Abstract
A carcinogen‐induced premalignant oral lesion model that progresses to oral cancer was used to examine the impact of blocking PD‐1 on cytokine expression and on progression of lesions to cancer. The results of this study show increased production of IL‐2 and the inflammatory cytokines IL‐6, IL‐17 and TNF‐α by spleen cells of lesion‐bearing mice that were treated with PD‐1 antibody for 1 week compared to cytokine production by spleen cells of lesion‐bearing mice treated with control antibody. Production of IFN‐γ increased at 3 weeks of PD‐1 antibody treatment, although production of the other Th1 and inflammatory mediators declined. By 5 weeks, levels of these cytokines declined for both control and PD‐1 antibody‐treated mice. Flow cytometric analysis for IFN‐γ‐expressing cells showed shifts in CD4+ cells expressing IFN‐γ consistent with the changes in cytokine secretion. Whether or not treatment generated reactivity to lesions or HNSCC was determined. Spleen cells from PD‐1 antibody‐treated mice were stimulated by lysates of premalignant lesion and HNSCC tongue tissues to produce increased levels of Th1 and select inflammatory cytokines early in the course of PD‐1 antibody treatment. However, with continued treatment, reactivity to lesion and HNSCC lysates declined. Analysis of clinical response to treatment suggested an early delay in lesion progression but, with continued treatment, lesions in PD‐1 antibody‐treated mice progressed to the same degree as in control antibody‐treated mice. Overall, these results show an early beneficial response to PD‐1 antibody treatment, which then fails with continued treatment and lesion progression.
Keywords: cytokines, head and neck cancer, HNSCC, immunotherapy, PD‐1, premalignant oral lesions, T cell
Short abstract
What's new?
Immunotherapy has become an increasingly attractive strategy in cancer treatment, but its impact on premalignant lesions as they progress to cancer is unknown. Using a mouse carcinogen‐induced premalignant oral lesion model, the authors showed an early increase in cytokine expression and stabilization of disease on treatment with antibodies against programmed cell death protein 1 (PD‐1). However, with continued PD‐1 antibody treatment, cytokine production declined and lesions progressed to cancer at a similar rate as observed in mice receiving control antibody treatment, suggesting a transient efficacy of PD‐1 treatment in these premalignant lesions.
Introduction
Patients with select premalignant oral lesions have a high incidence of developing squamous cell carcinoma of the head and neck (HNSCC). Despite surgical treatment or radiation plus chemotherapy treatment, the 5‐year survival of HNSCC patients has remained at about 50% for the last 30 years.1 Immune therapy is an alternative to current treatments, but is discouraged by the profound immune suppression induced by HNSCC.2, 3, 4, 5 A different strategy is to focus immunological treatment for patients with premalignant oral lesions that are at high risk of developing HNSCC. These high‐risk patients include those with thick leukoplakias on the floor of mouth, lip and tongue.6 Although vibrant immune reactivity is evident within premalignant oral lesions and adjacent lymph nodes, there is also evidence of immunological fatigue and a shift to immune inhibitory processes.7, 8, 9
Multiple mechanisms have been shown to contribute to the suppressed immune function in patients with HNSCC and in animal models of HNSCC. These include tumor production of immune inhibitory mediators and tumor induction of host immune suppressor cells. Among the immune suppressive cells induced by HNSCC are Treg, inhibitory tumor‐associated macrophages and fibroblasts, myeloid‐derived suppressor cells (MDSC) and the less mature CD34+ progenitor cells.10, 11, 12, 13, 14 More recently, attention has focused on mechanisms involving activation of immune checkpoint signaling such as through PD‐1 ligand and its PD‐1 receptor. PD‐1 functions to subvert immune reactivity through multiple approaches. Studies in animal models have shown that PD‐1 not only serves as an inhibitory pathway for T‐cells, but also is required for induction of immune inhibitory Treg.15, 16 Treg with upregulated PD‐1 exhibit increased levels of immune suppressive activity.17 Similarly, studies in humans have shown a role of the PD‐1 axis in T‐cell skewing toward Treg.18
HNSCC cells and tumor‐infiltrating lymphocytes have been shown to express PD‐L1 and PD‐1, respectively.12, 19 In addition to expression of PD‐L1 on HNSCC tumor, tumor‐associated macrophages also express increased levels of PD‐L1, which would enable engagement of PD‐1 on intratumoral T‐cells.20 In HNSCC patients, intratumoral Treg express higher levels of immune checkpoint receptors, including PD‐1 and this expression has been associated with their more pronounced immune inhibitory activity compared to that of circulating Treg.21 This increased expression of the PD‐1/PD‐L1 inhibitory pathway was seen in both HPV‐positive and HPV‐negative HNSCC.12, 19, 20 That the PD‐1/PD‐L1 axis mediates suppression was demonstrated in vitro by the increase in T‐cell proliferative response to activation on antibody blockade of PD‐1.19 Studies in a murine model of HNSCC further showed that antibody treatment to block PD‐1 reduced levels of both MDSC and tumor‐associated macrophages, and reduced tumor growth.12
A number of clinical trials have tested the effectiveness of antibodies to PD‐1. Studies involving patients with advanced melanoma showed clinical effectiveness of treatment with the anti‐PD‐1 antibody pembrolizumab.22, 23 In a trial involving recurrent or metastatic HNSCC patients for whom there were few treatment options, pembrolizumab was manageably tolerated and demonstrated clinical effectiveness, particularly in subjects with PD‐L1‐positive tumors.24 Similarly, pembrolizumab treatment of patients with PD‐L1‐positive advanced non‐small‐cell lung cancer prolonged their overall survival.25 Using a different PD‐1 blockade antibody, nivolumab, clinical efficacy was shown for patients with metastatic renal cell carcinoma and for patients with ovarian cancer.26, 27 Nivolumab also increased survival of patients with non‐squamous non‐small‐cell lung cancer that resisted prior chemotherapy.28 A meta‐analysis of clinical trials involving nivolumab‐based therapy for advanced melanoma showed that treatment prolonged patient progression‐free survival.29 In addition to clinical trials testing anti‐PD‐1 antibody treatment, antibodies to the PD‐1 ligand, PD‐L1 have also been tested. In a trial with non‐small‐cell lung cancer, treatment with the anti‐PD‐L1 antibody atezolizumab prolonged patient survival compared to patients that were treated with docetaxel.30 This was particularly the case for patients with elevated expression of PD‐L1. A separate study showed that atezolizumab treatment of patients with metastatic urothelial bladder cancer resulted in both immunological and clinical responses.31
Studies have been initiated to assess the effectiveness of combining treatment to block the PD‐1/PD‐L1 axis with other immune treatment approaches. For example, blockage of PD‐1 or PD‐L1 in a mouse model of epithelial ovarian cancer increased the effectiveness of tumor vaccination at stimulating tumor antigen‐specific T‐cells, reduced Treg and MDSC and induced tumor rejection.32 A murine model of cervical cancer that showed antibody treatment to block PD‐1 was not sufficient to stimulate T‐cell reactivity or to increase survival of tumor‐bearing mice, instead showed effectiveness when used in combination with agonistic antibody to the co‐stimulatory receptor OX40.33 Combining nivolumab (anti‐PD‐1) and ipilimumab (anti‐CTLA‐4) antibody treatments targeting two distinct immune checkpoints resulted in greater clinical response than when used alone.34, 35
While studies have shown increases in the PD‐L1/PD‐1 axis in the tumor environment, very few studies have examined when, in the process of tumor development, this immune inhibitory process appears. One such study showed increased expression of PD‐L1 within premalignant respiratory papillomas and suggested that this was indicative of immune exhaustion.36 Results of a study of patients with actinic cheilitis, an oral premalignant lesion that can progress to oral cancer, showed increased levels of PD‐1+ cells within the peripheral blood compared to that seen for controls, although levels were greater within the cancer tissue compared to levels in the premalignant lesions.37 Also shown in this study was PD‐L1 expression within premalignant lesions, although PD‐L1 expression was greater within HNSCC tissues.
