Abstract
Many bacteria can assemble functional amyloid fibers on their cell surface. The majority of bacterial amyloids contribute to biofilm or other community behaviors where cells interact with a surface or with another cell. Bacterial amyloids, like all functional amyloids, share structural and biochemical properties with disease-associated eukaryotic amyloids. The general ability of amyloids to bind amyloid-specific dyes, such as Congo red, and their resistance to denaturation have provided useful tools for scoring and quantifying bacterial amyloid formation. Here, we present basic approaches to study bacterial amyloids by focusing on the well-studied curli amyloid fibers expressed by Enterobacteriaceae. These methods exploit the specific tinctorial and biophysical properties of amyloids. The methods described here are straightforward and can be easily applied by any modern molecular biology lab for the study of other bacterial amyloids.
Keywords: Bacterial amyloids, Curli, Congo red dye, Western blot analysis, Plug western blot analysis, Overlay assay, Interbacterial complementation
1. Introduction
Amyloids are traditionally associated with protein misfolding and neurodegenerative diseases including Alzheimer’s disease, Parkinson’s disease, and Creutzfeldt-Jacob disease (1–3). However, a rapidly growing class of “functional amyloids” has recently been described, which indicates that the amyloid fold can be utilized by all walks of cellular life for a variety of functions (4–6). So far, most functional amyloids in bacteria perform physiological tasks on the cell surface including biofilm formation, adhesion, invasion of host cells, and host–pathogen interactions (7–14). For example, curli generated by Escherichia coli and Salmonella spp. (15, 16), chaplins formed by Streptomyces spp. (6, 17), and TasA fibers produced by Bacillus subtilis (11) are all functional amyloids utilized by microbes to promote interbacterial interactions. Unlike disease-associated eukaryotic amyloids, functional bacterial amyloids are assembled by highly regulated biosynthetic pathways (4). Bacterial amyloids exhibit the structural and biochemical properties of amyloids. Like all amyloids, functional amyloids bind dyes such as Congo red (CR) and Thioflavin T (6, 11, 15, 18). The structural analysis of bacterial amyloid fibers indicates a beta-sheet-rich secondary structure (11, 15, 17, 19). Amyloid fibers are also extraordinarily stable and resistant to SDS denaturation and Protease K digestion (17, 20, 21). These properties provide a toolbox for research on bacterial amyloids. Here we use curli, one of the best-characterized bacterial amyloids as an example to describe a few basic approaches to study bacterial amyloids.
Curli are extracellular amyloid fibers produced by many species of E. coli and Salmonella spp. strains (16, 22, 23). Purified curli fibers bind CR and induce a spectral red shift in absorbance (15). Colonies of curliated E. coli K-12 stain red on agar plates containing CR, whereas curli defective mutants are nonstained (15). Once CR interacts with curli, it also produces a bright red fluorescence that can be quantified with an excitation wavelength of 485 nm and an emission wavelength of 612 nm.
Curli fibers are composed of two structural components: the major curlin subunit CsgA (csg: curli-specific gene) and the minor subunit CsgB. The secretion of CsgA and CsgB requires the outer membrane lipoprotein CsgG and the periplasmic accessory factors CsgE and CsgF (4). Once incorporated into curli fibers, CsgA and CsgB are no longer soluble by SDS denaturation treatment (15). CsgB functions as a nucleator by templating the polymerization of CsgA in vivo. Without CsgB, CsgA proteins are secreted to the extracellular space in an SDS-soluble, unstructured form that can be detected in the agar (24, 25).
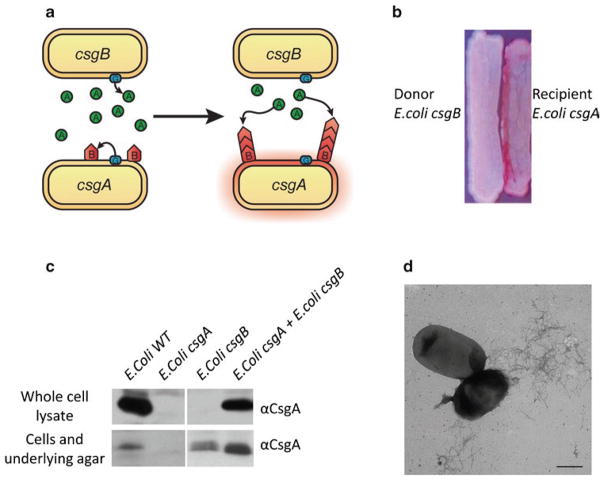
In this chapter, we describe basic approaches for analyzing the presence and/or integrity of curli fibers under physiological conditions. The CR-based assays described here are amendable to high-throughput screens that assess curli production. CR indicator plates can be used to screen for curli defective mutants and to identify genes important for curli regulation and assembly (26, 27). Western blot analysis of whole cell lysate is also useful to sort factors involved in curli amyloidogenesis (28–30). Curli produced by wild-type E. coli are cell associated and insoluble in SDS-sample buffer with boiling. Treatment of whole cell lysates with formic acid (FA) or hexafluoro-2-propanol (HFIP) dissociates the curli fibers into monomores of the major subunit CsgA. After chemical denaturation, CsgA can mobilize into an SDS–PAGE gel and can be identified as a band that migrates at about 17.5 kDa using anti-CsgA antibodies (15). We will also detail how a “plug” western blot assay can be used to differentiate between curli subunits that are unpolymerized from those that are cell-associated and polymerized (15, 25, 29). Finally, the overlay assay and interbacterial complementation provide ways to test CsgA polymerization templated by CsgB in vivo on the bacterial surface. Freshly purified CsgA or CsgA secreted by a csgB mutant assembles on a csgA mutant that presents CsgB on the cell surface (Figs. 1a and 2). The assays also help to identify the interacting domains of CsgA and CsgB responsible for the nucleation process. These assays can be carried out using common equipment and can be adapted to study other bacterial amyloids.
Fig. 1.
