Abstract
The Family 16 methyltransferases are a group of eukaryotic nonhistone protein methyltransferases. Sixteen of these have recently been described in yeast and human, but little is known about their sequence and structural features. Here we investigate one of these methyltransferases, Saccharomyces cerevisiae elongation factor methyltransferase 2 (Efm2), by site‐directed mutagenesis and truncation. We show that an active site‐associated tryptophan, invariant in Family 16 methyltransferases and at position 222 in Efm2, is important for methyltransferase activity. A second highly conserved tryptophan, at position 318 in Efm2, is likely involved in S‐adenosyl methionine binding but is of lesser consequence for catalysis. By truncation analysis, we show that the N‐terminal 50–200 amino acids of Efm2 are critical for its methyltransferase activity. As N‐terminal regions are variable among Family 16 methyltransferases, this suggests a possible role in determining substrate specificity. This is consistent with recently solved structures that show the core of Family 16 methyltransferases to be near‐identical but the N termini to be structurally quite different. Finally, we show that Efm2 can exist as an oligomer but that its N terminus is not necessary for oligomerisation to occur.
Keywords: lysine methylation, methyltransferase, Saccharomyces cerevisiae
Abbreviations
- AdoMet
S‐adenosyl methionine
- eEF2
eukaryotic elongation factor 2
- Efm2
elongation factor methyltransferase 2
- MTF16
methyltransferase Family 16
Protein methylation is a widespread post‐translational modification in the eukaryotic cell. While the methylation of histones has been widely characterised, methylation of nonhistone proteins has gained increased attention due to its roles in cellular signalling and disease 1, 2, 3, 4, 5, 6, 7. In order to expand the knowledge of nonhistone methylation, there have been many recent proteome‐scale studies to discover protein methylation sites 8, 9, 10, 11, 12, 13. However, the function of the vast majority of these sites remains elusive. One critical step in understanding the function of protein methylation is to identify and characterise the methyltransferases that catalyse it.
Protein methyltransferases can be separated into two main groups based on their methyltransferase domain: SET domain methyltransferases and seven‐beta‐strand methyltransferases, also called Class I methyltransferases 6. Additionally, there is one known protein methyltransferase with the SPOUT fold 14. All SET domain methyltransferases are protein methyltransferases that are specific to lysine 15, while seven‐beta‐strand methyltransferases are more diverse in their substrates. Seven‐beta‐strand methyltransferases, which make up the majority of all methyltransferases, are known to methylate DNA, RNA, metabolites and other small molecules, as well as proteins 16. It is therefore difficult to predict the substrate specificity of seven‐beta‐strand methyltransferases. Nonetheless, there have been many attempts to predict the substrate specificity of yeast methyltransferases based on features beyond their core fold 17, 18.
Recently, a subclass of seven‐beta‐strand methyltransferases has been discovered which has, so far, proven to be protein‐specific: the Family 16 group of methyltransferases. There are 16 protein methyltransferases in Saccharomyces cerevisiae and human which belong to this family, 12 of which have described substrates (Table 1). All members appear to exclusively methylate nonhistone proteins. The S. cerevisiae members target translation‐associated proteins. Efm2 and Efm3 methylate eukaryotic translation elongation factor 2 (eEF2), Efm6 and Efm7 methylate eukaryotic translation elongation factor 1A (eEF1A), while Rkm5 and Hpm1 methylate ribosomal proteins RPL1A/B and RPL3 19, 20, 21, 22, 23, 24, 25, 26. The human members target more diverse substrates: CaM‐KMT methylates calmodulin, VCP‐KMT methylates the valosin‐containing protein (VCP), HSPA‐KMT methylates a number of 70 kDa heat shock proteins, METTL22 methylates KIN17, ETFB‐KMT methylates electron transfer flavoprotein subunit beta and eEF2‐KMT, the orthologue of Efm3, methylates eEF2 21, 27, 28, 29, 30, 31, 32, 33. Interestingly, no other substrates have been described for each of these methyltransferases, despite attempts to find more 21, 28, 30, 34. The Family 16 methyltransferases therefore have very restricted substrate specificity. This is probably due to the fact that they recognise three‐dimensional aspects of their substrates and not just sequence motifs 24, 28, 35. It is not yet known, however, what aspect of the Family 16 methyltransferases determines their specificity.
Table 1.
Family 16 methyltransferases in yeast and human
| Name | Organism | Substrate and site | Methylation degree | UniProt accession | Ref(s) |
|---|---|---|---|---|---|
| Efm2 | Yeast | eEF2‐K613 | Di | P38347 | 19, 20 |
| Efm3 | Yeast | eEF2‐K509 | Tri | P47163 | 20, 21, 22 |
| Efm6 | Yeast | eEF1A‐K390 | Mono | P53970 | 23 |
| Efm7 | Yeast | eEF1A‐G2/K3 | Tri/Di | Q05874 | 24 |
| Rkm5 | Yeast | RPL1A/B‐K47 | Mono | Q12367 | 25 |
| Hpm1 | Yeast | RPL3‐H243 | Mono | P40481 | 26 |
| VCP‐KMT | Human | VCP‐K315 | Tri | Q9H867 | 28, 29 |
| HSPA‐KMT | Human | Hsp70s: HSPA1‐K561, HSPA5‐K585, HSPA8‐K561 | Tri | Q8WXB1 | 29, 30 |
| METTL22 | Human | KIN17‐K135 | Tri | Q9BUU2 | 29, 31 |
| eEF2‐KMT | Human | eEF2‐K525 | Tri | Q96G04 | 21 |
| CaM‐KMT | Human | Calmodulin‐K116 | Tri | Q7Z624 | 27 |
| ETFB‐KMT | Human | ETFB‐K200/K203 | Tri/Tri | Q8IXQ9 | 32, 33 |
| METTL18 | Human | – | – | O95568 | – |
| METTL21B | Human | – | – | Q96AZ1 | – |
| METTL21C | Human | – | – | Q5VZV1 | – |
| METTL23 | Human | – | – | Q86XA0 | – |
All Family 16 methyltransferases contain a [D/E]XX[Y/F] motif. This is important for methyltransferase activity, as evidenced, for example, by loss of activity of VCP‐KMT when the aspartate is mutated 28. Besides this, however, there have been no functional studies into the sequence features of Family 16 methyltransferases. We previously noted the presence of two highly conserved tryptophans in Efm2 19. Here, we use mutagenesis and structural models of Efm2 to show that one of these residues is important for methyltransferase activity, while the other is of lesser consequence. We also show that an extended N‐terminal region of Efm2, of about 200 residues, is also critical for its methyltransferase activity. We suggest that it may be involved in binding its substrate eEF2, and that this highly variable region among Family 16 methyltransferases may be responsible for their substrate specificity.