Using a carcinogen‐induced premalignant oral lesion model that progresses to oral cancer, we previously showed an increase in expression of PD‐1 on CD4+ and CD8+ cells of mice bearing premalignant oral lesions.8 The present study used this same model to examine the immunological and clinical impact of blocking the PD‐L1/PD‐1 axis in mice with premalignant oral lesions. The results of this study showed a transient early increase in cytokine expression and stabilization of disease on treatment with PD‐1 antibody. However, with continued PD‐1 antibody treatment, cytokine production declined and lesions progressed to cancer similar to that in mice receiving control antibody treatment.
Materials and Methods
Oral premalignant lesion model and treatment schedule
To induce premalignant oral lesions that progress to oral cancer, female C57BL/6 mice (Charles Rivers Laboratory, Wilmington, MA) were treated with 50 μg/mL 4‐nitroquinoline 1‐oxide (4NQO) in their drinking water starting at 2 month of age.38, 39 After approximately 8 weeks of 4NQO treatment, mice developed endoscopically visible premalignant lesions on the tongue. After an additional 8–9 weeks, tumors became detectable on the tongue. Development of premalignant oral lesions and HNSCC was monitored by sedating mice with inhaled isoflurane (Piramal Healthcare, Bethlehem, PA) and examining the oral cavities by endoscopy using a Stryker 1.9 mm × 30° scope and a Stryker 1088 HD camera (Stryker, Kalamazoo, MI). The identity of these premalignant oral lesions and HNSCC was confirmed histologically. Clinical assessments of lesions were quantitated in a blinded manner by counting the number of visible lesions, and giving lesions a gross pathologic score between 1 and 4 based on their horizontal and vertical size, and overall appearance.8 The following criteria were used for scoring: 1: flat macule, 2: raised papule, 3: raised plaque, 4: grossly exophytic.
Tissue culture medium
The culture medium used in all studies was 1× DMEM (Life Technologies, Grand Island, NY) containing 4.5 g/L d‐glucose and l‐glutamine. This medium was supplemented with 10% fetal bovine serum and 1× antibiotic antimycotic solution containing penicillin, streptomycin and amphotericin B (Sigma‐Aldrich, St. Louis, MO).
Antibody treatment of mice with premalignant oral lesions and immunological analyses
At the time that premalignant oral lesions were detected endoscopically on the tongues of 4NQO‐treated mice, treatment with IgG1 isotype control antibody or anti‐PD‐1 blocking antibody (muDX400) was initiated (Merck & Co., Kenilworth, NJ). Treatments consisted of i.p. injections at 4 day intervals of 200 μg of these mouse antibodies. After 1, 3 and 5 weeks of antibody treatment, subgroups of mice were sacrificed and their spleens collected for assessment of basal (unstimulated) cytokine production and cytokine production in response to a challenge of control lysates of normal tongue tissues or lysates of premalignant oral lesion or HNSCC tongue tissues. Aliquots of spleen cells were also immunostained for expression of IFN‐γ by CD4+ and CD8+ spleen cells both at the time of spleen cell collection and after culture with the lysate challenges. To assess the basal level of cytokine production by spleen cells of control‐ and anti‐PD‐1‐treated mice bearing premalignant oral lesions, spleen cells were cultured on anti‐CD3‐coated tissue culture wells as a density of 1 × 106/mL. Supernatants were collected after 24 hr for measurement of cytokine levels. To determine spleen cell responsiveness to premalignant lesions or HNSCC, lysates were prepared using tongue tissue from healthy control mice or from 4NQO‐treated mice bearing premalignant lesions or HNSCC on the tongue. The epithelial layers of tongue tissues were stripped by enzymatic digestion at 37°C with 0.23 U Liberase (Sigma‐Aldrich) for 4 hr. The dissociated tissues were then washed and lysed by sonication. After equalizing protein levels, lysates were added into anti‐CD3‐coated tissue culture plates containing spleen cells from 4NQO‐treated mice that received either isotype control or anti‐PD‐1 antibody. These spleen cell cultures were established in duplicate individually for each mouse, with a total of five mice being assessed in each group at each time point. After 3 days of culture, supernatants were collected and used for cytokine measurement by cytokine bead array. Cytokine levels in the lysates were also measured and found to be undetectable at the concentrations used in the spleen cell cultures.
Cytokine bead array
The levels of IFN‐γ, IL‐2, IL‐17A, IL‐4, IL‐6 and IL‐10 in cell culture supernatants were determined using a mouse cytometric bead array Th1/Th2/Th17 cytokine kits (BD Biosciences, San Jose, CA). A FACS Canto (BD Biosciences) flow cytometer was used to quantify cytokine profiles and relative amounts of each cytokine were calculated using FCAP Array Software (manufactured by Soft Flow Hungary Ltd. for BD Biosciences).