Interbacterial complementation between an E. coli csgA mutant and a csgB mutant. (a) A schematic presentation of interbacterial complementation. A csgB mutant (the donor) secretes soluble CsgA into the media, which assembles into curli fibers on the cell surface of an adjacent csgA mutant (the recipient) expressing CsgB. (b) A csgA mutant and a csgB mutant were streaked adjacent to each other on the YESCA CR plate and incubated at 26°C for 48 h. Colonies of the csgA mutant facing to the csgB mutant stained red. (c) Western blot analysis was used to detect formation of intercellular curli fibers between an E. coli csgA mutant and a csgB mutant. Overnight cultures of csgA and csgB mutants were mixed in a 1:1 ratio and spotted onto a YESCA plate and a thin YESCA plate, respectively. Whole cell lysates and the plugs were collected for western analysis. A wild-type E. coli, a csgA and a csgB mutant were used as controls. All of the samples were treated with HFIP and probed with anti-CsgA antibody. (d) A mixed culture of E. coli csgA and csgB mutants was spotted onto YESCA plate and grown for 48 h. Curli were detected by EM. Scar bar equals to 500 nm.
Fig. 2.
Purified CsgA assembles into curli fibers on CsgB expressing cells. (Figure adapted from Wang et al. (29)) (a) CR staining of CsgA−B+ and CsgA−B− overlaid with different concentrations of freshly purified CsgA. Only CsgA−B+ cells with CsgA proteins stained red. (b) Negative-stain EM of CsgA−B+ overlaid with freshly purified CsgA. Fibers were observed on the bacterial surface. Scar bar equals to 500 nm. (c) Negative-stain EM of CsgA−B− overlaid with purified CsgA. No fibers were detected. Scar bar equals to 500 nm.
2. Materials
Prepare all the solutions and media using ultrapure water. Prepare and store the reagents at room temperature (RT) unless otherwise indicated. Add antibiotics to media if needed.
2.1. Standard Growth Media for E. coli Curli Induction
Luria-Bertani (LB) agar plates: dissolve 5 g/L yeast extract, 10 g/L bacto tryptone, and 10 g/L sodium chloride in water.
YESCA agar plates: YESCA agar is used to induce curli production. Dissolve 1 g/L yeast extract, 10 g/L casamino acids, and 20 g/L agar in water. Pour 25 mL per Petri dish to make normal YESCA plates. For thin YESCA plates used in plug western blots, pour only 15 mL per Petri dish (see Note 1).
2.2. Congo Red Assays
For quantitative CR biochemical assays, one will need a Spectra max M2 plate reader (or equivalent) and opaque flat-bottom polystyrene 96-well plates for CR fluorescence quantification.
CR stock solution (10 mg/mL): dissolve 1 g CR in 100 mL water and filter sterilize. Store at 4°C (see Note 2).
Brilliant Blue G250 stock solution (10 mg/mL): dissolve 1 g Brilliant Blue G250 dye in 100 mL water and filter sterilize. Store at 4°C (see Note 3).
YESCA CR plates: dissolve 1 g/L yeast extract, 10 g/L casamino acids, and 20 g/L agar in water. After autoclave, add 5 mL CR stock and 100 μl Brilliant blue stock to make a final concentration of 50 μg/mL CR and 1 μg/mL Brilliant blue.
50 mM Potassium phosphate buffer (KPi), pH 7.2: dissolve 28.9 mmol KH2PO4 and 21.1 mmol K2HPO4 into pure water. Dilute to 1 L and filter sterilize.
2.3. Western Blot Analysis
In this experiment, you will need Pasteur pipettes with 8 mm diameter, Whatman filter paper, polyvinylidene difluoride (PVDF) and nitrocellulose (0.2 μM) membranes, protein electrophoresis apparatus, semidry and wet transfer apparatus, chemiluminescent substrates, and autoradiography cassettes.
50 mM KPi, pH 7.2.
HFIP or FA (see Note 4).
2× SDS sample buffer: mix 1 mL 1.25 M Tris pH 6.8, 1 mL β-mercaptoethanol, 200 μL 1% bromophenol blue, 2 mL glycerol, 6 mL 10% SDS, and 10 mL water. Bring the final volume up to 20 mL.
15-Well SDS–polyacrylamide gel: 15% separating gel and 3% stacking gel.
SDS running buffer: dissolve 30.27 g Tris base, 144.1 g glycine, and 10 g SDS into 1 L water to make a 10× stock. Dilute the stocking buffer by 10× into water to make 1× running buffer.
Semidry transfer buffer: dissolve 30.3 g Tris base, 7.5 g glycine, and 0.386 g dithiothreitol (DTT) in 1 L water to make a 10× stock solution. Mix 100 mL 10× stock buffer, 20 mL methanol, and 70 mL water to make 1 L of 1× semidry transfer buffer.
Wet transfer buffer: dissolve 5.53 g CAPS in a mixture of 100 mL methanol and 900 mL water. Use NaOH pellets to adjust the pH to 11.2 (see Note 5).
Tris-buffered saline–Tween-20 (TBS-T): dissolve 160 g NaCl, 4 g KCl, and 60 g Tris in water and adjust pH to 7.5 with HCl. Add 20 mL Tween-20 and add water to make a 1 L 20× stock. Dilute the stock in water to make 1× TBS-T buffer. Store the 20× stocking buffer and 1× buffer at 4°C.
Blocking solution: dissolve 1% dry fat-free milk and 1% BSA in 1× TBS-T buffer.
CsgA antibody (Proteintech, Chicago, IL) was raised in rabbits against purified CsgA proteins. CsgB antibody (Proteintech, Chicago, IL) was raised in rabbits against a peptide fragment of the second repeating units of CsgB. Save the antibodies at −20°C. Dilute antibodies by 1:10,000 for CsgA and 1:7,000 for CsgB into blocking solution for primary probing (see Note 6).
Anti-rabbit secondary antibody: peroxidase antibody produced in goat. Store the antibody at −20°C. Dilute the antibody by 1:7,000 into blocking solution before use.
