Abstract
Purpose
Peripheral retinal defocus has been implicated in myopia progression. The effect of commercially-available spherical soft contact lenses (SCLs) on peripheral defocus of adult myopic eyes was investigated.
Methods
Twenty-five young adults with spherical equivalent (SE) refractions between −0.50 D and −6.00 D were enrolled. Cycloplegic autorefraction (right eye) was measured centrally and ±20°, ±30°, and ±40° from the line of sight along the horizontal meridian using an autorefractor. Four commercially-available spherical SCLs (Biofinity, Acuvue2, PureVision2, and Air Optix Night & Day Aqua) were evaluated. SE defocus (M) was used to calculate relative peripheral defocus (RPD) while wearing each SCL and relative peripheral refraction (RPR) of the uncorrected eye. Spherical aberration (SA) changes due to each SCL were measured along the line of sight by aberrometry. Peripheral defocus was analyzed using repeated-measures analyses of variance (RM-ANOVA). The association between changes in axial SA and the change in peripheral defocus was evaluated using linear mixed models.
Results
The mean age (±SD) and central SE refractive error were 24.0 ± 1.3 years and −3.45 ± 1.42 D, respectively. PureVision2 did not change RPD (p=0.33). Significant myopic shifts on the temporal retina were found with three lenses: Acuvue 2 (−0.29 D at 30°; −0.80 D at 40°; both p≤0.01), Biofinity (−1.21 D at 40°; p=0.02), and Air Optix Night & Day Aqua (−0.23 D at 20°, −0.48 D at 30°, and −1.50 D at 40°; all p<0.004). All SCLs caused a negative change in SA. SCLs inducing less negative (more positive) SA changes were associated with a less hyperopic change in RPD.
Conclusions
Spherical SCL design can influence the peripheral defocus profile experienced by a myopic eye. Several, but not all, SCLs reduced peripheral hyperopia. Differences in how SCL types influence peripheral defocus may have implications for myopia progression.
Keywords: myopia, contact lens, aberrations, peripheral defocus, spherical aberration
The prevalence of myopia is increasing in the United States and has reached epidemic levels in parts of Asia, making myopia a serious health concern.1, 2 In the United States, the prevalence of myopia has increased from 25% in the 1970s to more than 40% today.3 At the current rate of progression, myopia is estimated to affect approximately 5 billion people by 2050, with almost 1 billion of those being high myopes (more myopic than −5.00 D).4 Myopia is a health concern because as it progresses, there is increased risk for ocular diseases such as chorioretinal atrophy, choroidal neovascularization, foveal retinoschisis, open angle glaucoma, and retinal detachment.5, 6
Animal studies have shown that ocular growth is dependent on visual feedback, meaning that the sign of defocus experienced by the retina is critical in determining the axial length of the eye. Monkeys reared experiencing full-field, lens-induced myopic defocus develop hyperopia, while full-field hyperopic defocus results in a myopic refractive error.7 Further studies have shown that defocus influences eye growth in localized retinal areas with different regions of the retina being able to respond independently to local defocus signals.8 Lens-induced defocus experiments in chicks showed that altering peripheral retinal defocus had a larger influence on axial elongation than central retinal defocus.9 Furthermore, hyperopic defocus on the peripheral retina accelerates axial eye growth in primates, even in the presence of clear, unrestricted central vision.10 Based on these results in animal models, peripheral defocus is suspected to be influential in human emmetropization, and peripheral myopic defocus is hypothesized to slow axial eye growth and myopia progression.
The evidence for the influence of peripheral defocus on eye growth in animal studies has encouraged researchers to explore novel optical corrections in an attempt to slow the progression of myopia. When measuring relative peripheral refraction (RPR) of the uncorrected eye, myopic eyes typically have relative peripheral hyperopic defocus in the horizontal meridian (a potential grow signal).11, 12 Longitudinal studies have evaluated whether the amount of hyperopic RPR of the uncorrected eye is associated with either myopia onset or progression, and these studies have not found a meaningful association.13–15 It is important to note that RPR (measurements of the uncorrected eye) does not completely describe what the eye experiences when wearing correction. Single vision minus-power spectacle lenses typically used to correct myopia increase peripheral hyperopic defocus.16–19 With animal research showing that myopic defocus is a stronger “stop” signal for eye growth versus the acceleration of growth associated with hyperopic defocus,9 it is hypothesized that optical corrections that induce myopic defocus are capable of slowing myopia progression in humans.
Optical interventions for myopia control have included under correction, gas permeable contact lenses, bifocal spectacles, orthokeratology, and bifocal or novel soft contact lenses. While neither under correction nor gas permeable contact lenses have been shown to slow myopia progression,20–23 progressive addition lens (PAL) studies have generally found clinically small reductions in myopia progression.24–28 Aside from PALs, there is some evidence that executive bifocals, which have a larger add area than standard bifocal spectacles, might be more effective at slowing myopia progression.29
Orthokeratology and bifocal soft contact lenses have shown promise for slowing myopia progression. Both of these optical treatments can induce a peripheral myopic shift in retinal defocus on multiple areas of the peripheral retina.30, 31 A reduction in axial eye growth ranging from 36 to 55 percent has been reported with orthokeratology treated eyes over a period of two years.32–35 The effect of bifocal soft contact lenses for myopia control is more varied, ranging from 25% to over 70% reduction in myopia progression, with some of these studies only reporting results over a one-year period.36–38 The more variable results with soft bifocal contact lenses compared to orthokeratology could be due to differences in lens design as well as compliance with wearing the contact lenses. For example, Lam et al. (2014) reported that myopia was slowed by 25% in a two-year randomized study, but noted a 46% reduction in children who wore lenses at least 5 hours per day.
Associations have also been found between peripheral defocus when wearing optical correction and the rate of myopia progression in children,19, 39 reinforcing the potential role of peripheral defocus in eye growth and myopia progression in humans. Orthokeratology is known to increase positive spherical aberration.40 A study of orthokeratology also found that eyes in which mid-peripheral corneal steepening after orthokeratology was greater (presumably causing greater myopic defocus on the peripheral retina) had a greater slowing of myopia progression.41 These studies support the concept that myopic defocus in the retinal periphery may act as a signal to slow myopia progression.
While optical interventions such as bifocal contact lenses and orthokeratology show promise for slowing myopia progression, it is important to understand how commonly prescribed standard optical corrections impact peripheral defocus in myopic eyes. Based on animal studies, one would expect standard spectacle corrections, which increase peripheral hyperopic defocus,17, 19 to potentially encourage axial elongation and myopia progression. Soft contact lenses are another commonly utilized correction option that come in a wide variety of optical profiles and in which lens manufacturers often include various amounts of spherical aberration by design, which can influence peripheral defocus. The purpose of this study was to determine the change in peripheral defocus in the horizontal meridian of myopic eyes when four commonly-prescribed, commercially-available, spherical soft contact lenses of the same labeled power were placed on the eye.
METHODS
Subjects
Twenty-five myopic young adults with spherical equivalent refractive error at the corneal plane of between −0.50 D and −6.00 D with less than −1.25 D of astigmatism participated in this study. This investigation adhered to the tenets of the Declaration of Helsinki and was approved by the University of Houston Committee for the Protection of Human Subjects. All subjects provided written informed consent prior to any testing.