Phenotypic analysis of spleen cells for expression of IFN‐γ
In order to detect intracellular cytokines, single‐cell suspensions of spleen cells were treated for 4 hr with brefeldin A solution (BD Bioscience) to prevent cytokine secretion. Nonspecific staining of cells was blocked with FBS and anti‐CD16/32 monoclonal antibodies. Cells were then cell surface stained with PerCP‐Cy5.5 CD4 and PE‐Cy7 CD8a antibodies, fixed and permeabilized with Cytofix/Cytoperm and then intracellularly stained with FITC IFN‐γ (BD Bioscience). The extent and frequency of positively stained cells was visualized by flow cytometry (FACSCanto, BD Bioscience).
Statistical analysis
Data are reported as means ± standard deviation or standard error of the mean, as appropriate. Spleen cell cytokine production was determined in duplicate for each mouse individually, with five mice being analyzed per group at each time point and all studies being repeated. The Mann‐Whitney U test was used to determine significance of differences in values between each of two parameters (GraphPad Prism version 6.03 for Windows, GraphPad Software, La Jolla, CA). Significance was reported in the 95% confidence interval.
Results
Transient increases in cytokine production by spleen cells of PD‐1‐treated mice bearing premalignant lesions
Levels of representative Th1, inflammatory and inhibitory cytokines secreted by spleen cells of premalignant lesion‐bearing mice that were treated with PD‐1 or isotype control antibody for 1, 3 or 5 weeks were measured. Spleen cells of mice that were treated for one week with PD‐1 antibody produced higher levels of the Th1 mediator IL‐2 (p < 0.01), but slightly lower levels of the Th1 mediator IFN‐γ (p < 0.05) than did spleen cells of isotype control‐treated mice (Fig. 1). However, after 3 weeks of treatment, production of both Th1 mediators by spleen cells from isotype control‐treated mice sharply declined. IL‐2 production by spleen cells of mice that were treated for 3 weeks with PD‐1 antibody also declined, although IFN‐γ production by these spleen cells increased (p < 0.02). At 5 weeks, spleen cells produced only minimal levels of IL‐2 and IFN‐γ, regardless of whether the mice had been treated with anti‐PD‐1 antibody or isotype control antibody.
Figure 1.
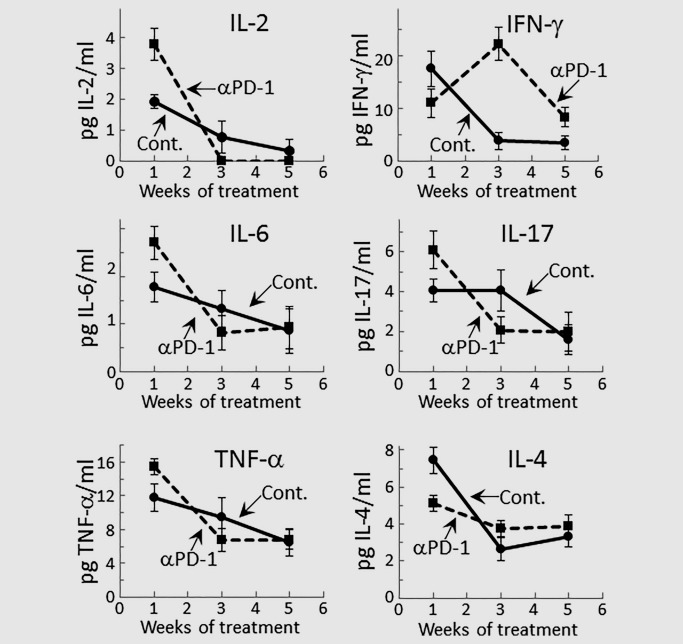
Early stimulation of cytokine production by PD‐1 antibody treatment of mice bearing premalignant oral lesions. Mice bearing 4NQO‐induced premalignant oral lesions were treated with either isotype control or anti‐PD‐1 (αPD‐1) antibodies. After 1, 3 and 5 weeks of treatment, sample groups of 5 mice each mouse were sacrificed and their spleens were cultured for measurement of secreted cytokine levels. Shown are levels of cytokines secreted into the supernatants by spleen cells from 5 mice per group per time point, with spleen cells from each mouse being cultured individually and assayed for cytokine levels in duplicate (mean ± SEM).
Our prior studies had shown increased production of inflammatory cytokines by immune cells of mice with premalignant oral lesions and a decline in this inflammatory state as lesions progress to HNSCC.8, 9 Therefore, the effect of PD‐1 antibody treatment on these inflammatory states was measured (Fig. 1). After one week of treatment with anti‐PD‐1 antibodies, production of the inflammatory mediators IL‐6 (p < 0.02), IL‐17 (p < 0.05) and TNF‐α (p < 0.05) was further increased over levels produced by spleen cells of isotype control‐treated lesion‐bearing mice. However, consistent with our prior studies, production of inflammatory mediators by spleen cells of control lesion‐bearing mice declined as lesions progressed. Treatment with PD‐1 antibodies did not temper this decline in production of inflammatory mediators and, in fact, the decline in production of inflammatory mediators tended to be accentuated in PD‐1 antibody‐treated lesion‐bearing mice.
Contrasting the higher levels of production of most of the cytokines measured during the early period of treatment with PD‐1 antibody, production of the inhibitory mediator IL‐4 by spleen cells of PD‐1 antibody‐treated mice was lower than by spleen cells of control antibody‐treated lesion‐bearing mice (p < 0.02; Fig. 1). With continued antibody treatment and as the lesions progressed, levels of IL‐4 produced by control lesion‐bearing mice declined to levels that were similar to levels produced by PD‐1 antibody‐treated mice. Levels of the inhibitory mediator IL‐10 were also measured, but the levels produced by spleen cells were below the level of detection, regardless of whether mice were treated with control or PD‐1 antibodies (data not shown). Overall, these results show that anti‐PD‐1 antibody treatment transiently stimulates production of Th1 and inflammatory cytokines by spleen cells of mice with newly developed premalignant oral lesions. As lesions progress, production of each of the categories of cytokines declines, regardless of whether lesion‐bearing mice were treated with isotype control antibodies or PD‐1 antibodies.