2.4. CsgA Purification and Overlay Assay
For CsgA purification as described here, you will need 0.22-μm polyethersulfone bottle-top filters, nickel–nitrilotriacetic acid agarose (Ni-NTA), sephadex G-25, BCA protein assay kit, Kontes 2.5 × 10 cm, and 1 × 30 cm glass columns, Nalgene powder funnel (150 mm with 2.5 cm spout), disposable poly-propylene columns, 30 kDa centrifugal filter units, spin desalt columns and 0.02-μm Anotop filters (Whatman).
LSR12/pMC1/pMC3: LSR12 is a complete curli deletion mutant of E. coli C600 (31). pMC1 was made by cloning CsgG into the NcoI and BamHI sites of pTRC99. pMC3 was made by cloning full-length C-terminal His6-tagged CsgA into the NdeI and EcoRI sites of pHL3 (15, 31).
NEB C2566/pNH11 (30): The plasmid pNH11 was made by cloning C-terminal His6-tagged CsgA without the Sec signal sequence into the pET11d vector.
Isopropyl β-d-1-thiogalactopyranoside stock (1 M): dissolve 4.76 g IPTG powder into 20 mL water and filter sterilize. Make 1 mL aliquots in 1.5-mL microcentrifuge tubes. Store at −20°C.
Guanidine hydrochloride (8 M): Dissolve 764.2 GdnHCl into 1 L 50 mM KPi. Adjust the pH to 7.2 with NaOH and bring up the final volume to 1 L.
50 mM KPi, pH 7.2. Store at 4°C.
100 mM Imidazole in 50 mM KPi, pH 7.2: dissolve 6.8 g imidazole into 1 L 10 mM KPi and filter sterilize. Store at 4°C.
12.5 mM Imidazole in 50 mM KPi, pH 7.2: dissolve 0.85 g imidazole into 1 L 50 mM KPi and filter sterilize. Store at 4°C.
125 mM imidazole in 50 mM KPi, pH 7.2: dissolve 8.51 g imidazole into 1 L 50 mM KPi and filter sterilize. Store at 4°C.
3. Methods
3.1. Congo Red Assays
Curliated bacteria stain red when grown on plates amended with CR. Therefore, it is easy to differentiate curli-producing strains from curli defective strains by assessing the colony color on plates containing CR (15).
3.1.1. CR Staining of Bacterial Colonies (Fig. 3a)
Fig. 3.
CR staining and fluorescence quantification. (a) Use of CR staining to screen for curli mutants. Colonies of the wild-type E. coli stained red on the YESCA CR agar plate, while the curli defective mutant, csgA, remained white. Curli mutants (36) showed dark red, pink, or light pink color on the YESCA CR plate. (b–d) Measurements of the fluorescence associated with bacteria for CR (Em/Ex: 485/612 nm). BW25113 wild-type and the csgA deletion mutant strains were recovered from YESCA plates after 48 h of growth at 26°C and resuspended in 1 mL of 50 mM KPi (pH 7.2) containing 0.5 μg/mL CR or 4.5 μg/mL DAPI. After wash, serial base two dilutions are prepared and the fluorescence was measured in a 96-well plates by triplicate. As a reference, 100 μL of 50 mM KPi were used. (b) Comparison of CR fluorescence of curli producing wild-type and curli deficient csgA strains. Nonlinear (exponential fit): R 2 = 0.970 and 0.778 for BW25113 and csgA, respectively. (c) To show that the BW25113 csgA mutant was present in the same amount as the wild-type BW25113 strain, bacteria were post-labeled with the unspecific DNA 4′,6-diamidino-2-phenylindole (DAPI) fluorochrome and the DAPI fluorescence is measured (Em/Ex: 350/460 nm). Nonlinear (exponential fit): R 2 = 0.865 and 0.867 for BW25113 and csgA, respectively. (d) Quantification of CR fluorescence for bacteria prestained on YESCA CR plates. The CR fluorescence of the wild-type strain was significantly higher (around three times) compared with the BW25113 csgA mutant. ***p < 0.0001 using student’s t test. Error bars: average SEM of at least six wells per sample. The plate reader used in this determination was an Infinite 200 with the Tecan-I application and automatic optimization of the gain.
Streak E. coli strains from −80°C frozen stock onto an LB plate and grow at 37°C overnight.
Pick single colonies, streak out on a YESCA CR agar plate (see Note 7).
Grow bacteria at 26°C for 2 days to induce curli production (see Note 8).
Check the color of colonies on the YESCA CR plate. Curliated bacteria stain red on YESCA CR agar, while curli defective mutants are usually pink or white (see Note 9).
3.1.2. Congo Red Fluorescence (see Notes 7 and 9)
-
Quantification of CR fluorescence by post-CR staining (Fig. 3b, c).
Streak E. coli strains from −80°C frozen stock to an LB plate and grow at 37°C overnight.
Inoculate single colonies in LB liquid broth and grow at 37°C overnight with shaking.
Spot 4 μL drops of overnight cultures on an YESCA agar plate without CR. Incubate the plate at 26°C for 48 h to induce curli production.
Recover bacteria cells from the YESCA plate and suspend them in 1 mL 50 mM KPi. Adjust the optical density at 600 nm to 1 U in 1 mL of 50 mM KPi.
Centrifuge bacteria at 12,000 rpm for 1.5 min and resus-pend them in 1 mL 50 mM KPi containing 0.5 μg/mL CR. Incubate at RT with shaking for 20 min.
Wash bacterial cells by centrifugation and resuspend them in 1 mL of 50 mM KPi buffer.
Prepare serial dilutions of bacterial suspension. Load 100 μL of each sample onto a 96-well opaque plate. Measure the CR fluorescence using a Spectra max M2 plate reader. Set the excitation wavelength at 485 nm and the emission at 612 nm (see Note 10). Use 100 μL of KPi as the blank.
-
Quantification of CR fluorescence for bacteria prestained on YESCA CR plates (Fig. 3d).
Spot 4 μL of overnight bacterial culture on the YESCA CR plate. Incubate the plate at 26°C for 48 h.