Materials and methods
Bioinformatic analysis
All yeast and human Family 16 methyltransferases were aligned using Clustal Omega 36. This alignment was then used to generate a sequence logo using Web logo 37. The domain structures of Family 16 proteins were visualised using CDvist 38. Efm2 was modelled with Swiss‐Model 39 based on the structure of METTL21D (PDB ID: 4LG1) and disorder predicted by pondr‐fit 40. Structures of METTL21A‐D were acquired from the RCSB Protein Data Bank (www.rcsb.org) 41 with IDs of PDB: 4LEC, 4QPN, 4MTL and 4LG1.
Expression and purification of eEF2, Efm2 and mutant Efm2
N‐terminal truncation mutants of Efm2 were generated by site‐directed ligase‐independent mutagenesis (SLIM) 42. Tryptophan‐to‐phenylalanine mutations in Efm2 were generated by site‐directed mutagenesis 43. Efm2 and mutated Efm2 were overexpressed and purified from Escherichia coli (Rosetta DE3), while eEF2 was overexpressed and purified from a ΔEFM2 yeast strain, according to previous methods 20.
In vitro methylation
In vitro methylation reactions were performed and analysed by SDS/PAGE and immunoblotting according to previous methods 20. Briefly, eEF2 was incubated with Efm2 (wild‐type or mutant) in the presence of 50 μm S‐adenosyl methionine (AdoMet) in 1× in vitro methylation buffer (50 mm HEPES‐KOH, 20 mm NaCl, 1 mm EDTA, pH 7.4) at 30 °C for 1 h, unless otherwise indicated. The antibodies used for immunoblotting were the methylated lysine antibody ab7315 (1 : 1000 dilution; Abcam, Cambridge, UK) and anti‐PentaHis HRP‐conjugated antibody (1 : 5000 dilution; Qiagen, Hilden, Germany, 34460). Ab7315 does not recognise the K509 trimethylation site on eEF2 (see negative controls in Figs 3 and 4), which is catalysed by Efm3 20, 21, 22.
Mass spectrometry
Samples were analysed on an Orbitrap Velos Pro (Thermo Fisher Scientific, Waltham, MA, USA) according to previous methods 44. Extracted ion chromatograms (XICs) for peptides were obtained using thermo xcalibur qual browser 2.2 SP1.48 by setting mass windows of ±10 ppm of the relevant m/z value, and applying a scan filter to only analyse MS1 scans. Methyl‐peptide identities were confirmed by comparison with a synthetic equivalent (see Fig. S1 for representative spectra), as done previously 45.
In vitro crosslinking
Efm2 and N‐terminal truncation mutants, in 25 mm sodium phosphate buffer, 100 mm NaCl, 20% (v/v) glycerol, 5 mm β‐mercaptoethanol, 0.2 m triethanolamine, were crosslinked with 330 μm dimethyl pimelimidate (Thermo Fisher Scientific) for 2 h at room temperature. For the 60‐min time course assay of Efm2 crosslinking, 410 μm dimethyl pimelimidate was added instead. SDS was added to a final concentration of 1% prior to addition of the crosslinker for a negative control. SDS sample buffer was added to quench the crosslinking reaction and samples were analysed by SDS/PAGE and immunoblotting as described above.
Results
Family 16 methyltransferases show conservation of two key tryptophans and variable N‐terminal regions
In order to better understand the potential significance of tryptophans W222 and W318 in Efm2, their conservation was investigated by aligning all yeast and human Family 16 methyltransferases and generating a sequence logo from this alignment (Fig. 1A, see Fig. S2 for entire sequence logo). W222 showed 100% conservation, while W318 was absent only in ETFB‐KMT (Fig. 1A). We then investigated the structural contexts of both these tryptophans in a homology‐predicted model of Efm2. Strikingly, both tryptophans are positioned at the location of methyl‐donor AdoMet binding (Fig. 1B). W222 is positioned near the active site, as has been noted previously 22, while W318 is positioned adjacent to the imidazole ring of AdoMet, and may therefore stabilise the binding of AdoMet via π‐stacking interactions between its indole group and the adenine group of AdoMet, as has been suggested to occur for Efm6 23. These relative positions of the tryptophans are also observable in the structures of METTL21A‐D and CaM‐KMT (Fig. S3). Overall, this strongly suggests an important role for these tryptophans in the activity of Efm2 and in Family 16 methyltransferases.
Figure 1.
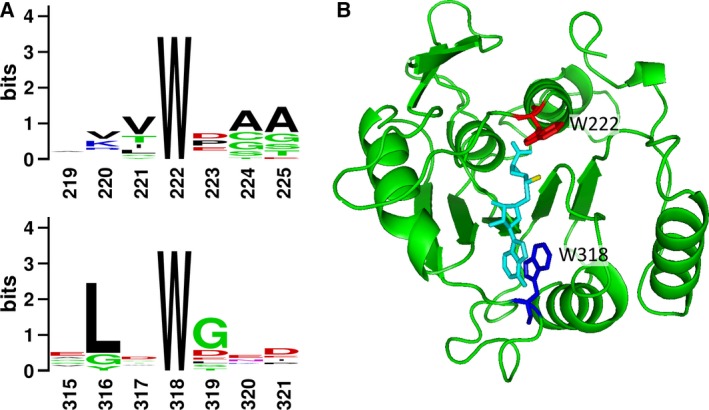
Two highly conserved tryptophans are situated near the location of AdoMet binding in Family 16 methyltransferases. (A) Sequence logo for all Family 16 methyltransferases from yeast and human, showing the relative conservation of W222 (top) and W318 (bottom) numbered relative to Efm2. The y‐axis shows the total conservation of each position (total height of each stack) and the relative conservation of each amino acid (relative heights of each letter). Maximum conservation is the maximum entropy for amino acid sequences (log2 of 20 amino acids = 4.3). W222 showed 100% conservation, while W318 was only absent in ETFB‐KMT. The full sequence logo is shown in Fig. S2. (B) The predicted structure of residues 192–406 Efm2 by homology modelling based upon the structure of VCP‐KMT (PDB ID: 4LG1). Efm2 is shown as a ribbon structure in green, with tryptophans 222 and 318 shown as stick structures in red and blue respectively; AdoMet is shown as a stick structure in cyan, with the donated methyl group shown in yellow. Visualised in pymol (The PyMOL Molecular Graphics System, Version 1.3 Schrödinger, LLC, New York, NY, USA).
