Microglia, resident immune cells of the brain, together with other glia populations (such as astrocytes, oligodendrocytes) form a very dynamic network which ensure the correct environment necessary for brain development and to maintain an homeostatic environment in the brain after birth. In addition, thoughout life, the neuronal population in the brain suffers from multiple different challenges that require microglia to react to and to execute different roles. In the context of diseases, activation of microglia can contribute to rather contrasting effects; either promoting neuronal cell death in the case of neurodegenerative diseases where chronic inflammation plays an important role during the demise of neuronal cell populations such as in Alzheimer disease and Parkinson disease, or promoting an environment that allows tumor cell growth and invasiveness in the case of glioma.1,2 Microglia cells are extremely plastic, and undergo a variety of structural and functional changes based on their location and current role.3 Despite the importance of microglia in the maintenance of CNS homeostasis and the pathogenesis of neurodegenerative diseases and brain tumors, the molecular mechanisms behind their polarization toward selective phenotypes are still unclear.
Caspase-3, member of family of cysteinyl aspartate-specific proteases, is best known as an executioner of apoptotic cell death and its activation is considered to be a commitment to cell death. However, in the last years, other roles for this and other “executioner” caspases have been uncovered including an unexpected novel function for this caspase in regulating microglia pro-inflammatory activation and associated neurotoxicity.4 Different pro-inflammatory stimulants in microglia cells induce the orderly activation of caspase-8, and thereafter caspase-3. In turn, active caspase-3 promotes, through a protein kinase-Cδ-dependent pathway, the pro-inflammatory activation of microglia in the absence of cell death.5 Knockdown or chemical inhibition of each of these caspases hindered the pro-inflammatory activation of microglia. Moreover, activation of these caspases was reported in microglia in the ventral mesencephalon of Parkinson disease and the frontal cortex of Alzheimer disease subjects.
A central question emerging from this finding is what prevents caspase-3 during the microglia activation from killing those cells? Caspase-3 activation occurs as a 2-step process, where the zymogen is first cleaved by upstream caspases, such as caspase-8, to form intermediate, yet still active, cytoplasmic p19/p12 complex; thereafter, autocatalytic processing generates the fully mature p17/p12 form of the enzyme which can relocate to the nuclear compartment. The induction of cellular inhibitor of apoptosis protein 2 (cIAP2) expression upon microglia activation prevents the conversion of caspase-3 p19 subunit to p17 subunit and is responsible for restraining caspase-3 in terms of activity and subcellular localization. Counteracting the repressive effect of cIAP2 on caspase-3 activation reduced the pro-inflammatory activation of microglia cells and promoted their death.6
Recently a new role for caspase-3 was reported in the control of microglia activation in the context of glioma expansion. Inhibition of basal caspase-3 activity in microglia was found to be associated with microglia polarization toward a tumor-supportive phenotype. Indeed, glioma-induced microglia conversion is coupled to a reduction of basal microglial caspase-3 activity and increased S-nitrosylation of mitochondria-associated caspase-3 through inhibition of thioredoxin-2 activity. Compelling evidence, including the generation of mice with specific ablation of microglial caspase-3, demonstrate that caspase-3 inhibition regulates microglial tumor-supporting function. Furthermore, nitric oxide synthase-2 (NOS2) activity originating from the glioma cells was identified as a driving stimulus in the control of microglial caspase-3 activity. Repression of glioma NOS2 expression in vivo led to reduction in both microglia recruitment and tumor expansion, whereas depletion of the microglial caspase-3 gene promoted tumor growth.7
These investigations uncover a key role for caspase-3 in the regulation of microglia phenotypes.5,7 Caspase-3 may work as a rheostat which controls the microglial cell fate in response to diverse stimuli, where elevated activity of the protease leads to cell death, but low activity and reduced basal caspase-3 activity regulate, respectively, the pro-inflammatory and the tumor-supporting microglial activation states (Fig. 1). Despite “killer” caspase-3 not being involved in the dismiss of the microglia, those caspase-3-dependent signaling pathways leading to pro-inflammatory or tumor-supporting activation of these cells are guilty by association for exacerbating their neurotoxicity or ability to favors tumor progression.