Transient increase in levels of CD4+ cells expressing IFN‐γ in spleens of premalignant lesion‐bearing mice that were treated with PD‐1 antibody
The studies above showing that treatment of mice with PD‐1 antibodies triggered a robust increase in spleen cell production of IFN‐γ at a time when production by spleen cells of control lesion‐bearing mice had declined prompted phenotypic analysis for expression of IFN‐γ by CD4+ and CD8+ T‐cells (Figs. 2 and 3). At 1 week of PD‐1 antibody treatment, levels of IFN‐γ‐expressing CD4+ were lower than levels of IFN‐γ‐expressing spleen cells from isotype control‐treated mice bearing premalignant oral lesions (p < 0.01). This was also seen for expression of IFN‐γ by CD8+ cells, although to a less prominent extent (p < 0.05). Continuing treatment with PD‐1 antibody for 3 weeks, increased levels of CD4+ cells that expressed IFN‐γ (p < 0.01). At this 3‐week time point, not only were levels of CD4+ cells expressing IFN‐γ increased, but overall levels of CD4+ cells were slightly increased (p < 0.05). While levels of CD8+ cells expressing IFN‐γ increased, this increase was not statistically significant. The increase in levels of CD4+ cells expressing IFN‐γ was not sustained as lesions progressed. After 5 weeks of treatment, levels of CD4+ cells expressing IFN‐γ declined. Thus, these phenotypic analyses paralleled the assessments of cytokine secretion and showed a transient, but not sustainable, increase in expression of the Th1 mediator IFN‐γ by CD4+ spleen cells of premalignant lesion‐bearing mice that were treated with PD‐1 antibody.
Figure 2.
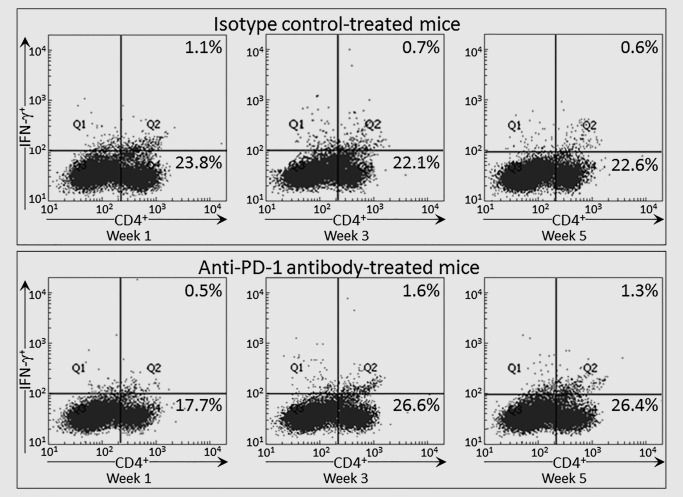
Transient increase in IFN‐γ expression by CD4+ spleen cells from PD‐1 antibody‐treated mice bearing premalignant oral lesions. Mice bearing 4NQO‐induced premalignant oral lesions were treated with either isotype control or PD‐1 antibodies. After 1, 3 and 5 weeks of treatment, sample mice were sacrificed and their spleens were immunostained extracellularly for CD4 and intracellularly for IFN‐γ. Shown are representative fluorescent dot‐blots of spleen cells from mice treated with isotype control antibody or anti‐PD‐1 antibody.
Figure 3.
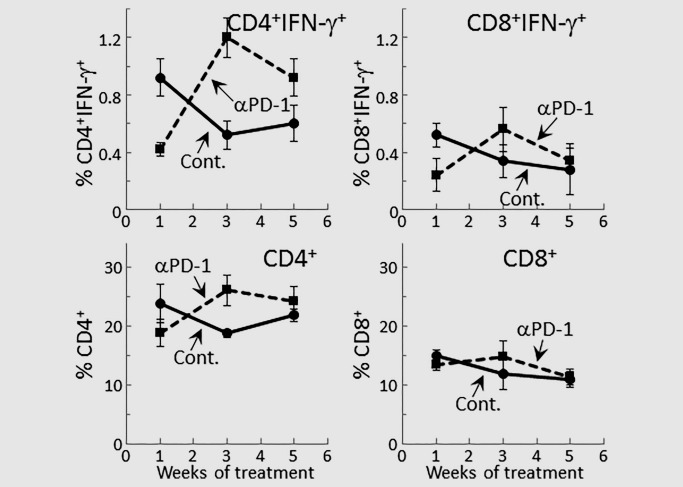
Transient increase in IFN‐γ expression by splenic T‐cells from PD‐1 antibody‐treated mice bearing premalignant oral lesions. Mice bearing 4NQO‐induced premalignant oral lesions were treated with either isotype control or PD‐1 antibodies. After 1, 3 and 5 weeks of treatment, sample mice were sacrificed and their spleens were immunostained extracellularly for CD4 and CD8, and intracellularly for IFN‐γ. Shown are percentages of positive‐staining cells from groups of 5 mice per group per each time point (mean ± SD).
Transient responsiveness of spleen cells from PD‐1‐treated mice bearing premalignant lesions to a challenge of premalignant lesion and HNSCC tongue lysates
Our prior studies had shown that 4NQO‐induced premalignant oral lesions and oral cancer share expression of a number of tumor antigens.40 Therefore, studies were conducted to determine if treatment of mice bearing premalignant oral lesions with PD‐1 antibody would stimulate their reactivity to premalignant lesions or HNSCC. Spleen cells were cultured with lysates of control, premalignant lesion or HNSCC tongue tissues and their responsiveness to these lysates was then measured by spleen cell cytokine production (Fig. 4). What was most prominent was an initial increase followed by a decline in production of Th1 and select inflammatory cytokines in response to the challenge with premalignant or HNSCC lysates by spleen cells of mice that received PD‐1 antibody treatments and, subsequently, by spleen cells of control antibody‐treated lesion‐bearing mice.
Figure 4.
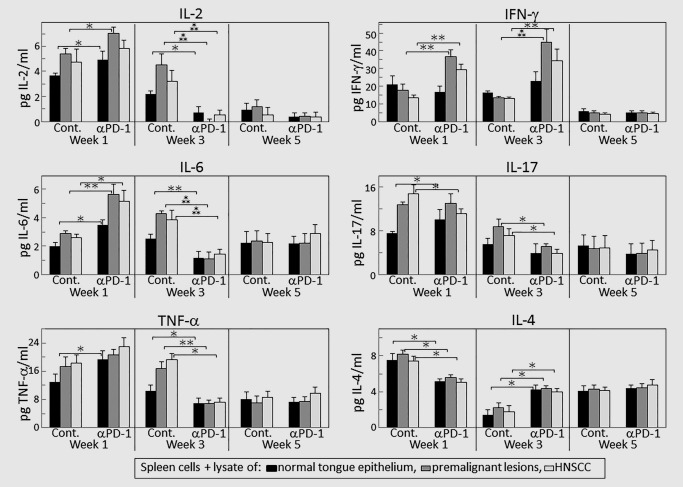
PD‐1 antibody treatment transiently increases select cytokine responses to lysates of premalignant oral lesion or HNSCC tongue tissues. Mice bearing 4NQO‐induced premalignant oral lesions were treated with either isotype control or PD‐1 antibodies. After 1, 3 and 5 weeks of treatment, sample mice were sacrificed. Their spleen cells were cultured with lysates of control normal tongue tissue or lysates of premalignant lesion or HNSCC tongue tissues. Shown are levels of cytokines secreted into the supernatants by spleen cells from 5 mice per group per time point, with spleen cells from each mouse being cultured individually and assayed for cytokine levels in duplicate (mean ± SEM). ✶=p < 0.05; ✶✶=p < 0.02; ✶✶✶=p < 0.01.