An examination that included a standardized manifest refraction (most plus/least minus to best visual acuity) and biomicroscopy was performed to determine eligibility. Subjects were not presbyopic, had no history of ocular surgery or trauma, were free from ocular disease, and did not wear rigid gas permeable contact lenses. All subjects had spherical equivalent corrected visual acuity of 20/25 or better to exclude subjects with meaningful amounts of amblyopia and to ensure that any astigmatism present would not degrade vision to a level below which a spherical contact lens might be prescribed.
Contact Lenses
The right eye of each subject was fitted with each of the following spherical soft contact lenses, in random order: Biofinity (comfilcon A; CooperVision); Acuvue 2 (etafilcon A; Johnson & Johnson Vision Care); PureVision2 (balafilcon A; Bausch + Lomb); and Air Optix Night & Day Aqua (lotrafilcon A; Alcon) (Table 1). The contact lens power chosen for each subject was based on the spherical equivalent of the manifest refraction after vertexing to the corneal plane. Each lens was fitted and allowed to settle for approximately five minutes prior to evaluating the lens fit. Lateral decentration of each lens was measured after lens settling using a reticule in one of the slit lamp oculars. Nasal and temporal lens overlap beyond the cornea was measured to determine horizontal lens decentration. The same contact lens power was used for each brand of contact lens fitted on a subject; no over-refraction was performed for each contact lens because subjects were already cyclopleged to allow for more accurate peripheral autorefraction measurements, described below.
Table 1.
Manufacturer-reported contact lens parameters (mm) of the lenses fitted
| Biofinity | Acuvue 2 | PureVision2 | Air Optix Night & Day Aqua | |
|---|---|---|---|---|
| Material | comfilcon A | etafilcon A | balafilcon A | lotrafilcon A |
| Base Curve | 8.6 | 8.3 | 8.6 | 8.4 |
| Overall Diameter | 14.0 | 14.0 | 14.0 | 13.8 |
| Center Thickness (−3.00D) | 0.08 | 0.08 | 0.07 | 0.08 |
| Optic Zone | * | * | 9.0 | 8.0 |
Not reported by the manufacturer
Autorefraction
Cycloplegic autorefraction of the right eye was performed 30 minutes after instilling the first of two drops of 1% tropicamide, separated by 5 minutes. Autorefraction was performed using a modified, open-field Grand Seiko WAM-5500 autorefractor (Grand Seiko Co.; Hiroshima, Japan) with each lens on the eye and with no lens on the eye. Measurements were made centrally (on-axis) and at ±20°, ±30°, and ±40° from the line of sight in the horizontal meridian of the eye. The side on which measurements began (nasal or temporal) and the order in which lenses were fit were randomized. The Biofinity, Acuvue2, and PureVision2 contact lenses were evaluated in random order on one visit over a period of approximately 90 minutes. Air Optix Night and Day Aqua was evaluated at a second visit. The testing protocol was the same at both visits. The autorefractor was centered within the entrance pupil for all measurements.42 Subjects turned their head (not the eye) to view a red LED target projected on a wall for all peripheral measurements to avoid contact lens decentration due to eye rotation. The examiner monitored the subject upon each new target position to ensure an appropriate head movement was made.
The first five autorefraction measurements collected at each retinal location that were within ±1.00 D of the mode for the sphere and cylinder powers of the measurements were transposed to vector form using previously reported methods and averaged to obtain the mean M, J0, and J45 vector components.43 RPR at each retinal location was calculated by subtracting the central spherical equivalent (M) of the unaided eye from the peripheral M component of the unaided eye. Relative peripheral defocus (RPD) was calculated in the same way using measurements made when the subject wore each contact lens.
Aberrometry Measurements
A Discovery System aberrometer (Innovative Visual Systems; Elmhurst, IL) was used to collect on-axis cycloplegic aberrometry measurements with each contact lens on the eye and with no lens on the eye. Five measurements each were made while the subject fixated the instrument’s internal target. The aberration analysis diameter was limited by the smallest pupil across all subjects. Zernike coefficients were calculated over a 7-mm pupil and averaged.44 The measured spherical aberration of the eye alone (C4,0) was subtracted from the spherical aberration measured while wearing each contact lens to determine the change in spherical aberration induced by each contact lens.
Sample Size
When designing this study, sample size was determined using reported standard deviations of repeated measurements using the Grand Seiko autorefractor. Applying the standard deviation of ±0.24 D from previously reported cycloplegic repeatability of the Grand Seiko,45 assuming a 2-sided alpha of 0.05 and power of 80%, a sample size of seven subjects was necessary to detect a 0.25 D difference in defocus. Applying the worst reported repeatability of the Grand Seiko available (standard deviation of ±0.44 D) under non-cycloplegic conditions,46 assuming a 2-sided alpha of 0.05 and power of 80%, a sample size of 24 subjects was adequate to detect a 0.25 D difference in defocus. A total of 25 subjects were enrolled in this study.
Data Analyses
Data analyses were conducted with STATA 13.1 (StataCorp; College Station, TX) and SAS 9.4 (SAS Institute Inc.; Cary, NC). Repeated-measures analyses of variance (RM-ANOVA) were used to evaluate whether differences existed in RPD, relative peripheral J0 and J45 while wearing each of the contact lenses versus the uncorrected eye (i.e., evaluating whether the optics of each contact lens caused a change in peripheral defocus and astigmatism). Retinal location and testing condition (each contact lens brand or uncorrected eye) were included as factors in the RM-ANOVA. Labeled contact lens spherical power was also included as a covariate to determine whether the magnitude of minus power in the lens influenced changes in RPD caused by the lens. RM-ANOVA including contact lens power as a covariate was also used to determine whether changes in spherical aberration depended on contact lens power. Benjamini-Hochberg corrected post-hoc t-tests were performed when appropriate to test for differences in defocus, J0, and J45 between the contact lens-corrected eye and the uncorrected eye and also to determine if lens decentration was significantly different than zero. A linear mixed effects regression model was used to determine whether the association between the on-axis change in spherical aberration caused by each contact lens and the change in peripheral defocus induced by the contact lens was modified by retinal eccentricity, while adjusting for the power of the contact lens. All tests were conducted using an alpha level of 0.05. We verified that the assumptions were met for all parametric testing performed.
RESULTS
The subjects had a mean age ± SD of 24.0 ± 1.3 years and an average spherical equivalent refractive error of −3.45 ± 1.42 D (range: −1.00 to −5.75 D). Of the 25 subjects, 18 (72%) were female.