Recover bacteria from the YESCA CR plate. Wash them in 50 mM KPi by centrifugation at 12,000 rpm for 1.5 min and resuspend them in 1 mL 50 mM KPi. Adjust the cell density to 1OD600/mL.
Load 100 μL bacteria suspensions onto a 96-well plate reader. Measure the CR fluorescence with a Spectra max M2 plate reader as described above.
3.1.3. Interbacterial Complementation (see Note 11)
Unpolymerized CsgA secreted out by an E. coli csgB mutant assemble into amyloid fibers on the surface of an adjacent E. coli csgA mutant that is expressing the nucleator protein CsgB, a process called inter-bacterial complementation (15). Interbacterial complementation provides a tool for analyzing the nucleation process during curli assembly. In this section, we define the E. coli csgB mutant that secretes out unpolymerized CsgA as the donor strain and the csgA mutant that expresses CsgB on the cell surface as the recipient strain.
To test the ability of E. coli mutants to nucleate CsgA secreted by the donor, streak out the donor from the top of the YESCA CR plate to the bottom and then streak the putative acceptor strains perpendicularly across the donor streak. Use an E. coli csgA mutant as a positive control. Incubate the plate at 26°C for 48 h. Bacteria that are able to accept CsgA from the donor and convert them to curli fibers stain red on CR plate when cross-streaked.
To analyze the secretion of CsgA or the properties of CsgA protein variants, streak out the recipient from the top of the YESCA CR plate to the bottom and cross-streak the putative donor strains perpendicularly over the recipient. Use an E. coli csgB mutant as the positive control. Incubate the plate at 26°C for 48 h. Bacteria donating functional CsgA stain red after cross-streaked over the recipient (see Notes 12 and 13).
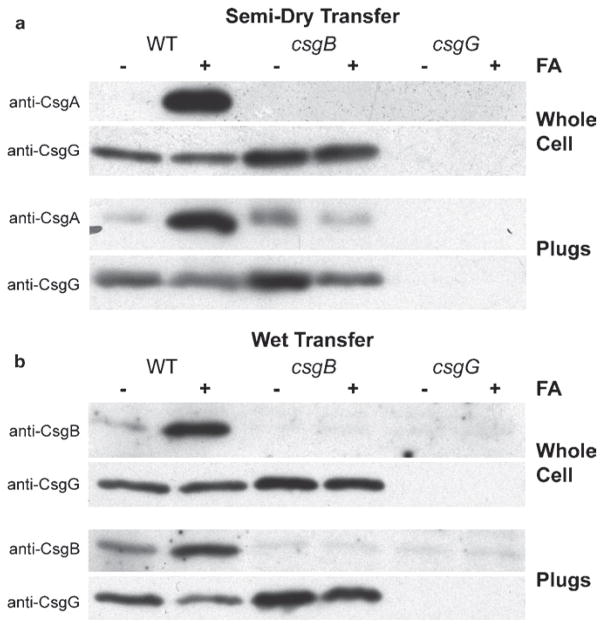
3.2. Western Blot Analysis of the Whole Cell Lysate (Fig. 4)
Fig. 4.
Western blot analysis of whole cell lysates and agar plugs. (a) Western blots of whole cell extracts and plugs of E. coli strains (36) that were transferred onto PVDF membranes and probed with polyclonal anti-CsgA antibodies. The csgB mutant strain did not produce curli fibers and soluble secreted CsgA was found in the agar plug. (b) Western blots of whole cell and plugs of E. coli strains (36) that were transferred onto nitrocellulose membranes and probed with polyclonal anti-CsgB antibodies. A strain lacking CsgG results in no CsgA or CsgB in whole cells or in the agar analysis.
Western blotting provides another way to study curli assembly. Curli fibers are resistant to SDS. HFIP/FA treatment of curli releases monomeric CsgA and CsgB that mobilize into the SDS–PAGE and can be probed by anti-CsgA or anti-CsgB antibodies, respectively.
Grow E. coli strains on the YESCA agar plate at 26°C for 48 h.
Scrape off bacteria cells from the YESCA agar using a sterile inoculating loop and suspend them in 1 mL 50 mM KPi. Normalize cells by optical cell density at 600 nm to 1OD600/mL.
Transfer 150 μL of normalized cell suspension into each of two new tubes per strain under analysis, one for HFIP/FA treatment and the other for the SDS only control.
Spin down bacteria cells at 16,000 × g for 3 min and remove the supernatant. Cell-associated curli fibers and protein aggregates should pellet.
To depolymerize curli fibers into monomers, resuspend one of the duplicate pellets in 70 μL 100% HFIP or FA briefly (see Notes and 14).
Immediately dry samples in a Speedvac at 45°C for 30 min (see Note 14).
Resuspend all pellets in 150 μL 2× SDS loading buffer. FA-treated samples may contain residual acid turning the sample buffer yellow. Adjust the pH by the addition of 1 μL at a time of 5 N NaOH until the loading buffer turns blue again. Boil samples at 95°C for 10 min (see Note 15).
Run 5–7 μL of each samples on a 15% SDS–polyacrylamide gel at 25 mA per gel.
Semidry transfer: Use for CsgA western blotting. After electrophoresis, transfer proteins from the SDS–polyacrylamide gel to a PVDF membrane by semidry transfer system. Each blotting stack should be made of three sheets of Whatman paper, the gel, a PVDF membrane followed by another three sheets of Whatman paper. The PVDF membrane needs to be pretreated with methanol, water, and transfer buffer. Transfer for 20 min at 10 V (see Note 16).
Wet transfer: Use for CsgB, which does not efficiently transfer using the semidry transfer system, probably due to its high pI. Use a similar blotting stack as it is for the semidry transfer, except use a nitrocellulose membrane instead of PVDF and use the wet transfer buffer, pH 11.2. Transfer in a wet transfer system for at 50 V for 3 h or 12 V overnight at 4°C (see Note 16).
Block the western blot with blocking solution for at least 1 h at RT or at 4°C overnight.
Probe the western blots with 1:10,000 diluted anti-CsgA or 1:7,000 diluted anti-CsgB polyclonal antibodies in blocking buffer at RT for 1 h (see Note 6).
