Although the majority of studies on cancer origin have pointed to the identification/characterization of driver genetic lesions, a major breakthrough is emerging, that is, profound biochemical/metabolic alterations occur in cancers. Importantly, these changes are not simply due to the need of microenvironment adaptation, but are associated to carcinogenesis/metastatization processes. Their unraveling will result crucial for developing new metabolism-targeting drugs and may hold promises for treating patients.
About ninety years ago, a link between metabolism and cancer was revealed1: essentially, increased glucose utilization through aerobic glycolysis (the Warburg effect) distinguishes cancer from normal cells. Even if aerobic glycolysis results in a minor ATP production from glucose, it fuels cells with three-carbon moieties for synthetic pathways (including serine and purine production). For long time, aerobic glycolysis has remained the main metabolic characteristic attributed to cancer cells, together with an increase in glutamine uptake and utilization. However, after a period of silence, the investigation resurgence into cancer metabolism has demonstrated significant rewiring of the main metabolic cellular pathways to fulfill the high energy and biomass cancer cell requirements. The role of mitochondria came on stage, in that other important metabolic pathways, occurring in mitochondria or at the intercross with the cytoplasm, resulted as playing a fundamental role in the metabolic profile of, at least, some type of cancer cells.2
Cancer mass is extraordinary heterogeneous in cell composition, although in a manner depending on cancer type and, probably, on the specific genetic lesion. For simplicity, in a single cancer we can distinguish highly proliferating cancer cells (frequently intrinsically genetically dishomogeneous) and cancer stem cells (CSCs) or tumor-initiating cells. CSCs represent a small population with self-renewal ability, slow proliferating rate, resistance to chemotherapy and radiotherapy and involvement in tumor relapse.
A number of data indicates that fast-growing relatively differentiated cancer cells are strongly dependent on aerobic glycolysis (the “Warburg Effect”). On the other hand, CSCs rely on a wide array of metabolic profile, exhibiting an extremely low3 or a relatively high2 content of mitochondria, the latter scenario implying high levels of oxidative phosphorylation. Altogether, these findings strongly indicate that a straightforward knowledge of cancer metabolism represents a central issue for a rational tumor therapy.
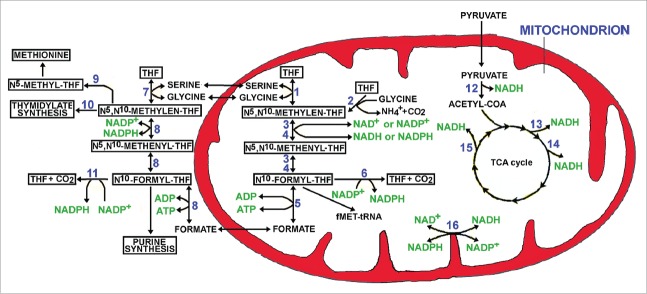
Although several metabolic alterations have been identified, the fate of glutamine in cancer seems strongly different from that occurring in normal cells, as probable consequence of specific enzyme pattern and genetic or epigenetic changes.4 However, data supporting an epigenetic effect of glutamine (and its metabolites) has been reported and, at present, this still appears as the question of “the chicken and the egg.” In addition, the metabolism of one-carbon moiety is of extreme interest being mainly localized in mitochondria, organelles frequently abundant in CSCs.5 Several actors have a role in this story: namely serine, glycine, formate, folate/tetrahydrofolate (F/THF), NADH/NAD+ and NADPH/NADP+. In addition, a number of important cytosolic pathways are involved, including the de novo purine biosynthesis, the thymidilate formation, the methionine cycle and others. Figure 1 summarizes the interplay between cytosol and mitochondrion, pointing in particular to THF pathway. It might be hypothesized that in CSCs, which live in a hypoxic microenvironment, significant metabolic alterations might occur. As an example, mitochondrial NADH (deriving from pyruvate dehydrogenase complex activity and Krebs cycle) might be converted into NADPH as consequence of the proton gradient (only partially used for ATP synthesis due to hypoxia) and nicotinamide nucleotide transhydrogenase (NNT) activity.6 The accumulation of NADPH might hamper a normal one-carbon moiety metabolism and the mitochondrial production of formate, necessary for cytoplasmic purine biosynthesis (Fig. 1).
Figure 1.