Refractive Changes Caused by Contact Lenses
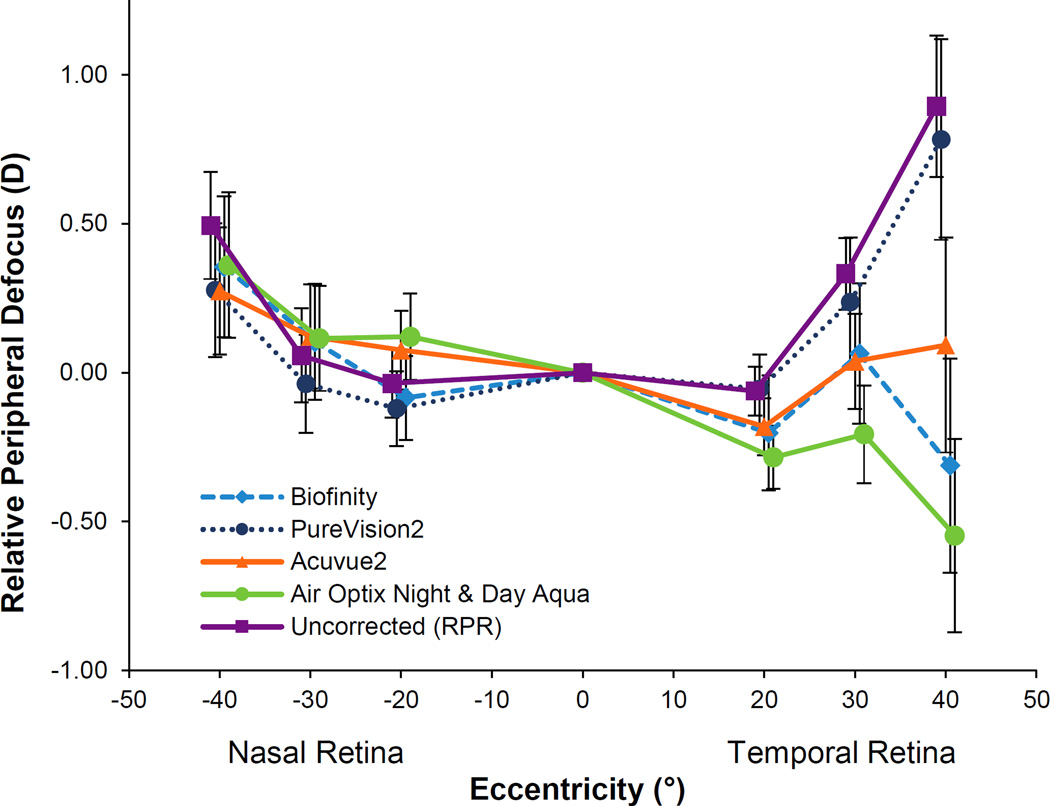
The mean RPR with no lens on the eye and the mean RPD with each of the four spherical soft lenses on the eye are shown in Figure 1. Differences in peripheral defocus at each location measured depended on the testing condition (testing condition by location interaction; p < 0.001). RPD while wearing a contact lenses was significantly different than RPR (no lens) for: Biofinity (p = 0.003); Acuvue2 (p = 0.001); and Air Optix Night & Day Aqua (p < 0.0001). RPD with PureVision2 was not different than RPR of the uncorrected eye (p = 0.33), meaning that PureVision2 did not change peripheral defocus.
Figure 1.
Relative peripheral defocus (M) while wearing each of the four soft contact lenses and relative peripheral refraction with no lens on the eye. Positive values represent hyperopic defocus and negative values represent myopic defocus. Error bars represent the SEM.
Compared to RPR, a statistically significant myopic shift was found with contact lenses at the following retinal locations: Biofinity at temporal 40° (−1.21 D), Acuvue2 at temporal 30° (−0.29 D) and temporal 40° (−0.80 D), and Air Optix Night & Day Aqua at temporal 20° (−0.23 D), temporal 30° (−0.48 D), and temporal 40° (−1.50 D) (all p < 0.05). RPD with contact lenses was not statistically different than RPR (uncorrected eye) along the nasal retina for any retinal location.
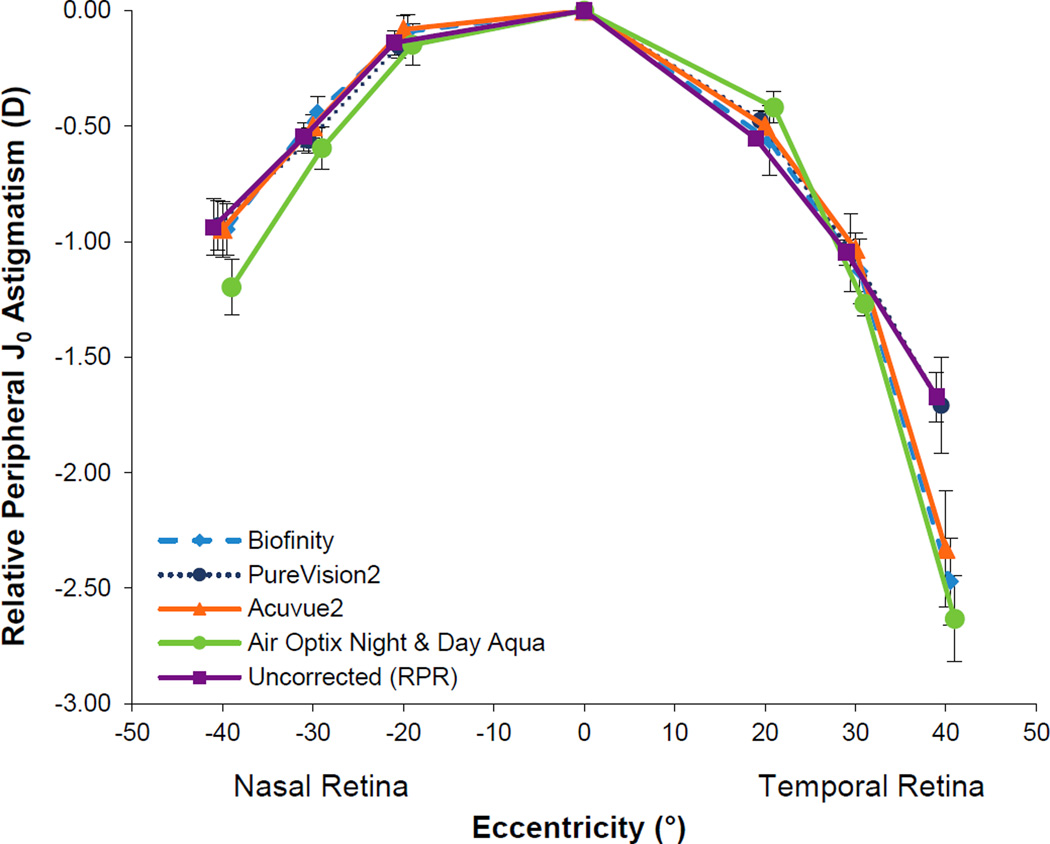
J0 astigmatism is plotted in Figure 2. Relative peripheral J0 astigmatism at each location depended on the testing condition (testing condition by location interaction; p<0.001). J0 astigmatism was found to significantly increase compared to that of the uncorrected eye at the 40° temporal retinal location with all contact lenses tested (all p < 0.03), except for PureVision2 (p = 0.97). There was also a significant difference between the J0 astigmatism of Air Optix Night & Day Aqua and that of the uncorrected eye at 40 degrees nasal retina (p = 0.03) and 30 degrees temporal retina (p < 0.001).
Figure 2.
Relative peripheral J0 astigmatism with the four soft contact lenses and with no lens on the eye. Error bars represent the SEM.