To investigate the prevalence of N‐terminal extensions beyond the core Family 16 methyltransferase domain, we visualised the domain architecture of all yeast and human Family 16 methyltransferases using CDvist (Fig. 2A). This showed that Efm2 has the longest N‐terminal extension. Interestingly, some of these methyltransferases, notably Efm6, Efm7, VCP‐KMT and HSPA‐KMT, have very short N‐terminal extensions, in sharp contrast with Efm2. The N‐terminal ~ 50 residues of Efm2 are predicted to be disordered, with the rest of the protein being predominantly ordered, as typified by the prediction made by Pondr‐fit (Fig. 2B).
Figure 2.
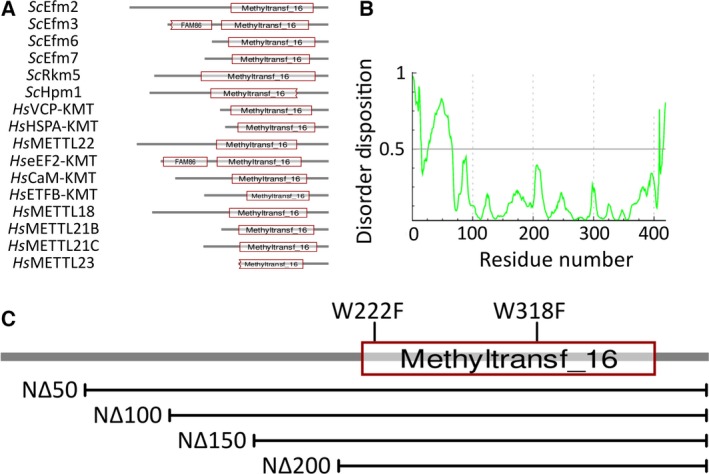
Efm2 has a long, partially disordered N‐terminal region separate from the core methyltransferase domain. (A) The domain architectures of all known Family 16 methyltransferases from yeast and human. A number of them have extended N‐terminal regions; Efm2 has the longest of all. Methyltransferase lengths are to scale; Efm2 is 419 residues long. Jagged edges of domains represent partial truncations. (B) The predicted disorder of Efm2 as determined by pondr‐fit. Disorder disposition > 0.5 represents disorder. (C) Mutagenesis of Efm2. Four N‐terminal truncation mutants and two point mutants were generated in order to characterise the N‐terminal region and the conserved tryptophans in Efm2. Note that all four N‐terminal truncations retain the core Family 16 methyltransferase domain.
We therefore sought to investigate the importance of the two conserved tryptophans and N‐terminal region in Efm2. We generated conservative tryptophan‐to‐phenylalanine point mutations for W222 and W318 and four N‐terminal truncations, as depicted in Fig. 2C, and investigated their methyltransferase activities compared to the wild‐type enzyme.
Conservative point mutations indicate the importance of two key tryptophan residues
Given that the tryptophans are likely to be critical for methyltransferase activity, we mutated them to the similarly hydrophobic and aromatic residue, phenylalanine. Immunoblotting of a time‐series assay of methyltransferase activity on eEF2, compared to wild‐type Efm2, showed a severe reduction in methyltransferase activity with the W222F mutant, while the W318F showed only a slight reduction in activity (Fig. 3A). LC‐MS/MS analysis of eEF2 from the 10‐min time‐point showed that wild‐type Efm2 produced predominantly dimethylation of K613 in the peptide DDFKAR (Fig. 3B). The W318F mutant produced slightly less dimethylation than the wild‐type, and had slightly more unmethylated eEF2 remaining (Fig. 3B). The W222F mutant, however, produced predominantly monomethylation (Fig. 3B). This could reflect a decreased rate of activity or a change in the degree of methylation the enzyme is capable of catalysing. The pronounced effect of the W222F mutation may be due to the proximity of W222 to the active site of Efm2, as mentioned above. The fact that the W318F mutant had an activity comparable to that of the wild‐type enzyme indicates either that W318 is not critical for binding AdoMet, or that the phenylalanine is sufficiently similar to the structure of tryptophan to bind AdoMet effectively.
Figure 3.
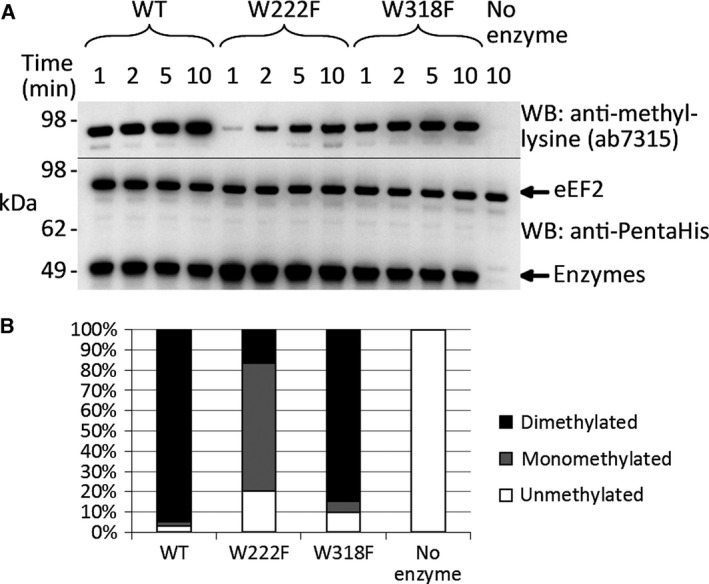
Tryptophan point mutants of Efm2 show reduced methyltransferase activity. eEF2 was incubated with wild‐type (WT), W222F or W318F Efm2 in the presence of AdoMet for 1, 2, 5 or 10 min, before reactions were resolved by SDS/PAGE and analysed by immunoblotting (A) or LC‐MS/MS (B). No enzyme was added for a negative control. (A) Immunoblotting with the anti‐methyl‐lysine antibody ab7315 (top) revealed markedly reduced methyltransferase activity of the W222F mutant, while the W318F mutant showed slightly reduced activity. An immunoblot with the anti‐PentaHis antibody was used as a loading control (bottom). (B) LC‐MS/MS of eEF2 from the 10‐min assay revealed that the wild‐type enzyme was able to produce almost complete dimethylation of K613 in the peptide DDFKAR, while the W222F mutant produced predominantly monomethylation and the W318F mutant produced nearly the same level of dimethylation as the wild‐type enzyme. The no enzyme control did not show any gain of methylation. The relative amount of each methylation state was determined by the area under the curve for each peak in the extracted ion chromatograms (XICs), which are shown in Fig. S4. W222 therefore appears to be important for methyltransferase activity, while W318 is not as important.