At 1 week after initiating treatments, spleen cells of mice treated with PD‐1 antibodies produced increased levels of the Th1 cytokines IL‐2 (p < 0.05) and IFN‐γ (p < 0.02), and increased levels of the inflammatory cytokine IL‐6 (p < 0.02) in response to a challenge with lysates from premalignant oral lesion and HNSCC tongue tissues (Fig. 4). For IFN‐γ, this response to the challenges was more prominent at 3 weeks of PD‐1 antibody treatment. With the exception of IFN‐γ, there were no longer Th1 or inflammatory cytokine responses by spleen cells of PD‐1‐treated mice to lysates of premalignant or HNSCC tissue lysates at Week 3 of PD‐1 antibody treatment. Spleen cells of control antibody‐treated mice also responded to lesion and HNSCC lysates by producing increased cytokine levels, but the stimulation was present at Week 1 for IL‐2 (p < 0.05), IL‐17 (p < 0.02) and, to lesser extents, for IL‐6 (p < 0.05) and TNF‐α (not significant), and persisted at 3 weeks for IL‐2 (p < 0.02), IL‐6 (p < 0.02) and TNF‐α (p < 0.05). Increases in production of these cytokines in response to lesion or HNSCC challenges were transient as they were absent at 5 weeks, regardless of whether mice were treated with isotype control or PD‐1 antibodies.
Spleen cell production of the inhibitory mediators IL‐4 and IL‐10 in response to lysates of lesion or HNSCC tissues were also measured (Fig. 4). Levels of IL‐10 were undetectable, regardless of which lysates were added to the spleen cell cultures. However, spleen cells from lesion‐bearing mice that were treated for 1 week with PD‐1 antibodies produced lower levels of IL‐4 than did spleen cells of mice that were treated with isotype control antibodies (p < 0.05). The addition of lysates from premalignant lesions or HNSCC had no influence on IL‐4 production by spleen cells of either of the groups of mice, regardless of the duration of the antibody treatments.
Overall, these studies indicated that treatment of premalignant lesion‐bearing mice with PD‐1 antibody increases their Th1 and select inflammatory cytokine responses to premalignant lesion and HNSCC lysates early in the period of treatment. However, stimulation to produce Th1 cytokines declines with prolonged treatment and stimulation to produce inflammatory cytokines declines more rapidly with continued treatment with PD‐1 antibody than the decline seen for mice treated with isotype control antibodies.
Phenotypic analysis of T‐cells expressing IFN‐γ in response to culture with lysates from premalignant lesion or HNSCC tissues
Because of the robust stimulation of IFN‐γ production by spleen cells of PD‐1‐treated mice in response to a challenge of lesion or HNSCC tissue lysates, aliquots of these spleen cells were also analyzed phenotypically for expression of IFN‐γ by CD4+ and CD8+ spleen cells. After culture with control normal tongue lysates, spleen cells of mice that were treated for one week with PD‐1 antibodies had lower levels of IFN‐γ‐expressing CD4+ cells than did spleen cultures of mice that were treated with isotype control antibodies. Levels of IFN‐γ expressing CD4+ cells were slightly increased (not significant) in cultures that were challenged with lysates of lesion or HNSCC tongue tissues (Fig. 5). The overall levels of CD4+ cells were also lower among cultures of spleen cells from the mice treated for one week with PD‐1 antibodies. Similar to what was seen for CD4+ cells, a slightly lower proportion of CD8+ cells from mice treated for 1 week with PD‐1 antibodies expressed IFN‐γ in cultures containing any of the lysates compared to CD8 + IFN‐γ+ cell levels in cultures of spleen cells of mice treated with isotype control antibodies. There were no differences in the overall proportions of CD8+ cells between the cultures of spleen cells of control and PD‐1‐treated groups.
Figure 5.
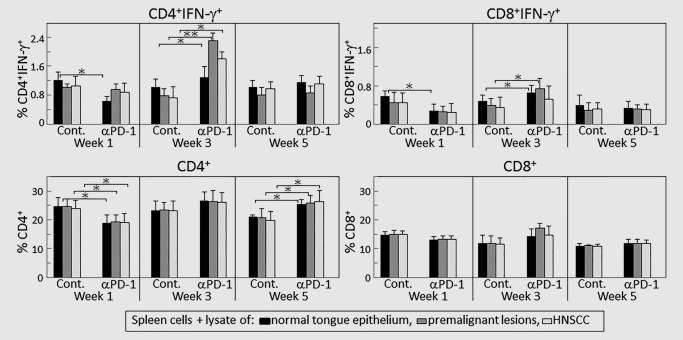
PD‐1 antibody treatment transiently increases expression of IFN‐γ by CD4+ cells in response to lysates of premalignant oral lesion or HNSCC tongue tissues. Mice bearing 4NQO‐induced premalignant oral lesions were treated with either isotype control or PD‐1 antibodies. After 1, 3 and 5 weeks of treatment, sample mice were sacrificed and their spleens were cultured with lysates of control normal tongue tissue or lysates of premalignant lesion or HNSCC tongue tissues. Cultures were then immunostained for CD4, CD8 and IFN‐γ. Shown are percentages of positive‐staining cells from 5 mice per group per time point, with spleen cells from each mouse being cultured and phenotyped individually (mean ± SD). ✶=p < 0.05; ✶✶=p < 0.02.
Contrasting to what was seen after one week of antibody treatment, at 3 weeks, levels of CD4+ cells expressing IFN‐γ in cultures containing normal tongue lysates were greater for spleen cells from PD‐1‐treated mice versus control antibody‐treated mice. In response to a challenge of premalignant lesion or HNSCC tissue lysates, levels of CD4+ cells expressing IFN‐γ were further increased in cultures of spleen cells from PD‐1‐treated mice (p < 0.02). Such a response to challenge was not seen in cultures of spleen cells from isotype control‐treated mice. After 3 weeks of antibody treatment, there were also slight increases in CD8+ cells expressing IFN‐γ among spleen cells from PD‐1‐treated mice, but there was no additional increase in IFN‐γ‐expressing CD8+ cells as a result of culturing with lesion or HNSCC lysates compared to culture with normal tongue tissue lysates.