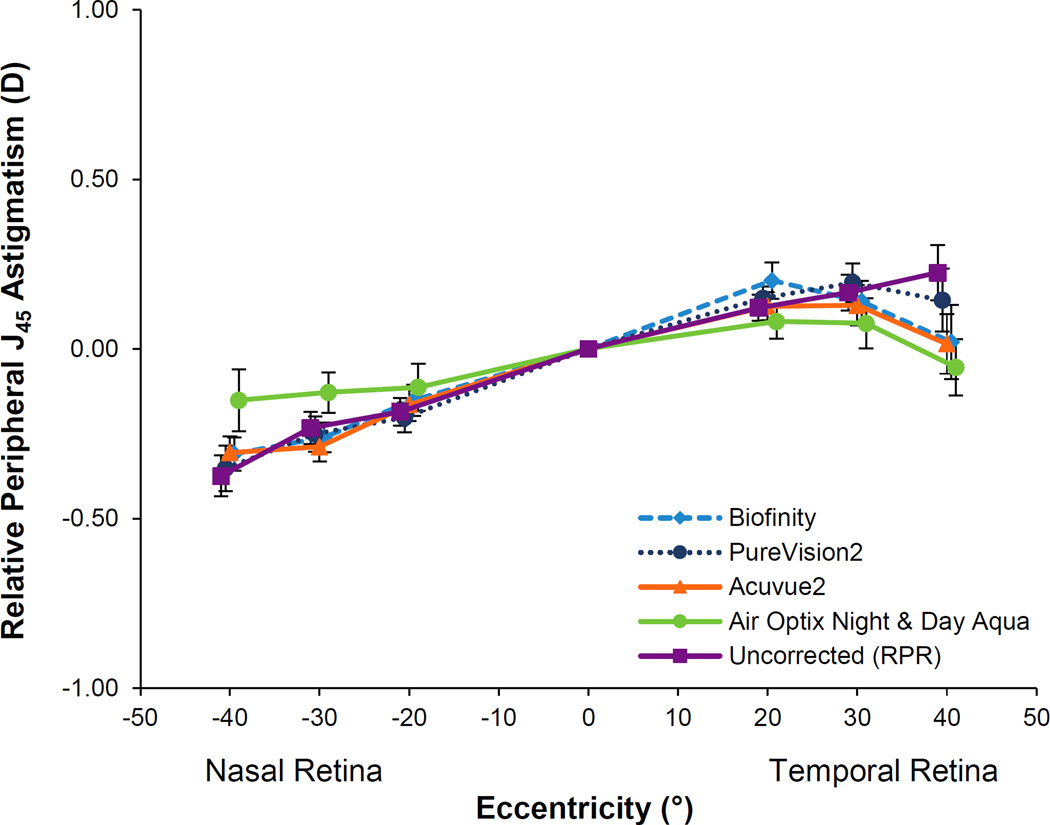
J45 astigmatism is plotted in Figure 3. Relative peripheral J45 astigmatism by location depended on the testing condition (testing condition by location interaction; p = 0.007). At the 40 degree temporal retinal location, relative peripheral J45 astigmatism with Air Optix Night & Day Aqua was significantly different than the uncorrected eye (p = 0.004). There were no other statistically significant differences in J45 astigmatism for any of the lenses compared to the uncorrected eye (all p ≥ 0.21).
Figure 3.
Relative peripheral J45 astigmatism with the four soft contact lenses and with no lens on the eye. Error bars represent the SEM.
The average amount of lateral lens decentration for each lens type was not significantly different than zero (mean decentration for each lens brand = 0.1 mm, all p>0.18). That being said, the direction of decentration differed among lens types (p=0.01). Biofinity, Acuvue2, and PureVision2 on average decentered 0.1 mm temporal on the cornea while Air Optix Night & Day Aqua decentered 0.1 mm nasal on the cornea (overall difference of 0.2 mm; p<0.05; Tukey’s HSD).
Influence of Contact Lens Power
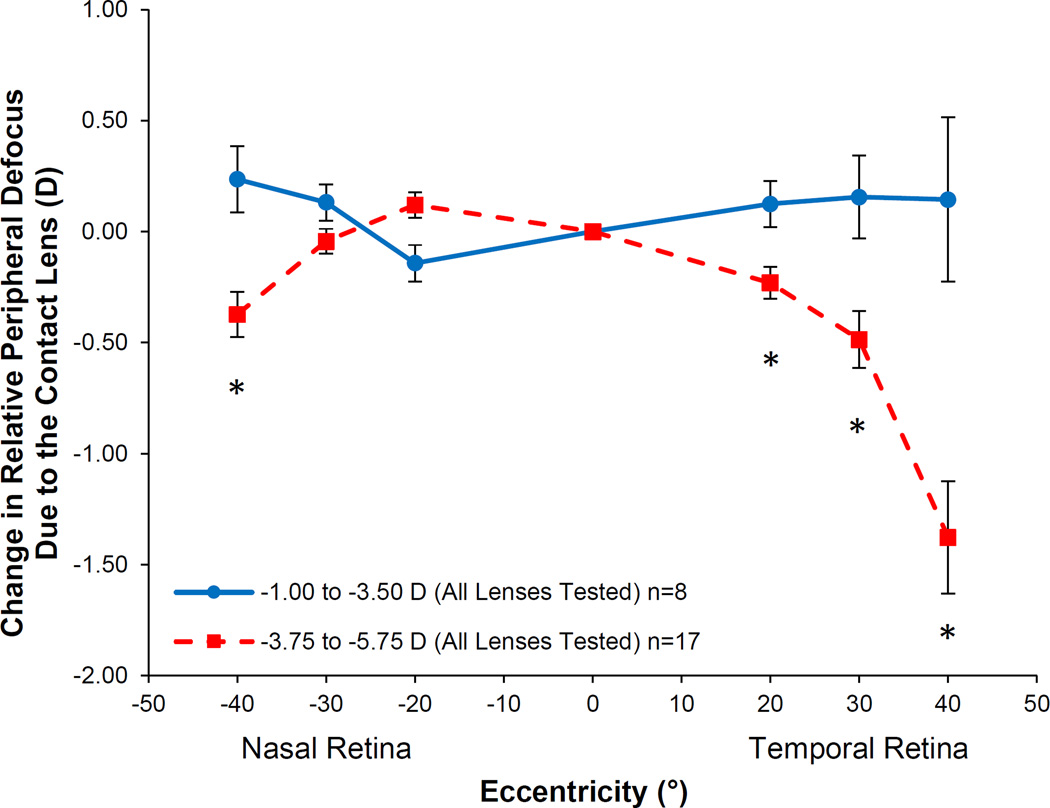
The change in RPD at each location due to the contact lens optics depended on the power of the contact lens (lens power by location interaction; p = 0.002). The change in RPD due to the power of the contact lens did not depend on the lens type (lens type by lens power by location interaction; p=0.58); therefore, defocus values at each location were averaged across lens types. In Figure 4, the defocus profile averaged across the four contact lenses are shown based on a split (more minus versus less minus power) to demonstrate the effect that contact lens power had on the change in peripheral defocus caused by the contact lenses. More negative SCL powers caused a more myopic change in RPD than less negative SCL powers at 40° nasal (−0.57 D), 20° temporal (−0.40 D), 30° temporal (−0.79 D), and 40° temporal (−1.38 D) on the retina (all p < 0.02).
Figure 4.
Change in relative peripheral defocus averaged across the four soft contact lenses split by contact lens power. Positive defocus represents a hyperopic image shift and negative defocus represents a myopic image shift. The asterisks denote eccentricities at which the two groups are significantly different from one another. Error bars represent the SEM.