Wash the blot with TBS-T and then probe with anti-rabbit secondary antibody at a dilution of 1:10,000 in blocking buffer at RT for 1 h.
Develop blot with chemiluminescent substrates.
3.3. Plug Western Blot Analysis (Fig. 4)
Western blotting of whole cell lysate is a powerful tool to study cell-associated fibers. However, it has its drawbacks. If no CsgA is detected by FA/HFIP treatment, it is possible that CsgA or CsgA mutants are secreted into the agar in an unpolymerized form. It is also likely that non-cell-associated fibers are formed in the agar, or CsgA is not expressed or is degraded. The plug western blot analysis provides an approach to distinguish between those possibilities.
Inoculate single colonies of E. coli strains to LB liquid broth and grow at 37°C overnight with shaking.
Equilibrate the overnight cultures by OD600.
Spot 4 μL of normalized cultures onto a thin YESCA agar plate.
Grow bacteria on the thin YESCA plate at 26°C for 48 h.
Use the wide end of a Pasteur pipette as a cookie cutter-like tool to remove an 8-mm circular plug including the bacteria and the underlying agar. Collect 2 plugs for each strain: one for HFIP/FA treatment and the other as an SDS only control. Put each plug into a 1.5-mL microcentrifuge tube.
For the HFIP/FA treatment group, suspend the plug in 100 μL HFIP or FA and briefly vortex. Dry the samples in a Speedvac at 45°C for 40 min, and then resuspend the pellets with 2× SDS loading buffer.
For the nontreated plugs, suspend plugs in 150 μL 2× SDS loading buffer.
Sonicate both HFIP/FA-treated samples and nontreated samples in a water-bath sonicator for 3 min (see Note 17).
Adjust the sample pH to neutral by 1 or 2 μL of 5 N NaOH if the SDS loading buffer turns yellow. Heat the samples at 95°C for 10 min before loading onto a 15% SDS–polyacrylamide gel (see Note 15).
Electrophorese the samples, transfer proteins to a PVDF membrane for detecting CsgA or nitrocellulose for detecting CsgB, probe, and develop the blot as described in Subheading 3.2.
3.4. CsgA Purification and Overlay Assay
There are two CsgA purification schemes in the published literature (see Note 18). In one of the approaches, both CsgA and CsgG are overexpressed in the absence of all other curli proteins. CsgA is secreted into the supernatant and is collected and purified by nickel affinity chromatography (15, 31). In an alternative purification method, CsgA without the Sec signal sequence can be purified from the cytoplasm using a denaturing protocol (10). C-terminal His6-tagged CsgA is referred as “CsgA” in this section.
3.4.1. CsgA Purified from Cell Supernatants (see Note 19)
Perform the purification in a 4°C cold room or a 4°C fridge. Store the columns and buffers at 4°C unless otherwise stated.
Start an overnight culture of LSR12/pMC1/pMC3 in 25 mL LB-Amp-Cm per 1 L of desired prep at 37°C.
Dilute the overnight culture into 1 L LB-Amp-Cm and incubate with shaking at 37°C to OD600 of ~1.0.
Induce with 0.25 mM IPTG and incubate with shaking for 45 min at 37°C.
Centrifuge the culture at 10,000 × g for 15 min at 4°C. Collect the supernatant.
Shift purification to 4°C.
Filter the supernatant over a 0.22-μm polyethersulfone bottle-top filter (see Note 20).
Prepare a column with 4 mL of Ni-NTA bead volume. Wash the column with 4 bed volumes (BV) of 50 mM KPi, pH 7.2.
Flow the filtrates over the column set to the maximum flow rate (see Note 21).
Wash the column with 10BV of 50 mM KPi, pH 7.2.
Elute the protein off the column with 2BV of 100 mM imidazole in 50 mM KPi, pH 7.2. Adjust the flow rate to 20 drops/ min. Collect elutes in 1 mL fractions and store in microcentrifuge tubes.
Combine fractions containing CsgA as measured by BCA in a 15-mL conical tube.
Equilibrate a Sephadex G-25 gel filtration column with 25 mL 50 mM KPi, pH 7.2.
Load 3–4 mL of protein onto the column. Elute 1 mL fractions into microcentrifuge tubes using 50 mM KPi, pH 7.2. Measure the protein concentration of each fraction by UV280 or BCA assay at RT. Combine the fractions that contain most of CsgA proteins, filter through a 0.02-μm Anotop filter, and measure the final concentration. Store on ice (see Notes 22 and 23).
3.4.2. CsgA Purified from Cell Lysates (see Note 19)
Start an overnight culture of NEB C2566/pNH11 in 10 mL LB Amp-Kan-Cm per 500 mL of desired prep at 37°C.
Dilute the overnight culture into 500 mL LB-Amp and incubate with shaking at 37°C to an OD600 of ~1.0.
Induce with 0.5 mM IPTG and incubate for 1 h at 37°C.
Collect bacteria by centrifugation at 5,000 × g for 20 min and discard the supernatant. The pellet can be stored at −80°C for future use.
Lyse bacterial cells by adding 50 mL 8 M GdnHCl to the pellet and incubate at 4°C overnight with stirring.
Centrifuge the GdnHCl bacterial suspension at 10,000 × g for 20 min to remove the insoluble portion of the lysate.
Incubate the resulting supernatant with Ni-NTA resin for 1 h at RT with rocking.
Load the supernatant with Ni-NTA onto a disposable polypro-pylene column.
Wash the beads with 10BV 50 mM KPi, pH 7.2 followed by 3BV 12.5 mM imidazole in 50 mM KPi, pH 7.2 to remove nonspecific bound proteins.
Elute the protein off the column with 6BV 125 μM imidazole in 50 mM KPi, pH 7.2. Collect 1 mL of elutes for each fraction in 1.5-mL microcentrifuge tubes. Store fractions on ice (see Note 22).
Measure the protein concentration from each fraction by BCA assay. Combine the fractions with proteins and load onto a 30 kDa centrifugal filter unites. Centrifuge at 7,500 × g for 10 min (see Note 24). The bottom part of the filter units contains CsgA monomers.