Efm2 N‐terminal truncations reveal the importance of the N terminus for methyltransferase activity
We investigated the unique extended N‐terminal region of Efm2 by generating four incremental 50 amino acid N‐terminal truncations (Fig. 2C) and testing their methyltransferase activity on eEF2 over 1 h. Surprisingly, immunoblotting suggested that the NΔ100, NΔ150 and NΔ200 truncations all showed no methyltransferase activity, while the NΔ50 truncation showed reduced activity compared to wild‐type Efm2 (Fig. 4A). LC‐MS/MS of eEF2 (Fig. 4B) confirmed the lack of activity observed for NΔ100, NΔ150 and NΔ200 and showed that NΔ50 produced predominantly monomethylation at K613 in the peptide DDFKAR, and markedly less dimethylation than wild‐type Efm2 produces in 10 min (see above). Together, these results indicate that the N‐terminal region of Efm2, particularly residues 50–200, is important for its methyltransferase activity.
Figure 4.
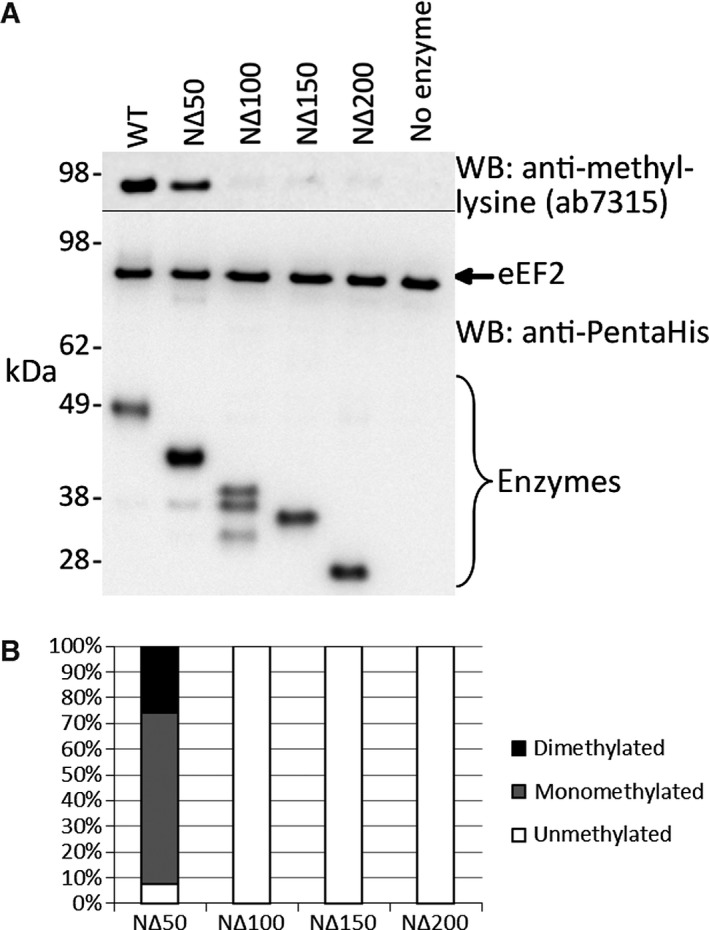
N‐terminal truncations of Efm2 show severely reduced methyltransferase activity. eEF2 was incubated with wild‐type (WT) or N‐terminally truncated Efm2 (NΔ50, NΔ100, NΔ150 and NΔ200) in the presence of AdoMet for 1 h, before reactions were resolved by SDS/PAGE and analysed by immunoblotting (A) or LC‐MS/MS (B). No enzyme was added for a negative control. (A) Immunoblotting with the anti‐methyl‐lysine antibody ab7315 (top) revealed markedly reduced methyltransferase activity of the NΔ50 mutant, while the NΔ100, NΔ150 and NΔ200 mutants showed no activity. An immunoblot with the anti‐PentaHis antibody was used as a loading control (bottom). (B) LC‐MS/MS of eEF2 revealed that the NΔ50 mutant produced predominantly monomethylation of K613 in the peptide DDFKAR, while the NΔ100, NΔ150 and NΔ200 mutants produced no methylation, in agreement with the immunoblot. The relative amount of each methylation state was determined by the area under the curve for each peak in the extracted ion chromatograms (XICs), which are shown in Fig. S5. The N terminus of Efm2, from residue 50 onwards, is therefore critical for its methyltransferase activity.
One possible explanation for this loss of methyltransferase activity is that the N‐terminal region is required for Efm2 to self‐interact and form oligomers necessary for its activity, as is the case for some other protein methyltransferases 46, 47. In order to investigate this possibility, we first tested whether wild‐type Efm2 is capable of forming oligomers. In vitro chemical crosslinking followed by SDS/PAGE revealed that wild‐type Efm2 forms a series of oligomeric states, as bands corresponding to the dimer (~ 96 kDa), trimer (~ 144 kDa) and tetramer (~ 192 kDa) were seen to gradually form over an hour of crosslinking (Fig. 5A). A negative control with 1% SDS added prior to crosslinking showed minimal formation of oligomeric bands, indicating that the crosslinking is due to the self‐interaction of Efm2, which is disturbed upon denaturation by SDS. We then tested the ability of the four N‐terminal truncations to form oligomers after 2 h of crosslinking, compared to wild‐type Efm2 and SDS controls. This showed that all four N‐terminal truncations were able to form the oligomeric states observed for wild‐type Efm2 (Fig. 5B). This indicates that the N‐terminal 200 residues of Efm2 are not critical for oligomerisation, and suggests that the effect on methyltransferase activity observed upon N‐terminal truncation may be due to a loss of interaction with its substrate eEF2.
Figure 5.