Influence of Spherical Aberration
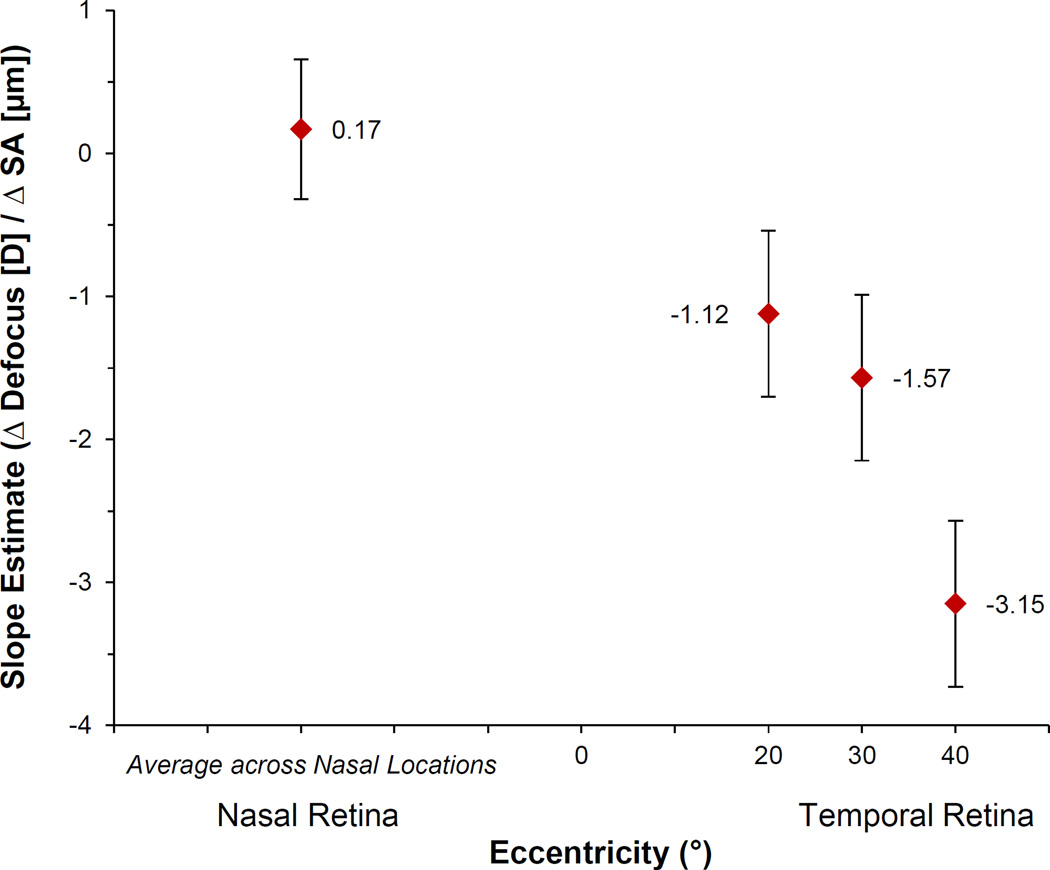
The mean on-axis change (± SD) in spherical aberration (C4,0) caused by each contact lens type over a 7-mm pupil was negative: Biofinity: −0.34 ± 0.12 µm; PureVision2: −0.33 ± 0.11 µm; Acuvue2: −0.21 ± 0.12 µm; and Air Optix Night & Day Aqua: −0.11 ± −0.09 µm. Across all lens designs, more negative contact lens powers were associated with more negative amounts of spherical aberration (p < 0.001). Because there were no significant differences between slope estimates for the association between the change in spherical aberration due to the lens and the change in peripheral defocus across the three nasal retinal locations, we simplified the model to include one combined slope for the nasal retinal locations. Overall, retinal location significantly modified the effect of the change in on-axis spherical aberration due to the contact lens on the change in peripheral defocus (p < 0.001), becoming more negative with increasing temporal retinal eccentricity. The slope estimates (± SE) shown in Figure 5 represent the effect of a one-micron change in spherical aberration caused by the contact lens (measured on axis) on the dioptric change in peripheral defocus at the nasal and three temporal retinal locations. The change in peripheral defocus associated with the mean on-axis change in spherical aberration caused by each contact lens brand is shown in Table 2 based on the slope estimate at the 40° temporal retinal location of −3.15 D/µm change in spherical aberration. Overall, contact lenses that induced less negative (more positive) changes in spherical aberration were associated with a less hyperopic change in RPD.
Figure 5.
Slope estimates from linear mixed model showing the influence of eccentricity on the dioptric change in defocus per 1 micron (µm) change in on-axis spherical aberration (SA) due to the contact lens. Error bars represent the SEM.
Table 2.
Dioptric change in defocus at 40° temporal retina per average change in on-axis spherical aberration (7 mm pupil) for each contact lens.
| Lens Type | Mean Δ SA (microns) | 40T Dioptric Δ/Avg Δ SA |
|---|---|---|
| Biofinity | −0.34 | 1.07 D |
| PureVision2 | −0.33 | 1.04 D |
| Acuvue2 | −0.21 | 0.66 D |
| Air Optix Night & Day Aqua | −0.11 | 0.35 D |
DISCUSSION
Depending on the retinal location measured, the four contact lenses evaluated in this study either induced a myopic shift in peripheral defocus or caused no significant change in the peripheral defocus experienced by the eye. None of the contact lenses caused a hyperopic shift. PureVision2 was the only contact lens that induced no change in peripheral defocus at any measurement location. Biofinity, Acuvue2, and Air Optix Night & Day Aqua caused a myopic shift on the temporal retina at greater eccentricities.
Previous studies evaluating the peripheral defocus profile induced by commercially-available soft contact lenses have found variable results. Peripheral myopic shifts have been reported with Acuvue 1-Day Moist, Acuvue2, and Air Optix Night & Day Aqua contact lenses.16, 47–49 A hyperopic shift in peripheral defocus has been reported with Proclear and Acuvue2 contact lenses.49, 50 Although a previous study of Biofinity reported no significant differences in peripheral defocus caused by the lens at most retinal locations, a sudden myopic shift in peripheral defocus at 40 degrees temporal on the retina was reported similar to the profile found in this study.30 The sudden myopic shift is hypothesized to be due to the edge of the optic zone of the contact lens, as described later below.
Of the lenses we tested, Acuvue2 is the only lens where our findings conflict with a previous report of the lens peripheral defocus profile. A study by de la Jara et al. reported a relative peripheral hyperopic shift in defocus produced by Acuvue2.49 In both our study and another study by Shen et al., a myopic shift in peripheral defocus was found with Acuvue2.48 Possible reasons for the discrepancy between our studies could include methodology (e.g., measurements made with versus without cycloplegia) and differences in lens fit and centration on the eye of study subjects.
At the 40 degree temporal retinal location, Biofinity, Acuvue2, and Air Optix Night & Day Aqua had a myopic shift with a corresponding increase in J0 astigmatism. The relative peripheral changes in J45 astigmatism were minimal. The increase in J0 astigmatism is hypothesized to be due to the size of the optic zone. The optic zones are reported by the manufacturer for only two of our study lenses: PureVision2 with a 9.0 mm optic zone and Air Optix Night & Day Aqua with an 8.0 mm optic zone.51 In evaluating schematic diagrams of the eye with a contact lens and utilizing the Grand Seiko measurement beam diameter of 2.3 mm,52 the autorefractor’s measurement beam at the 40 degree temporal retinal location is partially outside of the smaller optic zone of the Air Optix Night & Day Aqua. Because of its larger optic zone, PureVision2 does not result in measurements made outside of the optic zone when measuring at 40 degrees temporally on the retina, even after accounting for the contact lens centering over the optic axis of the eye rather than the visual axis upon which measurement eccentricity is based. The increase in astigmatism for three of the lenses tested is likely due to the instrument measuring partially through the junction of the optic zone and peripheral curve of the contact lens. The sudden increase in J0 astigmatism at the 40-degree temporal retinal location can account for a significant amount of the myopic shift in defocus at this peripheral location. This increase in astigmatism suggests that the optic zone size is an important factor to consider when determining the desired peripheral defocus profile to be induced by a contact lens.