Prewash a spin desalt column with 50 mM KPi by centrifugation at 1,000 × g for 2 min. Collect proteins from the bottom part of the 30 kDa filter units and load onto the desalting column. Centrifuge at 1,000 × g for 2 min (see Note 24).
Store purified protein on ice (see Note 22). Measure protein concentration by BCA assay or UV280.
3.4.3. Overlay Assay
Soluble amyloid proteins polymerize into amyloid fibers and this polymerization can be accelerated by its own fibers, a process known as seeding. The polymerization and seeding can be monitored in vitro by Thioflavin T fluorescence or EM. The overlay assay provides a mechanism to test the assembly of bacterial amyloids directly on the cell surface. It can be used to determine the amyloidogenic domains of bacterial amyloid proteins (28).
Spread a lawn of 50 μL desired bacterial suspension on the YESCA plate by sterile inoculation loop and incubate for 2 days at 26°C. Use E. coli K-12 csgA and csgBA mutant as the positive control and negative control, respectively.
Drip 10 μL of freshly purified CsgA on the bacterial lawn. Incubate at RT for 10 min.
Stain the plate with 10 mL of 0.5 mg/mL CR at RT for 5 min then wash with 50 mM KPi, pH 7.2.
Observe the CR stain at the spot where CsgA proteins were spotted. csgA mutant with CsgA proteins should strain red, while csgBA mutant with CsgA proteins will remain white (Fig. 2a). Assembly of curli fibers on bacterial surfaces can also be verified by electron microscopy (EM) (Fig. 2b, c).
Acknowledgments
We thank members of the Chapman laboratory for helpful discussions and review of this manuscript. This work was supported by the National Institutes of Health Grant AI073847.
Footnotes
Other than YESCA agar, T-agar (16), colonization factor antigen (CFA) agar (20), and LB agar without salt (32) are also used to induce curli production of Salmonella spp. and E. coli. LB agar with no salt contains 5 g/L yeast extract, 10 g/L bacto tryptone, and 1.5% agar.
Mix CR with water by stirring for at least an hour. Make sure CR is completely dissolved before filter sterilizing.
Brilliant blue is used to increase the color contrast of colonies on CR agar.
HFIP is a strong solvent that disaggregates curli subunits and does not result in acid hydrolysis. FA is also used to disassociate curli subunits. FA and HFIP are corrosive and cause burns. They evaporate at room temperature. Store FA and HFIP in a cool, well-ventilated place. Always use them under a chemical fume hood and wear appropriate protective equipment.
Here we use high-pH CAPS buffer because the CsgB protein has a high pI and cannot be transferred efficiently with normal buffer.
1:10,000 dilution rate of anti-CsgA antibody or 1:7,000–1:10,000 of anti-CsgB antibody gives good signal for western blotting. High concentration of antibody is not recommended as it may cause nonspecific binding. The anti-CsgA antibody also binds to an unknown nonspecific protein that migrates at around 40 kDa on SDS–PAGE.
For CR staining and fluorescence quantification, it is recommended to grow a curliated wild-type strain as a positive control and curli defective mutant such as a csgA mutant or a csgB mutant as a negative control.
Temperature and incubation time are important factors for CR assays. Most of the E. coli K-12 and Salmonella spp. lab strains produce curli at 26°C, whereas some clinical isolates produce curli at 37°C (33, 34). Although E. coli K-12 lab strains grown at 37°C do not produce curli, they may eventually take up CR and stain red. Incubation for longer than 2 days may also make bacteria take up or bind CR.
Most E. coli K-12 isolates have CR phenotypes that are completely dependent on curli fiber production. However, many bacterial strains can produce cellulose or other extracellular polysaccharides that bind to CR. The corresponding curli defective mutants of those strains may form pink, smooth colonies on YESCA CR plates. CR is able to interact and emit fluorescence with cellulose. Therefore, multiple approaches are recommended for the study of amyloids produced by bacteria with complex surface structures.
A wavelength scan of CR fluorescence is required to determine the ideal excitation and emission wavelength for CR quantification for the analysis of other bacterial amyloids.
Some bacteria do not participate in interbacterial complementation. Interbacterial complementation is not detected between S. enterica serovar Enteritidis 3b csgA and csgB mutants, probably due to the lipopolysaccharide O polysaccharide (35).
The curli mutants of cellulose-positive bacteria are pink on the YESCA CR agar, which makes the detection of interbacterial complementation difficult by CR staining. As an alternative, a mixture of desired bacteria can be collected and the intercellular curli formation can be assessed by EM or western blot analysis as mentioned in Subheadings 3.2 and 3.3 (Fig. 1c, d).
Another way to test interbacterial complementation is to streak the donor or recipient on the YESCA plate and, then make a parallel streak 3 mm away from the first streak. Incubate the plate at 26°C for 48 h (Fig. 21.1b). The recipient cells facing to donor stain red if intercellular curli are formed.
FA is capable of hydrolyzing proteins including curli subunits. Long treatment with FA may result in target protein loss. Speed-dry samples immediately after the FA treatment or use HFIP instead.
Make sure that samples are thoroughly dried after speed-vac step. If there is still liquid FA or HFIP left in the tube after 30 min in the Speedvac, dry the sample for longer. The remaining FA in bacterial pellets or plugs lowers the pH and turns the SDS-sample buffer yellow. The low pH of samples affects electrophoresis and boiling low pH sample increases the chance of acid hydrolysis. It is recommended to adjust the pH of sample buffer back to around pH 7 before loading samples onto SDS–PAGE.
We use the semidry transfer apparatus from FisherBiotech and a wet transfer system from Biorad. The voltage and transfer time may vary for different transfer systems.
We found that water bath sonication makes the results for the plug assay more consistent, probably by breaking up large chunks of dried agar.