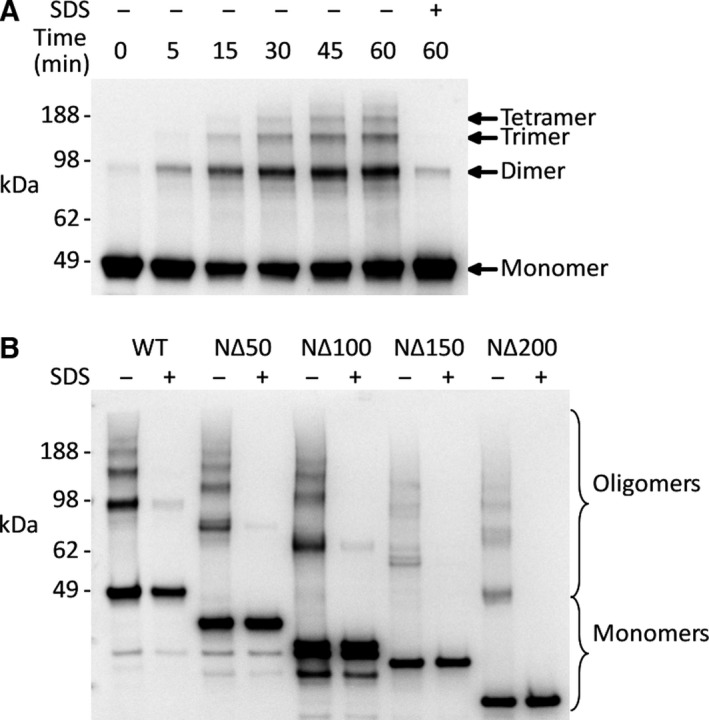
Efm2 and its N‐terminal truncation mutants form oligomeric states. Wild‐type (WT) Efm2 and its N‐terminal truncation mutants were incubated with or without SDS in the presence of the crosslinker dimethyl pimelimidate. An anti‐PentaHis immunoblot was used to detect all relevant species. (A) Time‐series crosslinking analysis of wild‐type Efm2 indicates that it forms oligomeric states. (B) Crosslinking of Efm2 N‐terminal truncation mutants for 2 h. SDS (1%) added prior to crosslinking was used as a negative control for all N‐terminal truncations.
Discussion
Elongation factor methyltransferase 2 is one of the Family 16 group of methyltransferases, a group of newly discovered eukaryotic enzymes that all methylate nonhistone proteins. Here we have investigated Efm2 by site‐directed mutagenesis and truncation in order to better understand Family 16 methyltransferases. We have shown that one completely conserved tryptophan (W222 in Efm2) is important for methyltransferase activity, while the other, which is present in all but one Family 16 methyltransferase, is of lesser consequence. This disparity may be explained by the fact that W222 is positioned at the active site, while W318 is not. The shift to monomethylation of K613 in eEF2, seen with the W222F mutation of Efm2, could indicate that W222 determines the degree of methylation by affecting the size of the active site and thus how many methyl groups can be added to a substrate. The SET domain methyltransferases use a similar mechanism to control the degree of substrate methylation 48. It could alternatively be that the W222F mutation reduced the rate of activity of Efm2, and thus the monomethylation was simply an intermediate in the formation of dimethylation. This would point to a distributive mechanism of action, as Efm2 would dissociate from eEF2 after the formation of monomethylation.
We have also shown that the N‐terminal 50–200 residues of Efm2 are essential for its methyltransferase activity. Interestingly, this correlates with the predicted structural order of this region of Efm2 from residue ~ 80 onwards. This suggests that the N‐terminal region of Efm2 may form tertiary structures that are critical for its specific recognition of eEF2; it also raises a broader possibility that the N‐terminal regions of Family 16 methyltransferases are critical for their specificity. This is best exemplified by the human METTL21 proteins (METTL21A‐D), which form a subgroup within Family 16 methyltransferases due to their similarity (Fig. 6A). METTL21A (HSPA‐KMT) and METTL21D (VCP‐KMT) methylate different proteins, while METTL21B and METTL21C have no known substrates. Alignment of the crystal structures of all four of these proteins demonstrates the remarkable similarity of their core methyltransferase domains, while revealing highly variable N‐terminal regions (Fig. 6B). It is therefore likely that these N‐terminal regions of the METTL21 proteins, and Family 16 methyltransferases in general, are important for determining their substrate protein specificity.
Figure 6.
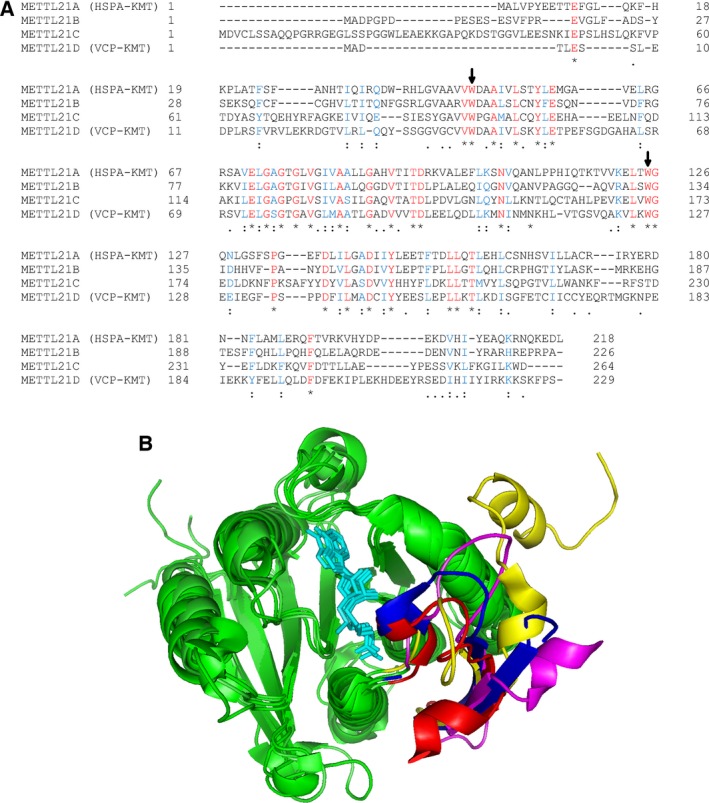
The human METTL21 proteins exemplify the variability of N‐terminal regions of Family 16 methyltransferases. (A) Alignment of human METTL21A (HSPA‐KMT), METTL21B, METTL21C and METTL21D (VCP‐KMT) using Clustal Omega with default settings 36. Asterisks (*) and red colour indicate identical residues; double dots (:) and blue colour indicate chemically similar residues; single dots (.) indicate dissimilar residues; dashes (–) indicate missing residues. The two conserved tryptophans are indicated with arrows. (B) Alignment of the structures of the METTL21s (PDB IDs: 4LEC, 4QPN, 4MTL, 4LG1), showing their highly similar core folds (green, AdoMet in cyan) and their highly dissimilar N‐terminal regions (residues 15–46 of METTL21A: red; residues 24–56 of METTL21B: blue; residues 45–91 of METTL21C: yellow; residues 11–42 of METTL21D: magenta). Visualised in pymol (The PyMOL Molecular Graphics System, Version 1.3 Schrödinger, LLC).