Similar to previously published peripheral defocus profiles with soft contact lenses on the eye,48, 50 there is a nasal-temporal asymmetry in our defocus profiles. The nasal retina remains unchanged from the uncorrected eye while the temporal retina shows a myopic shift with three of the four soft contact lenses. One frequently cited cause for this asymmetry is that lenses center on the cornea and thus sit over the optical axis of the eye as opposed to the visual axis (the difference being angle alpha). One might also wonder whether contact lens decentration was a potential contributing factor to asymmetry in this study. If a contact lens consistently decentered in a particular direction, this could influence the peripheral defocus profile caused by the lens. While the average lens decentration did not differ significantly from zero, the Air Optix Night & Day Aqua contact lens decentered nasally on average while the other three lenses decentered temporally on average (difference of 0.2 mm). Though these contact lenses decentered in opposite directions, there was little difference in the defocus profiles, which is likely because of the small differences in centration between lenses measured in this study. The measured increase in J0 astigmatism that corresponds to the myopic shift in defocus at the 40 degree temporal location provides convincing evidence that measurements are occurring outside of the controlled optics (i.e., optic zone) of the contact lens and are responsible for the most peripheral asymmetric myopic shifts measured in this study.
The strengths of the current study versus previously published studies that evaluated spherical contact lenses on eye include our sample size, use of cycloplegia to avoid accommodation differences between lenses, the extent of the measurements into the periphery (40°), and the evaluation of multiple lenses on the same eye. Several previous studies had smaller sample sizes of between 10 to 11 subjects,16, 47, 48 only evaluated one soft contact lens design,16, 47, 48, 50 or did not measure as far into the periphery with maximum measurement locations of 20 degrees,47 30 degrees,16, 48 and 35 degrees50 from the line of sight. While most studies have used open-field autorefraction to measure defocus, Shen at al. used aberrometry,48 which can make direct comparisons between studies more difficult. A previous study by de la Jara et al. (2014) evaluated four different lens types using a contralateral eye design out to 40 degrees under non-cycloplegic conditions;49 advantages of the present study include the use of cycloplegia and evaluating additional lenses up to higher myopic values (−5.75 D in our study versus −4.25 D). Additionally, our work demonstrated that although most contact lenses caused a myopic change in peripheral defocus to some extent, design decisions by the manufacturer can result in no change in peripheral defocus (PureVision 2).
The power of the contact lens had a significant effect on the change in peripheral defocus caused by the contact lens. On average, across all four contact lenses, a higher power (more minus) contact lens was associated with a greater myopic shift in the periphery. This finding of more negative contact lens powers causing a greater myopic shift in peripheral defocus has been reported before with the Biofinity lens.30 Our findings demonstrate that in addition to variations in contact lens designs between manufacturers, the power of the contact lens can have a significant influence on the change in peripheral defocus caused by a contact lens of any brand. It is also important to note that while more minus power contact lenses in this study increased peripheral myopic defocus, more minus power spectacle lenses have been previously reported to increase peripheral hyperopic defocus.17 Based on these results, it seems that spherical soft contact lenses may provide a more favorable peripheral defocus profile than spectacle lenses from a myopia progression standpoint.
Though multiple contact lens manufacturers report that their lenses include aspheric optics, it is not always clear what goal the manufacturer is trying to achieve. Because spherical aberration is rotationally symmetric, it can be manipulated when designing a spherical contact lens. On average, the general population has positive spherical aberration,53 so one might infer that manufacturers designing aspheric soft contact lenses are either attempting to control induced spherical aberration in the contact lens or have the goal of eliminating the eye’s inherent spherical aberration in an attempt to improve visual quality. The average change in spherical aberration caused by the contact lenses measured in this study was negative, which would contribute to a more hyperopic shift in the periphery. Despite finding that the lenses in this study contained negative spherical aberration, we observed myopic changes in peripheral defocus in three of the lenses. That being said, a closer evaluation of the influence of spherical aberration suggests that any hyperopic shift was outweighed by the large impact of contact lens power on peripheral defocus. For example, when considering Air Optix Night & Day Aqua, our model results show that at the 40 degree temporal retina, the average spherical aberration induced by this particular lens brand accounts for approximately a 0.35 D hyperopic shift in peripheral defocus. When looking at the effect of prescribed contact lens power on peripheral defocus (Figure 4), higher powered (more minus) lenses are approximately 1.5 D more myopic at 40 degrees temporal retina than lower powered contact lenses. This shows that although negative spherical aberration in this study contributed to a hyperopic shift in the defocus profile, the myopic shifts associated with increasingly negative contact lens power were greater and produced an overall net myopic shift on the temporal retina for three of the four contact lenses.
Other groups have reported that the labeled contact lens power provided by the manufacturer may not always match the power measured by lensometry. Factors that can cause differences between labeled and measured power include the power profile across the optic zone, the diameter of the lens stop chosen to measure the lens, and differences in osmolarity between the manufacturer’s lens packaging solution and other solutions in which the lens is immersed.49, 54, 55 One might wonder whether the results would be different had we used measured contact lens power rather than the labeled power that most practitioners use in practice. Although lensometry was not performed on our lenses, we objectively determined contact lens power by calculating the change in central autorefraction between the bare eye and when each contact lens was on the eye. As sensitivity analyses, linear mixed regression analyses were performed using these objective contact lens powers, and the results were consistent in revealing the significant relationship between peripheral defocus and contact lens power and the significant relationship between peripheral defocus and spherical aberration when controlling for lens power.
A limitation of this study is that the profiles shown are specific to the four soft contact lenses evaluated. The profiles measured differed among the lenses tested demonstrating that design decisions made by manufacturers can yield differences in how the lens changes peripheral defocus when worn on the eye. As shown in this study, based on the manufacturer’s lens design, some lenses may result in no change in peripheral defocus (PureVision2) while others might cause a myopic change at multiple locations. It is also important for the clinician to note that these profiles represent the average change in defocus caused by the four minus powered lenses measured in this study. Because of variability in ocular shape, the final type of defocus experienced by the retina can be different between two eyes. Measuring peripheral defocus is the only way to definitively know the type and amount of defocus experienced by the peripheral retina of a particular eye. It is also still unclear how much of a myopic change in peripheral defocus is needed to cause a clinically meaningful change in the rate of myopia progression. Longitudinal studies are needed to answer this question.
CONCLUSIONS
Overall, there were differences in the change in peripheral defocus caused by each spherical soft contact lens. The differences in the defocus profiles seen between different brands of contact lenses are likely due to differences in contact lens optical design (including aspheric optics and optic zone diameter) and the influence of prescribed contact lens power. If peripheral defocus influences myopia progression, the influence of these lens design parameters should be kept in mind when designing contact lenses. Additionally, eye care providers should be aware of the potential differences in peripheral defocus caused by different contact lenses.