Both CsgA purification approaches have advantages and drawbacks. Purification of CsgA from bacterial supernatant does not required denaturation of the target protein. However, the whole process is time consuming and typically takes 2 days. The denaturing method takes only half a day, but the final yield is typically lower. CsgA proteins purified by both methods have the same biochemical properties and form fibers with same morphologies and with similar kinetics.
Collect ~100 μL of sample during each of the purification steps (e.g., the load, the wash, and elutes) and store on ice. Run an SDS–PAGE of the samples after purification to make sure that the protein is pure. Also, an expression test is required for new expression strains to make sure that protein is expressed in the tested conditions.
To remove bacteria that remain in the supernatant after centrifugation, filter the supernatant through the 0.22-μm filter. Filtered supernatant can be stored at 4°C overnight. However, longer storage is not recommended.
Depending on the column size and the particulates in the supernatant, it can take upwards of 10 h to gravity flow 1 L of supernatant through the nickel affinity column. Use of high-flow Ni-NTA and addition of a powder funnel on top of the column both greatly accelerate the loading process.
Purified CsgA forms fibers at RT in 1–2 h, so it is important to keep the protein on ice to slow the aggregation process. Start the overlay assay immediately after the purification. CsgA cannot be stored at −20°C or −80°C because CsgA precipitates out of solution during the thawing process.
To purify CsgA from cell supernatants under denaturing conditions, equilibrate the Ni-NTA gel with 5BV 8 M GdHCl in 10 mM KPi, pH 7.2 at RT after supernatant binding and washing with 5BV 10 mM KPi, pH 7.2. Elute with 3BV 8 M GdHCl in 50 mM KPi, pH 2. Use BCA assay or UV280 to detect and pool CsgA-containing fractions, which can be stored at RT for several days. Desalt using Sephadex G-25 at RT using cold columns and 4°C 50 mM KPi, pH 7.2.
Check the manuals that accompany the 30 kDa centrifuge units and desalting columns. The speed and time for centrifugation may vary from product to product.
References
- 1.Cooper GJ, Willis AC, Clark A, Turner RC, Sim RB, Reid KB. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc Natl Acad Sci USA. 1987;84:8628–8632. doi: 10.1073/pnas.84.23.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebro-vascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- 3.Prusiner SB. Molecular biology and pathogenesis of prion diseases. Trends Biochem Sci. 1996;21:482–487. doi: 10.1016/s0968-0004(96)10063-3. [DOI] [PubMed] [Google Scholar]
- 4.Barnhart MM, Chapman MR. Curli biogenesis and function. Annu Rev Microbiol. 2006;60:131–147. doi: 10.1146/annurev.micro.60.080805.142106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gebbink MF, Claessen D, Bouma B, Dijkhuizen L, Wosten HA. Amyloids--a functional coat for microorganisms. Nat Rev Microbiol. 2005;3:333–341. doi: 10.1038/nrmicro1127. [DOI] [PubMed] [Google Scholar]
- 6.Elliot MA, Karoonuthaisiri N, Huang J, Bibb MJ, Cohen SN, Kao CM, Buttner MJ. The chaplins: a family of hydrophobic cell-surface proteins involved in aerial mycelium formation in Streptomyces coelicolor. Genes Dev. 2003;17:1727–1740. doi: 10.1101/gad.264403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tukel C, Nishimori JH, Wilson RP, Winter MG, Keestra AM, van Putten JP, Baumler AJ. Toll-like receptors 1 and 2 cooperatively mediate immune responses to curli, a common amyloid from enterobacterial biofilms. Cell Microbiol. 2010 doi: 10.1111/j.1462-5822.2010.01485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gophna U, Barlev M, Seijffers R, Oelschlager TA, Hacker J, Ron EZ. Curli fibers mediate internalization of Escherichia coli by eukaryotic cells. Infect Immun. 2001;69:2659–2665. doi: 10.1128/IAI.69.4.2659-2665.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Austin JW, Sanders G, Kay WW, Collinson SK. Thin aggregative fim-briae enhance Salmonella enteritidis biofilm formation. FEMS Microbiol Lett. 1998;162:295–301. doi: 10.1111/j.1574-6968.1998.tb13012.x. [DOI] [PubMed] [Google Scholar]
- 10.Cegelski L, Pinkner JS, Hammer ND, Cusumano CK, Hung CS, Chorell E, Aberg V, Walker JN, Seed PC, Almqvist F, Chapman MR, Hultgren SJ. Small-molecule inhibitors target Escherichia coli amyloid biogenesis and biofilm formation. at Chem Biol. 2009;5:913–919. doi: 10.1038/nchembio.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romero D, Aguilar C, Losick R, Kolter R. Amyloid fibers provide structural integrity to Bacillus subtilis biofilms. Proc Natl Acad Sci USA. 2010;107:2230–2234. doi: 10.1073/pnas.0910560107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vidal O, LR, Prigent-Combaret C, Dorel C, Hooreman M, Lejeune P. Isolation of an Escherichia coli K-12 mutant strain able to form biofilms on inert surfaces: involvement of a new ompR allele that increases curli expression. J Bacteriol. 1998;180:2442–2449. doi: 10.1128/jb.180.9.2442-2449.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johansson C, Nilsson T, Olsen A, Wick MJ. The influence of curli, a MHC-I-binding bacterial surface structure, on macrophage-T cell interactions. FEMS Immunol Med Microbiol. 2001;30:21–29. doi: 10.1111/j.1574-695X.2001.tb01545.x. [DOI] [PubMed] [Google Scholar]
- 14.Tükel CRM, Humphries AD, Wilson RP, Andrews-Polymenis HL, Gull T, Figueiredo JF, Wong MH, Michelsen KS, Akçelik M, Adams LG, Bäumler AJ. CsgA is a pathogen-associated molecular pattern of Salmonella enterica serotype Typhimurium that is recognized by Toll-like receptor 2. Mol Microbiol. 2005;58:289–304. doi: 10.1111/j.1365-2958.2005.04825.x. [DOI] [PubMed] [Google Scholar]