Efm2 was shown to form a number of oligomeric states. Several protein methyltransferases have been previously shown to form oligomers, including the arginine methyltransferases Hmt1 46, CARM1 49, PRMT1 47, PRMT3 50, 51, PRMT5 52, PRMT8 53 and some lysine methyltransferases such as G9a, GLP 54 and SU(VAR)3‐9 55. The oligomerisation of Efm2 may point to a processive mechanism of action, as a dimer of Efm2 could achieve dimethylation without detachment from eEF2, as is the case for many arginine methyltransferases 56. This is the first demonstration that a Family 16 methyltransferase forms oligomers, and it would be interesting to investigate whether other Family 16 methyltransferases also oligomerise.
While Family 16 methyltransferases have only been discovered recently, their biological and medical significance is already becoming apparent. Efm2 and Efm3 both act on eEF2 in yeast, and deletion of either methyltransferase has been shown to increase sensitivity to translational inhibitors 21, 22. Both were also shown to have potential effects on translational fidelity 21, 22. In human, VCP methylation by VCP‐KMT appears to affect its ATPase activity and the methyltransferase was shown to be important for cell migration and invasion, suggesting a role in cancer metastasis 28, 29. METTL22‐mediated methylation of Kin17 appears to regulate its association with chromatin 31, while CaM‐KMT appears to be important for normal body growth and somatosensory development 57. HSPA‐KMT‐mediated methylation of HSPA8 was shown to reduce its affinity for α‐Syn, a protein whose aggregation is associated with Parkinson's disease 30. HSPA1 methylation, also catalysed by HSPA‐KMT, was recently found to correlate with cancer outcome 58. Even some Family 16 methyltransferases without known substrates have been associated with disease. METTL21B has been linked to multiple sclerosis 59, 60, 61, mutation of METTL23 has been linked to intellectual disability 62, 63 and METTL21C has been found to be important in muscle cell differentiation and bone cell viability, and thereby is associated with osteoporosis and sarcopenia 64. It will therefore be of great interest to discover the targets of these methyltransferases, and to further characterise these and all other Family 16 methyltransferases, in order to better understand their biological functions and roles in disease.
Author contributions
JJH and MRW conceived of the study. JJH, MAE and GHS performed experiments. JJH and MRW drafted the manuscript, with input from all other authors.
Supporting information
Fig. S1. Example spectra showing mono‐ and dimethylation of K613 in peptide DDFKAR from eEF2.
Fig. S2. Full sequence logo of human and yeast Family 16 methyltransferases.
Fig. S3. Structures of Family 16 methyltransferases show identical placement of conserved tryptophans.
Fig. S4. Extracted ion chromatograms for Fig. 3B.
Fig. S5. Extracted ion chromatograms for Fig. 4B.
Acknowledgements
Mass spectrometric results were obtained at the Bioanalytical Mass Spectrometry Facility within the Analytical Centre of the University of New South Wales.
References
- 1. Biggar KK and Li SS (2015) Non‐histone protein methylation as a regulator of cellular signalling and function. Nat Rev Mol Cell Biol 16, 5–17. [DOI] [PubMed] [Google Scholar]
- 2. Zhang X, Huang Y and Shi X (2015) Emerging roles of lysine methylation on non‐histone proteins. Cell Mol Life Sci 72, 4257–4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hamamoto R, Saloura V and Nakamura Y (2015) Critical roles of non‐histone protein lysine methylation in human tumorigenesis. Nat Rev Cancer 15, 110–124. [DOI] [PubMed] [Google Scholar]
- 4. Wei H, Mundade R, Lange KC and Lu T (2014) Protein arginine methylation of non‐histone proteins and its role in diseases. Cell Cycle 13, 32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moore KE and Gozani O (2014) An unexpected journey: lysine methylation across the proteome. Biochim Biophys Acta 1839, 1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clarke SG (2013) Protein methylation at the surface and buried deep: thinking outside the histone box. Trends Biochem Sci 38, 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang J and Berger SL (2008) The emerging field of dynamic lysine methylation of non‐histone proteins. Curr Opin Genet Dev 18, 152–158. [DOI] [PubMed] [Google Scholar]
- 8. Bremang M, Cuomo A, Agresta AM, Stugiewicz M, Spadotto V and Bonaldi T (2013) Mass spectrometry‐based identification and characterisation of lysine and arginine methylation in the human proteome. Mol BioSyst 9, 2231–2247. [DOI] [PubMed] [Google Scholar]
- 9. Guo A, Gu H, Zhou J, Mulhern D, Wang Y, Lee KA, Yang V, Aguiar M, Kornhauser J, Jia X et al (2014) Immunoaffinity enrichment and mass spectrometry analysis of protein methylation. Mol Cell Proteomics 13, 372–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu H, Galka M, Mori E, Liu X, Lin YF, Wei R, Pittock P, Voss C, Dhami G, Li X et al (2013) A method for systematic mapping of protein lysine methylation identifies functions for HP1beta in DNA damage response. Mol Cell 50, 723–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cao XJ, Arnaudo AM and Garcia BA (2013) Large‐scale global identification of protein lysine methylation in vivo . Epigenetics 8, 477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moore KE, Carlson SM, Camp ND, Cheung P, James RG, Chua KF, Wolf‐Yadlin A and Gozani O (2013) A general molecular affinity strategy for global detection and proteomic analysis of lysine methylation. Mol Cell 50, 444–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu Z, Cheng Z, Sun M, Wan X, Liu P, He T, Tan M and Zhao Y (2015) A chemical proteomics approach for global analysis of lysine monomethylome profiling. Mol Cell Proteomics 14, 329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Young BD, Weiss DI, Zurita‐Lopez CI, Webb KJ, Clarke SG and McBride AE (2012) Identification of methylated proteins in the yeast small ribosomal subunit: a role for SPOUT methyltransferases in protein arginine methylation. Biochemistry 51, 5091–5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dillon SC, Zhang X, Trievel RC and Cheng X (2005) The SET‐domain protein superfamily: protein lysine methyltransferases. Genome Biol 6, 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Martin JL and McMillan FM (2002) SAM (dependent) I AM: the S‐adenosylmethionine‐dependent methyltransferase fold. Curr Opin Struct Biol 12, 783–793. [DOI] [PubMed] [Google Scholar]