The increases in IFN‐γ‐expressing T‐cells in responses to lesion or HNSCC lysate challenges that were seen after 3 weeks were not sustained with continued PD‐1 antibody treatment. At 5 weeks of treatment, levels of CD4+ and CD8+ cells expressing IFN‐γ were similar among spleen cells of control and PD‐1 antibody‐treated mice and this was not altered by a challenge with premalignant lesion or HNSCC lysates.
PD‐1 antibody treatment transiently stabilizes lesion progression but is not sufficient to sustain protection against progression of premalignant oral lesions to HNSCC
The clinical effectiveness of treating mice bearing premalignant oral lesions with PD‐1 antibodies was tested by endoscopic examination of the oral cavity (Fig. 6). Mice were initiated on 4NQO treatment and, once premalignant oral lesions became endoscopically detectable, they were divided into groups of 15 mice each and initiated on treatment with isotype control antibody or PD‐1 antibody. After 2 weeks of antibody treatment, mice receiving anti‐PD‐1 treatment appeared to have a slight clinical response in that the appearance of the premalignant lesions on the tongue was stabilized compared to the progressive development in isotype control‐treated mice (p < 0.05 on days 18 and 22). However, by 4 weeks of antibody treatment, lesions in PD‐1 antibody‐treated mice progressed and, while still being less advanced than lesions of control antibody‐treated mice (p < 0.05 on Day 28), they became more similar to lesions in control mice. By 5 weeks, when the mice were sacrificed, lesions could not be distinguished between the mice that received control or PD‐1 antibody treatment. Thus, despite an early stabilization of oral lesions by PD‐1 antibody treatment, with time, there was no clinical difference in progressive development of lesions between the PD‐1 or isotype control antibody treated groups.
Figure 6.
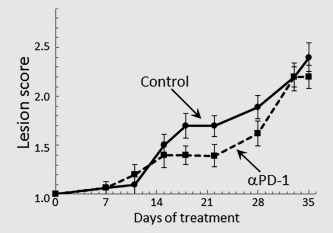
Clinical response of mice with premalignant oral lesions to treatment with isotype control antibodies or PD‐1 antibodies. Mice with endoscopically detectable premalignant oral lesions were divided into groups of 15 mice each and initiated on treatment with isotype control antibody or PD‐1 antibody. Progression of lesions toward cancer development was monitored by endoscopic examination of the oral cavity. These endoscopic images were quantitated in a blinded manner by counting the number of visible lesions, and giving lesions a gross pathologic score between 1 and 4 based on their horizontal and vertical size, and overall appearance. The following criteria were used for scoring: 1: flat macule, 2: raised papule, 3: raised plaque, 4: grossly exophytic. Shown are means ± SD of clinical analyses.
Discussion
Immunotherapy using antibodies targeting checkpoint proteins has become of increasing interest and a number of clinical trials using these antibodies for several different cancer types have shown clinical responses.22, 23, 24, 25, 26, 27, 28 However, studies with animal models and cancer patients have also shown that combining antibody blockade of the PD‐1/PD‐L1 axis with other forms of immunotherapy can lead to more meaningful clinical responses.32, 33, 34, 35 Approaches to block checkpoint signals have not been previously tested for their capacity to prevent premalignant lesion progression to cancer, despite indications of subjects with premalignant lesions having increased expression of PD‐L1 and PD‐1+ cells36, 37. The current study tested the immunological and clinical effects of PD‐1 blocking antibody treatment in a mouse 4NQO carcinogen‐induced premalignant oral lesion model that progresses to oral cancer. This model represents the stages of carcinogenesis‐induced cancer described for tobacco products.41 In addition, similar to what has been shown for human subjects with premalignant lesions, we had previously shown that mice bearing 4NQO‐induced premalignant oral lesions have increased expression of PD‐1 on their CD4+ and CD8+ T‐cells.8 The results of this study showed an early increase in spleen cell secretion of the Th1 cytokine IL‐2 and the inflammatory cytokines IL‐6, IL‐17 and TNF‐α after one week of treatment with antibody against PD‐1, and an increase of IFN‐γ after 3 weeks of treatment. Assessment of T‐cell expression of IFN‐γ showed a parallel increase in levels of CD4+ cells expressing IFN‐γ. With continued treatment, cytokine production declined and lesions progression was similar for both groups of mice.
A goal of immunotherapy is to generate immune reactivity against the tumor. Our prior studies had shown that both human lesions and the premalignant oral lesions that develop in this 4NQO carcinogenesis model express similar tumor antigens as are expressed on the respective oral cancers.40, 42 Thus, studies determined if reactivity to either premalignant oral lesions or HNSCC was generated by administering antibodies to PD‐1. This was accomplished by measuring cytokine secretion by spleen cells in response to culture with either control lysates of normal tongue tissue or with lysates of lesion‐ or HNSCC‐containing tongue tissues. Secretion of Th1 cytokines and select inflammatory cytokines by spleen cells of mice treated for one week with PD‐1 antibody was increased in response to lysate of premalignant and HNSCC tongue tissues. Increases were also seen for spleen cells from isotype control antibody‐treated mice, but these increases were less prominent than for PD‐1 antibody‐treated mice, with the exception of section of IL‐17. Secretion of the inhibitory mediator IL‐4 was lower from spleen cells of mice that were treated for one week with PD‐1 antibody, and it was not affected by exposure to lysates.
While 1 week of treatment with PD‐1 antibody led to increased secretion of Th1 and select inflammatory cytokines and increased responsiveness to premalignant lesion or HNSCC lysates, these effects were diminished with continued treatment and as lesions progressed toward development of HNSCC. With the exception of IFN‐γ, secretion of Th1 and inflammatory cytokines by spleen cells declined for both the control and PD‐1 antibody‐treated mice at 3 weeks, although the decline occurred more prominently for spleen cells of mice that were treated with PD‐1 antibody. By 5 weeks of treatment, spleen cell cytokine secretion declined for both the control and PD‐1 antibody‐treated groups and there were no increases in secretion of any of the cytokines by exposure to lysates of lesions or HNSCC.
Consistent with the early increase and subsequent decline in secretion of Th1 and inflammatory cytokines seen for mice treated with PD‐1 antibody, there appeared to be an early clinical response in these mice. Between weeks 2 and 3 of treatment, endoscopic evaluation of the oral cavities showed a slowing of lesion progression with the appearance of stabilization. However, by Week 4 of treatment, the lesions of mice that were treated with PD‐1 antibodies were approaching the appearance of lesions in the isotype control antibody‐treated mice.