- 15.Chapman MR, Robinson LS, Pinkner JS, Roth R, Heuser J, Hammar M, Normark S, Hultgren SJ. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science. 2002;295:851–855. doi: 10.1126/science.1067484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collinson SK, Emody L, Muller KH, Trust TJ, Kay WW. Purification and characterization of thin, aggregative fimbriae from Salmonella enteritidis. J Bacteriol. 1991;173:4773–4781. doi: 10.1128/jb.173.15.4773-4781.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Claessen D, Rink R, de Jong W, Siebring J, de Vreugd P, Boersma FG, Dijkhuizen L, Wosten HA. A novel class of secreted hydrophobic proteins is involved in aerial hyphae formation in Streptomyces coeli-color by forming amyloid-like fibrils. Genes Dev. 2003;17:1714–1726. doi: 10.1101/gad.264303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Jong W, WH, Dijkhuizen L, Claessen D. Attachment of Streptomyces coelicolor is mediated by amyloidal fimbriae that are anchored to the cell surface via cellulose. Mol Microbiol. 2009;73:1128–1140. doi: 10.1111/j.1365-2958.2009.06838.x. [DOI] [PubMed] [Google Scholar]
- 19.Shewmaker F, MR, Thurber KR, McPhie P, Dyda F, Tycko R, Wickner RB. The functional curli amyloid is not based on in-register parallel beta-sheet structure. J Biol Chem. 2009;284:25065–25076. doi: 10.1074/jbc.M109.007054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collinson SK, Doig PC, Doran JL, Clouthier S, Trust TJ, Kay WW. Thin, aggregative fimbriae mediate binding of Salmonella enteritidis to fibronectin. J Bacteriol. 1993;175:12–18. doi: 10.1128/jb.175.1.12-18.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collinson SK, Parker JM, Hodges RS, Kay WW. Structural predictions of AgfA, the insoluble fimbrial subunit of Salmonella thin aggregative fimbriae. J Mol Biol. 1999;290:741–756. doi: 10.1006/jmbi.1999.2882. [DOI] [PubMed] [Google Scholar]
- 22.Zogaj X, Bokranz W, Nimtz M, Romling U. Production of cellulose and curli fimbriae by members of the family Enterobacteriaceae isolated from the human gastrointestinal tract. Infect Immun. 2003;71:4151–4158. doi: 10.1128/IAI.71.7.4151-4158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olsen A, Jonsson A, Normark S. Fibronectin binding mediated by a novel class of surface organelles on Escherichia coli. Nature. 1989;338:652–655. doi: 10.1038/338652a0. [DOI] [PubMed] [Google Scholar]
- 24.Loferer H, Hammar M, Normark S. Availability of the fibre subunit CsgA and the nucleator protein CsgB during assembly of fibronectin-binding curli is limited by the intracellular concentration of the novel lipoprotein CsgG. Mol Microbiol. 1997;26:11–23. doi: 10.1046/j.1365-2958.1997.5231883.x. [DOI] [PubMed] [Google Scholar]
- 25.Hammer ND, Schmidt JC, Chapman MR. The curli nucleator protein, CsgB, contains an amyloidogenic domain that directs CsgA polymerization. Proc Natl Acad Sci USA. 2007;104:12494–12499. doi: 10.1073/pnas.0703310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hammar M, AA, Bian Z, Olsén A, Normark S. Expression of two csg operons is required for production of fibronectin- and congo red-binding curli polymers in Escherichia coli K-12. Mol Microbiol. 1995;18:661–670. doi: 10.1111/j.1365-2958.1995.mmi_18040661.x.. [DOI] [PubMed] [Google Scholar]
- 27.Weiss-Muszkat M, Shakh D, Zhou Y, Pinto R, Belausov E, Chapman MR, Sela S. Biofilm formation by and multicellular behavior of Escherichia coli O55:H7, an atypical enteropathogenic strain. Appl Environ Microbiol. 2010;76:1545–1554. doi: 10.1128/AEM.01395-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Chapman MR. Sequence determinants of bacterial amyloid formation. J Mol Biol. 2008;380:570–580. doi: 10.1016/j.jmb.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Hammer ND, Chapman MR. The molecular basis of functional bacterial amyloid polymerization and nucleation. J Biol Chem. 2008;283:21530–21539. doi: 10.1074/jbc.M800466200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Zhou Y, Ren JJ, Hammer ND, Chapman MR. Gatekeeper residues in the major curlin subunit modulate bacterial amyloid fiber biogenesis. Proc Natl Acad Sci USA. 2010;107:163–168. doi: 10.1073/pnas.0908714107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Smith DR, Jones JW, Chapman MR. In vitro polymerization of a functional Escherichia coli amyloid protein. J Biol Chem. 2007;282:3713–3719. doi: 10.1074/jbc.M609228200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Werner Bokranz XW, Tschäpe Helmut, Römling Ute. Expression of cellulose and curli fimbriae by Escherichia coli isolated from the gastrointestinal tract. J Med Microbiol. 2005;54:1171–1182. doi: 10.1099/jmm.0.46064-0. [DOI] [PubMed] [Google Scholar]
- 33.Kikuchi T, Mizunoe Y, Takade A, Naito S, Yoshida S. Curli fibers are required for development of biofilm architecture in Escherichia coli K-12 and enhance bacterial adherence to human uroepithelial cells. Microbiol Immunol. 2005;49:875–884. doi: 10.1111/j.1348-0421.2005.tb03678.x. [DOI] [PubMed] [Google Scholar]
- 34.Bian Z, Brauner A, Li Y, Normark S. Expression of and cytokine activation by Escherichia coli curli fibers in human sepsis. J Infect Dis. 2000;181:602–612. doi: 10.1086/315233. [DOI] [PubMed] [Google Scholar]
- 35.White AP, GD, Collinson SK, Banser PA, Kay WW. Extracellular polysaccharides associated with thin aggregative fimbriae of Salmonella enterica serovar enteritidis. J Bacteriol. 2003;185:5398–5407. doi: 10.1128/JB.185.18.5398-5407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol. 2006;2 doi: 10.1038/msb4100050. 2006 0008. [DOI] [PMC free article] [PubMed] [Google Scholar]