- 17. Wlodarski T, Kutner J, Towpik J, Knizewski L, Rychlewski L, Kudlicki A, Rowicka M, Dziembowski A and Ginalski K (2011) Comprehensive structural and substrate specificity classification of the Saccharomyces cerevisiae methyltransferome. PLoS One 6, e23168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Szczepinska T, Kutner J, Kopczynski M, Pawlowski K, Dziembowski A, Kudlicki A, Ginalski K and Rowicka M (2014) Probabilistic approach to predicting substrate specificity of methyltransferases. PLoS Comput Biol 10, e1003514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Couttas TA, Raftery MJ, Padula MP, Herbert BR and Wilkins MR (2012) Methylation of translation‐associated proteins in Saccharomyces cerevisiae: Identification of methylated lysines and their methyltransferases. Proteomics 12, 960–972. [DOI] [PubMed] [Google Scholar]
- 20. Zhang L, Hamey JJ, Hart‐Smith G, Erce MA and Wilkins MR (2014) Elongation factor methyltransferase 3–a novel eukaryotic lysine methyltransferase. Biochem Biophys Res Commun 451, 229–234. [DOI] [PubMed] [Google Scholar]
- 21. Davydova E, Ho AY, Malecki J, Moen A, Enserink JM, Jakobsson ME, Loenarz C and Falnes PO (2014) Identification and characterization of a novel evolutionarily conserved lysine‐specific methyltransferase targeting eukaryotic translation elongation factor 2 (eEF2). J Biol Chem 289, 30499–30510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dzialo MC, Travaglini KJ, Shen S, Roy K, Chanfreau GF, Loo JA and Clarke SG (2014) Translational roles of elongation factor 2 protein lysine methylation. J Biol Chem 289, 30511–30524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jakobsson ME, Davydova E, Malecki J, Moen A and Falnes PO (2015) Saccharomyces cerevisiae eukaryotic elongation factor 1A (eEF1A) is methylated at Lys‐390 by a METTL21‐like methyltransferase. PLoS One 10, e0131426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hamey JJ, Winter DL, Yagoub D, Overall CM, Hart‐Smith G and Wilkins MR (2016) Novel N‐terminal and lysine methyltransferases that target translation elongation factor 1A in yeast and human. Mol Cell Proteomics 15, 164–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Webb KJ, Al‐Hadid Q, Zurita‐Lopez CI, Young BD, Lipson RS and Clarke SG (2011) The ribosomal l1 protuberance in yeast is methylated on a lysine residue catalyzed by a seven‐beta‐strand methyltransferase. J Biol Chem 286, 18405–18413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Webb KJ, Zurita‐Lopez CI, Al‐Hadid Q, Laganowsky A, Young BD, Lipson RS, Souda P, Faull KF, Whitelegge JP and Clarke SG (2010) A novel 3‐methylhistidine modification of yeast ribosomal protein Rpl3 is dependent upon the YIL110W methyltransferase. J Biol Chem 285, 37598–37606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Magnani R, Dirk LM, Trievel RC and Houtz RL (2010) Calmodulin methyltransferase is an evolutionarily conserved enzyme that trimethylates Lys‐115 in calmodulin. Nat Commun 1, 43. [DOI] [PubMed] [Google Scholar]
- 28. Kernstock S, Davydova E, Jakobsson M, Moen A, Pettersen S, Maelandsmo GM, Egge‐Jacobsen W and Falnes PO (2012) Lysine methylation of VCP by a member of a novel human protein methyltransferase family. Nat Commun 3, 1038. [DOI] [PubMed] [Google Scholar]
- 29. Cloutier P, Lavallee‐Adam M, Faubert D, Blanchette M and Coulombe B (2013) A newly uncovered group of distantly related lysine methyltransferases preferentially interact with molecular chaperones to regulate their activity. PLoS Genet 9, e1003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jakobsson ME, Moen A, Bousset L, Egge‐Jacobsen W, Kernstock S, Melki R and Falnes PO (2013) Identification and characterization of a novel human methyltransferase modulating Hsp70 protein function through lysine methylation. J Biol Chem 288, 27752–27763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cloutier P, Lavallee‐Adam M, Faubert D, Blanchette M and Coulombe B (2014) Methylation of the DNA/RNA‐binding protein Kin17 by METTL22 affects its association with chromatin. J Proteomics 100, 115–124. [DOI] [PubMed] [Google Scholar]
- 32. Rhein VF, Carroll J, He J, Ding S, Fearnley IM and Walker JE (2014) Human METTL20 methylates lysine residues adjacent to the recognition loop of the electron transfer flavoprotein in mitochondria. J Biol Chem 289, 24640–24651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Malecki J, Ho AY, Moen A, Dahl HA and Falnes PO (2015) Human METTL20 is a mitochondrial lysine methyltransferase that targets the beta subunit of electron transfer flavoprotein (ETFbeta) and modulates its activity. J Biol Chem 290, 423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fusser M, Kernstock S, Aileni VK, Egge‐Jacobsen W, Falnes PO and Klungland A (2015) Lysine methylation of the valosin‐containing protein (VCP) is dispensable for development and survival of mice. PLoS One 10, e0141472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cobb JA and Roberts DM (2000) Structural requirements for N‐trimethylation of lysine 115 of calmodulin. J Biol Chem 275, 18969–18975. [DOI] [PubMed] [Google Scholar]
- 36. Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Soding J et al (2011) Fast, scalable generation of high‐quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7, 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Crooks GE, Hon G, Chandonia JM and Brenner SE (2004) WebLogo: a sequence logo generator. Genome Res 14, 1188–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Adebali O, Ortega DR and Zhulin IB (2015) CDvist: a webserver for identification and visualization of conserved domains in protein sequences. Bioinformatics 31, 1475–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Arnold K, Bordoli L, Kopp J and Schwede T (2006) The SWISS‐MODEL workspace: a web‐based environment for protein structure homology modelling. Bioinformatics 22, 195–201. [DOI] [PubMed] [Google Scholar]
- 40. Xue B, Dunbrack RL, Williams RW, Dunker AK and Uversky VN (2010) PONDR‐FIT: a meta‐predictor of intrinsically disordered amino acids. Biochim Biophys Acta 1804, 996–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN and Bourne PE (2000) The Protein Data Bank. Nucleic Acids Res 28, 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chiu J, Tillett D, Dawes IW and March PE (2008) Site‐directed, Ligase‐Independent Mutagenesis (SLIM) for highly efficient mutagenesis of plasmids greater than 8 kb. J Microbiol Methods 73, 195–198. [DOI] [PubMed] [Google Scholar]