Acknowledgments
NIH/NEI grant support: T35-EY007088 (KEM) and P30-EY07551. The authors thank Chris Kuether for his assistance with instrument modifications.
Footnotes
These data were presented, in part, at annual meetings of the American Academy of Optometry (Denver 2014 and New Orleans 2015) and at the 15th International Myopia Conference (2015) in Wenzhou, China.
REFERENCES
- 1.Lin LL, Shih YF, Tsai CB, et al. Epidemiologic study of ocular refraction among schoolchildren in Taiwan in 1995. Optom Vis Sci. 1999;76:275–281. doi: 10.1097/00006324-199905000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet. 2012;379:1739–1748. doi: 10.1016/S0140-6736(12)60272-4. [DOI] [PubMed] [Google Scholar]
- 3.Vitale S, Ellwein L, Cotch MF, et al. Prevalence of refractive error in the United States, 1999–2004. Arch Ophthalmol. 2008;126:1111–1119. doi: 10.1001/archopht.126.8.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123:1036–1042. doi: 10.1016/j.ophtha.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Saw SM, Gazzard G, Shih-Yen EC, et al. Myopia and associated pathological complications. Ophthalmic Physiol Opt. 2005;25:381–391. doi: 10.1111/j.1475-1313.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 6.Cho BJ, Shin JY, Yu HG. Complications of Pathologic Myopia. Eye Contact Lens. 2016;42:9–15. doi: 10.1097/ICL.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 7.Smith EL, 3rd, Hung LF. The role of optical defocus in regulating refractive development in infant monkeys. Vision Res. 1999;39:1415–1435. doi: 10.1016/s0042-6989(98)00229-6. [DOI] [PubMed] [Google Scholar]
- 8.Wallman J, Gottlieb MD, Rajaram V, et al. Local retinal regions control local eye growth and myopia. Science. 1987;237:73–77. doi: 10.1126/science.3603011. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Wildsoet C. The effect of two-zone concentric bifocal spectacle lenses on refractive error development and eye growth in young chicks. Invest Ophthalmol Vis Sci. 2011;52:1078–1086. doi: 10.1167/iovs.10-5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith EL, 3rd, Hung LF, Huang J. Relative peripheral hyperopic defocus alters central refractive development in infant monkeys. Vision Res. 2009;49:2386–2392. doi: 10.1016/j.visres.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seidemann A, Schaeffel F, Guirao A, et al. Peripheral refractive errors in myopic, emmetropic, and hyperopic young subjects. J Opt Soc Am (A) 2002;19:2363–2373. doi: 10.1364/josaa.19.002363. [DOI] [PubMed] [Google Scholar]
- 12.Mutti DO, Sholtz RI, Friedman NE, et al. Peripheral refraction and ocular shape in children. Invest Ophthalmol Vis Sci. 2000;41:1022–1030. [PubMed] [Google Scholar]
- 13.Mutti DO, Sinnott LT, Mitchell GL, et al. Relative peripheral refractive error and the risk of onset and progression of myopia in children. Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error (CLEERE) Study Group. Invest Ophthalmol Vis Sci. 2011;52:199–205. doi: 10.1167/iovs.09-4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sng CC, Lin XY, Gazzard G, et al. Change in peripheral refraction over time in Singapore Chinese children. Invest Ophthalmol Vis Sci. 2011;52:7880–7887. doi: 10.1167/iovs.11-7290. [DOI] [PubMed] [Google Scholar]
- 15.Atchison DA, Li SM, Li H, et al. Relative peripheral hyperopia does not predict development and progression of myopia in children. Invest Ophthalmol Vis Sci. 2015;56:6162–6170. doi: 10.1167/iovs.15-17200. [DOI] [PubMed] [Google Scholar]
- 16.Backhouse S, Fox S, Ibrahim B, et al. Peripheral refraction in myopia corrected with spectacles versus contact lenses. Ophthalmic Physiol Opt. 2012;32:294–303. doi: 10.1111/j.1475-1313.2012.00912.x. [DOI] [PubMed] [Google Scholar]
- 17.Lin Z, Martinez A, Chen X, et al. Peripheral defocus with single-vision spectacle lenses in myopic children. Optom Vis Sci. 2010;87:4–9. doi: 10.1097/OPX.0b013e3181c078f1. [DOI] [PubMed] [Google Scholar]
- 18.Tabernero J, Vazquez D, Seidemann A, et al. Effects of myopic spectacle correction and radial refractive gradient spectacles on peripheral refraction. Vision Res. 2009;49:2176–2186. doi: 10.1016/j.visres.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Berntsen DA, Barr CD, Mutti DO, et al. Peripheral defocus and myopia progression in myopic children randomly assigned to wear single vision and progressive addition lenses. Invest Ophthalmol Vis Sci. 2013;54:5761–5770. doi: 10.1167/iovs.13-11904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adler D, Millodot M. The possible effect of undercorrection on myopic progression in children. Clin Exp Optom. 2006;89:315–321. doi: 10.1111/j.1444-0938.2006.00055.x. [DOI] [PubMed] [Google Scholar]
- 21.Chung K, Mohidin N, O’Leary DJ. Undercorrection of myopia enhances rather than inhibits myopia progression. Vision Res. 2002;42:2555–2559. doi: 10.1016/s0042-6989(02)00258-4. [DOI] [PubMed] [Google Scholar]
- 22.Walline JJ, Jones LA, Mutti DO, et al. A randomized trial of the effects of rigid contact lenses on myopia progression. Arch Ophthalmol. 2004;122:1760–1766. doi: 10.1001/archopht.122.12.1760. [DOI] [PubMed] [Google Scholar]
- 23.Katz J, Schein OD, Levy B, et al. A randomized trial of rigid gas permeable contact lenses to reduce progression of children’s myopia. Am J Ophthalmol. 2003;136:82–90. doi: 10.1016/s0002-9394(03)00106-5. [DOI] [PubMed] [Google Scholar]
- 24.Gwiazda J, Hyman L, Hussein M, et al. A randomized clinical trial of progressive addition lenses versus single vision lenses on the progression of myopia in children. Invest Ophthalmol Vis Sci. 2003;44:1492–1500. doi: 10.1167/iovs.02-0816. [DOI] [PubMed] [Google Scholar]
- 25.Hasebe S, Ohtsuki H, Nonaka T, et al. Effect of progressive addition lenses on myopia progression in Japanese children: a prospective, randomized, double-masked, crossover trial. Invest Ophthalmol Vis Sci. 2008;49:2781–2789. doi: 10.1167/iovs.07-0385. [DOI] [PubMed] [Google Scholar]
- 26.Edwards MH, Li RW, Lam CS, et al. The Hong Kong progressive lens myopia control study: study design and main findings. Invest Ophthalmol Vis Sci. 2002;43:2852–2858. [PubMed] [Google Scholar]