This transient response of mice bearing premalignant oral lesions to PD‐1 blockade that was shown in the present study has some consistencies with other studies involving cancer patients and animal cancer models, although immunological analyses at different treatment time points have generally not been conducted. One consistency is demonstrations that blocking PD‐1 increased progression‐free survival of patients with melanoma, although the rates of complete responses were low.23 Similarly, blocking PD‐1 in patients with non‐small‐cell lung cancer prolonged progression‐free survival, but cancer nevertheless progressed in almost all patients.25 The most direct consistency with the present study's demonstration of a transient immunological response to PD‐1 blockade is demonstration of acquired resistance to PD‐1 antibody treatment in melanoma patients.43 This development of resistance was shown to be associated with alterations interferon receptor signaling and in antigen presentation. Resistance to PD‐1 antibody treatment was also shown to be associated with suppression of interferon signaling in a mouse lung cancer model.44 A study with a lung cancer mouse model and with cancer patients showed that development of resistance to PD‐1 blockade could be attributed to upregulation of other immune checkpoints.45
It is presently not possible to compare the results of this study to results of other studies, either in human subjects or in animal models, since antibodies targeting checkpoint proteins have not been tested as a treatment approach for premalignant oral lesions. Our prior studies had shown increased immune activity and increases in inflammatory mediators in both humans with premalignant oral lesions and in the carcinogen‐induced lesion model used in the present studies.8, 9, 46 These studies also showed that this increased reactivity associated with lesions is replaced with an immune inhibitory environment as oral cancers are developing. It is possible that the early stimulation seen by PD‐1 antibody treatment in this study is a bolstering of the immune stimulated environment of premalignant lesions. More difficult to explain is the underlying cause for the more rapid decay in this immune activity in mice receiving PD‐1 antibody treatment compared to the mice receiving control treatment. It is important to note that, in the HNSCC environment, there are multiple mechanisms contributing to the immune dysfunction including inhibitory mediators such and PGE2 produced directly by the tumor cells as well as tumor‐induced immune inhibitory cells such as Treg, MDSC and the less mature CD34+ progenitor cells, infiltrating macrophages and endothelial cells.10, 11, 12, 13, 14 Studies have not, however, been conducted to determine at what point and in what sequence during progression of premalignant oral lesions to HNSCC these various inhibitory mediators and cells overcome the immune defenses. Also, not yet assessed is the immunological status within the lesion environment of mice treated with PD‐1 antibodies versus control antibodies. It is possible that the immune stimulation seen early in the course of PD‐1 antibody treatment is more prominent in the tumor environment. Such studies are currently ongoing, but are challenging due to the small nature of the lesions and tumors in the mouse model.
The early effectiveness of PD‐1 antibody at stimulating immune reactivity and slowing lesion progression would suggest that PD‐1 antibody treatment could be most effective when used at the earliest stages possible in the process that leads to cancer. However, it is also possible that treatment with PD‐1 antibody to interrupt one immune inhibitory mechanism leads to increased prominence of other immune inhibitory mechanisms that are protective to developing tumors. This would support use of multi‐armed immunotherapeutic approaches that target more than one checkpoint inhibitory axis or inhibitory mechanism for a more effective approach to stop progression of premalignant oral lesions to cancer.
Author contributions: M.R.I.Y. planned and designed the studies, while C.A.L. conducted the laboratory analyses. Both authors contributed to data analysis and to the preparation of the manuscript.
References
- 1. Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975‐2013. Bethesda, MD: National Cancer Institute; 2016. Available at: http://seer.cancer.gov/csr/1975_2013/ [Google Scholar]
- 2. Lathers DMR, Achille N, Kolesiak K, et al. Increased levels of immune inhibitory CD34+ progenitor cells in the peripheral blood of patients with node positive head and neck cancer and the ability of the CD34+ cells to differentiate into antigen presenting dendritic cells. Clin Cancer Res 2001;125:205–12. [DOI] [PubMed] [Google Scholar]
- 3. Mulligan JK, Day TA, Gillespie MB, et al. Secretion of vascular endothelial growth factor by oral squamous cell carcinoma cells skews endothelial cells to suppress T‐cell functions. Hum Immunol 2009;70:375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Walker DD, Reeves TD, de Costa AM, et al. Immunological modulation by 1a,25‐dihydroxyvitamin D3 in patients with squamous cell carcinoma of the head and neck. Cytokine 2012;58:448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wanebo HJ, Blackinton D, Kouttab N, et al. Contribution of serum inhibitory factors and immune cellular defects to the depressed cell‐mediated immunity in patients with head and neck cancer. Am J Surg 1993;166:389–94. [DOI] [PubMed] [Google Scholar]
- 6. Neville BW, Day TA. Oral cancer and precancerous lesions. CA Cancer J Clin 2002;52:195–215. [DOI] [PubMed] [Google Scholar]
- 7. Dadian G, Riches PG, Henderson DC, et al. Immune changes in peripheral blood resulting from locally directed interleukin‐2 therapy in squamous cell carcinoma of the head and neck. Eur J Cancer 1993;29:34 [DOI] [PubMed] [Google Scholar]
- 8. De Costa AM, Schuyler CA, Walker DD, et al. Characterization of the evolution of immune phenotype during the development and progression of squamous cell carcinoma of the head and neck. Cancer Immunol Immunother 2011;61:927–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Woodford D, Johnson SD, Costa D, et al. An inflammatory cytokine milieu is prominent in premalignant oral lesions, but subsides when lesions progress to squamous cell carcinoma. J Clin Cell Immunol 2014;5:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Takahashi H, Sakakura K, Kawabata‐Iwakawa R, et al. Immunosuppressive activity of cancer‐associated fibroblasts in head and neck squamous cell carcinoma. Cancer Immunol Immunother 2015;64:1407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sun W, Li WJ, Fu QL, et al. Functionally distinct subsets of CD4+ regulatory T cells in patients with laryngeal squamous cell carcinoma are indicative of immune deregulation and disease progression. Oncol Rep 2015;33:354–62. [DOI] [PubMed] [Google Scholar]