- 43. Edelheit O, Hanukoglu A and Hanukoglu I (2009) Simple and efficient site‐directed mutagenesis using two single‐primer reactions in parallel to generate mutants for protein structure‐function studies. BMC Biotechnol 9, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hart‐Smith G, Low JK, Erce MA and Wilkins MR (2012) Enhanced methylarginine characterization by post‐translational modification‐specific targeted data acquisition and electron‐transfer dissociation mass spectrometry. J Am Soc Mass Spectrom 23, 1376–1389. [DOI] [PubMed] [Google Scholar]
- 45. Hart‐Smith G, Chia SZ, Low JK, McKay MJ, Molloy MP and Wilkins MR (2014) Stoichiometry of Saccharomyces cerevisiae lysine methylation: insights into non‐histone protein lysine methyltransferase activity. J Proteome Res 13, 1744–1756. [DOI] [PubMed] [Google Scholar]
- 46. Weiss VH, McBride AE, Soriano MA, Filman DJ, Silver PA and Hogle JM (2000) The structure and oligomerization of the yeast arginine methyltransferase, Hmt1. Nat Struct Biol 7, 1165–1171. [DOI] [PubMed] [Google Scholar]
- 47. Zhang X and Cheng X (2003) Structure of the predominant protein arginine methyltransferase PRMT1 and analysis of its binding to substrate peptides. Structure 11, 509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang X, Yang Z, Khan SI, Horton JR, Tamaru H, Selker EU and Cheng X (2003) Structural basis for the product specificity of histone lysine methyltransferases. Mol Cell 12, 177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Teyssier C, Chen D and Stallcup MR (2002) Requirement for multiple domains of the protein arginine methyltransferase CARM1 in its transcriptional coactivator function. J Biol Chem 277, 46066–46072. [DOI] [PubMed] [Google Scholar]
- 50. Tang J, Gary JD, Clarke S and Herschman HR (1998) PRMT 3, a type I protein arginine N‐methyltransferase that differs from PRMT1 in its oligomerization, subcellular localization, substrate specificity, and regulation. J Biol Chem 273, 16935–16945. [DOI] [PubMed] [Google Scholar]
- 51. Zhang X, Zhou L and Cheng X (2000) Crystal structure of the conserved core of protein arginine methyltransferase PRMT3. EMBO J 19, 3509–3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rho J, Choi S, Seong YR, Cho WK, Kim SH and Im DS (2001) Prmt5, which forms distinct homo‐oligomers, is a member of the protein‐arginine methyltransferase family. J Biol Chem 276, 11393–11401. [DOI] [PubMed] [Google Scholar]
- 53. Lee J, Sayegh J, Daniel J, Clarke S and Bedford MT (2005) PRMT8, a new membrane‐bound tissue‐specific member of the protein arginine methyltransferase family. J Biol Chem 280, 32890–32896. [DOI] [PubMed] [Google Scholar]
- 54. Tachibana M, Ueda J, Fukuda M, Takeda N, Ohta T, Iwanari H, Sakihama T, Kodama T, Hamakubo T and Shinkai Y (2005) Histone methyltransferases G9a and GLP form heteromeric complexes and are both crucial for methylation of euchromatin at H3‐K9. Genes Dev 19, 815–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Eskeland R, Czermin B, Boeke J, Bonaldi T, Regula JT and Imhof A (2004) The N‐terminus of Drosophila SU(VAR)3‐9 mediates dimerization and regulates its methyltransferase activity. Biochemistry 43, 3740–3749. [DOI] [PubMed] [Google Scholar]
- 56. Cheng X, Collins RE and Zhang X (2005) Structural and sequence motifs of protein (histone) methylation enzymes. Annu Rev Biophys Biomol Struct 34, 267–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Haziza S, Magnani R, Lan D, Keinan O, Saada A, Hershkovitz E, Yanay N, Cohen Y, Nevo Y, Houtz RL et al (2015) Calmodulin methyltransferase is required for growth, muscle strength, somatosensory development and brain function. PLoS Genet 11, e1005388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jakobsson ME, Moen A, Davidson B and Falnes PO (2015) Hsp70 (HSPA1) lysine methylation status as a potential prognostic factor in metastatic high‐grade serous carcinoma. PLoS One 10, e0140168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gandhi KS, McKay FC, Cox M, Riveros C, Armstrong N, Heard RN, Vucic S, Williams DW, Stankovich J, Brown M et al (2010) The multiple sclerosis whole blood mRNA transcriptome and genetic associations indicate dysregulation of specific T cell pathways in pathogenesis. Hum Mol Genet 19, 2134–2143. [DOI] [PubMed] [Google Scholar]
- 60. Alcina A, Fedetz M, Fernandez O, Saiz A, Izquierdo G, Lucas M, Leyva L, Garcia‐Leon JA, Abad‐Grau Mdel M, Alloza I et al (2013) Identification of a functional variant in the KIF5A‐CYP27B1‐METTL1‐FAM119B locus associated with multiple sclerosis. J Med Genet 50, 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shahijanian F, Parnell GP, McKay FC, Gatt PN, Shojoei M, O'Connor KS, Schibeci SD, Brilot F, Liddle C, Batten M et al (2014) The CYP27B1 variant associated with an increased risk of autoimmune disease is underexpressed in tolerizing dendritic cells. Hum Mol Genet 23, 1425–1434. [DOI] [PubMed] [Google Scholar]
- 62. Reiff RE, Ali BR, Baron B, Yu TW, Ben‐Salem S, Coulter ME, Schubert CR, Hill RS, Akawi NA, Al‐Younes B et al (2014) METTL23, a transcriptional partner of GABPA, is essential for human cognition. Hum Mol Genet 23, 3456–3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bernkopf M, Webersinke G, Tongsook C, Koyani CN, Rafiq MA, Ayaz M, Muller D, Enzinger C, Aslam M, Naeem F et al (2014) Disruption of the methyltransferase‐like 23 gene METTL23 causes mild autosomal recessive intellectual disability. Hum Mol Genet 23, 4015–4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Huang J, Hsu YH, Mo C, Abreu E, Kiel DP, Bonewald LF, Brotto M and Karasik D (2014) METTL21C is a potential pleiotropic gene for osteoporosis and sarcopenia acting through the modulation of the NF‐kappaB signaling pathway. J Bone Miner Res 29, 1531–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Example spectra showing mono‐ and dimethylation of K613 in peptide DDFKAR from eEF2.
Fig. S2. Full sequence logo of human and yeast Family 16 methyltransferases.
Fig. S3. Structures of Family 16 methyltransferases show identical placement of conserved tryptophans.
Fig. S4. Extracted ion chromatograms for Fig. 3B.
Fig. S5. Extracted ion chromatograms for Fig. 4B.


