surfactant treatment for neonatal respiratory distress syndrome. Cochrane Database Syst Rev 1999; 4: CD001456. [DOI] [PubMed] [Google Scholar]
- 7. Stevens TP, Harrington EW, Blennow M, Soll F. Early surfactant administration with brief ventilation vs. selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. Cochrane Database Syst Rev 2007; 4: CD003063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Verder H, Albertsen P, Ebbesen F, Greisen G, Robertson B, Bertelsen A, et al. Nasal continuous positive airway pressure and early surfactant therapy for respiratory distress syndrome in newborns less than 30 weeks' gestation. Pediatrics 1999; 103: e24. [DOI] [PubMed] [Google Scholar]
- 9. Isayama T, Iwami H, McDonald S, Beyene J. Association of noninvasive ventilation strategies with mortality and bronchopulmonary dysplasia among preterm infants. A systematic review and meta‐analysis. JAMA 2016; 316: 611–24. [DOI] [PubMed] [Google Scholar]
- 10. Sweet DG, Carnielli V, Greisen G, Hallmann M, Ozek E, Plavka R, et al. European Consensus guidelines on the management of neonatal respiratory distress syndrome in Preterm Infants – 2013 update. Neonatology 2013; 103: 353–68. [DOI] [PubMed] [Google Scholar]
- 11. Soll RF. Prophylactic versus selective use of surfactant in prevention of morbidity and mortality in preterm infants. Cochrane Rev Update Neonatol 2012; 102: 169–71. [Google Scholar]
- 12. Gluck L, Kulovich MV, Borer RC, Brenner PH, Anderson GG, Spellacy WN. Diagnosis of respiratory distress syndrome by amniocentesis. Am J Obstet Gynecol 1971; 109: 440–5. [DOI] [PubMed] [Google Scholar]
- 13. Borer RC, Kuhns LR, Holt JF, Poznanski AK, Bednarek FT. Accuracy of gastric aspirate lecithin/sphingomyelin ratio and chest radiographs in the diagnosis of respiratory distress syndrome. Pediatr Res 1974; 8: 444. [Google Scholar]
- 14. Blumenfeld TA, Driscoll JM, James LS. Lecithin/sphingomyelin ratios in tracheal and pharyngeal aspirates in respiratory distress syndrome. J Pediatr 1974; 85: 403–7. [DOI] [PubMed] [Google Scholar]
- 15. Spillman T, Cotton DB. Current perspectives in assessment of fetal pulmonary surfactant status with amniotic fluid. Crit Rev Clin Lab Sci 1989; 27: 341–89. [DOI] [PubMed] [Google Scholar]
- 16. Kulovich MV, Hallman MB, Gluck L. The lung profile I. Normal pregnancy. Am J Obstet Gynecol 1979; 135: 57–63. [PubMed] [Google Scholar]
- 17. Gabbe SG. Recent advances in the assessment of fetal lung maturity. J Reprod Med 1979; 23: 228–43. [PubMed] [Google Scholar]
- 18. Brace RA. Physiology of amniotic fluid volume regulation. Clin Obstet Gynecol 1997; 40: 280–9. [DOI] [PubMed] [Google Scholar]
- 19. Verder H, Ebbesen F, Linderholm B, Robertson B, Eschen C, Arrøe M, et al. Prediction of respiratory distress syndrome by microbubble stability test on gastric aspirate in newborns less than 32 weeks' gestation. Acta Paediatr 2003; 92: 728–33. [DOI] [PubMed] [Google Scholar]
- 20. Verder H, Ebbesen F, Brandt J, Dahl M, Esberg G, Eschen C, et al. Lamellar body counts on gastric aspirates for prediction of respiratory distress syndrome. Acta Paediatr 2011; 100: 175–80. [DOI] [PubMed] [Google Scholar]
- 21. Verder H, Ebbesen F, Fenger‐Grøn J, Henriksen TB, Andreasson B, Bender L, et al. Early surfactant guided by lamellar body counts on gastric aspirate in very preterm infants. Neonatology 2013; 104: 116–22. [DOI] [PubMed] [Google Scholar]
- 22. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 1955; 37: 911–7. [DOI] [PubMed] [Google Scholar]
- 23. Höskuldsson AT. Common framework for linear regression. Chemom Intell Lab Syst 2015; 146: 250–62. [Google Scholar]
- 24. Jessen TE, Höskuldsson AT, Bjerrum PJ, Verder H, Sørensen L, Bratholm PS, et al. Simultaneous determination of glucose, triglycerides, urea, cholesterol, albumin and total protein in human plasma by Fourier transform infrared spectroscopy: direct clinical biochemistry without reagents. Clin Biochem 2014; 47: 1306–12. [DOI] [PubMed] [Google Scholar]
- 25. Altman DG. Practical statistics for medical research. London: Chapman and Hall, 1991. [Google Scholar]
- 26. Barr PA, Jenkins PA, Baum JD. Lecithin‐sphingomyelin ratio in hypopharyngeal aspirate of newborn infants. Arch Dis Child 1975; 50: 856–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bender T, Stone L, Amenta J. Diagnostic power of lecithin/sphingomyelin ratio and fluorescence polarization assays for respiratory distress syndrome compared by relative operating characteristics curves. Clin Chem 1994; 40: 541–5. [PubMed] [Google Scholar]
- 28. Worthington D, Smith BT. The site of amniocentesis and the lecithin‐sphingomyelin ratio. Obstet Gynecol 1978; 52: 552–4. [PubMed] [Google Scholar]
- 29. Stichtenoth GS, Walter G, Lange L, Lange R, Raith M, Herting E. Surface tension of airway aspirates Withdrawn during neonatal resuscitation reflects lung maturity. Pediatr Pulmonol 2014; 49: 751–6. [DOI] [PubMed] [Google Scholar]
- 30. Liu K‐Z, Dembinski TC, Mantsch HH. Rapid determination of fetal lung maturity from infrared spectra of amniotic fluid. Am J Obstet Gynecol 1998; 178: 234–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Development of the algorithms for quantification of phosphatidylcholine (PC16:0/16:0) and sphingomyelin (SM) by FTIR.
Figure S1. Calibration result according to the developed algorithm for determination of the concentration of PC16:0/16:0.
Figure S2. Calibration result according to the developed algorithm for determination of the concentration of sphingomyelin.
Table S1. Comparison of L/S‐values measured on the same cases by mass spectrometry and by infrared spectroscopy.