Figure 1.
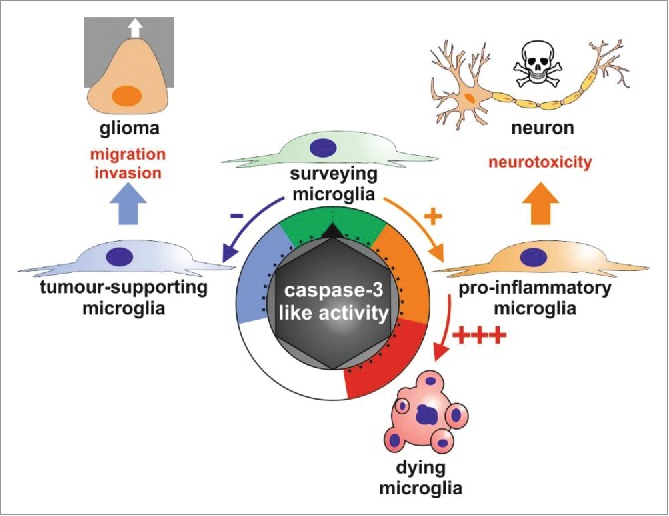
Schematic representation describing the role of caspase-3-like activity as a rheostat regulating microglia polarization. The microglial activation states range from the surveying phenotype with a basal caspase-3 like activity, the tumor supporting phenotype with protease activity lower than the basal one, and the pro-inflammatory phenotype exhibiting a modest but significant induction for caspase-3 like activity. Robust induction of this caspase-3 like activity can lead to microglial cell death.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Ransohoff RM. How neuroinflammation contributes to neurodegeneration. Science 2016; 353:777-83; PMID:27540165; http://dx.doi.org/ 10.1126/science.aag2590 [DOI] [PubMed] [Google Scholar]
- [2].Hambardzumyan D, Gutman DH, Kettenmann H. The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci 2016; 19:20-7; PMID:26713745; http://dx.doi.org/ 10.1038/nn.4185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Shemer A, Erny D, Jung S, Prinz M. Microglia plasticity during health and disease: An immunological perspective. Trends Immunol 2015; 36:614-24; PMID:26431939; http://dx.doi.org/ 10.1016/j.it.2015.08.003 [DOI] [PubMed] [Google Scholar]
- [4].Venero JL, Burguillos MA, Brundin P, Joseph B. The executioners sing a new song: killer caspases activate microglia. Cell Death Differ 2011; 18:1679-91; PMID:21836616; http://dx.doi.org/ 10.1038/cdd.2011.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Burguillos MA, Deierborg T, Kavanagh E, Persson A, Hajji N, Garcia-Quintanilla A, Cano J, Brundin P, Englund E, Venero JL, et al. Caspase signalling controls microglia activation and neurotoxicity. Nature 2011; 472:319-24; PMID:21389984; http://dx.doi.org/ 10.1038/nature09788 [DOI] [PubMed] [Google Scholar]
- [6].Kavanagh E, Rodhe J, Burguillos MA, Venero JL, Joseph B. Regulation of caspase-3 processing by cIAP2 controls the switch between pro-inflammatory activation and cell death in microglia. Cell Death Dis 2014; 5:e1565; PMID:25501826; http://dx.doi.org/ 10.1038/cddis.2014.514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Shen X, Burguillos MA, Osman AM, Frijhoff J, Carrillo-Jiménez A, Kanatani S, Augsten M, Saidi D, Rodhe J, Kavanagh E, et al. Glioma-induced inhibition of caspase-3 in microglia promotes a tumor-supportive phenotype. Nat Immunol 2016; 17:1282-90; PMID:27618552; http://dx.doi.org/ 10.1038/ni.3545 [DOI] [PubMed] [Google Scholar]