- 12. Yu GT, Bu LL, Huang CF, et al. PD‐1 blockade attenuates immunosuppressive myeloid cells due to inhibition of CD47/SIRPα axis in HPV negative head and neck squamous cell carcinoma. Oncotarget 2015;6:42067–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Walsh JE, Clark AM, Day TA, et al. Use of 1a,25‐dihydroxyvitamin D3 treatment to stimulate immune infiltration into head and neck squamous cell carcinoma. Hum Immunol 2010;71:659–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mulligan JK, Rosenzweig SA, Young MR. Tumor secretion of VEGF induces endothelial cells to suppress T cell functions through the production of PGE2 . J Immunother 2010;33:126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen X, Fosco D, Kline DE, et al. PD‐1 regulates extrathymic regulatory T‐cell differentiation. Eur J Immunol 2014;44:2603–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wainwright DA, Chang AL, Dey M, et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA‐4 and PD‐L1 in mice with brain tumors. Clin Cancer Res 2014;20:5290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yan Z, Zhuansun Y, Chen R, et al. Immunomodulation of mesenchymal stromal cells on regulatory T cells and its possible mechanism. Exp Cell Res 2014;324:65–74. [DOI] [PubMed] [Google Scholar]
- 18. Rabe H, Nordstrom I, Andersson K, et al. Staphylococcus aureus convert neonatal conventional CD4+ T cells into FOXP3+ CD25+ CD127low T cells via the PD‐1/PD‐L1 axis. Immunology 2014;141:467–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Malm IJ, Bruno TC, Fu J, et al. Expression profile and in vitro blockade of PD‐1 in HPV‐negative head and neck squamous cell carcinoma. Head Neck 2015;37:1088–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lyford‐Pike S, Peng S, Young GD, et al. Evidence for a role of the PD‐1:PD‐L1 pathway in immune resistance of HPV‐associated head and neck squamous cell carcinoma. Cancer Res 2013;73:1733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jie HB, Gildener‐Leapman N, Li J, et al. Intratumoral regulatory T cells upregulate immunosuppressive molecules in head and neck cancer patients. Br J Cancer 2013;109:2629–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Robert C, Ribas A, Wolchok JD, et al. Anti‐programmed‐death‐receptor‐1 treatment with pembrolizumab in ipilimumab‐refractory advanced melanoma: a randomised dose‐comparison cohort of a phase 1 trial. Lancet 2014;348:1109–17. [DOI] [PubMed] [Google Scholar]
- 23. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015;372:2521–32. [DOI] [PubMed] [Google Scholar]
- 24. Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE‐012): an open‐label, multicentre, phase 1b trial. Lancet Oncol 2016;17:956–65. [DOI] [PubMed] [Google Scholar]
- 25. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): a randomised controlled trial. Lancet 2016;387:1540–50. [DOI] [PubMed] [Google Scholar]
- 26. George S, Motzer RJ, Hammers HJ, et al. Safety and efficacy of nivolumab in patients with metastatic renal cell carcinoma treated beyond progression: a subgroup analysis of a randomized clinical trial. JAMA Oncol, in press. doi:10.1001/jamaoncol.2016.0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hamanishi J, Mandai M, Ikeda T, et al. Safety and antitumor activity of anti‐PD‐1 antibody, nivolumab, in patients with platinum‐resistant ovarian cancer. J Clin Oncol 2015;33:4015–22. [DOI] [PubMed] [Google Scholar]
- 28. Borghaei H, Paz‐Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015;373:1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jin C, Zhang X, Zhao K, et al. The efficacy and safety of nivolumab in the treatment of advanced melanoma: a meta‐analysis of clinical trials. OncoTargets Ther 2016;9:1571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non‐small‐cell lung cancer (POPLAR): a multicentre, open‐label, phase 2 randomised controlled trial. Lancet 2016;387:1837–46. [DOI] [PubMed] [Google Scholar]
- 31. Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti‐PD‐L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014;515:558–62. [DOI] [PubMed] [Google Scholar]
- 32. Duraiswamy J, Freeman GJ, Coukos G. Therapeutic PD‐1 pathway blockade augments with other modalities of immunotherapy T‐cell function to prevent immune decline in ovarian cancer. Cancer Res 2013;73:6900–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guo Z, Wang X, Cheng D, et al. PD‐1 blockade and OX40 triggering synergistically protects against tumor growth in a murine model of ovarian cancer. PLoS One 2014;9:e89350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013;369:122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Larkin J, Chiarion‐Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hatam LJ, Devoti JA, Rosenthal DW, et al. Immune suppression in premalignant respiratory papillomas: enriched functional CD4+Foxp3+ regulatory T cells and PD‐1/PD‐L1/L2 expression. Clin Cancer Res 2012;18:1925–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Malaspina TS, Gasparoto TH, Costa MR, et al. Enhanced programmed death 1 (PD‐1) and PD‐1 ligand (PD‐L1) expression in patients with actinic cheilitis and oral squamous cell carcinoma. Cancer Immunol Immunother 2011;60:965–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tang XH, Knudsen B, Bemis D, et al. Oral cavity and esophageal carcinogenesis modeled in carcinogen‐treated mice. Clin Cancer Res 2004;10:301–13. [DOI] [PubMed] [Google Scholar]
- 39. De Costa AM, Justis DN, Schuyler CA, et al. Administration of a vaccine composed of dendritic cells pulsed with premalignant oral lesion lysate to mice bearing carcinogen‐induced premalignant oral lesions stimulates a protective immune response. Int Immunopharmacol 2012;13:322–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Young MRI. Use of carcinogen‐induced premalignant oral lesions in a dendritic cell‐based vaccine to stimulate immune reactivity against both premalignant oral lesions and oral cancer. J Immunother 2008;31:148–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kanojia D, Vaidya MM. 4‐Nitroquinoline‐1‐oxide induced experimental oral carcinogenesis. Oral Oncol 2006;42:655–67. [DOI] [PubMed] [Google Scholar]
- 42. Young MR, Neville BW, Chi AC, et al. Oral premalignant lesions induce immune reactivity to both premalignant oral lesions and head and neck squamous cell carcinoma. Cancer Immunol Immunother 2007;56:1077–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zaretsky JM, Garcia‐Diaz A, Shin DS, et al. Mutations associated with acquired resistance to PD‐1 blockade in melanoma. N Engl J Med 2016;375:819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang X, Schoenhals JE, Li A, et al. Suppression of type I IFN signaling in tumors mediates resistance to anti‐PD‐1 treatment that can be overcome by radiotherapy. Cancer Res, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Koyama S, Akbay EA, Li YY, et al. Adaptive resistance to therapeutic PD‐1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun 2016;7:10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Johnson SD, Levingston C, Young MR. Premalignant oral lesion cells elicit increased cytokine production and activation of T‐cells. Anticancer Res 2016;36:3261–70. [PMC free article] [PubMed] [Google Scholar]