Cytosolic and mitochondrial tetrahydrofolate (THF) metabolism. The numbers in the figure correspond to the following enzymes: 1) SHMT2, serine hydroxymethyltransferase 2; 2) GLDC, Glycine dehydrogenase (decarboxylating), mitochondrial; 3) MTHFD2 methylenetetrahydrofolate dehydrogenase (NADP+ dependent) 2 (expressed in normal cells and employing both NAD+ and NADP+); 4) MTHFD2L, MTHFD2-like (employing mostly NAD+) and expressed predominantly in cancer cells; 5) MTHFD1L, formyltetrahydrofolate synthetase; 6) ALDH1L2, Aldehyde Dehydrogenase 1 Family Member L2; 7) SHMT1 serine hydroxymethyltransferase 1; 8) MTHD1, trifunctional enzyme with the following activityies methylenetetrahydrofolate dehydrogenase, methenyltetrahydrofolate cyclohydrolase and formate–tetrahydrofolate ligase; 9) MTHFR, Methylene tetrahydrofolate reductase; 10) TYMS, thymidylate synthase; 11) ALDH1L1, Aldehyde Dehydrogenase 1 Family Member L1; 12) PDC, Pyruvate dehydrogenase complex; 13) IDH3, Isocytric dehydrogenase 3; 14) KGDHC, α-ketoglutarate dehydrogenase complex; 15) MDH, Malate dehydrogenase; 16) NNT, Nicotinamide Nucleotide Transhydrogenase.
In this scenario, Cipolleschi and colleagues added new information by investigating the effects of several metabolic intermediates on the G1-S transition in a CSCs model, i.e. AH130 hepatoma cells. In a previous study, the authors demonstrated that pyruvate (the main mitochondrial source of NADH) arrests cell proliferation and increases NADPH/NADP+ ratio, while folate or purine remove this block.3 More recently, methotrexate (MTX) effect on pyruvate addition was studied.7 Surprisingly, a MTX hormetic-like effect was observed with growth stimulation at low levels and classic antiproliferative activity exerted at higher concentrations. Folate (and its derivatives) removed MTX inhibition, while the compound (but not TFH2/TFH4) enhances the low dose MTX stimulatory effect. Finally, serine and glycine hampered AH130 G1-S transition both by itself and in MTX-stimulated model. These observations suggest that the folate and MTX, under specific conditions, should be employed with caution.7 Most importantly, Cipolleschi and colleagues' studies confirm that, at least in an experimental CSC model, alterations of one-carbon metabolism (obtained by treatment with key amino- or keto-acids) result in change of nicotinamide coenzymes reduced status, affect purine synthesis and, finally cell growth. This message appears to confirm the view that CSC metabolism might represent a new and unexpected target for anticancer therapy. A novel road is now opened.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol 1927; 8:519-30; PMID:19872213; http://dx.doi.org/ 10.1085/jgp.8.6.519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Farnie G, Sotgia F, Lisanti MP. High mitochondrial mass identifies a sub-population of stem-like cancer cells that are chemo-resistant. Oncotarget 2015; PMID:26421710; 24200966:30472-86; http://dx.doi.org/ 10.18632/oncotarget.5401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cipolleschi MG, Marzi I, Santini R, Fredducci D, Vinci MC, D'Amico M, Rovida E, Stivarou T, Torre E, Dello Sbarba P, et al.. Hypoxia-resistant profile implies vulnerability of cancer stem cells to physiological agents, which suggests new therapeutic targets. Cell Cycle 2014; 13:268-78; PMID:24200964; http://dx.doi.org/ 10.4161/cc.27031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Altman BJ, Stine ZE, Dang CV. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer 2016; 16:619-34; PMID:27492215; http://dx.doi.org/ 10.1038/nrc.2016.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yang M, Vousden KH. Serine and one-carbon metabolism in cancer. Nat Rev Cancer 2016; 16:650-62; PMID:27634448 http://dx.doi.org/ 10.1038/nrc.2016.81 [DOI] [PubMed] [Google Scholar]
- [6].Gameiro PA, Laviolette LA, Kelleher JK, Iliopoulos O, Stephanopoulos G. Cofactor balance by nicotinamide nucleotide transhydrogenase (NNT) coordinates reductive carboxylation and glucose catabolism in the tricarboxylic acid (TCA) cycle. J Biol Chem 2013; 288:12967-77; PMID:23504317; http://dx.doi.org/ 10.1074/jbc.M112.396796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cipolleschi MG, Marzi I, Rovida E, Olivotto M, Dello Sbarba P. Low-dose methotrexate enhances cycling of highly anaplastic cancer cells. Cell Cycle 2017; PMID:27841718; http://dx.doi.org/ 10.1080/15384101.2016.1252883 [DOI] [PMC free article] [PubMed] [Google Scholar]