- 27.Gwiazda J, Chandler DL, Cotter SA, et al. Progressive-addition lenses versus single-vision lenses for slowing progression of myopia in children with high accommodative lag and near esophoria. Correction of Myopia Evaluation Trial (COMET) 2 Study Group for the Pediatric Eye Disease Investigator Group. Invest Ophthalmol Vis Sci. 2011;52:2749–2757. doi: 10.1167/iovs.10-6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berntsen DA, Sinnott LT, Mutti DO, et al. A randomized trial using progressive addition lenses to evaluate theories of myopia progression in children with a high lag of accommodation. Invest Ophthalmol Vis Sci. 2012;53:640–649. doi: 10.1167/iovs.11-7769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng D, Woo GC, Drobe B, et al. Effect of bifocal and prismatic bifocal spectacles on myopia progression in children: three-year results of a randomized clinical trial. JAMA Ophthalmol. 2014;132:258–264. doi: 10.1001/jamaophthalmol.2013.7623. [DOI] [PubMed] [Google Scholar]
- 30.Berntsen DA, Kramer CE. Peripheral defocus with spherical and multifocal soft contact lenses. Optom Vis Sci. 2013;90:1215–1224. doi: 10.1097/OPX.0000000000000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang P, Swarbrick H. Peripheral refraction in myopic children wearing orthokeratology and gas-permeable lenses. Optom Vis Sci. 2011;88:476–482. doi: 10.1097/OPX.0b013e31820f16fb. [DOI] [PubMed] [Google Scholar]
- 32.Cho P, Cheung SW, Edwards M. The longitudinal orthokeratology research in children (LORIC) in Hong Kong: a pilot study on refractive changes and myopic control. Curr Eye Res. 2005;30:71–80. doi: 10.1080/02713680590907256. [DOI] [PubMed] [Google Scholar]
- 33.Kakita T, Hiraoka T, Oshika T. Influence of overnight orthokeratology on axial elongation in childhood myopia. Invest Ophthalmol Vis Sci. 2011;52:2170–2174. doi: 10.1167/iovs.10-5485. [DOI] [PubMed] [Google Scholar]
- 34.Walline JJ, Jones LA, Sinnott LT. Corneal reshaping and myopia progression. Br J Ophthalmol. 2009;93:1181–1185. doi: 10.1136/bjo.2008.151365. [DOI] [PubMed] [Google Scholar]
- 35.Cho P, Cheung SW. Retardation of myopia in Orthokeratology (ROMIO) study: a 2-year randomized clinical trial. Invest Ophthalmol Vis Sci. 2012;53:7077–7085. doi: 10.1167/iovs.12-10565. [DOI] [PubMed] [Google Scholar]
- 36.Lam CS, Tang WC, Tse DY, et al. Defocus Incorporated Soft Contact (DISC) lens slows myopia progression in Hong Kong Chinese schoolchildren: a 2-year randomised clinical trial. Br J Ophthalmol. 2014;98:40–45. doi: 10.1136/bjophthalmol-2013-303914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walline JJ, Greiner KL, McVey ME, et al. Multifocal contact lens myopia control. Optom Vis Sci. 2013;90:1207–1214. doi: 10.1097/OPX.0000000000000036. [DOI] [PubMed] [Google Scholar]
- 38.Aller TA, Liu M, Wildsoet CF. Myopia control with bifocal contact lenses: a randomized clinical trial. Optom Vis Sci. 2016;93:344–352. doi: 10.1097/OPX.0000000000000808. [DOI] [PubMed] [Google Scholar]
- 39.Sankaridurg P, Holden B, Smith E, 3rd, et al. Decrease in rate of myopia progression with a contact lens designed to reduce relative peripheral hyperopia: one-year results. Invest Ophthalmol Vis Sci. 2011;52:9362–9367. doi: 10.1167/iovs.11-7260. [DOI] [PubMed] [Google Scholar]
- 40.Berntsen DA, Barr JT, Mitchell GL. The effect of overnight contact lens corneal reshaping on higher-order aberrations and best-corrected visual acuity. Optom Vis Sci. 2005;82:490–497. doi: 10.1097/01.opx.0000168586.36165.bb. [DOI] [PubMed] [Google Scholar]
- 41.Zhong Y, Chen Z, Xue F, et al. Corneal power change is predictive of myopia progression in orthokeratology. Optom Vis Sci. 2014;91:404–411. doi: 10.1097/OPX.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 42.Fedtke C, Ehrmann K, Ho A, et al. Lateral pupil alignment tolerance in peripheral refractometry. Optom Vis Sci. 2011;88:570–579. doi: 10.1097/OPX.0b013e31821041e2. [DOI] [PubMed] [Google Scholar]
- 43.Thibos LN, Wheeler W, Horner D. Power vectors: an application of Fourier analysis to the description and statistical analysis of refractive error. Optom Vis Sci. 1997;74:367–375. doi: 10.1097/00006324-199706000-00019. [DOI] [PubMed] [Google Scholar]
- 44.American National Standards Institute (ANZI) Ophthalmics - Methods for Reporting Optical Aberrations of the Eye; ANSI Z80.28–2004. New York: ANZI; 2004. [Google Scholar]
- 45.Bailey MD, Twa MD, Mitchell GL, et al. Repeatability of autorefraction and axial length measurements after laser in situ keratomileusis. J Cataract Refract Surg. 2005;31:1025–1034. doi: 10.1016/j.jcrs.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 46.Cleary G, Spalton DJ, Patel PM, et al. Diagnostic accuracy and variability of autorefraction by the Tracey Visual Function Analyzer and the Shin-Nippon NVision-K 5001 in relation to subjective refraction. Ophthalmic Physiol Opt. 2009;29:173–181. doi: 10.1111/j.1475-1313.2008.00627.x. [DOI] [PubMed] [Google Scholar]
- 47.Kwok E, Patel B, Backhouse S, et al. Peripheral refraction in high myopia with spherical soft contact lenses. Optom Vis Sci. 2012;89:263–270. doi: 10.1097/OPX.0b013e318242dfbf. [DOI] [PubMed] [Google Scholar]
- 48.Shen J, Clark CA, Soni PS, et al. Peripheral refraction with and without contact lens correction. Optom Vis Sci. 2010;87:642–655. doi: 10.1097/OPX.0b013e3181ea16ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de la Jara PL, Sankaridurg P, Ehrmann K, et al. Influence of contact lens power profile on peripheral refractive error. Optom Vis Sci. 2014;91:642–649. doi: 10.1097/OPX.0000000000000273. [DOI] [PubMed] [Google Scholar]
- 50.Kang P, Fan Y, Oh K, et al. Effect of single vision soft contact lenses on peripheral refraction. Optom Vis Sci. 2012;89:1014–1021. doi: 10.1097/OPX.0b013e31825da339. [DOI] [PubMed] [Google Scholar]
- 51.Thompson T. Tyler's Quarterly Soft Contact Lens Parameter Guide. Little Rock, AR: Tyler's Quarterly; 2015. [Google Scholar]
- 52.Fedtke C, Ehrmann K, Holden BA. A review of peripheral refraction techniques. Optom Vis Sci. 2009;86:429–446. doi: 10.1097/OPX.0b013e31819fa727. [DOI] [PubMed] [Google Scholar]
- 53.Salmon TO, van de Pol C. Normal-eye Zernike coefficients and root-mean-square wavefront errors. J Cataract Refract Surg. 2006;32:2064–2074. doi: 10.1016/j.jcrs.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 54.Lum E, Perera I, Ho A. Osmolality and buffering agents in soft contact lens packaging solutions. Cont Lens Anterior Eye. 2004;27:21–26. doi: 10.1016/j.clae.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 55.Papas E, Dahms A, Carnt N, et al. Power profiles and short-term visual performance of soft contact lenses. Optom Vis Sci. 2009;86:318–323. doi: 10.1097/OPX.0b013e318198959e. [DOI] [PubMed] [Google Scholar]